Abstract
We are aimed to systematically assess the worldwide trend in incidence of childhood type 1 diabetes mellitus (CT1DM) from 1965 to 2012 and to discuss whether climate affect incidence of CT1DM. We searched the relevant literatures in detail to judge the effect of different climates on incidence of CT1DM. The climates included Mediterranean, monsoon, oceanic, continental, savanna, and rainforest. According to different climates, we further researched relevant factor such as sunshine durations and latitudes. The overall incidence of CT1DM in 72 countries was 11.43 (95% CI 10.31–12.55) per 100,000 children/yr. The incidence of CT1DM in Oceanic climate [10.56 (8.69–12.42)] is highest compared with other climates; the incidence in 40°–66°34′N/S [14.71 (12.30–17.29)] is higher than other latitude groups; the incidence in sunshine durations with 3–4 hours per day [15.17 (11.14–19.20)] is highest compared with other two groups; the incidence of CT1DM from 2000 to 2012 [19.58 (14.55–24.60)] is higher than other periods; all p < 0.01. Incidence of CT1DM was increasing from 1965 to 2012, but incidence in Oceanic climate is higher than other climates. Furthermore, it is higher in centers with higher latitude and lower sunshine durations. The climates might play a key role in inducing CT1DM.
Introduction
The worldwide variation in the incidence of type 1 diabetes mellitus (TIDM) among children has been confirmed to be increased over the past 50 years1–3, especially among children of 10–14 years of age4. Childhood type 1 diabetes mellitus (CTIDM) is a syndrome caused by β-cell destruction that results in progressive or acute insulin deficiency5,6.
While we know that children with diabetes aged less than 7 years are at high risk of cognitive dysfunction, and poor glycaemic control might induce hypoglycaemia that could influence the developing nervous system7,8. Furthermore, immunosuppressive drugs for CT1DM treatment have kidney toxicity and other side effects9.
No clear evidence of a correlation between the CT1DM and climates had emerged from human or animal studies. Previous studies indicated the milk consumption10,11, dietary habit12,13, socioeconomic14, latitude15, familial predisposition16, drinking water17 or radiation18 might be important factor for CT1DM.
It is vital therefore to conduct this study to confirm the various climates in relation to the incidence of CT1DM.
Methods
Data collection
This study is supported by the Guilin Medical University Ethnic Committee Board. Articles published between Jan 1, 1965 and Jan 31, 2017 that were systematically searched in the databases: the PubMed, the Chinese National Knowledge Infrastructure (CNKI), Library of Congress, and Web of Science. All potentially relevant articles in reference lists of included articles were screened as full-text. For missing information or ambiguous, the corresponding author of this study was contacted with authors of relevant articles by email. For duplicated duplications, we only included the latest articles in our analysis. More than 3,600 publications reporting the incidence of CT1DM were identified.
Eligibility Criteria
Relevant studies for incidence of CT1DM in various countries were included in final analysis if the following strict criteria were met: (1) patients younger than 19 years old diagnosed with T1DM; (2) the number of cases was three or more; (3) the study period was more than a year; (4) T1DM was diagnosed according to World Health Organization definition. Studies met the following criteria were excluded: duplication (the same articles in different database); case reports, and comments; the studies not meeting criteria of inclusion. Eligibility assessment was independently conducted by 2 authors, with all inconsistent questions solved by discussion with other authors.
Description of the data
Incidence data were extracted either from the text or from the tables in the publications. There was no incidence rate of the original articles were presented in the figures. Altogether 87 studies from 72 countries met the inclusion criteria and were finally included in this study (Table 1). In 78 studies the children aged from 0 to 14 years and in 9 studies from 0 to 12, 15, 17, 19 years. The time period of the researches ranged from 1 to 30 years. The degree of case-ascertainment ranged from 85 to 100%. The researches included in this study were from the period 1965 to 2012.
Table 1.
The characteristics of worldwide incidence (per 100,000 children/yr) of childhood type 1 diabetes mellitus.
| Regions and Centers | Study periods | Age-group (years old) | Main climate type | Boy* | Girl* | Total | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| case (n) | Incidence | case (n) | Incidence | case (n) | Incidence | |||||
| Africa | ||||||||||
| Algeria | ||||||||||
| Oran | 1980–1989 | 0–14 | ① | 505 | 8.1 | 44 | ||||
| 1990 | 0–14 | 9 | 4.4 | 14 | 7 | 23 | 5.7 (3.62–5.82) | 4 | ||
| 1979–1988 | 0–14 | 4.7 | 45 | |||||||
| 1990–1999 | 0–14 | 7.7 | 9.6 | 8.6 (7.6–9.8) | 46 | |||||
| Libya | ||||||||||
| Benghazi | 1981–1990 | 0–19 | ② | 121 | 8.3 (6.9–10.0) | 130 | 9.2 (7.7–11.0) | 251 | 8.8 (7.8–10.0) | 47 |
| 1991–1999 | 0–14 | 7.8 | 10.3 | 9 (8.0–10.2) | 46 | |||||
| Mauritius | 1986–1990 | 0–14 | ③ | 1.8 | 2.4 | 2.1 | 48 | |||
| 1990–1994 | 0–14 | 10 | 1.3 | 11 | 1.5 | 21 | 1.4 (0.83–2.07) | 4 | ||
| Sudan | 1991–1995 | 0–14 | ② | 534 | 10.1 (9.0–12.8) | 49 | ||||
| Khartoum | ② | 196 | 31.8 (28.4–35.2) | 50 | ||||||
| Gezira | 1990 | 0–14 | ② | 17 | 5.6 | 12 | 4.4 | 29 | 5(3.74–6.54) | 4 |
| Sultanate of Oman | 1993 | 0–14 | ② | 3.23 | 1.99 | 2.45 | 51 | |||
| 1994 | 0–14 | 2.91 | 1.95 | 2.62 | 51 | |||||
| Tunisia | ||||||||||
| Beja | 1990–1994 | 0–14 | ② | 22 | 9 | 16 | 6.5 | 38 | 7.8 (5.47–10.68) | 4 |
| 1990–1999 | 0–14 | 8.4 | 6.9 | 7.7 (6.1–9.6) | 46 | |||||
| Gafsa | 1990–1994 | 0–14 | ② | 31 | 10 | 22 | 7.5 | 53 | 8.8 (6.59–11.51) | 4 |
| 1990–1999 | 0–14 | 9.5 | 7.5 | 8.5 (6.9–10.3) | 46 | |||||
| Kairoan | 1991–1993 | 0–14 | ② | 7.3 | 7.8 | 7.6 (5.6–10.0) | 46 | |||
| Monastir | 1990–1994 | 0–14 | ② | 15 | 4.7 | 16 | 5.2 | 31 | 4.9 (3.35–6.96) | 4 |
| 1990–1999 | 0–14 | 6.6 | 5.1 | 5.8 (4.6–7.3) | 46 | |||||
| Tanzania | ||||||||||
| Dar es Salaam | 1982–1991 | 0–14 | ④ | 0.8 | 0.9 | 86 | 0.8 | 52 | ||
| Asia | ||||||||||
| China | ||||||||||
| Beijing | 1990–1994 | 0–14 | ⑤ | 38 | 0.7 | 52 | 1.1 | 90 | 0.9 (0.72–1.09) | 4 |
| 1995–2000 | 0–14 | 0.93 (0.65–1.22) | 1.60 (1.42–1.78) | 1.25 (1.07–1.43) | 53 | |||||
| 2001–2005 | 0–14 | 1.37 (1.26–1.48) | 2.07 (1.62–2.51) | 1.70 (1.48–1.91) | 53 | |||||
| 2006–2010 | 0–14 | 2.05 (1.45–2.63) | 2.48 (1.81–3.15 | 2.25 (1.64–2.85) | 53 | |||||
| Chang Chun | 1990–1994 | 0–14 | ⑤ | 7 | 0.6 | 11 | 1.1 | 18 | 0.8 (0.49–1.30) | 46 |
| Changsha | 1990–1994 | 0–14 | ⑤ | 10 | 0.6 | 7 | 0.2 | 17 | 0.2 (0.2–0.4) | 46 |
| Dalian | 1990–1994 | 0–14 | ⑤ | 10 | 1.1 | 11 | 1.2 | 21 | 1.1 (0.7–1.7) | 46 |
| Guilin | 1991–1994 | 0–14 | ⑤ | 2 | 0.6 | 3 | 1 | 5 | 0.8 (0.2–2.0) | 46 |
| Hainan | 1990–1994 | 0–14 | ⑤ | 6 | 0.1 | 11 | 0.2 | 17 | 0.2 (0.1–0.2) | 46 |
| Harbin | 1990–1996 | 0–14 | ⑤ | 18 | 0.6 | 17 | 0.6 | 35 | 0.6 (0.4–0.8) | 46 |
| Hong-Kong | 1986–1990 | 0–14 | ⑤ | 1.5 | 2.4 | 22 | 2 | 54 | ||
| 1990–1994 | 0–14 | 4 | 0.6 | 13 | 2.1 | 17 | 1.3 (0.77–2.17) | 4 | ||
| 1990–1995 | 0–14 | 0.6 | 1.9 | 1.3 (0.8–1.9) | 46 | |||||
| 1997 | 0–14 | 218 | 1.4 | 55 | ||||||
| Huhehot | 1990–1994 | 0–14 | ⑥ | 10 | 1.1 | 6 | 0.7 | 16 | 0.9 (0.5–1.5) | 46 |
| Jilin | 1990–1994 | 0–14 | ⑥ | 8 | 0.4 | 14 | 0.8 | 22 | 0.6 (0.4–0.9) | 46 |
| Jinan | 1990–1995 | 0–14 | ⑤ | 12 | 0.5 | 11 | 0.4 | 23 | 0.4 (0.3–0.6) | 46 |
| Lanzhou | 1991–1994 | 0–14 | ⑥ | 5 | 0.4 | 3 | 0.2 | 8 | 0.3 (0.1–0.5) | 46 |
| Nanjing | 1990–1994 | 0–14 | ⑤ | 7 | 0.3 | 13 | 0.7 | 20 | 0.5 (0.3–0.8) | 46 |
| Nanning | 1990–1994 | 0–14 | ⑤ | 4 | 0.7 | 10 | 0.7 | 14 | 0.7 (0.5–0.9) | 46 |
| Shanghai | 1980–1991 | 0–14 | ⑤ | 35 | 0.55 (0.38–0.76) | 40 | 0.67 (0.45–0.91) | 75 | 0.61 (0.48–0.77) | 56 |
| 1989–1993 | 0–14 | 28 | 0.78 (0.52–1.12) | 30 | 0.88 (0.59–1.25) | 58 | 0.83 (0.61–1.04) | 57 | ||
| 1990–1994 | 0–14 | 24 | 0.4 | 23 | 0.5 | 47 | 0.5 (0.3–0.7) | 46 | ||
| 1997–2011 | 0–14 | 306 | 3.1 (2.8–3.4) | 316 | 3.2 (2.8–3.5) | 622 | 3.1 (2.9–3.3) | 39 | ||
| Sichuan | 1990–1994 | 0–14 | ⑤ | 9 | 1.8 | 13 | 2.7 | 22 | 2.3 (1.4–3.3) | 46 |
| Tie Ling | 1990–1994 | 0–14 | ⑤ | 5 | 0.2 | 3 | 0.1 | 8 | 0.2 (0.1–0.3) | 46 |
| Wuhan | 1990–1994 | 0–14 | ⑤ | 13 | 5.2 | 9 | 3.8 | 22 | 4.5 (2.8–7.0) | 46 |
