Abstract
Aim
To determine the rate of abdominal lymph node metastasis after radical surgery for esophageal cancer and define the radiotherapy target area.
Methods
Of the 1593 patients who underwent R0 radical esophagectomy for thoracic esophageal squamous cell carcinoma (TE-SCC), 148 developed abdominal lymph node (LN) metastases within three years of surgery. During that time interval, patients were examined by various imaging methods (enhanced computer tomography, magnetic resonance imaging, and positron emission tomography–CT) at set time points. The emerging recurrence pattern, preferred sites for abdominal metastasis, and correlation with added clinical factors were carefully recorded, to permit for delineation of a target area for radiotherapy.
Results
We found postoperative metastatic abdominal LNs in 9.3% of the patients treated for esophageal cancer. Lesions in the upper, middle, and lower esophageal segments metastasized to abdominal LNs at 2.3%, 7.8%, and 26.6% (P < 0.0001), respectively. Of all cases, 4.8% had fewer than two affected LNs, while 20.1% had more than three metastatic LNs (P< 0.0001). The metastasis rates of negative and positive celiac LNs were 4.6% and 22.7%, respectively. Abdominal LN metastasis rates for the following LNs: 16a2 and 16a1 of para-aortic, celiac artery, posterior surface of the pancreatic head and common hepatic artery were 64.9%, 41.2%, 37.8%, 32.4%, and 20.9%, respectively. The overall rate of metastasis to these groups of LNs was 91.9%.
Conclusion
This study determined that stations 16a1 and 16a2 of the para-aortic, truncus coeliacus, posterior surface of the pancreatic head, and arteria hepatica communis lymph nodes were the preferred sites for abdominal LN metastasis, thus defining target areas for postoperative radiotherapy.
Introduction
Local metastasis to abdominal lymph nodes (LNs) is the main cause for failure to respond to radical surgery in patients with esophageal cancers, mostly due to the high recurrence frequency (8.4%–20%) [1–5]. Previous studies [1,6] reported that postoperative radiotherapy had a positive impact on patient survival after radical surgeries for stage III and LN-positive esophageal cancers, by reducing the rate of metastasized supraclavicular and upper mediastinal LNs. However, the overall rate of metastasis to LNs remained unchanged, since the target area for postoperative radiotherapy did not include the abdominal region [7]. Rates and patterns of postsurgery abdominal LN metastasis have been described in previous systematic studies [1, 8], but radiotherapy solutions have not been proposed. In this study, we examined 148 patients with abdominal LN metastasis after R0 radical surgery for thoracic esophageal squamous cell carcinoma (TE-SCC) and we analyzed their relapse patterns and sites, to provide references for designing an appropriate target area for postoperative radiotherapy.
Materials and methods
Ethics statement
Study participants voluntarily agreed to participate in the study and provided written informed consent prior to enrollment. The study was approved by the Ethics Committee of the Teaching Hospital of Fujian Medical University and Fujian Provincial Cancer Hospital.
Recruitment of participants
Our study is a retrospective study. Of the 2510 thoracic esophageal squamous cell carcinoma patients, who underwent radical R0 surgery at the Fujian Provincial Tumor Hospital in China, between February 2005 and April 2013, we selected 1593 to participate in this study. Participants were instructed to return periodically to the observing hospital for follow-up evaluations. Specifically, participants were examined every 3 months in the first year, then every 6 months in second and third year, and annually thereafter until the completion of the study, in April 2015. Abdominal LN metastases were detected by regular abdominal enhanced CT, MRI, and PET-CT (in some cases). Only the corresponding and first authors had access to information that could identify individual participants during or after data collection.
Inclusion criteria
a. No retroperitoneal LN or distant hematogenous metastases detected by enhanced computer tomography (CT), during the presurgical chest and abdomen examination.
b. More than 15 LNs dissected during the neck/chest/abdomen three-field or chest/abdomen two-field lymphadenectomy.
Exclusion criteria
a. Less than15 dissected LNs or palliative excision.
b. Postoperative pathology report indicating areas characteristic of nonsquamous cell carcinoma.
c. Unreliable imaging information regarding specific metastasis sites.
d. Preoperative neoadjuvant radio- or chemoradiotherapy.
