Abstract
Hepatocellular carcinoma (HCC) presentation is heterogeneous necessitating a variety of therapeutic interventions with varying efficacies and associated prognoses. Poor prognostic patients often undergo non-curative palliative interventions including transarterial chemoembolization (TACE), sorafenib, chemotherapy, or purely supportive care. The decision to pursue one of many palliative interventions for HCC is complex and an economic evaluation comparing these interventions has not been done. This study evaluates the cost-effectiveness of non-curative palliative treatment strategies such as TACE alone or TACE+sorafenib, sorafenib alone, and non-sorafenib chemotherapy compared with no treatment or best supportive care (BSC) among patients diagnosed with HCC between 2007 and 2010 in a Canadian setting. Using person-level data, we estimated effectiveness in life years and quality-adjusted life years (QALYs) along with total health care costs (2013 US dollars) from the health care payer’s perspective (3% annual discount). A net benefit regression approach accounting for baseline covariates with propensity score adjustment was used to calculate incremental net benefit to generate incremental cost-effectiveness ratio (ICER) and uncertainty measures. Among 1,172 identified patients diagnosed with HCC, 4.5%, 7.9%, and 5.6%, received TACE alone or TACE+sorafenib, sorafenib, and non-sorafenib chemotherapy clone, respectively. Compared with no treatment or BSC (81.9%), ICER estimates for TACE alone or TACE+sorafenib was $6,665/QALY (additional QALY: 0.47, additional cost: $3,120; 95% CI: -$18,800-$34,500/QALY). The cost-effectiveness acceptability curve demonstrated that if the relevant threshold was $50,000/QALY, TACE alone or TACE+sorafenib, non-sorafenib chemotherapy, and sorafenib alone, would have a cost-effectiveness probability of 99.7%, 46.6%, and 5.5%, respectively. Covariates associated with the incremental net benefit of treatments are age, sex, comorbidity, and cancer stage. Findings suggest that TACE with or without sorafenib is currently the most cost-effective active non-curative palliative treatment approach to HCC. Further research into new combination treatment strategies that afford the best tumor response is needed.
Introduction
Liver cancer is the sixth most common cancer and the second leading cause of cancer-related death worldwide [1]. In Canada, hepatocellular carcinoma (HCC) incidence continues to rise and five-year relative survival rates remain poor (~20%) [2]. HCC accounts for the majority (~72%) of primary liver cancers in Canada [2]. Risk factors for HCC include cirrhosis, chronic hepatitis B or C infection, HIV co-infection, alcoholic- and non-alcoholic fatty liver disease, diabetes, obesity, and smoking [3,4].
Transarterial chemoembolization (TACE) is the standard of care for patients with intermediate-stage disease [5,6], and survival times and time to progression appear longer in patients with the combination of sorafenib and TACE [7–9]. Sorafenib, a multikinase inhibitor, as an oral form of systemic therapy for patients with advanced HCC has shown improved survival and time to progression in patients with advanced HCC; however, its use remains substantial financial burden [10–12]. There is mixed evidence regarding the cost-effectiveness of sorafenib using decision analytic Markov models. Studies using data from the SHARP trial [10] determined that sorafenib is cost-effective compared to best supportive care (BSC) with an incremental cost-effectiveness ratio (ICER) within the established willingness-to-pay threshold between $50,000 and $100,000/life year (LY) gained [13,14]. The Italian SOFIA study concluded that dose-adjusted sorafenib is cost-effective compared to BSC in intermediate and advanced HCC with an ICER of less than a threshold of €38,000/quality-adjusted life year (QALY) gained (~$50,000/QALY) [15]. Though, another study found that sorafenib is not a cost-effective treatment option for Chinese patients with advanced HCC [16].
Historically, HCC was diagnosed after developing symptoms which correlates with advanced-stage disease, limited therapeutic options, and poor prognosis [3,7]. Patients with a poor prognosis often undergo non-curative palliative treatments, including TACE, sorafenib, chemotherapy, or purely supportive care. The decision to pursue any of the aforementioned therapies is multifactorial and a systematic comparison of the financial impact of these interventions has not been done.