| Wulumuqi | 1990–1994 | 0–14 | ⑥ | 5 | 0.9 | 4 | 0.8 | 9 | 0.8 (0.3–1.7) | 46 |
| Zhengzhou | 1991–1994 | 0–14 | ⑤ | 2 | 0.2 | 8 | 1 | 10 | 0.6 (0.3–1.1) | 46 |
| Zunyi | 1990–1995 | 0–14 | ⑤ | 1 | 0 | 2 | 0.1 | 3 | 0.1 (0.0–0.2) | 46 |
| India | ||||||||||
| Karnataka | 0–15 | ⑤ | 3.7 | 4 | 58 | |||||
| Israel | 1975–1980 | 0–14 | ① | 4.4 | 6.7 | 296 | 5.5 | 59 | ||
| 1989–1990 | 0–14 | 64 | 4.4 (3.4–5.6) | 92 | 6.7 (5.4–8.2) | 156 | 5.5 (4.7–6.5) | 59 | ||
| 1990–1993 | 0–14 | 5.5 | 6.6 | 6 (5.4–6.7) | 46 | |||||
| 1990–1993 | 0–17 | 201 | 7.0 (6.1–8.0) | 206 | 7.6 (6.6–8.7) | 407 | 7.3 (6.6–8.0) | 60 | ||
| 1990–1994 | 0–14 | 167 | 5.5 | 194 | 6.6 | 361 | 6.0 (5.42–6.67) | 4 | ||
| Japan | 1986–1990 | 0–14 | ⑤ | 522 | 1.2 (1.1–1.3) | 738 | 1.8 (1.7–1.9) | 1260 | 1.5 (1.4–1.6) | 61 |
| Chiba | 1990–1993 | 0–14 | ⑤ | 27 | 1.2 | 34 | 1.6 | 61 | 1.4 (1.1–1.8) | 46 |
| Hokkaido | 1974–1986 | 0–14 | ⑤ | 1.3 | 2.1 | 283 | 1.7 | 62 | ||
| 1990–1993 | 0–14 | 45 | 2.2 | 44 | 2.1 | 89 | 2.2 (1.7–2.6) | 46 | ||
| Okinawa | 1990–1993 | 0–14 | ③ | 6 | 1 | 11 | 1.8 | 17 | 1.4 (0.8–2.2) | 46 |
| Kuwait | 1992–1993 | 0–14 | ② | 47 | 16.58 (12.2–22.1) | 39 | 14.11(10.0–19.3) | 86 | 15.36 (12.4–19.1) | 63 |
| 1992–1994 | 0–14 | 82 | 19.2 | 71 | 17.3 | 153 | 18.3 (15.5–21.4) | 4 | ||
| 1992–1999 | 0–14 | 21.7 | 22.9 | 22.3 (20.5–24.2) | 46 | |||||
| Pakistan | ||||||||||
| Karachi | 1990 | 0–14 | ② | 9 | 0.5 | 16 | 0.9 | 25 | 0.7 (0.44–0.99) | 4 |
| 1990–1999 | 0–14 | 0.4 | 0.5 | 0.5 (0.3–0.5) | 46 | |||||
| Republic of Korea | ||||||||||
| Seoul | 1985–1988 | 0–14 | ⑤ | 0.6 | 0.8 | 71 | 0.7 | 64 | ||
| 1990–1991 | 0–14 | ⑤ | 1.1 | 1.2 | 1.1 (0.9–10.4) | 46 | ||||
| Russia | ||||||||||
| Novosibirsk | 1983–1989 | 0–14 | ⑥ | 4.6 | 4.9 | 4.7 | 65 | |||
| 1990–1994 | 0–14 | 90 | 5.7 | 101 | 6.4 | 191 | 6.0 (5.18–6.94) | 4 | ||
| 1990–1999 | 0–14 | 6.8 | 7.1 | 6.9 (6.3–7.6) | 46 | |||||
| Saudi Arabia | 1986–1997 | 0–14 | ② | 19 | 9.9 (5.4–17.7) | 27 | 14.8 (8.9–23.9) | 46 | 12.3 (8.4–17.9) | 66 |
| Al-Madinah | 2004–2009 | 0–12 | ② | 170 | 22.2 (19.1–25.7) | 249 | 33.0 (29.1–37.3) | 419 | 27.6 (25.0–37.3) | 67 |
| Eastern | 1990–2007 | 0–14 | ② | 195 | 243 | 438 | 27.52 (26.7–28.3) | 68 | ||
| Europe | ||||||||||
| Austria | 1979–1993 | 0–14 | ③ | 7.8 | 45 | |||||
| 1989–1990 | 0–14 | 107 | 7.9 (6.5–9.3) | 98 | 7.5 (6.1–9.2) | 205 | 7.7 (6.7–8.8) | 59 | ||
| 1990–1994 | 0–14 | 348 | 9.8 | 312 | 9.3 | 660 | 9.6 (8.84–10.31) | 4 | ||
| 1990–1999 | 0–14 | 10.3 | 9.5 | 9.9 (9.4–10.4) | 46 | |||||
| 2000–2005 | 0–14 | 610 | 14.8 (13.6–16.0) | 561 | 14.3 (13.2–15.5) | 1171 | 14.6 (13.7–15.4) | 69 | ||
| Belgium | ||||||||||
| Antwerpen | 1989–1990 | 0–14 | ③ | 15 | 9.2 (5.2–15.3) | 16 | 10.4 (5.9–16.9) | 31 | 9.8 (6.7–13.9) | 59 |
| 1990–1994 | 0–14 | 44 | 10.5 | 51 | 12.8 | 95 | 11.6 (9.40–14.41) | 4 | ||
| 1990–1999 | 0–14 | 10.7 | 12.8 | 11.7 (10.2–13.5) | 46 | |||||
| Belarus | ||||||||||
| Gomel | 1976–1999 | 0–14 | ⑥ | 433 | 4.6 (4.4–4.8) | 70 | ||||
| Bosnia and Herzegovina | ||||||||||
| Tuzla | 1990–1998 | 0–14 | ⑥ | 22 | 3.39 (1.8–4.9) | 21 | 3.37 (1.7–5.0) | 43 | 3.38 (2.3–4.5) | 71 |
| Bulgaria | ||||||||||
| Sofia | 1987–1991 | 0–14 | ⑥ | 6.7 | 72 | |||||
| East | 1974–1995 | 0–14 | ⑥ | 6.3 | 45 | |||||
| Varna | 1990–1994 | 0–14 | ① | 82 | 5.9 | 100 | 7.6 | 182 | 6.8 (5.80–7.83) | 4 |
| 1990–1999 | 0–14 | 7.9 | 8.3 | 8.1 (7.4–9.0) | 46 | |||||
| West-Bulgaria | 1990–1994 | 0–14 | ③ | 131 | 9.9 | 125 | 10 | 256 | 9.9 (8.71–11.21) | 4 |
| 1990–1999 | 0–14 | 11.6 | 9.8 | 10.7 (9.8–11.6) | 46 | |||||
| Croatia | 1995–2003 | 0–14 | ① | 369 | 9.26 (8.30–10.21) | 323 | 8.47 (7.54–9.41) | 692 | 9.05 (8.38–9.72) | 25 |
| Zagreb | 1988–1992 | 0–14 | ⑥ | 7.7 | 6.7 | 72 | 7.2 | 73 | ||
| Czech Republic | 1990–1997 | 0–14 | ③ | 814 | 10.0 (9.4–10.7) | 790 | 10.2 (9.5–11.0) | 1604 | 10.1 (9.6–10.6) | 74 |
| 1995–1999 | 0–14 | 12.6 | 12.7 | 12.7 (11.9–13.5) | 46 | |||||
| 1990–2001 | 0–14 | 2644 | 11.4 (11.0–11.9) | 75 | ||||||
| Denmark | ||||||||||
| 3 countries | 1989–1994 | 0–14 | ③ | 34 | 21.5 (14.9–30.1) | 32 | 21.4 (14.7–30.3) | 66 | 21.5 (16.6–27.3) | 59 |
| Four countries | 1990–1994 | 0–14 | ③ | 96 | 16.4 | 81 | 14.5 | 177 | 15.5 (13.3–17.9) | 4 |
| 1990–1999 | 0–14 | 17.1 | 16.2 | 16.6 (14.9–18.4) | 46 | |||||
| Estonia | 1983–1990 | 0–14 | ③ | 149 | 6.3(5.3–7.4) | 142 | 6.3 (5.3–7.5) | 291 | 10.1 (8.9–11.4) | 76 |
| 1991–1998 | 0–14 | 153 | 6.7 (5.7–7.9) | 157 | 7.2 (6.1–8.4) | 210 | 12.3 (11.0–13.8) | 76 | ||
| 1990–1994 | 0–14 | 85 | 9.9 | 93 | 11.2 | 178 | 10.5 (9.05–12.20) | 4 | ||
| 1990–1999 | 0–14 | 12.6 | 10.9 | 11.7 (10.6–13.0) | 46 | |||||
| Finland | 1987–1992 | 0–14 | ③ | 1113 | 37.6 (35.5–39.9) | 949 | 33.5 (31.5–35.8) | 2062 | 35.7 (34.1–37.2) | 77 |
| 1983–1990 | 0–14 | 1447 | 35.9 (34.1–37.8) | 1198 | 31.2 (29.5–33.0) | 2645 | 34.6 (33.3–36.0) | 76 | ||
| 1987–1991 | 0–14 | 1728 | 35.4 (33.9–37.4) | 78 | ||||||
| 1991–1998 | 0–14 | 1654 | 1497 | 3151 | 40.8 (39.4–42.2) | 76 | ||||
| 1990–1994 | 0–14 | 915 | 37 | 853 | 36 | 1768 | 36.5 (34.8–38.3) | 4 | ||
| 1990–1999 | 0–14 | 41.9 | 39.9 | 40.9 (39.6–42.2) | 46 | |||||
| 2 regions | 1989–1990 | 0–14 | ③ | 84 | 47.0 (37.5–58.1) | 67 | 38.8 (30.5–50.0) | 151 | 42.9 (36.6–50.6) | 59 |
| France | 1988 | 0–19 | ③ | 96 | 7.86 (6.63–9.09) | 79 | 6.96 (5.76–8.16) | 175 | 7.41 (6.55–8.27) | 79 |
| 1997 | 0–19 | 117 | 10.48 (6.13–11.83) | 93 | 8.68 (7.39–9.97) | 210 | 9.58 (8.64–10.52) | 79 | ||
| Four regions | 1989–1990 | 0–14 | ③ | 134 | 7.8 (6.6–9.3) | 127 | 7.8 (6.5–9.2) | 261 | 7.8 (6.9–8.8) | 59 |
| 1990–1994 | 0–14 | 372 | 8.7 | 337 | 8.3 | 709 | 8.5 (7.9–9.1) | 46 | ||
| FYR Macedonia | 1995–1999 | 0–14 | ⑥ | 4.9 | 3.5 | 4.2 (3.4–5.2) | 46 | |||
| Germany | 1999–2003 | 0–14 | ③ | 19.9 (19.0–20.7) | 18.9 (18.1–19.8) | 12335 | 19.4 (18.7–20.1) | 80 | ||
| 2004–2008 | 0–14 | 23.5 (22.5–24.5) | 22.4 (21.4–23.3) | 13299 | 22.9 (24.6–28.0) | 80 | ||||
| Düsseldorf | 1995–1999 | 0–14 | ③ | 14.8 | 16.1 | 15.4 (13.8–17.2) | 46 | |||
| Baden-Württemberg | 1990–1994 | 0–14 | ③ | 463 | 11 | 440 | 10.9 | 903 | 11.0 (10.3–11.7) | 4 |
| 1990–1999 | 0–14 | 12.7 | 12.6 | 12.6 (12.1–13.2) | 46 | |||||
| 1987–2003 | 0–14 | 14.1 (13.7–14.6) | 81 | |||||||
| 1999–2003 | 0–14 | 17.4(16.6–18.1) | 16.5 (15.9–17.2) | 1492 | 17.0 (16.4–17.6) | 80 | ||||
| 2004–2008 | 0–14 | 22.7 (21.9–23.6) | 21.7 (20.8–22.5) | 1832 | 25.4 (24.1–26.8) | 80 | ||||
| North Rhine-Westphalia | 1999–2003 | 0–14 | ③ | 21.8 (21.1–22.5) | 20.8 (20.0–21.5) | 3112 | 21.3 (20.7–21.9) | 80 | ||
| 2004–2008 | 0–14 | 25.0 (24.1–25.8) | 23.8 (23.0–24.6) | 3295 | 24.4 (23.8–25.0) | 80 | ||||
| Saxony | 1999–2003 | 0–14 | ③ | 15.8 (14.7–16.9) | 15.0 (14.0–16.1) | 411 | 17.7 (15.9–19.6) | 80 | ||
| 2004–2008 | 0–14 | 20.8 (19.4–22.2) | 19.8 (18.5–21.1) | 445 | 20.3 (19.1–25.5) | 80 | ||||
| Greece | 1992 | 0–14 | ① | 6.7 | 6.5 | 137 | 6.6 | 82 | ||
| Attica | 1990–1994 | 0–14 | ① | 149 | 10.2 | 124 | 9.1 | 273 | 9.7 (8.55–10.92) | 4 |
| 1990–1999 | 0–14 | 11 | 9 | 10 (9.2–10.9) | 46 | |||||
| Athens region | 1989–1990 | 0–14 | ① | 72 | 10.9 (8.5–13.7) | 50 | 7.7 (5.7–10.2) | 122 | 9.3 (7.7–11.1) | 59 |
| Northen 5 regions | 1989–1990 | 0–14 | ① | 9 | 5.3 (2.4–10.1) | 6 | 3.8 (1.4–8.2) | 15 | 4.6 (2.6–7.5) | 59 |
| Hungary | 1978–1987 | 0–14 | ⑥ | 1060 | 6.1 (4.7–7.3) | 83 | ||||
| Eighteen countries | 1989–1990 | 0–14 | ⑥ | 132 | 7.7 (6.4–9.1) | 124 | 7.5 (6.3–9.0) | 256 | 7.6 (6.7–8.6) | 59 |
| 1990–1994 | 0–14 | 337 | 8.7 | 360 | 9.6 | 697 | 9.1 (8.43–9.81) | 4 | ||
| 1990–1999 | 0–14 | 9.6 | 9.8 | 9.7 (9.2–10.2) | 46 | |||||
| Italy | 1990–2003 | 0–14 | ① | 2840 | 13.13 (12.66–13.62) | 2340 | 11.35 (10.90–11.82) | 5180 | 12.26 (11.93–12.60) | 84 |
| Lazio | 1989–1990 | 0–14 | ① | 66 | 7.2 (5.5–9.2) | 51 | 5.8 (4.4–7.7) | 117 | 6.5 (5.4–7.8) | 59 |
| 1990–1994 | 0–14 | 164 | 8 | 162 | 8.3 | 326 | 8.1 (7.28–9.07) | 4 | ||
| 1990–1999 | 0–14 | 8.9 | 8.6 | 8.8 (8.1–9.4) | 46 | |||||
| Lombardia | 1989–1990 | 0–14 | ① | 110 | 7.6 (6.3–9.2) | 83 | 5.9 (4.7–7.3) | 193 | 6.8 (5.8–7.8) | 59 |
| 1990–1994 | 0–14 | 239 | 7.6 | 204 | 6.8 | 443 | 7.2 (6.55–7.92) | 4 | ||
| 1990–1995 | 0–14 | 7.2 | 6.5 | 6.9 (6.3–7.5) | 46 | |||||
| Marche | 1990–1994 | 0–14 | ① | 55 | 10.5 | 44 | 8.9 | 99 | 9.7 (7.90–11.84) | 4 |
| 1990–1999 | 0–14 | 10.5 | 9.7 | 10.1 (8.8–11.6) | 46 | |||||
| Pavia | 1990–1994 | 0–14 | ① | 17 | 11.6 | 17 | 11.9 | 34 | 11.7 (8.08–16.44) | 4 |
| 1990–1999 | 0–14 | 12.3 | 12.5 | 12.4 (9.7–15.6) | 46 | |||||