Diagnostic criteria
Target areas for postoperative adjuvant radio- or chemoradiotherapy included the supraclavicular draining LNs, the upper mediastinal draining LNs, the anastomosis, and the original esophageal bed [6,9,10].
Metastatic abdominal LNs were considered those with a transverse diameter larger than 10 mm [11,12]. Categorization standards for esophageal cancers put forth by the 7th edition of the American Joint Committee on Cancer (AJCC) were ambiguous, leading to the decision to use the abdominal LNs classification standards for gastric carcinoma [13]: No. 8 (Hepatic arterial lymph nodes), No. 9 (celiac artery LNs), No. 10 (splenic hilar LNs, including those adjacent to the splenic artery and distal to the pancreatic tail; those adjacent to the roots of the short gastric arteries; and those along the left gastro-epiploic artery and proximal to its first gastric branch), No. 11 (proximal splenic artery LNs from its origin to halfway between its origin and the pancreatic tail end), No. 12 (hepato-duodenal ligament LNs), No. 13 (LNs on the posterior surface of the pancreatic head), No. 14 (LNs along the superior mesenteric vein), No. 16 (16b1 = para-aortic LNs in the diaphragmatic aortic hiatus; 16a1 = para-aortic LNs between the upper margin of the celiac artery origin and the lower border of the left renal vein; 16b1 = LNs adjacent to the abdominal aorta from the lower left renal vein to upper inferior mesentery artery; 16b1 = para-aortic LNs between the lower border of the left renal vein and the upper border of the inferior mesenteric artery origin), No. 18 (LNs along the inferior border of the pancreatic body), No. 19 (infra-diaphragmatic LNs predominantly along the subphrenic artery), and No. 20 (para-esophageal LNs in the diaphragmatic esophageal hiatus).
Statistics
All data were analyzed using the SPSS15.0 software (SPSS Inc., Chicago, IL, USA). A chi-square test was used for statistical data comparison. The Kaplan–Meier method was adopted to calculate the survival rate, and the log-rank method was used to compare survival curves between groups. A Cox regression model with stepwise selection was used to perform multivariate analyses. P–values lower than 0.05 were considered statistically significant.
Results
917 cases were excluded based on the following: 1)239 cases with esophageal cancer palliative resection or the number of surgical lymph node dissection was <15; 2) 136 cases with postoperative pathology report of non-squamous cell carcinoma; 3)170 cases of incomplete imaging data that prohibited accurate location of abdominal lymph node metastasis; 4) 372 cases with preoperative neoadjuvant chemotherapy or radiotherapy and chemotherapy.Following exclusion, 1593 patients were selected to participate in this study, with a median follow up duration of 43.5 months (95%CI: 38.4–48.6 months).
Patterns of postoperative abdominal lymph node metastasis
148 presented with abdominal LN metastases at 1.1–74.4 months after surgery, with a median of 10.7 months. The abdominal metastasis rate post radical resection was 9.3%. Of all patients, 39.2% exhibited only abdominal LN metastases, 23.6% developed abdominal and other LN metastases, 19.6% had abdominal LN and hematogenous metastases, and 17.6% exhibited regional LN and hematogenous metastases. (Table 1).
Table 1. Pattern of postoperative abdominal lymph node metastasis (%, cases/all samples).