In this study, we aim to evaluate the cost-effectiveness of alternative non-curative palliative treatment interventions of TACE alone or TACE+sorafenib, sorafenib alone, and non-sorafenib chemotherapy alone compared with no treatment or BSC (i.e. symptom management to improve quality of life but no specific antineoplastic therapy) using person-level data from the Canadian health care perspective. Our cost-effectiveness analysis uses a net benefit regression approach and accounts for the fact that patients do not randomly receive non-curative palliative treatments in the real-world. Results from this study will examine the utility of TACE alone or TACE and sorafenib combination therapy as an anticancer agent and help inform health policy regarding the treatment of intermediate or advanced HCC.
Materials and methods
Study design and population
We identified all eligible HCC cases aged 18 years and older in Ontario diagnosed between January 1, 2007 and December 31, 2010. These HCC cases were used to estimate the cost, effectiveness, and cost-effectiveness of alternative non-curative palliative treatment strategies compared with no treatment or BSC to provide an estimate of the trade-off between extra cost and extra benefit as well as utilizing net benefit regression framework to estimate the incremental net benefit (INB). HCC cases were identified through the Ontario Cancer Registry (OCR). The International Statistical Classification of Disease and Related Health Problems, 9th Revision (ICD-9) site code 155.0, in combination with histology codes 8170–8175 of the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) were used to identify cases of primary liver cancer. Cases of primary liver cancer were identified only using ICD-9 coding due to the lack of ICD-10 C22 code in the dataset. Patients who had death dates before or on the HCC diagnosis date during the study period were excluded. Furthermore, potential curative treatments such as radiofrequency ablation, surgical resection, liver transplantation, and percutaneous ethanol injection for small HCC or unresectable liver cancer treatment were also excluded.
Data sources and study variables
The OCR is a provincial population-based cancer registry that contains information on all new cases of cancer (except for non-melanoma skin cancers) in Ontario since 1964 [17]. The OCR includes data for the date and stage of HCC diagnosis, age, sex, birth location, urban or rural residence, cause of death, and date of death. As in previous studies [18–21], we linked the OCR cohort to the Ontario Health Insurance Plan (OHIP) database, the Canadian Institute for Health Information Discharge Abstract Database, the Ontario Drug Benefit (ODB) program database, and the Canadian census data to provide person-level information on sociodemographic, screening, staging, treatment, and clinical factors. The OHIP physician billing claims contain service and diagnosis information for outpatient visits. The Discharge Abstract Database contains information relating to in-hospital procedures and diagnoses. The ODB dataset contains information regarding prescription medications (including sorafenib) dispensed to all adults aged 65 years and older and those receiving social assistance. Although there are some variances in different health care services, the system provides free access to hospital and emergency department visits, physician services, homecare, co-payments for long-term care placements, and prescription medications for those aged 65 years and older.
Area-level socio-economic status was quantified using median neighbourhood household income. Median neighbourhood household income was determined through linking of postal codes to Canadian census data; income was categorized into quintiles corresponding to income status of neighbourhoods. The income quintile 1 represents the lowest 20% of neighborhoods and income quintile 5 represents the most well-off 20% of neighbourhoods [18].
Where possible, hospitalization records from the date of diagnosis were used to assign each patient and control subject a baseline Charlson–Deyo comorbidity index. If patients did not have a hospitalization record at their diagnosis date, baseline comorbidity was determined by looking back 2 years into the hospitalization data to find the most recent hospitalization record; the comorbidity score from that hospitalization was then applied [18–21]. The Charlson–Deyo comorbidity index at baseline was marked as “missing” if the individual had no hospitalization records at diagnosis or during the 2 years before diagnosis. Comorbidity was adjusted for each hospitalization after baseline. The Charlson–Deyo comorbidity index was calculated using methods previously described [22,23]; an ICD-10 coding algorithm was applied to the diagnostic field codes from the hospitalization data (excluding diagnoses for liver disease, metastatic cancer, diabetes, and HIV). Conditions were weighted and then summed up to provide an overall comorbidity index value for a given episode, which was then categorized into one of five groups (0, 1, 2, ≥ 3, or no hospitalization record) representing different degrees of comorbidity.