| Sardinia | 1989–1990 | 0–14 | ① | 126 | 33.5 (27.9–39.9) | 95 | 26.9 (21.7–32.9) | 221 | 30.2 (26.4–34.4) | 59 |
| 1990–1994 | 0–14 | 337 | 43.6 | 211 | 29.5 | 548 | 36.8 (33.72–39.98) | 4 | ||
| 1990–1998 | 0–14 | 45 | 30.6 | 37.8 (35.5–40.3) | 46 | |||||
| Eastern Sicily | 1989–1990 | 0–14 | ① | 29 | 11.2 (7.5–16.1) | 23 | 9.0 (5.7–13.5) | 52 | 10.1 (7.5–13.2) | 59 |
| 1990–1994 | 0–14 | 75 | 13.4 | 53 | 9.9 | 128 | 11.7 (9.8–13.9) | 46 | ||
| Turin | 1984–1991 | 0–14 | ① | 116 | 8.42 (6.99–10.10) | 111 | 8.42 (6.95–10.19) | 227 | 8.42 (7.37–9.62) | 85 |
| 1990–1994 | 0–14 | 86 | 11.9 | 69 | 10.1 | 155 | 11.0 (9.32–11.15) | 4 | ||
| 1990–1999 | 0–14 | 11.7 | 10.3 | 11 (9.8–12.3) | 46 | |||||
| Roman and Lazio region | 1989–1993 | 0–14 | ① | 7.9(6.8–9.2) | 7.8(6.7–9.1) | 7.9 (7.1–8.8) | 86 | |||
| Liguria | 1989–1998 | 0–14 | ① | 126 | 14.15 (11.9–16.9) | 93 | 10.88 (8.9–13.3) | 219 | 12.56 (11.0–14.3) | 87 |
| Iceland | 1970–1979 | 0–14 | ③ | 31 | 9.3 (6.3–13.2) | 21 | 6.6 (4.1–10.1) | 52 | 8.0 (8.4–13.8) | 88 |
| 1980–1989 | 0–14 | 34 | 10.5 (7.3–14.7) | 34 | 11.1 (7.6–15.5) | 68 | 10.8 (8.4–13.8) | 88 | ||
| Latvia | 1983–1990 | 0–14 | ③ | 229 | 6.6 (5.8–7.5) | 227 | 6.7 (5.8–7.6) | 456 | 6.6 (5.8–7.3) | 76 |
| 1991–1998 | 0–14 | 242 | 6.9 (6.0–7.8) | 263 | 7.7 (6.8–8.7) | 505 | 7.4 (6.6–8.2) | 76 | ||
| 1990–1992 | 0–14 | 59 | 7 | 47 | 5.7 | 106 | 5.9 (5.06–6.98) | 4 | ||
| 1990–1999 | 0–14 | 7.8 | 7 | 7.4 (6.6–8.3) | 46 | |||||
| Lithuania | 1983–1990 | 0–14 | ③ | 143 | 9.7 (8.2–11.4) | 132 | 9.5 (7.9–11.3) | 275 | 6.8 (6.2–7.5) | 76 |
| 1991–1998 | 0–14 | 162 | 12.5 (10.7–14.6) | 139 | 10.9 (9.1–12.8) | 301 | 7.8 (7.1–8.5) | 76 | ||
| 1990–1994 | 0–14 | 162 | 7.7 | 145 | 7.1 | 307 | 7.4 (6.57–8.25) | 4 | ||
| 1990–1999 | 0–14 | 7.6 | 8.2 | 7.9 (7.3–8.5) | 46 | |||||
| 1983–2000 | 0–14 | 543 | 7.3 (6.7–7.9) | 557 | 7.8 (7.1–8.4) | 1100 | 7.5 (7.1–8.0) | 89 | ||
| Luxemburg | 1977–1986 | 0–14 | ③ | 12.1 | 12.6 | 16 | 12.4 | 59 | ||
| 1989–1990 | 0–14 | 8 | 12.1 (5.2–23.9) | 8 | 12.6 (5.4–24.8) | 16 | 12.4 (7.1–20.1) | 59 | ||
| 1990–1994 | 0–14 | 22 | 12.6 | 17 | 10.2 | 39 | 11.4 (8.14–15.59) | 4 | ||
| 1990–1999 | 0–14 | 10.3 | 12.2 | 11.3 (9.0–13.9) | 46 | |||||
| The Netherlands | ||||||||||
| Five regions | 1989–1990 | 0–14 | ③ | 30 | 11.2 (7.6–16.0) | 28 | 10.8 (7.2–15.7) | 58 | 11.0 (8.4–14.3) | 59 |
| 1990–1994 | 0–14 | 178 | 12.9 | 175 | 13.2 | 353 | 13 (11.7–14.4) | 46 | ||
| Macedonia | 1985–1991 | 0–14 | 2.4 | 2.5 | 112 | 2.5 | 90 | |||
| Malta | 1980–1987 | 0–14 | ⑥ | 43 | 12.7 (9.6–15.8) | 47 | 14.6 (11.3–17.9) | 90 | 13.6 (11.0–16.2) | 91 |
| 2006–2010 | 0–14 | 41 | 40 | 81 | 24.68 (21.98–27.43) | 92 | ||||
| Montenegro | 1997–2006 | 0–14 | ③ | 90 | 12.6 (10.1–15.5) | 94 | 14.3 (11.5–17.5) | 184 | 13.4 (11.5–15.5) | 93 |
| Norway | 1973–1982 | 0–14 | ③ | 1914 | 20.5 | 94 | ||||
| 2004–2012 | 0–14 | 33.9 (32.2–35.7) | 31.4 (29.7–33.2) | 32.7 (31.5–34.0) | 95 | |||||
| Eight countries | 1989–1990 | 0–14 | 87 | 22.3 (17.9–27.6) | 71 | 19.3 (15.1–24.3) | 158 | 20.8 (17.7–24.3) | 59 | |
| 1990–1994 | 0–14 | 222 | 22.4 | 187 | 19.9 | 409 | 21.2 (19.18–23.29) | 4 | ||
| 1990–1999 | 0–14 | 21.6 | 19.9 | 20.8 (19.4–22.1) | 46 | |||||
| Poland | ||||||||||
| 9 western provinces | 1989–1990 | 0–14 | ⑥ | 80 | 5.3 (4.6–6.5) | 84 | 5.8 (4.6–7.2) | 164 | 5.5 (4.7–6.4) | 59 |
| 3 cities | 1989–1990 | 0–14 | ⑥ | 51 | 5.7 (4.2–7.5) | 51 | 6.0 (4.5–7.9) | 102 | 5.8 (4.8–7.1) | 59 |
| Cracow | 1990–1999 | 0–14 | ⑥ | 7.5 | 7.6 | 7.6 (7.0–8.2) | 46 | |||
| Upper Silesia | 1995–1999 | 0–14 | ⑥ | 8 | 9.5 | 8.8 (7.9–9.7) | 46 | |||
| Wielkopolska | 1990 | 0–14 | ⑥ | 28 | 4.1 | 40 | 6 | 68 | 5 (3.88–6.36) | 4 |
| Portugal | ||||||||||
| 3 regions combined | 1989–1990 | 0–14 | ① | 17 | 10.1 (5.9–16.1) | 8 | 4.9 (2.1–9.6) | 25 | 7.5 (4.8–11.0) | 59 |
| Algarve | 1990–1994 | 0–14 | ① | 26 | 16.3 | 19 | 12.9 | 45 | 14.6 (10.62–19.64) | 4 |
| Coimbra | 1990–1994 | 0–14 | ① | 19 | 9.4 | 19 | 9.9 | 38 | 9.7 (6.76–13.36) | 4 |
| 1990–1999 | 0–14 | 10.1 | 9.1 | 9.6 (7.6–12.2) | 46 | |||||
| Madeira Island | 1990–1994 | 0–14 | ③ | 10 | 6.9 | 11 | 7.5 | 21 | 7.2 (4.46–11.05) | 4 |
| 1990–1999 | 0–14 | 7.1 | 6.8 | 6.9 (5.0–9.4) | 46 | |||||
| Portalegre | 1990–1994 | 0–14 | ① | 9 | 15.9 | 14 | 26.7 | 23 | 21.3 (13.29–31.89) | 4 |
| Romania | ||||||||||
| Bucharest | 1989–1990 | 0–14 | ⑥ | 22 | 4.6 (2.9–6.9) | 25 | 5.7 (3.7–8.4) | 47 | 5.1 (3.8–6.8) | 59 |
| 1990–1994 | 0–14 | 52 | 4.2 | 65 | 5.9 | 117 | 5.0 (4.14–6.05) | 4 | ||
| 1990–1999 | 0–14 | 4.7 | 5.9 | 5.3 (4.7–6.1) | 46 | |||||
| Silesian | 1989–2005 | 0–14 | ⑥ | 720 | 10.01 (8.58–11.45) | 665 | 9.72 (8.32–11.31) | 1385 | 9.87 (8.45–11.47) | 96 |
| Slovakia | 1990–1994 | 0–14 | ⑥ | 261 | 7.9 | 289 | 9.1 | 550 | 8.5 (7.81–9.25) | 4 |
| 1990–1999 | 0–14 | 9.7 | 9.7 | 9.7 (9.2–10.3) | 46 | |||||
| 2000 | 0–14 | 81 | 15.04 | 66 | 12.83 | 147 | 13.96 (11.35–15.72) | 97 | ||
| Slovenia | 1989–1990 | 0–14 | ① | 23 | 5.2 (3.3–10.4) | 33 | 7.7 (5.3–10.9) | 56 | 6.5 (4.9–8.4) | 59 |
| 1990–1994 | 0–14 | 70 | 6.8 | 88 | 9 | 158 | 7.9 (6.68–9.23) | 4 | ||
| 1990–1998 | 0–14 | 142 | 8.28 (6.9–9.6) | 157 | 9.63 (8.1–11.1) | 299 | 8.94 (7.9–9.9) | 71 | ||
| 1990–1999 | 0–14 | 8.3 | 9.5 | 8.9 (8.0–9.9) | 46 | |||||
| Spain | ||||||||||
| Catalonia | 1989–1990 | 0–14 | ① | 151 | 10.5 (8.8–12.3) | 146 | 10.6 (9.0–12.5) | 297 | 10.6 (9.4–11.9) | 59 |
| 1990–1994 | 0–14 | 358 | 12.5 | 338 | 12.6 | 696 | 12.5 (11.55–13.50) | 4 | ||
| 1990–1999 | 0–14 | 12.6 | 12.3 | 12.4 (11.7–13.1) | 46 | |||||
| Biscay | 1990–2013 | 0–14 | ③ | 199 | 10.4 (8.9–11.8) | 200 | 11.5 (9.5–12.6) | 399 | 10.7 (9.6–11.7) | 98 |
| Extremadura | 2003–2007 | 0–14 | ① | 104 | 24.9 (20.1–29.7) | 104 | 26.2 (21.2–31.6) | 208 | 25.5 (22.1–29.0) | 99 |
| Madrid | 1985–1988 | 0–14 | ⑥ | 11.3 | 10.5 | 501 | 10.9 | 100 | ||
| Cordoba | 1991–1992 | 0–14 | ① | 21 | 6.2 | 26 | 7.9 | 47 | 7 (5.20–9.26) | 4 |
| Sweden | 1978–1987 | 0–14 | ③ | 2012 | 25 | 1824 | 23.8 | 3838 | 24.4 | 101 |
| 1990–1994 | 0–14 | 1135 | 28.1 | 1031 | 26.9 | 2166 | 27.5 (26.36–28.67) | 4 | ||
| 1990–1999 | 0–14 | 30.5 | 29.4 | 30 (29.1–30.8) | 46 | |||||
| 1983–2000 | 0–14 | 4171 | 29.2 (28.3–30.1) | 3860 | 28.5 (27.6–29.4) | 8031 | 28.9 (28.2–29.5) | 89 | ||
| 2002–2004 | 0–14 | 42.9 (38.7–47.7) | 42.1 (37.6–46.7) | 2046 | 42.5 (39.3–45.7) | 102 | ||||
| 2005–2007 | 0–14 | 46.7 (41.6–51.5) | 41.2 (36.0–45.6) | 2029 | 43.9 (40.7–47.3) | 102 | ||||
| Serbia | ||||||||||
| Belgrade | 1982–1992 | 0–14 | ⑥ | 126 | 7.6 (6.4–9.1) | 133 | 8.6 (7.2–10.2) | 289 | 8.1 (7.1–9.2) | 103 |
| 1982–2005 | 0–14 | 372 | 10.6 (9.5–11.8) | 330 | 10.5 (9.4–11.7) | 702 | 10.6 (9.8–11.4) | 104 | ||
| Switzerland | 1995–1999 | 0–14 | ① | 13.3 | 10.7 | 12 (11.2–12.9) | 46 | |||
| Turkey | ||||||||||
| Diyarbakir | 2010–2011 | 0–14 | ⑥ | 24 | 8.7 | 17 | 5.7 | 41 | 7.2 | 105 |
| UK | ||||||||||
| Scotland | 1976–1983 | 0–14 | ③ | 20 | 19.4 | 1856 | 19.7 | 62 | ||
| 1990 | 0–14 | 16 | 32.5 | 7 | 15 | 23 | 24.0 (15.22–36.01) | 4 | ||
| 1990–1999 | 0–14 | 26.8 | 25.9 | 26.4 (25.4–27.4) | 46 | |||||
| Leicestershire | 1971–1980 | 0–14 | 10.6 (5.1–17.1) | 106 | ||||||
| 1990–1994 | 0–14 | ③ | 70 | 15.4 | 66 | 15.3 | 136 | 15.3 (12.85–18.07) | 4 | |
| Northern Ireland | 1989–1990 | 0–14 | ③ | 71 | 17.8 (13.9–22.5) | 59 | 15.4 (11.7–19.8) | 130 | 16.6 (13.9–19.7) | 59 |
| 1990–1994 | 0–14 | 202 | 20.1 | 185 | 19.3 | 387 | 19.7 (17.81–21.79) | 4 | ||
| 1990–1999 | 0–14 | 21.5 | 21.2 | 21.3 (19.9–22.8) | 46 | |||||
| Oxford | 1985–1995 | 0–14 | ③ | 572 | 19.9 (18.3–21.5) | 465 | 17.2 (15.6–18.7) | 1037 | 18.6 (17.4–19.8) | 107 |
| 1989–1990 | 0–14 | 90 | 17.8 (14.3–21.9) | 71 | 14.9 (11.7–18.8) | 161 | 16.4 (13.9–19.1) | 59 | ||
| 1990–1994 | 0–14 | 266 | 20.1 | 191 | 15.3 | 457 | 17.8 (16.18–19.46) | 4 | ||
| Plymouth | 1990–1994 | 0–14 | ③ | 63 | 16.5 | 65 | 18.1 | 128 | 17.3 (14.41–20.53) | 4 |
| 1990–1999 | 0–14 | 17.1 | 20.8 | 19 (16.8–21.2) | 46 | |||||
| Yorkshire | 1978–2007 | 0–14 | ③ | 2662 | 18.1 (17.6–18.7) | 108 | ||||
| 1990–1999 | 0–14 | 18.9 | 18.1 | 18.5 (17.5–19.5) | 46 | |||||
| Tayside | 1980–1983 | 0–14 | ③ | 19.7 | 22.1 | 64 | 20 | 62 | ||
| Bradford | 1978–1998 | 0–14 | ③ | 142 | 12.4 (10.4–14.4) | 147 | 13.6 (11.4–15.8) | 289 | 13.0 (11.5–14.5) | 109 |
| Far the south-west England | 1975–1996 | 0–14 | ③ | 228 | 13.63 (12.00–15.47) | 260 | 16.29 (14.49–18.38) | 488 | 14.93 (13.58–16.16) | 110 |
| North America | ||||||||||
| Canada | ||||||||||
| Newfoundland and Labrador | 1995–2002 | 0–19 | ⑥ | 400 | 77.3 (69.9–85.3) | 494 | 100.2 (91.6–109.4) | 894 | 88.6 (74.0–105.4) | 111 |