| Metastasis pattern | Overall | Upper thoracic esophageal cancer | Middle thoracic esophageal cancer | Lower thoracic esophageal cancer |
|---|---|---|---|---|
| Abdominal lymph node metastasis only | 39.2 (58/148) | 0.0 (0/6) | 41.4 (36/87) | 40.0 (22/55) |
| Abdominal and other lymph node metastasis | 23.6 (35/148) | 50.0 (3/6) | 19.5 (17/87) | 27.3 (15/55) |
| Abdominal lymph node and hematogenous metastasis | 19.6 (29/148) | 16.7 (1/6) | 19.5 (18/87) | 18.2 (10/55) |
| Regional lymph node and hematogenous metastasis | 17.6 (26/148) | 33.3 (2/6) | 18.4 (16/87) | 14.5 (8/55) |
Rates of postoperative abdominal metastasis at specific lymphatic sites
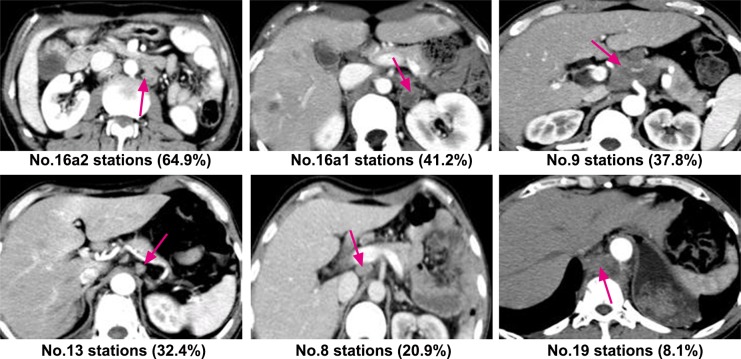
Of the 148 patients, seven had exceptionally high rates of postoperative abdominal LN metastasis, ranging from 6.8% to 64.9%, while the rest were below 5%. Abdominal metastasis rates at16a2 and 16a1 of the para-aortic, celiac artery, posterior surface of the pancreatic head, and common hepatic artery were 64.9%, 41.2%, 37.8%, 32.4%, and 20.9%, respectively. The overall metastasis rate in the above groups was 91.9%, as shown in Table 2.The illustration in Fig 1 shows specific sites with high rates of postoperative abdominal lymph node metastasis.
Table 2. Site distribution of postoperative abdominal LN metastasis in patients with TE-SSC (%, cases/all samples).
| LN category | Overall | Upper thoracic esophageal cancer | Middle thoracic esophageal cancer | Lower thoracic esophageal cancer |
|---|---|---|---|---|
| No. 8 | 20.9 (31/148) | 16.7 (1/6) | 23.0 (20/87) | 18.2 (10/55) |
| No. 9 | 37.8 (56/148) | 33.3 (2/6) | 36.8 (32/87) | 40.0 (22/55) |
| No. 10 | 1.4 (2/148) | 0.0 (0/6) | 2.3 (2/87) | 0.0 (0/55) |
| No. 11 | 4.1 (6/148) | 0.0 (0/6) | 5.7 (5/87) | 1.8 (1/55) |
| No. 12 | 3.4 (5/148) | 0.0 (0/6) | 4.6 (4/87) | 1.8 (1/55) |
| No. 13 | 32.4 (48/148) | 33.3 (2/6) | 27.8 (24/87) | 40.0 (22/55) |
| No. 14 | 6.8 (10/148) | 16.7 (1/6) | 4.6 (4/87) | 9.1 (5/55) |
| No. 15 | 1.4 (2/148) | 0.0 (0/6) | 1.1 (1/87) | 1.8 (1/55) |
| No. 16 | 79.7 (118/148) | 66.7 (4/6) | 79.3 (69/87) | 81.8 (45/55) |
| 16a1 | 41.2 (61/148) | 50.0 (3/6) | 41.4 (36/87) | 40.0 (22/55) |
| 16a2 | 64.9 (96/148) | 33.3 (2/6) | 65.5 (57/87) | 67.3 (37/55) |
| 16b1 | 6.8 (10/148) | 0.0 (0/6) | 10.3 (9/87) | 1.8 (1/55) |
| 16b2 | 0.7 (1/148) | 0.0 (0/6) | 0.0 (0/87) | 1.8 (1/55) |
| No. 18 | 2.7 (4/148) | 0.0 (0/6) | 3.4 (3/87) | 1.8 (1/55) |
| No. 19 | 8.1 (12/148) | 16.7 (1/6) | 5.7 (5/87) | 10.9 (6/55) |
| No. 20 | 1.4 (2/148) | 0.0 (0/6) | 1.1 (1/87) | 1.8 (1/55) |
Fig 1. Specific sites with high rates of postoperative abdominal lymph node metastasis.