Patients diagnosed with diabetes, HIV, and covariates that denote liver disease stage measured before HCC diagnosis were identified from the Discharge Abstract Database and OHIP using ICD-9 and ICD-10 codes. The study also included viral hepatitis cases identified through OHIP data; defined as subjects having at least two viral hepatitis visits (OHIP diagnostic code ‘070’) within the 4-year interval before the HCC diagnosis date ─ to cover as much available OHIP data as possible. Indicators of liver disease stage were categorized exclusively as: 1) viral hepatitis; 2) no cirrhosis; 3) cirrhosis; 4) alcoholic liver disease (ALD)+cirrhosis; 5) viral hepatitis+cirrhosis; 6) decompensated cirrhosis (i.e. cirrhosis and any recorded ascites, esophageal varices, or hepatic encephalopathy); 7) ALD+decompensated cirrhosis; 8) viral hepatitis+decompensated cirrhosis; and 9) ALD+viral hepatitis+decompensated cirrhosis.
To identify patients who received screening ultrasonography, we identified all abdominal ultrasonography performed on patients before HCC diagnosis utilizing OHIP fee codes [20]. We obtained exclusive data regarding receipt of abdominal ultrasound screening (at least 4.5 months apart from previous ultrasound), which was defined as receiving one or more ultrasound screening annually for 2 years before HCC diagnosis (i.e. routine screening), at least one screen either within 12 months or between 12–24 months before HCC diagnosis (i.e. inconsistent screening), and no screening before HCC diagnosis.
Classification of malignant tumors based on TNM staging [extent of the tumor (T), extent of spread to the lymph nodes (N), and presence of metastasis (M)] [24] was used in the OCR from 2004 onwards. TACE, non-sorafenib chemotherapy, and BSC were identified from the OHIP database and sorafenib was identified from the ODB database.
HCC treatment strategies
Mutually exclusive non-curative palliative treatment for HCC considered over the study period include: i) TACE alone or TACE+sorafenib; ii) sorafenib alone; iii) non-sorafenib chemotherapy alone; and iv) no treatment or BSC. The initial date of the first non-curative palliative treatment was considered the index date of treatment for HCC patients. Procedure codes used to identify diabetes, HIV, indicators of liver disease stage, HCC screening, and treatments can be found elsewhere [20,21].
Measuring effectiveness
Life expectancy for each age is the average period that a person may expect to live, according to the age-specific mortality rates for all causes [25]. LYs, QALYs, potential years of life lost (PYLL, a measure of premature mortality) and quality-adjusted life years lost (QALYL) were used to measure effectiveness. This study followed patients according to their death status until the end of year 2011. For those who died in or before 2011, age at death was calculated by adding years between diagnosis and death to the age at diagnosis. Age at diagnosis was recorded in the OCR data as a categorical variable: below 60, 60–69, 70–79, or 80 years and above, which was assumed to be 55, 65, 75, or 85 years, respectively in our analysis. To estimate age at death for patients who were still alive by the end of 2011, we first calculated the expected year of death using the year of diagnosis and the expected length of survival (i.e. period from diagnosis to death) according to stage at diagnosis from the published literature (e.g. early-stage I: 5 years; intermediate-stage II: 4 years; and advanced-stage III or IV: 3 years survival) [26,27]. If the expected year of death was 2011 or earlier, given the patient was still alive by the end of 2011, we assumed 2012 to be the most likely year of death. Accordingly, age at death could be estimated based on age at diagnosis and years between death and diagnosis. Subsequently, PYLL for each patient was determined using Ontario life tables which provided the standard life expectancy based on sex and age at death of an individual person [28].
QALYL consisted of two parts: 1) PYLL was weighted by the average health state utility should the person be still alive and without disease; and 2) number of years between diagnosis and death weighted by the quality of life according to stage of cancer (from normal utility to utility of HCC stage I, II, III, IV). Although we developed the year-specific model and considered treating stage as time-dependent, only stage at diagnosis was available in the database; we could not obtain whether patients progressed beyond their disease stage at diagnosis. Mean health state utilities of HCC by stage were derived from published literature and assumption for base case analysis and the lower and upper bounds for sensitivity analyses are shown in the S1 Table.