| Edmonton | 1990–1996 | 0–14 | ⑥ | 23 | 23.6 | 23.3 (20.5–26.4) | 46 | |||
| Calgary | 1990–1999 | 0–14 | ⑥ | 20.3 | 20.9 | 20.6 (18.5–22.7) | 46 | |||
| Prince Edward Island | 1975–1986 | 0–14 | ⑥ | 27 | 20.8 | 92 | 23.9 | 62 | ||
| 1990–1993 | 0–14 | 17 | 28 | 12 | 20.8 | 29 | 24.5 (16.38–35.16) | 4 | ||
| The Avalon Peninsula | 1987–2002 | 0–14 | ⑥ | 140 | 36.15 | 134 | 35.69 | 274 | 35.93 | 112 |
| Montreal | 1971–1985 | 0–14 | ⑥ | 9.6 | 10 | 919 | 9.8 | 62 | ||
| 1971–1983 | 0–14 | 9 | 9.1 | 9.0 (7.7–10.6) | 113 | |||||
| Alberta | 1990–1994 | 0–14 | ⑥ | 87 | 23.4 | 88 | 24.7 | 175 | 24.0 (20.62–27.82) | 4 |
| Manitoba | 1991–1993 | 0–14 | ⑥ | 21.4 | 20.7 | 21.1 (17.1–25.9) | 4 | |||
| USA | ||||||||||
| Allegheny, PA | 1990–1994 | 0–14 | ⑥ | 112 | 19.1 | 94 | 16.4 | 206 | 17.8 (15.45–20.33) | 4 |
| Chicago, IL | 190–1994 | 0–14 | ⑥ | 131 | 10.2 | 169 | 13.3 | 300 | 11.7 (10.47–13.12) | 4 |
| 1994–2003 | 0–17 | 617 | 16.0 (14.6–17.6) | 749 | 20.1 (18.3–22.1) | 1366 | 18.1 (16.9–19.3) | 114 | ||
| Jefferson, AL | 1990–1994 | 0–14 | ⑥ | 50 | 14.6 | 51 | 15.4 | 101 | 15.0 (12.21–18.22) | 4 |
| 1990–1995 | 0–14 | 14.1 | 15.1 | 14.6 (12.2–18.2) | 46 | |||||
| Colorado | ⑥ | |||||||||
| non-Hispanics | 1978–1988 | 0–17 | 654 | 16.4 (15.1–17.7) | 544 | 14.5 (13.3–15.7) | 115 | |||
| Hispanics | 1978–1988 | 0–17 | 56 | 7.1 (5.4–9.3) | 79 | 10.5 (8.4–13.1) | 115 | |||
| North Dakota | 1980–1986 | 0–14 | ⑥ | 21.6 | 16.2 | 204 | 18.9 | 116 | ||
| Wisconsin (part) | 1970–1979 | 0–14 | ⑥ | 20.2 | 16.2 | 166 | 18.2 | 62 | ||
| Rochester | 1965–1979 | 0–14 | ⑥ | 15.8 | 18.4 | 38 | 17.1 | 62 | ||
| Philadelphia | 1985–1989 | 0–14 | ⑥ | 11.3 | 14.8 | 215 | 13.4 | 117 | ||
| San Diego | 1978–1981 | 0–14 | ① | 9.6 | 9.1 | 48 | 9.4 | 62 | ||
| South America | ||||||||||
| Argentina | ||||||||||
| Avellaneda | 1985–1990 | 0–14 | ④ | 30 | 6.7 | 118 | ||||
| 1990–1994 | 0–14 | 11 | 5.6 | 15 | 7.5 | 26 | 6.5 (4.31–9.51) | 4 | ||
| 1990–1996 | 0–14 | 5.3 | 7.2 | 6.3 (5.7–11.1) | 46 | |||||
| Corrientes | 1992–1994 | 0–14 | ④ | 4 | 2.9 | 8 | 5.7 | 12 | 4.3 (2.21–7.51) | 4 |
| 1992–1999 | 0–14 | 4.7 | 8.5 | 6.6 (5.0–8.7) | 46 | |||||
| Tierra del Fuego | 1993–1994 | 0–14 | ③ | 4 | 20.2 | 0 | 4 | 8.0 (2.18–17.60) | 4 | |
| 1993–1996 | 0–14 | 14.2 | 6.3 | 10.3 (5.5–18.5) | 46 | |||||
| Brazil | ||||||||||
| Sao Paulo | 1987–1991 | 0–14 | ⑤ | 5.8 | 9.5 | 52 | 7.6 | 119 | ||
| 1990–1992 | 0–14 | 15 | 6.9 | 19 | 9.1 | 34 | 8 (5.53–1.14) | 4 | ||
| Passo Fundo | 1996–1999 | 0–14 | ⑤ | 5.4 | 8.7 | 7 (4.1–11.9) | 46 | |||
| Chile | 1990–1991 | 0–14 | ② | 2.2 | 2.8 | 52 | 2.5 | 120 | ||
| Santiago | 1990–1992 | 0–14 | ① | 66 | 1.7 | 56 | 1.5 | 122 | 1.6 (1.28–2.04) | 4 |
| 1990–1999 | 0–14 | 3.6 | 3.9 | 3.7 (3.4–4.0) | 46 | |||||
| Colombia | 1990 | 0–14 | ⑦ | 4.7 | 2.9 | 3.8 (2.9–4.9) | 4 | |||
| Cali | 1995–1999 | 0–14 | ⑦ | 0.4 | 0.5 | 0.5 (0.3–0.7) | 46 | |||
| Bogota | 1990 | 0–14 | ⑦ | 35 | 4.7 | 21 | 2.9 | 56 | 3.8 (2.88–4.93) | 4 |
| Paraguay | 1990–1994 | 0–14 | ④ | 45 | 1 | 34 | 0.8 | 79 | 0.9 (0.71–1.11) | 4 |
| 1990–1999 | 0–14 | 1 | 0.8 | 0.9 (0.8–1.0) | 46 | |||||
| Peru | ||||||||||
| Lima | 1990–1991 | 0–14 | ② | 0.2 | 0.6 | 0.4 (0.22–0.61) | 4 | |||
| 1990–1994 | 0–14 | 0.4 | 0.6 | 0.5 (0.4–0.64) | 46 | |||||
| Uruguay | ||||||||||
| Montevideo | 1992 | 0–14 | ⑤ | 13 | 8.3 | 13 | 8.3 | 26 | 8.3 (5.38–12.10) | 4 |
| Venezuela | ||||||||||
| Caracas | 1992 | 0–14 | ④ | 18 | 0.1 | 25 | 0.2 | 43 | 0.1 (0.09–0.18) | 4 |
| Central America and West Indies | ||||||||||
| Antigua | 1989–1993 | 0–19 | ③ | 3.5 (0.9–8.8) | 121 | |||||
| Barbados | 1982–1991 | 0–14 | ③ | 37 | 5 | 122 | ||||
| 1989–1993 | 0–19 | 2.6 (1.3–4.6) | 121 | |||||||
| 1990–1993 | 0–14 | 3 | 2.4 | 2 | 1.6 | 5 | 2.0 (0.32–6.36) | 4 | ||
| Cuba | 1978–1990 | 0–14 | ⑦ | 2.5 | 2.8 | 267 | 2.7 | 62 | ||
| 1990–1994 | 0–14 | 152 | 2.5 | 197 | 3.4 | 349 | 2.9 (2.63–3.24) | 4 | ||
| 1990–1999 | 0–14 | 2.1 | 2.5 | 2.3 (2.2–2.5) | 46 | |||||
| Dominican Republic | 1990–1993 | 0–14 | ③ | 3 | 6.6 | 2 | 4.9 | 5 | 5.7 (1.53–14.65) | 4 |
| 1995–1999 | 0–14 | 0.7 | 0.3 | 0.5 (0.4–0.7) | 46 | |||||
| Mexico | ||||||||||
| Mexico city | 1984–1986 | 0–14 | ⑥ | 0.4 | 0.7 | 100 | 0.6 | 62 | ||
| Veracruz | 1990–1993 | 0–14 | ④ | 3 | 6 | 9 | 1.5 (0.70–2.94) | 4 | ||
| Puerto Rico (USA) | 1985–1994 | 0–14 | ⑦ | 18.0 (17.6–18.3) | 123 | |||||
| 1990–1994 | 0–14 | 398 | 16.2 | 445 | 18.7 | 843 | 17.4 (16.25–18.63) | 4 | ||
| 1990–1999 | 0–14 | 15.8 | 17.8 | 16.8 (16.0–17.6) | 46 | |||||
| Virgin Islands (USA) | 1990–1994 | 0–14 | ③ | 9 | 14.7 | 7 | 11.5 | 16 | 13.1 (7.64–21.01) | 4 |
| 1990–1996 | 0–14 | 14 | 12.8 (8.1–18.8) | 46 | ||||||
| Oceania | ||||||||||
| Australia | 2000–2011 | 0–14 | ② | 6049 | 24.2 (23.6–24.8) | 5526 | 23.0 (22.4–23.7) | 11575 | 23.6 (23.2–24.0) | 124 |
| West | 1985–1992 | 0–14 | ④ | 14.9 | 45 | |||||
| 1985–2002 | 0–14 | 560 | 15.6 (13.7–17.5) | 584 | 17.3 (15.3–19.4) | 1144 | 16.5 (14.7–18.2) | 24 | ||
| 1985–2010 | 0–14 | 17.7 (16.9–19.3) | 18.5 (17.4–19.8) | 18.1 (17.5–19.2) | 125 | |||||
| New South Wales | 1990–1993 | 0–14 | ① | 335 | 13.1 | 387 | 15.9 | 722 | 14.5 (13.42–15.55) | 4 |
| 1990–1996 | 0–14 | 17.0 (14.1–20.6) | 18.6 (15.4–22.3) | 17.8 (15.6–20.3) | 26 | |||||
| New Zealand | ||||||||||
| Auckland | 1978–1985 | 0–14 | ③ | 9 | 10.5 | 233 | 9.8 | 62 | ||
| 1990–1994 | 0–14 | 65 | 12.3 | 70 | 13.6 | 135 | 12.9 (10.87–15.28) | 4 | ||
| 1990–1996 | 0–14 | 12.9 | 14.6 | 13.7 (12.0–15.7) | 46 | |||||
| Canterbury | 1981–1986 | 0–14 | ③ | 10.2 | 12.9 | 39 | 11.6 | 62 | ||
| 1990–1994 | 0–14 | 43 | 23.9 | 35 | 19.8 | 78 | 21.9 (17.33–27.32) | 4 | ||
| 1990–1999 | 0–14 | 23.8 | 20.8 | 22.3 (19.1–25.9) | 46 | |||||
Data showed as mean (95% CI); *represented boy vs. girl, p > 0.05, p derived from t-test; ①, Mediterranean climate; ②, Desert Climate; ③, Oceanic climate; ④, Savanna climate; ⑤, Monsoon climate; ⑥, Continental climate; ⑦, Rainforest climate.
Quality assessment
All abstracts ascertained initial search were screened and the researches in violation of inclusion criteria were excluded by two authors. Full-texts were posteriorly accessed by another two authors, in case of disagreement, a third professor was invited to evaluate such studies and the consensus was achieved via discussion. If original data was missing, the corresponding author of this study was contacted with alone tailored application forms by email.
Climate Style, latitude, and sunshine durations
Mediterranean climate, monsoon climate, oceanic climate, continental climate, savanna climate and rainforest climate were included in this study. Climate style met the announcement of national climate center, and the missing information was searched in the climate of the countries of the world19. Latitude of every center was identified by Google Earth’s high-resolution satellite image20, and if the countries didn’t have centers records, we would extract the latitude of the capital. Sunshine durations of the capital in each country was ascertained by average sunshine durations timetable around the world21. Mediterranean climate is the climate typical of the lands around the Mediterranean Sea from the largest areas, but it is also found in sections of Asia, in most coastal California, and in parts of Southern and West of Australia. Monsoon is currently defined as a seasonal changing in atmospheric precipitation and circulation associated with the asymmetric heating of land and sea. Oceanic climate is the typical of west coasts in higher middle latitudes of regions, with few extremes of temperature and a relatively narrow annual temperature range, and generally features cool summers and winters. Continental climate is referred to climates with significantly annual variation in temperature, which tended to occur in the middles of continents, mostly occur in the mainland China and the eastern U.S.22.
Statistical methods
The incidence of CT1DM for our study was obtained from the individual studies as it was researched in these publications. The incidence rates were calculated per 100, 000 people a year. Age standardization of the incidence rates was calculated using 5-years intervals with age groups 0–4 years, 5–9 years, and 10–14 years as the standard. The latitude groups 0°–23°26′N/S, 23°26′–40°N/S, and 40°–66°34′N/S as the study standard according to the tropic of Cancer/Capricorn, the Arctic/Antarctic circle, and westerlies, which based on geographic meteorology.
Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Continuous data that accord with a normal distribution were presented as mean [95% confidence interval (CI)], with least significant difference in parameters between two groups were analyzed by t-test, and the one-way ANOVA was used to assess the multiple groups for continuous variables in normal distribution. A p < 0.05 is considered to be statistically significant difference.
Results
Description of the included studies
After initial screening and removal of duplicates, we reviewed 3602 articles in full, of which 87 eligible studies on the incidence of CT1DM in various countries were included in this study (Table 1). Included studies on incidence of CT1DM entailed 118 records for centers in 72 countries. The numbers of records were available for North America (n = 17), South America (n = 10), Asia (n = 30), Europe (n = 47), Oceania (n = 3), Central America and West Indies (n = 2), and Africa (n = 9). The numbers of records were obtainable for Mediterranean climate (n = 22), Monsoon climate (n = 22), Oceanic climate (n = 22), Continental climate (n = 34), Desert climate (n = 11), Savanna climate (n = 5), and Rainforest climate (n = 2). The specific characteristics of included articles are displayed in Tables 1 and 2.
Table 2.
The characteristics of incidence of childhood type 1 diabetes mellitus (per 100,000 children/yr) in different age-groups.
| Countries and centers | Search periods | 0–4 years old* | 5–9 years old** | 10–14 years old | |||
|---|---|---|---|---|---|---|---|
| Case (n) | Incidence | Case (n) | Incidence | Case (n) | Incidence | ||
| Australia | 2000–2011 | 2402 | 14.9 (14.3–15.5) | 4007 | 24.7 (23.9–25.4) | 5166 | 31.0 (30.2–31.9) |
| West | 1985–2002 | 249 | 11.0 (9.2–12.8) | 437 | 18.8 (16.3–21.3) | 458 | 19.6 (17.6–21.6) |
| 1985–2010 | 11.0 (10.3–12.6) | 21.1 (19.5–22.6) | 25.5 (20.8–23.9) | ||||
| New South Wales | 1990–1996 | 10.8 (7.9–14.4) | 17.8 (14.1–22.4) | 25.0 (20.4–30.5) | |||
| Belarus | 1976–1999 | 2.7 | 5.2 | 9.3 | |||
| Canada | |||||||
| Newfoundland and Labrador | 1995–2002 | 59 | 29.6 (22.6–38.3) | 213 | 90.5 (78.9–103.6) | 348 | 127.4 (114.4–141.5) |
| The Avalon Peninsula | 1987–2002 | 58 | 24.95 | 95 | 37.01 | 121 | 43.62 |
| China | |||||||
| Shanghai | 1980–1991 | 16 | 0.26 (0.15–0.42) | 41 | 1.25 (0.89–1.70) | 18 | 0.62 (0.37–0.98) |
| 1989–1993 | 15 | 0.56 (0.32–0.93) | 28 | 1.02 (0.68–1.47) | 15 | 0.94 (0.52–1.55) | |
| Hong Kong | 1997 | 43 | 0.9 | 84 | 1.5 | 91 | 1.7 |
| Beijing | 1995–2000 | 0.41 (0.20–0.61) | 1.47 (1.07–1.90) | 1.49 (1.21–1.73) | |||
| 2001–2005 | 0.79 (0.65–0.93) | 1.79 (1.43–2.15) | 2.22 (1.91–2.53) | ||||
| 2006–2010 | 0.92 (1.81–3.15) | 2.83 (1.68–3.85) | 2.99 (1.93–4.04) | ||||
| Croatia | 1995–2003 | 134 | 5.77 (4.79–6.74) | 255 | 9.80 (8.60–11.01) | 303 | 11.13 (9.88–12.38) |
| Czech | 1990–1997 | 5.9 (5.3–6.7) | 10.5 (9.7–11.5) | 13.1 (12.2–14.1) | |||
| Germany | |||||||
| 1999–2003 | 14.5 (14.0–15.1) | 21.5 (20.1–22.9) | 22.2 (20.8–23.7) | ||||
| 2004–2008 | 17.1 (16.5–17.8) | 25.4 (23.8–27.1) | 26.3 (24.6–28.0) | ||||
| Baden-Württemberg | 1987–2003 | 5.8 (2.5–9.3) | 3.4 (0.8–6.0) | 2.7 (0.3–5.1) | |||
| 1999–2003 | 12.7 (11.9–13.5) | 18.8 (17.7–19.9) | 19.4 (18.3–20.6) | ||||
| 2004–2008 | 16.6 (15.6–17.6) | 24.6 (23.3–25.9) | 25.4 (24.1–26.8) | ||||
| North Rhine-Westphalia | 1999–2003 | 15.9 (15.1–16.8) | 23.5 (22.5–24.6) | 24.4 (23.3–25.5) | |||
| 2004–2008 | 18.2 (17.3–19.2) | 27.0 (25.8–28.2) | 28.0 (26.8–29.2) | ||||
| Saxony | 1999–2003 | 11.5 (10.3–12.8) | 17.0 (15.3–18.9) | 17.7 (15.9–19.6) | |||
| 2004–2008 | 15.2 (13.7–16.8) | 22.4 (20.3–24.8) | 23.2 (21.0–25.7) | ||||
| Italy | |||||||
| Rome and Lazio Region | 1989–1993 | 78 | 0.3 (5.0–7.9) | 130 | 9.8 (8.3–11.6) | 122 | 7.5 (6.2–9.0) |
| Turin | 1984–1991 | 40 | 5.49 (3.92–7.47) | 62 | 7.30 (5.69–9.49) | 125 | 11.17 (9.49–13.49) |
| Liguria | 1989–1998 | 50 | 9.01 (6.7–11.9) | 72 | 13.03 (10.2–16.4) | 97 | 15.01 (12.2–18.3) |
| Apulia | 2009–2013 | 149 | 20.1 (16.8–23.3) 1–4years | 296 | 29.7 (26.3–33.1) | 299 | 28.2 (25.0–31.4) |
| Jordanian | 19992–1996 | 39 | 1.3 | 90 | 3.2 | 146 | 5.5 |
| Kuwait | 1992–1993 | 27 | 12.83 (8.46–18.74) | 30 | 15.71 (10.60–22.46) | 29 | 18.29 (12.25–26.34) |
| Libya | |||||||
| Benghazi | 1981–1990 | 21 | 2.2 (1.4–3.4) | 54 | 7.2 (5.3–9.5) | 90 | 14.8 (12.0–18.4) |
| Lithuania | 1983–2000 | 185 | 4.0 (3.5–4.6) | 395 | 8.0 (7.2–8.8) | 520 | 10.5 (9.6–11.5) |
| Saudi Arabia | 1986–1997 | 8 | 7.1 (3.6–13.2) | 13 | 7.1 (3.7–13.2) | 25 | 24.1 (15.9–35.7) |
| Ai-Madinah | 2004–2009 | 115 | 17.1 (14.2–20.5) | 178 | 30.9 (26.6–35.7) | 126 | 46.5 (38.9–55.2) |
| Serbia | |||||||
| Belgrade | 1982–1992 | 40 | 3.9 (2.8–5.3) | 98 | 8.9 (7.3–10.9) | 121 | 11.2 (9.3–13.4) |
| 1982–2005 | 108 | 5.5 (4.5–6.7) | 256 | 11.9 (10.5–13.5) | 346 | 15.4 (13.8–17.1) | |
| Silesian | 1989–2005 | 5.33 (4.31–6.55) | 9.86 (8.45–11.45) | 13.20 (11.53–15.05) | |||
| Slovenia | 1990–1998 | 59 | 6.17 (4.5–7.7) | 103 | 9.20 (7.4–10.9) | 137 | 10.79 (9.0–12.6) |
| Bosnia and Herzegovina | |||||||
| Tuzla | 1990–1998 | 3 | 0.80 (0–1.7) | 18 | 4.68 (2.5–6.8) | 22 | 5.16 (2.8–7.5) |
| Slovakia | 2000 | 10.5 | 12.57 | 17.97 | |||
| Spain | |||||||
| Extremadure | 2003–2007 | 48 | 18.5 (10.1–30.3) | 66 | 25.2 (20.1–29.4) | 94 | 31.8 (25.8–34.1) |
| Biscay | 1990–2013 | 57 | 5.1 (3.8–6.5) | 168 | 14.6 (12.4–16.8) | 174 | 13.2 (11.3–15.2) |
| Sultanate of Oman | 1993 | 1.54 | 2.32 | 3.69 | |||
| 1994 | 0.97 | 2.79 | 4.22 | ||||
| Sweden | 1978–1987 | 759 | 15.7 | 1345 | 25.8 | 1734 | 30.6 |
| 1983–2000 | 1816 | 19.5 (18.6–20.4) | 2961 | 31.7 (30.6–32.8) | 3254 | 35.4 (34.2–36.6) | |
| 2002–2004 | 408 | 28.7 (23.9–33.5) | 765 | 50.9 (44.5–57.0) | 873 | 46.7 (41.5–52.2) | |
| 2005–2007 | 387 | 25.2 (20.8–29.6) | 676 | 47.9 (41.6–54.1) | 966 | 56.5 (50.5–62.9) | |
| Turkey | |||||||
| Diyarbakir | 2010–2011 | 8 | 4.3 | 17 | 9.1 | 16 | 8.4 |
| UK | |||||||
| Leicestershire | 1971–1980 | 6.3 (1.3–8.9) | 10.9 (1.6–19.5) | 15.1 (5.9–23.7) | |||
| Yorkshire | 1978–2007 | 807 | 11.7 (10.9–12.5) | 1330 | 18.6 (17.6–19.6) | 1774 | 23.7 (22.6–24.8) |
| Bradford | 1978–1998 | 70 | 9.3 (7.1–11.5) | 88 | 12.1 (9.6–14.7) | 131 | 17.9 (14.9–21.0) |
| Far the south-west | 1975–1996 | 96 | 9.35 (7.57–11.42) | 170 | 15.81 (13.52–18.37) | 222 | 19.02 (16.44–21.51) |
| USA | |||||||
| Chicago | 1994–2003 | 178 | 8.1 (7.0–9.5) | 340 | 15.3 (13.7–17.2) | 560 | 28.1 (25.5–30.9) |
Data showed as mean (95% CI); *represented 0–4 years old vs. 5–9 years old, p > 0.05; 0–4 years old vs. 10–14 years old, p < 0.01; **represented 5–9 years old vs. 10–14 years old, p > 0.05, p derived from one-way ANOVA.
Incidence of CT1DM
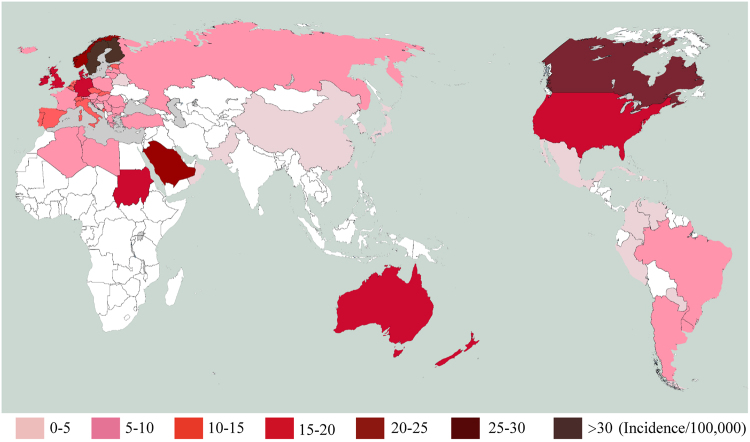
The average incidence of CT1DM in 70 countries showed in Fig. 1.
Figure 1.
Incidence of childhood type 1 diabetes mellitus in 72 countries (the first author independently created map by software-Adobe Illustrator CS5 and Adobe Photoshop CS5, and the copyright of map belongs to first author).
Overall incidence of CT1DM
Overall incidence of CT1DM was 11.43 (10.31–12.55) per 100,000 children/yr, in addition, boy, 11.42 (10.23–12.61) per 100,000 children/yr; girl, 11.11 (9.94–12.27) per 100,000 children/yr. There no significant difference existed between two groups of gender (p > 0.05) (Table 1).
Incidence of CT1DM in different regions
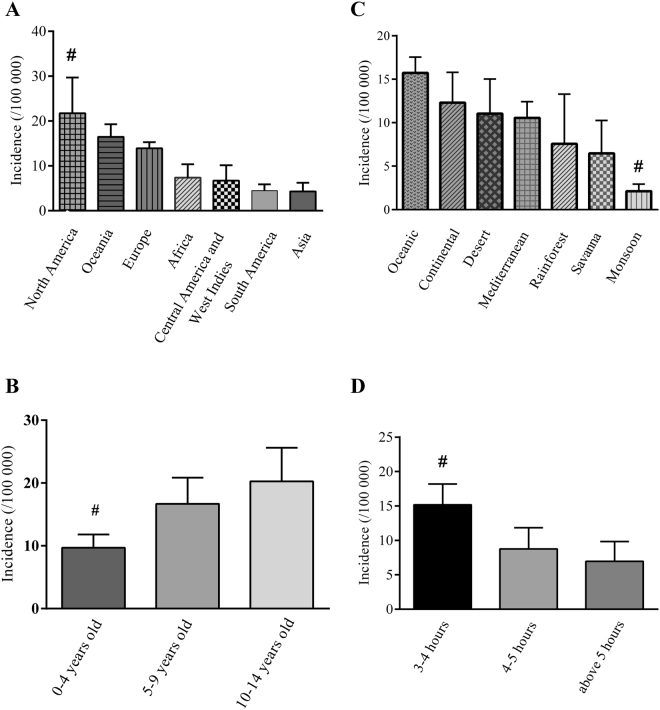
Overall incidence in different regions was indicated as following: Europe, 13.93 (12.59–15.27) per 100,000 children/yr; Asia, 4.31 (2.37–6.26) per 100,000 children/yr; North America, 21.75 (13.79–29.70) per 100,000 children/yr; South America, 4.47 (3.06–5.88) per 100,000 children/yr; Africa, 7.38 (4.37–10.39) per 100,000 children/yr; Central America and West Indies, 6.71 (3.27–10.16) per 100,000 children/yr; and Oceanic, 16.47 (13.67–19.27) per 100,000 children/yr; North America vs. other regions showed p < 0.01 excluded Oceania (Fig. 2A).