Correlation between clinical factors and postoperative rates of abdominal LN metastasis
The rates of abdominal metastasis from upper, middle, and lower esophageal thoracic cancers were 2.3%, 7.8%, and 26.6% (P< 0.0001), respectively. In patients with postoperative pathology of T1/2, the rate of abdominal metastasis was 8.7% and in those with T3/4, it was 9.5% (P< 0.0001). The rate of abdominal metastatic LNs in cases with other metastatic LNs was13.7% and in those without other metastases, it was 3.7% (P< 0.0001). 4.8% of cases had fewer than two abdominal lymphatic metastases and 20.1% had more than three (P< 0.0001). 4.6% of cases were negative for metastatic celiac LNs, while 22.7% were found to be positive. Abdominal metastasis in patients with and without postoperative adjuvant radio- or chemoradiotherapy occured at rates of 18.2% and 16.0%, respectively (P = 0.478) (Table 3). We analyzed the above six clinical factors and post-abdominal lymph node metastasis by multivariate regression analysis. The results indicated that lesion site, celiac lymph node metastasis, number of metastasic lymph nodes independently predict abdominal metastases (Table 4).
Table 3. Relevance of confounding clinical factors for the incidence of abdominal lymph node metastasis in TE-SCC patients (%, cases/all samples).
| Factor | All patients | With abdominal lymph node metastasis | χ2 value | P -value |
|---|---|---|---|---|
| Sample size | 1593 | 148 | ||
| Lesion site | 92.041 | < 0.0001 | ||
| Upper segment of chest | 16.7 (266/1593) | 2.3 (6/266) | ||
| Middle segment of chest | 70.3 (1120/1593) | 7.8 (87/1120) | ||
| Lower segment of chest | 13.0 (207/1593) | 26.6 (55/207) | ||
| Postoperative T classification | 0.274 | 0.601 | ||
| T1/2 | 30.9 (493/1593) | 8.7 (43/493) | ||
| T3/4 | 69.1 (1100/1593) | 9.5 (105/1100) | ||
| Presence of lymph node metastasis | 46.900 | < 0.0001 | ||
| No | 44.2 (704/1593) | 3.7 (26/704) | ||
| Yes | 55.8 (889/1593) | 13.7 (122/889) | ||
| Number of metastasis lymph node | 91.631 | < 0.0001 | ||
| 0–2 | 70.6 (1125/1593) | 4.8 (54/1125) | ||
| ≥ 3 | 29.4 (468/1593) | 20.1 (94/468) | ||
| Celiac lymph nodes | 118.859 | < 0.0001 | ||
| Negative | 73.9 (1178/1593) | 4.6 (54/1178) | ||
| Positive | 26.1 (415/1593) | 22.7 (94/415) | ||
| Adjuvant therapy | 0.504 | 0.478 | ||
| No | 83.8 (1335/1593) | 9.1 (121/1335) | ||
| Yes | 16.2 (258/1593) | 10.5 (27/258) |
Table 4. Multivariate regression analysis of clinical factors and abdominal lymph node metastasis in TE-SCC.
| Factor | Chi-Square | P-value |
|---|---|---|
| Lesion site | 39.458 | 0.000 |
| Celiac lymph node metastasis | 9.650 | 0.002 |
| Number of metastasis lymph node | 17.433 | 0.000 |
| Postoperative T classification | 2.179 | 0.140 |
| Adjuvanttherapy | 27.546 | 0.100 |
| Presence of lymph node metastasis | 0.015 | 0.901 |
Discussion
Metastasis to lymph nodes is commonly complicating the outcome of esophageal cancers [11] and abdominal metastases, in particular, are responsible for failure of complete remission after radical surgery. Xiao et al.[1] reported an overall rate of metastasis of 8.4% after TE-SCC (41/486), while Liu et al.[8] reported a 14.8% rate (38/256). We determined an overall rate of 9.3% (148/1593), consistent with the reports from both publications [1,8].
According to previous studies [1–8,14], the metastasis rates vary greatly with the site of the cancerous lesion, ranging from 0%–8.3%, 8.1%–13.9%, and 26.8%–40.8% (P< 0.0001) for the upper, middle, and lower esophageal segments, respectively (Table 5). In this study, we also found the metastasis rates of the upper, middle, and lower esophageal segments to be higher with each segment, indicating increasing incidence for distally located lesions. One explanation for this observation could be the difference in the spreading mechanisms employed by cancers from different segments. Upper-segment esophageal cancers commonly metastasize to supraclavicular and upper mediastinal LNs and rarely to abdominal nodes. For middle-segment and lower-segment esophageal cancers, abdominal LN metastases were observed more frequently, while supraclavicular and upper mediastinal ones were quite rare[9].
Table 5. Rates of postoperative abdominal lymph node metastasis reported in other studies (%, cases/all samples).
| Reference | Overall rate | Upper thoracic esophageal cancer | Middle thoracic esophageal cancer | Lower thoracic esophageal cancer |
|---|---|---|---|---|
| Cai WJ et al.[3]* | 20.0 (28/140) | 0.0 (0/7) | 12.8 (11/86) | 36.2 (17/47) |
| Doki Y et al.[4]* | 16.7 (30/180) | 3.6 (1/28) | 8.1 (7/86) | 33.3 (22/66) |
| Ge H et al.[5]* | 14.5 (32/220) | 5.2 (3/58) | 15.7 (16/102) | 23.3 (14/60) |
| Zhang WC et al.[14]* | 20.0 (39/195) | 8.3 (2/24) | 13.9 (17/122) | 40.8 (20/49) |
| Liu WJ et al.[13] † | 14.8 (38/256) | 3.1 (1/32) | 12.1 (19/157) | 26.8 (18/67) |
| Current study† | 9.3 (148/1593) | 2.3 (6/266) | 7.8 (87/1120) | 26.6 (55/207) |
*Analysis for patients with lymph node metastasis after esophagectomy only.
†Analysis for the whole group with lymph node metastasis.
Our data showed that retroperitoneal LN metastasis was the major contributing factor for abdominal LN metastasis after radical surgery for esophageal cancers. The highest rates of metastasis were at stations 16a1 and 16a2 of the para-aortic, celiac artery, posterior surface of the pancreatic head, and common hepatic artery, ranging from highest to lowest. LNs in the vicinity of the cardia and arteria gastrica sinistra, which are frequently metastatic before the surgery, showed no signs of postoperative metastasis. This can be attributed to the complete removal of those LNs during surgery, as they are readily exposed and easy to access. Meanwhile LNs in the truncus coeliacus, on the posterior surface of the pancreatic head, and adjacent to arteria hepatica communis are difficult to access and cannot be easily removed. Therefore, these sites should be the targeted by postoperative radiotherapy.