Figure 2.
Incidence of childhood type 1 diabetes mellitus in different regions, age-groups, climates, and sunshine durations. (A, Incidence of childhood type 1 diabetes mellitus in different regions: #indicated North America vs. other regions excluded Oceania, all p < 0.01; B, Incidence of childhood type 1 diabetes mellitus in three age-groups: #represented 0–4 years old vs. 10–14 years old, p < 0.01; C, Incidence of childhood type 1 diabetes mellitus in seven kinds of climates: *represented Monsoon climate vs. other climates excluded Savanna climate and Rainforest climate, all p < 0.01; D, Incidence of childhood type 1 diabetes mellitus in three sections of sunshine durations: #showed 3–4 hours/day vs. other two sections, both p < 0.01; all p derived from one-way ANOVA).
Incidence of CT1DM in different age-groups
Incidence of CT1DM in different age-groups as following: 0–4 years old, 9.70 (7.60–11.81) per 100,000 children/yr; 5–9 years old, 16.68 (12.51–20.86) per 100,000 children/yr; 10–14 years old, 20.27 (14.94–25.60) per 100,000 children/yr; 0–4 years old vs. 5–9 years old, p > 0.05; 5–9 years old vs. 10–14 years old, p > 0.05; 0–4 years old vs. 10–14 years old, p < 0.001 (Table 2, Fig. 2B).
Incidence of CT1DM in different climates type
Different gender for CT1DM incidence of different climates was displayed as follow: Monsoon climate: boy, 1.56 (0.95–2.16) per 100,000 children/yr; girl, 2.10 (1.28–2.92) per 100,000 children/yr; Oceanic climate: boy, 16.31 (14.29–18.33) per 100,000 children/yr; girl, 15.32 (13.51–17.12) per 100,000 children/yr; and the incidence of CT1DM of different genders in other climates showed in Table 3, all p > 0.05. Furthermore, overall incidence of different climates was presented as following: Mediterranean climate, 10.56 (5.69–12.42) per 100,000 children/yr; Monsoon climate, 2.12 (1.29–2.94) per 100,000 children/yr; Oceanic climate, 15.73 (13.93–17.54) per 100,000 children/yr; Continental climate, 12.30 (13.93–17.54) per 100,000 children/yr; Desert climate, 11.04 (7.06–15.02) per 100,000 children/yr; Savanna climate, 6.47 (2.68–10.26) per 100,000 children/yr; Rainforest climate, 7.58 (1.86–13.29) per 100,000 children/yr; pairwise comparison, Monsoon climate vs. other climates that excluding Savanna climate and Rainforest climate, all p < 0.01 (Fig. 2C).
Table 3.
The incidence of childhood type 1 diabetes mellitus (per 100,000 children/yr) with different gender in different climate.
| Incidence | P* | |||
|---|---|---|---|---|
| Boy | Girl | Total | ||
| Mediterranean Climate | 11.46 (9.11–13.81) | 10.58 (8.81–12.35) | 10.56 (8.69–12.42) | >0.05 |
| Desert Climate | 9.03 (5.76–12.29) | 9.18 (5.39–12.98) | 11.04 (7.06–15.02) | |
| Oceanic Climate | 16.31 (14.29–18.33) | 15.32 (13.51–17.12) | 15.73 (13.93–17.54) | |
| Monsoon Climate | 1.56 (0.95–2.16) | 2.10 (1.28–2.92) | 2.12 (1.29–2.94 | |
| Continental Climate | 12.34 (8.98–15.69) | 12.75 (8.77–16.73) | 12.30 (13.93–17.54) | |
| Savanna Climate | 5.47 (1.01–9.93) | 6.74 (1.96–11.52) | 6.47 (2.68–10.26) | |
| Rainforest Climate | 6.11 (0.88–11.35) | 6.44 (0.30–12.58) | 7.58 (1.86–13.29) | |
Data showed as Mean (95% CI); *represented boy vs. girl, all p > 0.05, p derived from the t-test.
Incidence of CT1DM in countries with different sunshine durations
Incidence of CT1DM in countries with different sunshine durations as following: 3–4 hours/day, 15.17 (11.14–19.20) per 100,000 children/yr; 4–5 hours/day, 8.77 (5.71–11.84) per 100,000 children/yr; above 5 hours/day, 6.96 (4.07–9.85) per 100,000 children/yr; 3–4 hours/day vs. other sunshine durations, p < 0.01; 4–5 hours/day vs. above 5 hours/day, p > 0.05 (Fig. 2D).
Incidence of CT1DM in centers with different latitude
Incidence of CT1DM in centers with different latitude as following: 0°–23°26′N/S: 4.98 (2.14–8.83) per 100,000 children/yr; 23°26′–40° N/S: 7.83 (6.01–9.84) per 100,000 children/yr; 40°–66°34′N/S: 14.71 (12.30–17.29) per 100,000 children/yr; 40°–66°34′N/S vs. other latitude, both p < 0.01; 0°–23°26′N/S vs. 23°26′–40° N/S, p > 0.05 (Fig. 3).
Figure 3.
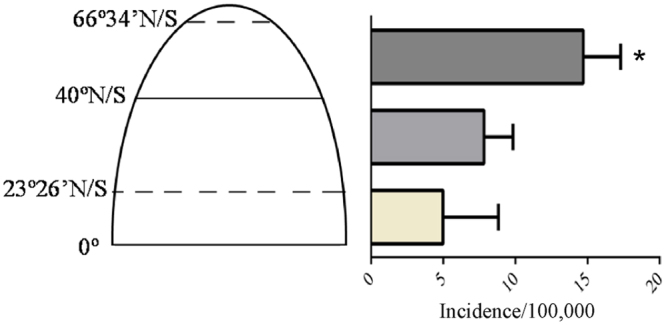
Incidence of childhood type 1 diabetes mellitus in three latitude sections (*expressed 40°–66°34′N/S vs. other two latitude sections, both p < 0.01, p derived from the one-way ANOVA).
Incidence of CT1DM during different periods
Incidence of CT1DM during different periods as following: 1965 to 1979, 9.44 (8.22–10.66) per 100,000 children/yr; 1980 to 1989, 10.79 (8.33–13.26) per 100,000 children/yr; 1990 to 1999, 11.50 (10.04–12.95) per 100,000 children/yr; 2000 to 2012, 19.58 (14.55–24.60) per 100, 000 children/yr; 2000 to 2012 vs. other two groups, p < 0.01; 1965 to 1990 vs. 1990 to 1999, p > 0.05 (Fig. 4).
Figure 4.
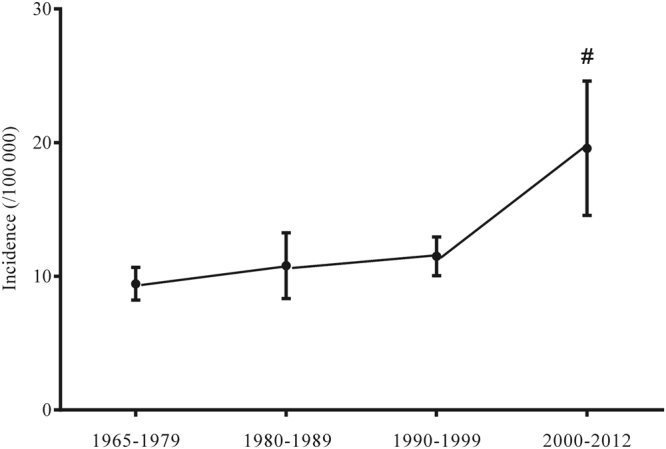
Incidence of childhood type 1 diabetes mellitus among four stages (*revealed 2000 to 2012 vs. other two stages, all p < 0.01, p derived from the one-way ANOVA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Discussion
This study performed firstly systematic estimates of CT1DM incidence among various climates, regions, genders, age-groups, latitude, and sunshine durations. The total countries based on this research consisted of 32% of the all countries in the world.
The worldwide incidence of CT1DM was increasing between 1965 and 2012 according to this study. Interestingly, the results of this study suggested there no significant difference was found in CT1DM incidence trended in boys compared with girls in this study. There are consistent results on the difference in incidence of diabetes by gender. Haynes et al. and Stipancic et al.23,24 displayed a significant increase incidence of T1DM in both boys and girls, and no significant difference was found in boys versus girls. However, others found a higher incidence in girls25–27. Adverse to our findings, Casu et al.28 expressed that a higher incidence of T1DM in boys. These divergences might depend on difference in sample size and statistical analysis.
In addition, our study illustrated a significantly increased incidence of T1DM in North America. Most countries in North America are developed countries with a higher per capita GDPs. Muntoni et al.29 showed that countries with a higher per capita GDPs tended to have higher T1DM incidence. Populations in wealthier countries typically drank more milk or eat more cheese than in poorer countries30,31. A high frequency of intake of milk or foods rich in protein may induce the occurrence and development of diabetes in humans10–12,32. Furthermore, these foods and dinks contain higher proportion of carbohydrate. Studies manifested that dietary carbohydrate could exacerbate postprandial glucose responses, which may be play a key role in blood glucose control33,34. Therefore, the higher proportion of carbohydrate may be also a major factor in development of diabetes in these regions.
Furthermore, the incidence of CT1DM of Asia has been increasing in recent years, although lower compared with Europe and America. Especially, the result of this study indicated the CT1DM incidence was higher in inland regions with continental climates compared with monsoon climates in China. The study stated the incidence of Huhehot is about 11 times in Hainan4. The higher incidence existed in inland region with high latitude that plays an important role in reducing childhood insulin-dependent diabetes mellitus (IDDM)35. Recent years, the per capita milk consumption and protein intake are increasing, especially in Xinjiang36 or Nnner Mongolia37. However, the overall incidence is low in China may based on individual’s diet habit and environmental factors38, which may resulted in a lack of public awareness, so could lead to a low quality of life of children in China.
As well, the incidence of CT1DM in regions with higher latitude and lower sunshine durations was higher than low latitude with high sunshine durations. In this study, the average incidence of CT1DM in Finland was 38.11 from 1965 to 1999, in which, latitude was 60°10′ N and the average amount of sunshine durations was only 3.18 hours a day. Eurodiab ACE Study Group39 had reported a 3-fold incidence increase of childhood IDDM was observed with the increasing latitude in Europe, and a similar result was reported within China40. In December, the northern Finland only has 2 hours of sunshine durations every day. Although there exists 23 hours of daylight per day in June, the most of the year exposure to daylight, Vitamin D production in the skin, is low by contrast with southern areas. Vitamin D supplementation is, thus, possibly more significant in this populations than others41. In this research, children lacked of adequate Vitamin D, who lived in higher latitude with low sunshine durations. Vitamin D is an immunosuppressive agent42, and the study believed the adequate Vitamin D supplementation for children might inhibit autoimmune reaction via damaging the β cells of pancreas and reduce the increasing trend in T1DM41. On the contrary, Vitamin D deficiency might induce CT1DM.
Last but not least, the incidence of CT1DM in centers or countries with oceanic climate was higher than other climates. The oceanic climate generally features long, but relatively mild winters and cool and short summers, which have a mean temperature below 22 °C in the warmest month43. In coastal areas of the higher middle latitudes (45–60° latitude), the prevailing onshore flow creates the basic structure of most oceanic climates. The previous studies reported the incidence rates of T1DM were associated with geographic variables such as average annual temperature35. Muntoni et al.29 indicated that countries or centers with lower annual temperatures tended to induce high incidence rate of CT1DM.
Nevertheless, this study just researched the incidence of CT1DM in 0–14 years old. Incidence data in older age groups exist from a few individuals. Furthermore, the incidence of childhood is unavailable after 2012 in this study. As well, the incidence of gender missing from Table 1 revealed the populations where development of the new register strategy was desired. Therefore, the continuous community-based registries are needed to access the T1DM incidence in the world, and further research is needed to find out the primary factor to identify prevention measures to stop the increased incidence of CT1DM.
Conclusions
In this study, the worldwide incidence of CT1DM was increasing, especially in countries with oceanic climates. Compared with previous researches, other than milk consumption, per capita GDPs, and genders, we found the climates included latitude and sunshine durations might play a key role in inducing CT1DM, which affected the lifestyle and dietary habit of individuals.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 81471054], Guangxi Natural Science Foundation [grant number 2015GXNSFBA139169] and the Innovation Project of Guangxi Graduate Education [grant number JGY2015128].
Author Contributions
Yin-ling Chen collected and analyzed data, wrote the first daft, and created the map. Yong-cheng Huang and Yong-chao Qiao designed the study and directed statistical analyses of the data. Wei Ling, Yan-hong Pan, Li-jun Geng, and Jian-long Xiao analyzed and interpreted the data. Xiao-Xi Zhang and Hai-Lu Zhao designed the study, and revised the submission. All authors contributed to the discussion, and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiao-xi Zhang, Email: mike_527@163.com.
Hai-lu Zhao, Email: zhaohailu9@126.com.
References
- 1.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 2.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 3.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J. Diabetes Complicat. 2005;19:238–46. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Karvonen M, et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes care. 2000;23:1516–26. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes A. Hyperglycemic crises in patients with diabetes mellitus. Diabetes care. 2001;24:1988–96. doi: 10.2337/diacare.24.11.1988. [DOI] [PubMed] [Google Scholar]
- 6.Gale EA. The discovery of type 1 diabetes. Diabetes. 2001;50:217–26. doi: 10.2337/diabetes.50.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills, T. Pharniaceutrie Rationalis or the Exercitation of the Operation of Medicines in Humane Bodies: The Works of Thomas Willis. London: Dring, Harper and Leigh (1679).
- 9.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virtanen SM, Laara E, Hypponen E, et al. Cow’s milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes. 2000;49:912–7. doi: 10.2337/diabetes.49.6.912. [DOI] [PubMed] [Google Scholar]
- 11.Verge CF, et al. Environmental factors in childhood IDDM. A population-based, case-control study. Diabetes care. 1994;17:1381–9. doi: 10.2337/diacare.17.12.1381. [DOI] [PubMed] [Google Scholar]
- 12.Dahlquist GG, Blom LG, Persson LA, Sandstrom AI, Wall SG. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ. 1990;300:1302–6. doi: 10.1136/bmj.300.6735.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pundziute-Lycka A, Persson LA, Cedermark G, et al. Diet, growth, and the risk for type 1 diabetes in childhood: a matched case-referent study. Diabetes care. 2004;27:2784–9. doi: 10.2337/diacare.27.12.2784. [DOI] [PubMed] [Google Scholar]
- 14.West R, et al. Epidemiologic survey of juvenile-onset diabetes in Montreal. Diabetes. 1979;28:690–3. doi: 10.2337/diab.28.7.690. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc. Soc. Exp. Biol. Med. 2000;223:230–3. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Nakamura K, Umeda E, et al. Familial predisposition of type 1 diabetes mellitus in Japan, a country with low incidence. Japan Diabetes Society Data Committee for ChildhoodDiabetes. JPEM. 2001;14(Suppl 1):589–95. doi: 10.1515/jpem.2001.14.s1.589. [DOI] [PubMed] [Google Scholar]
- 17.Parslow RC, et al. Incidence of childhood diabetes mellitus in Yorkshire, northern England, is associated with nitrate in drinking water: an ecological analysis. Diabetologia. 1997;40:550–6. doi: 10.1007/s001250050714. [DOI] [PubMed] [Google Scholar]
- 18.Duthie MS, Kimber I, Norval M. The effects of ultraviolet radiation on the human immune system. Brit. J. Dermatol. 1999;140:995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 19.Library of Congress Country Studies. Climate of the countries of the world. https://geographic.org/climate/climate_of_countries.html (2004).
- 20.Navinfo Data. Tencent-GS. http://www.gpsspg.com/maps.htm (2016).
- 21.Min, D. H. Sunshine durations in cities around the world. http://www.doc88.com/p-9991671143207.html (2013).
- 22.The free encyclopedia-Wikipedia. Continental Climate. https://en.wikipedia.org/wiki/Continental_climate (2009).
- 23.Haynes A, Bower C, Bulsara MK, Jones TW, Davis EA. Continued increase in the incidence of childhood Type 1 diabetes in a population-based Australian sample (1985–2002) Diabetologia. 2004;47:866–70. doi: 10.1007/s00125-004-1385-8. [DOI] [PubMed] [Google Scholar]
- 24.Stipancic G, et al. Incidence and trends of childhood Type 1 diabetes in Croatia from 1995 to 2003. Diabetes Res. Clin. Pr. 2008;80:122–7. doi: 10.1016/j.diabres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Craig ME, Howard NJ, Silink M, Chan A. The rising incidence of childhood type 1 diabetes in New South Wales, Australia. JPEM. 2000;13:363–72. doi: 10.1515/JPEM.2000.13.4.363. [DOI] [PubMed] [Google Scholar]
- 26.Ionescu-Tirgoviste C, Guja C, Calin A, Mota M. An increasing trend in the incidence of type 1 diabetes mellitus in children aged 0–14 years in Romania–ten years (1988–1997) EURODIAB study experience. JPEM. 2004;17:983–91. doi: 10.1515/JPEM.2004.17.7.983. [DOI] [PubMed] [Google Scholar]
- 27.Crow YJ, Alberti KG, Parkin JM. Insulin dependent diabetes in childhood and material deprivation in northern England, 1977–86. BMJ. 1991;303:158–60. doi: 10.1136/bmj.303.6795.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casu A, Pascutto C, Bernardinelli L, Songini M. Type 1 diabetes among sardinian children is increasing: the Sardinian diabetes register for children aged 0–14 years (1989–1999) Diabetes care. 2004;27:1623–9. doi: 10.2337/diacare.27.7.1623. [DOI] [PubMed] [Google Scholar]
- 29.Muntoni S, Cocco P, Aru G, Cucca F. Nutritional factors and worldwide incidence of childhood type 1 diabetes. Am. J. Clin. Nutr. 2000;71:1525–9. doi: 10.1093/ajcn/71.6.1525. [DOI] [PubMed] [Google Scholar]
- 30.Singh GM, Micha R, Khatibzadeh S, et al. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PloS one. 2015;10:e0124845. doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Villegas A, Martinez JA, Prattala R, et al. A systematic review of socioeconomic differences in food habits in Europe: consumption of cheese and milk. Eur. J. Clin. Nutr. 2003;57:917–29. doi: 10.1038/sj.ejcn.1601626. [DOI] [PubMed] [Google Scholar]
- 32.Elliott RB, Martin JM. Dietary protein: a trigger of insulin-dependent diabetes in the BB rat? Diabetologia. 1984;26:297–9. doi: 10.1007/BF00283653. [DOI] [PubMed] [Google Scholar]
- 33.Accurso A, Bernstein RK, Dahlqvist A, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr. & Metab. 2008;5:9. doi: 10.1186/1743-7075-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the american diabetes association. Diabetes care. 2004;27:2266–71. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 35.Geographic patterns of childhood insulin-dependent diabetes mellitus Diabetes Epidemiology Research International Group. Diabetes. 1988;37:1113–9. doi: 10.2337/diab.37.8.1113. [DOI] [PubMed] [Google Scholar]
- 36.Zhao GS, Yuan XY, Gong BQ, Wang SZ, Cheng ZH. Nutrition, metabolism, and hypertension. A comparative survey between dietary variables and blood pressure among three nationalities in China. J. Clin. Hypertens. 1986;2:124–31. [PubMed] [Google Scholar]
- 37.Ohno Y, Hirai K, Sowa S, Oka S, Murai Y. Food and nutrient intakes among nomads living in three different areas of Inner Mongolia, China. Asia Pac. J. Clin. Nutr. 2005;14:7–18. [PubMed] [Google Scholar]
- 38.Zhao Z, Sun C, Wang C, et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997–2011. Acta diabetologica. 2014;51:947–53. doi: 10.1007/s00592-014-0590-2. [DOI] [PubMed] [Google Scholar]
- 39.Variation and trends in incidence of childhood diabetes in Europe EURODIAB ACE Study Group. Lancet. 2000;355:873–6. doi: 10.1016/S0140-6736(99)07125-1. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Wang K, Li T, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes care. 1998;21:525–9. doi: 10.2337/diacare.21.4.525. [DOI] [PubMed] [Google Scholar]
- 41.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 42.Lemire J. 1,25-Dihydroxyvitamin D3–a hormone with immunomodulatory properties. Z. Rheumatol. 2000;59(Suppl 1):24–7. doi: 10.1007/s003930070034. [DOI] [PubMed] [Google Scholar]
- 43.“Whittier, Alaska, United States climate summary”. Weatherbase. Retrieved 9 March (2017).
- 44.Bessaoud K, et al. Epidemiology of juvenile insulin dependent diabetes mellitus in Algeria (Department of Oran). Rev. Epidem. Sante. Publ. 1990;38:91–99. [PubMed] [Google Scholar]
- 45.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 46.Group DP. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet. Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 47.Kadiki OA, Moawad SE. Incidence and prevalence of type 1 diabetes in children and adolescents in Benghazi, Libya. Diabet. Med. 1993;10:866–9. doi: 10.1111/j.1464-5491.1993.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 48.Tuomilehto J, et al. Incidence of IDDM in Mauritian children and adolescents from 1986 to 1990. Diabetes Care. 1993;16:1588–91. doi: 10.2337/diacare.16.12.1588. [DOI] [PubMed] [Google Scholar]
- 49.Elamin A, Ghalib M, Eltayeb B, Tuvemo T. High incidence of type 1 diabetes mellitus in Sudanese children, 1991–1995. Ann. Saudi. Med. 1997;17:478–80. doi: 10.5144/0256-4947.1997.478. [DOI] [PubMed] [Google Scholar]
- 50.Elamin A, Omer MI, Zein K, Tuvemo T. Epidemiology of childhood type I diabetes in Sudan, 1987–1990. Diabetes Care. 1992;15:1556–9. doi: 10.2337/diacare.15.11.1556. [DOI] [PubMed] [Google Scholar]
- 51.Soliman AT, al-Salmi IS, Asfour MG. Epidemiology of childhood insulin-dependent diabetes mellitus in the Sultanate of Oman. Diabet. Med. 1996;13:582–6. doi: 10.1002/(SICI)1096-9136(199606)13:6<582::AID-DIA114>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 52.Tuomilehto-Wolf E, Tuomilehto J. HLA antigens in insulin-dependent diabetes mellitus. Ann. Med. 1991;23:481–8. doi: 10.3109/07853899109150507. [DOI] [PubMed] [Google Scholar]
- 53.Gong C, et al. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: a retrospective multicenter study based on hospitalization data. Diabetes Technol. Ther. 2015;17:159–65. doi: 10.1089/dia.2014.0205. [DOI] [PubMed] [Google Scholar]
- 54.Wong GW, Leung SS, Oppenheimer SJ. Epidemiology of IDDM in southern Chinese children in Hong Kong. Diabetes Care. 1993;16:926–8. doi: 10.2337/diacare.16.6.926. [DOI] [PubMed] [Google Scholar]
- 55.Huen KF, et al. Epidemiology of diabetes mellitus in children in Hong Kong: the Hong Kong childhood diabetes register. J. Pediatr. Endocrinol. Metab. 2000;13:297–302. doi: 10.1515/JPEM.2000.13.3.297. [DOI] [PubMed] [Google Scholar]
- 56.Fu H, et al. Shanghai, China, has the lowest confirmed incidence of childhood diabetes in the world. Diabetes Care. 1994;17:1206–8. doi: 10.2337/diacare.17.10.1206. [DOI] [PubMed] [Google Scholar]
- 57.Shen SX, et al. The incidence of insulin-dependent diabetes mellitus in urban districts of Shanghai (1989–1993) J. Pediatr. Endocrinol. Metab. 1996;9:469–73. doi: 10.1515/jpem.1996.9.4.469. [DOI] [PubMed] [Google Scholar]
- 58.Kumar KM. Incidence trends for childhood type 1 diabetes in India. Indian J. Endocrinol. Metab. 2015;19:S34–5. doi: 10.4103/2230-8210.155378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green A, Gale EA, Patterson CC. Incidence of childhood-onset insulin-dependent diabetes mellitus: the EURODIAB ACE Study. Lancet. 1992;339:905–9. doi: 10.1016/0140-6736(92)90938-Y. [DOI] [PubMed] [Google Scholar]
- 60.Shamis I, Gordon O, Albag Y, Goldsand G, Laron Z. Ethnic differences in the incidence of childhood IDDM in Israel (1965–1993). Marked increase since 1985, especially in Yemenite Jews. Diabetes Care. 1997;20:504–8. doi: 10.2337/diacare.20.4.504. [DOI] [PubMed] [Google Scholar]
- 61.Kida K, et al. Incidence of Type 1 diabetes mellitus in children aged 0-14 in Japan, 1986–1990, including an analysis for seasonality of onset and month of birth: JDS study. The Data Committee for Childhood Diabetes of the Japan Diabetes Society (JDS) Diabet. Med. 2000;17:59–63. doi: 10.1046/j.1464-5491.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 62.Rewers M, LaPorte RE, King H, Tuomilehto J. The Diabetes Epidemiology Research International Study Group—DERI: Trends in the prevalance and incidence of diabetes: insulin-dependent diabetes mellitus in childhood. World Health Stat. 1988;41:179. [PubMed] [Google Scholar]
- 63.Shaltout AA, et al. High incidence of childhood-onset IDDM in Kuwait. Kuwait Study Group of Diabetes in Childhood. Diabetes Care. 1995;18:923–7. doi: 10.2337/diacare.18.7.923. [DOI] [PubMed] [Google Scholar]
- 64.Ko KW, Yang SW, Cho NH. The incidence of IDDM in Seoul from 1985 to 1988. Diabetes Care. 1994;17:1473–5. doi: 10.2337/diacare.17.12.1473. [DOI] [PubMed] [Google Scholar]
- 65.Podar T, Laporte RE, Tuomilehto J, Shubnikov E. Risk of childhood type 1 diabetes for Russians in Estonia and Siberia. Int. J. Epidemiol. 1993;22:262–7. doi: 10.1093/ije/22.2.262. [DOI] [PubMed] [Google Scholar]
- 66.Kulaylat NA, Narchi H. A twelve year study of the incidence of childhood type 1 diabetes mellitus in the Eastern Province of Saudi Arabia. J. Pediatr. Endocrinol. Metab. 2000;13:135–40. doi: 10.1515/JPEM.2000.13.2.135. [DOI] [PubMed] [Google Scholar]
- 67.Habeb AM, et al. High incidence of childhood type 1 diabetes in Al-Madinah, North West Saudi Arabia (2004-2009) Pediatr Diabetes. 2011;12:676–81. doi: 10.1111/j.1399-5448.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- 68.Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi. Med. J. 2010;31:413–8. [PubMed] [Google Scholar]
- 69.Schober E, Rami B, Waldhoer T. Steep increase of incidence of childhood diabetes since 1999 in Austria. Time trend analysis 1979-2005. A nationwide study. Eur. J. Pediatr. 2008;167:293–7. doi: 10.1007/s00431-007-0480-5. [DOI] [PubMed] [Google Scholar]
- 70.Martinucci ME, et al. Incidence of childhood type 1 diabetes mellitus in Gomel, Belarus. J. Pediatr. Endocrinol. Metab. 2002;15:53–7. doi: 10.1515/JPEM.2002.15.1.53. [DOI] [PubMed] [Google Scholar]
- 71.Bratina, N. U., Tahirovic, H., Battelino, T. & Krzisnik, C. Incidence of childhood-onset Type I diabetes in Slovenia and the Tuzia region (Bosnia and Herzegovina) in the period 1990–1998. Diabetologia. 44 (Suppl 3) B27–31 (abstract) (2001). [DOI] [PubMed]
- 72.Atanasova, M. et al. Incidence of diabetes mellitus type 1 in Bulgarian children. Diabetologia. 35 (Suppl 1) A131 (Abstract) (1992).