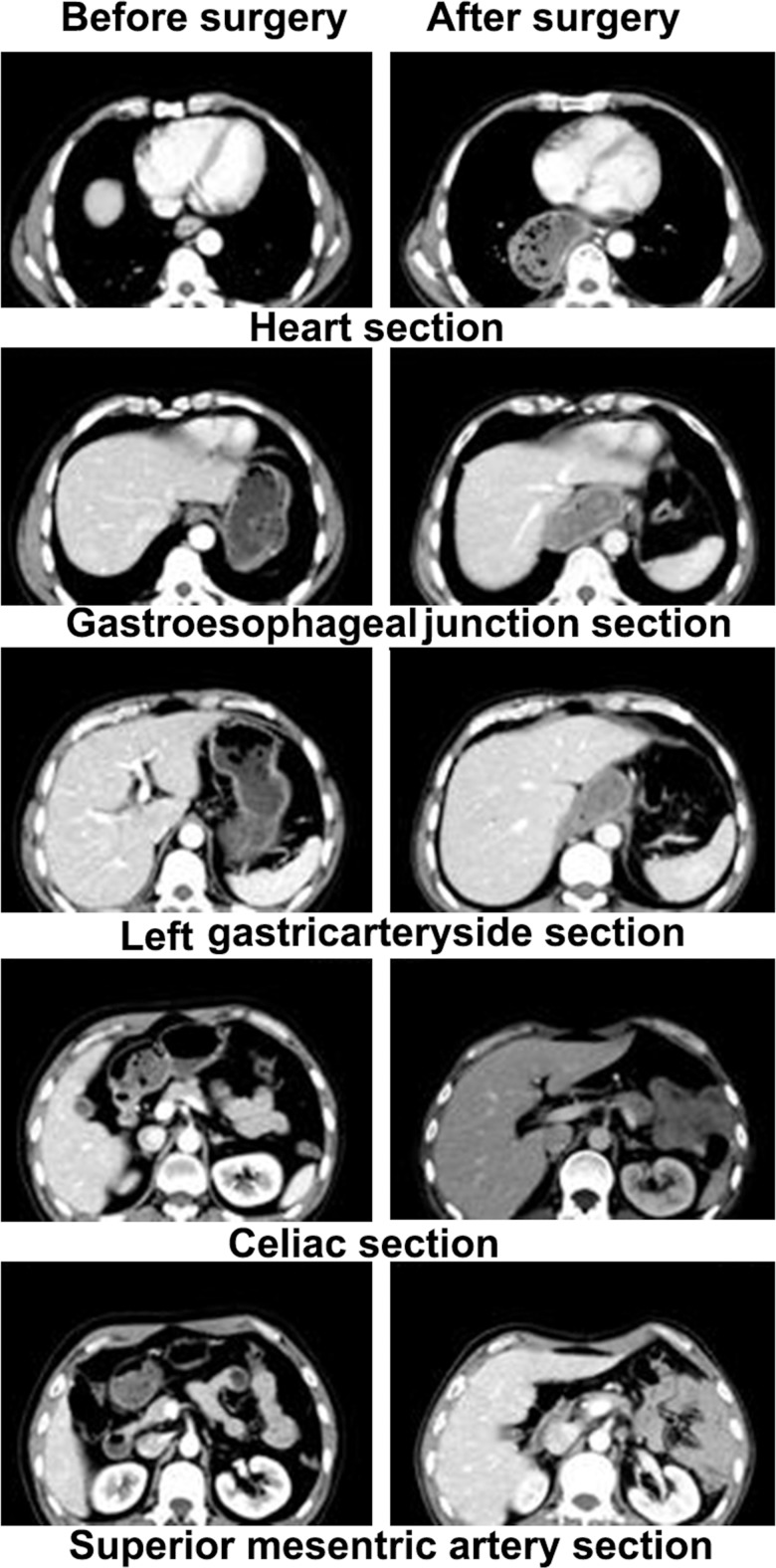
To date, there are no standards that define an abdominal target area for postoperative radiotherapy, but several options can be recommended. The large T target area includes the supraclavicular draining LNs, mediastinal LN, the anastomosis, and the original esophagus bed [1,6,15]; the small T target area includes the supraclavicular draining LNs, upper mediastinal draining LNs, the anastomosis, and the original esophagus bed [6]; and the conventional target area includes the esophagus bed, the subcarinal LNs, and the left gastric LNs [16]. However, regardless of the chosen target area, no significant reduction in the rates of abdominal metastasis was observed in cases subjected to postoperative radiotherapy (P> 0.05). Our results confirmed these previously published conclusions[1,6,15,16]. There are two possible explanations for this: first, that the draining retroperitoneal LNs were not included in the target area, and second, that the anatomical structure of the inferior mediastinum and epigastrium changed postsurgery, and thus, the corresponding LNs were obscured by gastrointestinal tissue. Fig 2 shows the anatomical structures of the inferior mediastinum and epigastrium before and after the radical surgery.
Fig 2. CT images of the inferior mediastinum and epigastrium before and after the radical surgery.
Previous studies reported improved survival rates after adjuvant radio- chemotherapy in esophageal cancer patients with more than 3 metastatic LNs [10, 17]. We showed a drastic increase in the abdominal lymphatic metastasis rates in cases with more than three affected nodes (4-fold increase) or with metastatic celiac LNs (almost 5-fold increase). Therefore, were commend adjuvant radiotherapy to the retroperitoneal draining LNs only for those cases. The lower esophageal segment could also be included when necessary in the radiotherapy target area. Conformal radiotherapy should be used to reduce the adverse gastrointestinal effects.
One of the shortcomings of this study was that the diagnosis of postoperative abdominal lymph node metastasis was dependent on regular abdominal enhanced CT, MRI, and PET-CT imaging. However, CT diagnosis of abdominal lymph nodes has an overall accuracy of 62–64% [18] and MRI has sensitivity of 71% ± 18.22, specificity of 29% ± 18.07, and accuracy of 58% ± 19.72 in this regard [19]. PET/CT for diagnosis of abdominal LN metastasis has the sensitivity, specificity, positive predictive value (PPV) and negative predictive value of FDG of 40%, 95%, 91% and 56%, respectively [20]. Most TE-SCC patients with postoperative recurrence received non-surgical treatment which limits available pathological data comparable to surgical treatment causing some deficiencies in the pathology diagnosis. In this study, strict image quality control may help to alleviate this deficiency to a certain extent. Since this study had a smaller sample size, further studies are expected to determine whether the abdominal draining LNs should be included in the postoperative radiotherapy target area. In particular, multi-center prospective large sample studies are required to compare the survival data between patients receiving preventive radiation therapy including the abdominal target area and those not containing the abdominal target area.
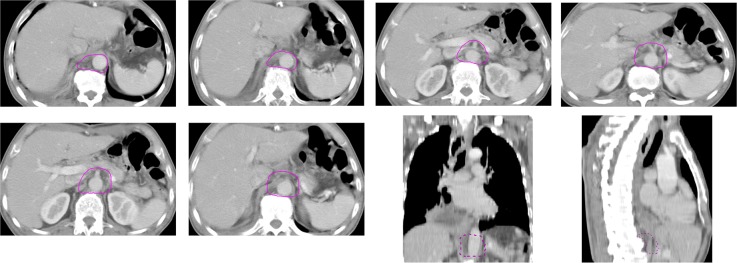
We concluded that the lymph nodes at stations 16a1 and 16a2 of the para-aortic, truncus coeliacus, posterior surface of the pancreatic head, and arteria hepatica communis were the major sites for abdominal metastasis after radical surgery for esophageal cancers. Hence, we recommend these regions to be included in the radiotherapy target area (Fig 3). We also recommend that in TE-SCC cases with more than three metastatic LNs and positive for celiac lymphatic metastases, radiotherapy should be employed following the radical resection and the target area should be adjusted conformingly.
Fig 3. Proposed postoperative target area for radiotherapy.
The lymph nodes at stations 16a1 and 16a2 of the para-aortic, truncus coeliacus, posterior surface of the pancreatic head, and arteria hepatica communis were the major sites for abdominal metastasis after radical surgery for esophageal cancers. Hence, we recommend these regions to be included in the radiotherapy target area.
Supporting information
(XLS)
(JPG)
(DOCX)
(DOCX)
Acknowledgments
This study is supported by grants from the Natural Science Foundation of Fujian Province (2015J01377), the Key Project of Science and Technology Foundation of Fujian Province (2011Y0014), and the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C.
This research did not increase the risk and economic burden of patients; the patients’ rights were fully protected; the project design was conducted in line with scientific and ethical principles. The institutional review board approved this project.
All participants in this study have provided informed written consent prior to enrollment.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Data Availability
All relevant data are within the paper and its Supporting Information Files.
Funding Statement
This study is supported by grants from the Natural Science Foundation of Fujian Province (2015J01377), the Key Project of Science and Technology Foundation of Fujian Province (2011Y0014) to Junqiang Chen, and the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C.