- 73.Roglic G, et al. Incidence of IDDM during 1988–1992 in Zagreb, Croatia. Diabetologia. 1995;38:550–4. doi: 10.1007/BF00400723. [DOI] [PubMed] [Google Scholar]
- 74.Cinek O, et al. Type 1 diabetes mellitus in Czech children diagnosed in 1990–1997: a significant increase in incidence and male predominance in the age group 0–4 years. Collaborators of the Czech Childhood Diabetes Registry. Diabet. Med. 2000;17:64–9. doi: 10.1046/j.1464-5491.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 75.Cinek O, Sumnik Z, Vavrinec J. Continuing increase in incidence of childhood-onset type 1 diabetes in the Czech Republic 1990–2001. Eur. J. Pediatr. 2003;162:428–9. doi: 10.1007/s00431-003-1211-1. [DOI] [PubMed] [Google Scholar]
- 76.Podar, T. et al. Increasing incidence of childhood-onset type I diabetes in 3 Baltic countries and Finland 1983–1998. Diabetologia. 44 (Suppl 3) B17–20 (abstract) (2001). [DOI] [PubMed]
- 77.Tuomilehto J, et al. Increase in incidence of insulin-dependent diabetes mellitus among children in Finland. Int. J. Epidemiol. 1995;24:984–92. doi: 10.1093/ije/24.5.984. [DOI] [PubMed] [Google Scholar]
- 78.Karvonen M, et al. Regional differences in the incidence of insulin-dependent diabetes mellitus among children in Finland from 1987 to 1991. Childhood Diabetes in Finland (DiMe) Study Group. Ann. Med. 1997;29:297–304. doi: 10.3109/07853899708999351. [DOI] [PubMed] [Google Scholar]
- 79.Charkaluk ML, Czernichow P, Levy-Marchal C. Incidence data of childhood-onset type I diabetes in France during 1988-1997: the case for a shift toward younger age at onset. Pediatr. Res. 2002;52:859–62. doi: 10.1203/00006450-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Bendas A, et al. Trends in Incidence Rates during 1999–2008 and Prevalence in 2008 of Childhood Type 1 Diabetes Mellitus in Germany–Model-Based National Estimates. PLoS One. 2015;10:e0132716. doi: 10.1371/journal.pone.0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ehehalt S, et al. Baden-Wurttemberg DI-sG. Continuous rise in incidence of childhood Type 1 diabetes in Germany. Diabet. Med. 2008;25:755–7. doi: 10.1111/j.1464-5491.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 82.Dacou-Voutetakis C, Karavanaki K, Tsoka-Gennatas H. National data on the epidemiology of IDDM in Greece. Cases diagnosed in 1992. Hellenic Epidemiology Study Group. Diabetes Care. 1995;18:552–4. doi: 10.2337/diacare.18.4.552. [DOI] [PubMed] [Google Scholar]
- 83.Soltesz G, Madacsy L, Bekefi D, Danko I. Rising incidence of type 1 diabetes in Hungarian children (1978–1987). Hungarian Childhood Diabetes Epidemiology Group. Diabet. Med. 1990;7:111–4. doi: 10.1111/j.1464-5491.1990.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 84.Bruno G, et al. Age-period-cohort analysis of 1990–2003 incidence time trends of childhood diabetes in Italy: the RIDI study. Diabetes. 2010;59:2281–7. doi: 10.2337/db10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruno G, et al. Comparison of incidence of insulin-dependent diabetes mellitus in children and young adults in the Province of Turin, Italy, 1984–91. Piedmont Study Group for Diabetes Epidemiology. Diabet. Med. 1997;14:964–9. doi: 10.1002/(SICI)1096-9136(199711)14:11<964::AID-DIA493>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 86.Sebastiani L, et al. A 5-year (1989–1993) prospective study of the incidence of IDDM in Rome and the Lazio region in the age-group 0–14 years. Diabetes Care. 1996;19:70–3. doi: 10.2337/diacare.19.1.70. [DOI] [PubMed] [Google Scholar]
- 87.Cotellessa M, et al. High incidence of childhood type 1 diabetes in Liguria, Italy, from 1989 to 1998. Diabetes Care. 2003;26:1786–9. doi: 10.2337/diacare.26.6.1786. [DOI] [PubMed] [Google Scholar]
- 88.Helgason T, Danielsen R, Thorsson AV. Incidence and prevalence of type 1 (insulin-dependent) diabetes mellitus in Icelandic children 1970-1989. Diabetologia. 1992;35:880–3. doi: 10.1007/BF00399936. [DOI] [PubMed] [Google Scholar]
- 89.Pundziute-Lycka A, Dahlquist G, Urbonaite B, Zalinkevicius R. Swedish Childhood Diabetes Study G, Lithuanian Childhood Diabetes Study G. Time trend of childhood type 1 diabetes incidence in Lithuania and Sweden, 1983–2000. Acta. Paediatr. 2004;93:1519–24. doi: 10.1111/j.1651-2227.2004.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 90.Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993;36:883–92. doi: 10.1007/BF02374468. [DOI] [PubMed] [Google Scholar]
- 91.Schranz AG, Prikatsky V. Type 1 diabetes in the Maltese Islands. Diabet. Med. 1989;6:228–31. doi: 10.1111/j.1464-5491.1989.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 92.Formosa N, Calleja N, Torpiano J. Incidence and modes of presentation of childhood type 1 diabetes mellitus in Malta between 2006 and 2010. Pediatr. Diabetes. 2012;13:484–8. doi: 10.1111/j.1399-5448.2011.00839.x. [DOI] [PubMed] [Google Scholar]
- 93.Samardzic M, Marinkovic J, Kocev N, Curovic N, Terzic N. Increasing incidence of childhood type 1 diabetes in Montenegro from 1997 to 2006. Pediatr. Diabetes. 2010;11:412–6. doi: 10.1111/j.1399-5448.2009.00617.x. [DOI] [PubMed] [Google Scholar]
- 94.Joner G, Sovik O. Increasing incidence of diabetes mellitus in Norwegian children 0–14 years of age 1973–1982. Diabetologia. 1989;32:79–83. doi: 10.1007/BF00505178. [DOI] [PubMed] [Google Scholar]
- 95.Skrivarhaug T, Stene LC, Drivvoll AK, Strom H, Joner G. Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57:57–62. doi: 10.1007/s00125-013-3090-y. [DOI] [PubMed] [Google Scholar]
- 96.Jarosz-Chobot P, Deja G, Polanska J. Epidemiology of type 1 diabetes among Silesian children aged 0–14 years, 1989–2005. Acta. Diabetol. 2010;47:29–33. doi: 10.1007/s00592-009-0094-7. [DOI] [PubMed] [Google Scholar]
- 97.Michalkova D, Minarik P, Hlava P, Camajova J, Nazarov V. Trends in the incidence of childhood-onset type 1 diabetes in Slovakia 1985–2000. Cent. Eur. J. Public Health. 2004;12:75–7. [PubMed] [Google Scholar]
- 98.Fernandez-Ramos C, Arana-Arri E, Jimenez-Huertas P, Vela A, Rica I. Incidence of childhood-onset type 1 diabetes in Biscay, Spain, 1990–2013. Pediatr. Diabetes. 2017;18:71–6. doi: 10.1111/pedi.12354. [DOI] [PubMed] [Google Scholar]
- 99.Gimeno Benitez A, Luengo Perez LM, Suero Villa P, Suero Villa S, Sanchez Vega J. [Incidence of childhood type I diabetes in Extremadura, Spain, 2003–2007] Semergen. 2014;40:177–82. doi: 10.1016/j.semerg.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 100.Serrano Rios M, et al. Incidence of type 1 (insulin-dependent) diabetes mellitus in subjects 0–14 years of age in the Comunidad of Madrid, Spain. Diabetologia. 1990;33:422–4. doi: 10.1007/BF00404093. [DOI] [PubMed] [Google Scholar]
- 101.Nystrom L, Dahlquist G, Rewers M, Wall S. The Swedish childhood diabetes study. An analysis of the temporal variation in diabetes incidence 1978–1987. Int. J. Epidemiol. 1990;19:141–6. doi: 10.1093/ije/19.1.141. [DOI] [PubMed] [Google Scholar]
- 102.Berhan Y, Waernbaum I, Lind T, Mollsten A, Dahlquist G. Swedish Childhood Diabetes Study G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60:577–81. doi: 10.2337/db10-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vlajinac HD, et al. Insulin dependent diabetes mellitus: incidence in childhood in Belgrade 1982–92. J. Epidemiol. Community Health. 1995;49:107–8. doi: 10.1136/jech.49.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sipetic S, et al. Rising incidence of type 1 diabetes in Belgrade children aged 0–14 years in the period from 1982 to 2005. J. Endocrinol. Invest. 2013;36:307–12. doi: 10.3275/8619. [DOI] [PubMed] [Google Scholar]
- 105.Demirbilek H, Ozbek MN, Baran RT. Incidence of type 1 diabetes mellitus in Turkish children from the southeastern region of the country: a regional report. J. Clin. Res. Pediatr. Endocrinol. 2013;5:98–103. doi: 10.4274/Jcrpe.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burden AC, Hearnshaw JR, Swift PG. Childhood diabetes mellitus: an increasing incidence. Diabet. Med. 1989;6:334–6. doi: 10.1111/j.1464-5491.1989.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 107.Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EA. Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford region: time trend analysis. The Bart’s-Oxford Study Group. BMJ. 1997;315:713–7. doi: 10.1136/bmj.315.7110.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harron KL, et al. Incidence rate trends in childhood type 1 diabetes in Yorkshire, UK 1978–2007: effects of deprivation and age at diagnosis in the South Asian and non-South Asian populations. Diabet. Med. 2011;28:1508–13. doi: 10.1111/j.1464-5491.2011.03413.x. [DOI] [PubMed] [Google Scholar]
- 109.Feltbower RG, et al. Trends in the incidence of childhood diabetes in south Asians and other children in Bradford, UK. Diabet. Med. 2002;19:162–6. doi: 10.1046/j.1464-5491.2002.00691.x. [DOI] [PubMed] [Google Scholar]
- 110.Zhao HX, et al. Incidence of childhood-onset Type 1 diabetes mellitus in Devon and Cornwall, England, 1975–1996. Diabet. Med. 1999;16:1030–5. doi: 10.1046/j.1464-5491.1999.00175.x. [DOI] [PubMed] [Google Scholar]
- 111.Alaghehbandan R, Collins KD, Newhook LA, MacDonald D. Childhood type 1 diabetes mellitus in Newfoundland and Labrador, Canada. Diabetes Res. Clin. Pract. 2006;74:82–9. doi: 10.1016/j.diabres.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Newhook LA, et al. High incidence of childhood type 1 diabetes in the Avalon Peninsula, Newfoundland, Canada. Diabetes Care. 2004;27:885–8. doi: 10.2337/diacare.27.4.885. [DOI] [PubMed] [Google Scholar]
- 113.Amos, A. F., McCarty, D. J. & Zimmet, P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet. Med. 14(Suppl 5) S1–85 (abstract) (1997). [PubMed]
- 114.Smith TL, Drum ML, Lipton RB. Incidence of childhood type I and non-type 1 diabetes mellitus in a diverse population: the Chicago Childhood Diabetes Registry, 1994 to 2003. J. Pediatr. Endocrinol. Metab. 2007;20:1093–107. doi: 10.1515/JPEM.2007.20.10.1093. [DOI] [PubMed] [Google Scholar]
- 115.Kostraba JN, et al. Incidence of insulin-dependent diabetes mellitus in Colorado. Epidemiology. 1992;3:232–8. doi: 10.1097/00001648-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Karvonen M, et al. Diabetes Metab. Rev. 1997. Sex difference in the incidence of insulin-dependent diabetes mellitus: an analysis of the recent epidemiological data. World Health Organization DIAMOND Project Group; pp. 275–91. [DOI] [PubMed] [Google Scholar]
- 117.Lipman TH. The epidemiology of type I diabetes in children 0–14 yr of age in Philadelphia. Diabetes Care. 1993;16:922–5. doi: 10.2337/diacare.16.6.922. [DOI] [PubMed] [Google Scholar]
- 118.Sereday MS, Marti ML, Damiano MM, Moser ME. Establishment of a registry and incidence of IDDM in Avellaneda, Argentina. Diabetes Care. 1994;17:1022–5. doi: 10.2337/diacare.17.9.1022. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira SR, et al. Population-based incidence of IDDM in the state of Sao Paulo, Brazil. Diabetes Care. 1993;16:701–4. doi: 10.2337/diacare.16.5.701. [DOI] [PubMed] [Google Scholar]
- 120.Carrasco, E., Lopez Garcia de los Rios, M., an Vargas, N. Incidencia de diabetes mellitus insulinodependiente en menores de 15 anos.periodo 90–91. Santiago, Chile. Rev. Soc. Argent. Diabetes. 26 (Suppl) 14–15 (abstract) (1992).
- 121.Tull ES, et al. Incidence of childhood-onset IDDM in black African-heritage populations in the Caribbean. The Caribbean African Heritage IDDM Study (CAHIS) Group. Diabetes Care. 1997;20:309–10. doi: 10.2337/diacare.20.3.309. [DOI] [PubMed] [Google Scholar]
- 122.Jordan OW, Lipton RB, Stupnicka E, Cruickshank JK, Fraser HS. Incidence of type I diabetes in people under 30 years of age in Barbados, West Indies, 1982–1991. Diabetes Care. 1994;17:428–31. doi: 10.2337/diacare.17.5.428. [DOI] [PubMed] [Google Scholar]
- 123.Frazer de Llado TE, Gonzalez de Pijem L, Hawk B. Incidence of IDDM in children living in Puerto Rico. Puerto Rican IDDM Coalition. Diabetes Care. 1998;21:744–6. doi: 10.2337/diacare.21.5.744. [DOI] [PubMed] [Google Scholar]
- 124.Haynes A, Bulsara MK, Bower C, Jones TW, Davis EA. Regular peaks and troughs in the Australian incidence of childhood type 1 diabetes mellitus (2000–2011) Diabetologia. 2015;58:2513–6. doi: 10.1007/s00125-015-3709-2. [DOI] [PubMed] [Google Scholar]
- 125.Haynes A, Bulsara MK, Bower C, Jones TW, Davis EA. Cyclical variation in the incidence of childhood type 1 diabetes in Western Australia (1985–2010) Diabetes Care. 2012;35:2300–2. doi: 10.2337/dc12-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.