References
- 1.Xiao ZF,Yang ZY,Miao YJ,Wang LH,Yin WB,Gu XZ et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005; 62: 82–90 [doi: 10.1016/j.ijrobp.2004.08.046 ] [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004; 198: 205–211 [doi: 10.1016/j.jamcollsurg.2003.10.005 ] [DOI] [PubMed] [Google Scholar]
- 3.Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol.2010; 96: 104–107 [doi: 10.1016/j.radonc.2010.04.029 ] [DOI] [PubMed] [Google Scholar]
- 4.Doki Y,Ishikawa O,Takachi K,Miyashiro I,Sasaki Y,Ohigashi H, et al. Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg 2005; 29: 700–707 [doi: 10.1007/s00268-005-7596-4 ] [DOI] [PubMed] [Google Scholar]
- 5.Ge H,Jiang Y,Liu JS. Postoperative radiotherapy target volume of thoracic esophageal squamous cell cancer. Chinese Clinical Oncology 2005; 10: 526–527, 544 [Google Scholar]
- 6.Chen J, Zhu J, Pan J, Zhu K, Zheng X, Chen M, Wang J, Liao Z. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg 2010; 90: 435–442 [doi: 10.1016/j.athoracsur.2010.04.002 ] [DOI] [PubMed] [Google Scholar]
- 7.Xiao Z, Yang Z, Liang J, Miao Y, Wang M, Yin W et al. Clinical value of prohylacic radiotherapy after curative resection of esophageal carcinoma. Chinese Clinical Oncology 2002; 6: 608–611 [PubMed] [Google Scholar]
- 8.Liu WJ, Kong L, Yu JM, Li MH, Hu M, Shi F et al. Pattern of Recurrence Following Complete Resection of Thoracic Esophageal Squamous Cell Carcinoma and Significance of Adjuvant Therapy. Chin J Clin Oncol 2010; 37: 1358–1361 [Google Scholar]
- 9.Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu J, Xiao J, Ying M. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2009; 36: 480–486 [doi: 10.1016/j.ejcts.2009.03.056 ] [DOI] [PubMed] [Google Scholar]
- 10.Chen J,Pan J,Liu J,Li J,Zhu K,Zheng X, et al. Postoperative radiation therapy with or without concurrent chemotherapy for node-positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2013; 86: 671–677. doi: 10.1016/j.ijrobp.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Chen Y, Fu X, Zhao K Jiang GL. Proposed revision of CT-based cervical and thoracic lymph node levels for esophageal cancer in UICC 7th version. Radiother Oncol 2014; 113: 175–181 [doi: 10.1016/j.radonc.2014.11.022 ] [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Miyazaki T, Nakajima M, Fukuchi M, Manda R Kuwano H. Value of positron emission tomography in the diagnosis of recurrent oesophageal carcinoma. Br J Surg 2004; 91: 1004–1009 doi: 10.1002/bjs.4595 [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: 7th ed. Springer, 2009 [Google Scholar]
- 14.Zhang WC, Wang QF, Xiao ZF, Yang LH, Liu XY. Patterns of failure after complete resection of thoracic esophageal squamous cell carcinoma: implications for postoperative radiation therapy volumes. Chin J Radiat Oncol 2012; 21: 38–41 [Google Scholar]
- 15.Teniere P, Hay JM, Fingerhut A Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991; 173: 123–130 [] [PubMed] [Google Scholar]
- 16.Qiao XY, Wang W, Zhou ZG, Gao XS Chang JY. Comparison of efficacy of regional and extensive clinical target volumes in postoperative radiotherapy for esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2008; 70: 396–402 [doi: 10.1016/j.ijrobp.2007.06.031 ] [DOI] [PubMed] [Google Scholar]
- 17.Bédard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer 2001, 91: 2423–2430 [] [PubMed] [Google Scholar]
- 18.Kim HJ,Kim AY,Oh ST,Kim JS,Kim KW,Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning[J]. Radiology,2005,236(3):879–885. doi: 10.1148/radiol.2363041101 [DOI] [PubMed] [Google Scholar]
- 19.Heye T,Kuntz C,Düx M,Encke J,Palmowski M,Autschbach F, et al. New coil concept for endoluminal MR imaging: initial results in staging of gastric carcinoma in correlation with histopathology [J]. Eur Radiol,2006,16(11):2401–2409. doi: 10.1007/s00330-006-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer[J]. Eur J Nucl Med Mol Imaging,2006,33(2):148–155. doi: 10.1007/s00259-005-1887-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(JPG)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information Files.