Measuring costs
Full details of data sources and estimation of direct health care costs associated with HCC have been previously published [19]. The total costs of health care services included outpatient visits, emergency department visits, acute inpatient hospitalizations, same-day surgery, prescription medications, homecare visits, continuing care, and long-term care. Costs associated with outpatient physician visits and laboratory tests in Ontario were estimated from the Physicians Claims History Database of the OHIP. Costs for emergency department visits and same-day surgery were estimated using the National Ambulatory Care Reporting System database [29]. Costs of hospitalization, emergency department visits, and same-day surgery for a particular year were estimated using the Resource Intensity Weight methodology developed by the Canadian Institute for Health Information [29–31]. Prescription medication costs were obtained from the ODB Program [29]. Costs associated with home care, continuing care, and long-term care were estimated from the Ontario Home Care database, Continuing Care Reporting System, and ODB Program. Costs were adjusted for inflation to 2013 Canadian dollars using the Statistics Canada Consumer Price Index for health care and personal items for Ontario [32]. Purchasing Power Parity for Gross Domestic Product was used to convert 2013 Canadian dollars to 2013 U.S. dollars [33]. Effects and costs were discounted at 3% annually as a base case to capture time preference given variation in follow-up time [34].
Statistical analysis
The net benefit regression framework [35] was used to evaluate the real-world cost-effectiveness of alternative non-curative palliative treatment strategies compared with no treatment or BSC among patients diagnosed with HCC from 2007 to 2010. In the first step, the net benefit value for each person (NBi) was calculated using the formula: willingness-to-pay threshold (λ)*Ei ‒ Ci, where Ei is the observed incremental effect (i.e. LY or QALY gained) and Ci is the incremental cost, for the ith person. Various values of λ for an additional effect were explored ranging from $0 to $500,000. NBi differs by various levels of λ; therefore, the person-level net benefit is denoted as NB(λ)i.
The net benefit regression (i.e. multiple linear regression) involved fitting a linear regression model while adjusting for the relevant covariates (dummy variables), including sociodemographic characteristics: age (< 60, 60–69, 70–79, ≥ 80 years); sex (male, female); income quintile (Q1-lowest to Q5-highest); residence (urban, rural); birth country (Canada, outside of Canada); clinical characteristics: Charlson-Deyo comorbidity index (0, 1, 2, ≥ 3); diabetes; HIV; liver disease stage (i.e. viral hepatitis; no cirrhosis; cirrhosis; ALD+cirrhosis; viral hepatitis+cirrhosis; decompensated cirrhosis; ALD+decompensated cirrhosis; viral hepatitis+decompensated cirrhosis; and ALD+viral hepatitis+decompensated cirrhosis); receipt of ultrasound screening 2 years before HCC diagnosis (routine screening, inconsistent screening, no screening); stage at diagnosis (early-stage I, intermediate-stage II, advanced-stage III-IV); and index year of HCC diagnosis (2007, 2008, 2009, 2010). Additionally, we adjusted for propensity score to minimize bias related to the non-random allocation of palliative treatment [36,37]. The propensity score for an individual is the conditional probability of assignment to having a palliative treatment of HCC given the observed individual covariates. Here, it was derived by fitting a logistic regression model with HCC non-curative palliative treatment as the dependent variable and the aforementioned covariates as independent variables. This approach allows the adjustment of how covariates may affect the estimate of the intervention’s INB (i.e. the marginal impact on ICER) [35]. To examine this, we employed an empirical model that interacts three treatment dummy variables with the covariates as follows:
where: NB(λ)i is the person-level NB; α is an intercept term; Ti is a treatment dummy, indicating whether person i received treatment (i.e. Ti = 1) or no treatment (i.e. Ti = 0); β is the coefficient estimate for the aforementioned covariates of interest x; γ is an interaction term between subject characteristic and the treatment indicator; and ε is a stochastic error term assumed to be normally distributed. The regression coefficient δ on the treatment dummy provides the estimate of the INB of treatment versus no treatment corresponding to a certain level of λ adjusted for the covariates. Treatment is defined to be cost-effective, at a certain level of λ, if the corresponding INB is positive (i.e. INB > 0). The INB was displayed visually by plotting the INB and its 95% confidence intervals (CIs) over the range of willingness-to-pay values.
Threshold values of the variance inflation factors were evaluated in the context of several other factors that influence the variance of regression coefficients [38]. We eliminated interaction terms if there was no statistical significance or if the variance inflation factor values exceeded 10 (i.e. indicating severe multicollinearity), which can reduce the variance of the regression coefficients. All covariates were included in the model because they were considered to be significant correlates of the outcome (theoretical justification) or were significantly different between the treatments (statistical validation). The final net benefit model comprised of:
The final step was assessing uncertainties and constructing cost-effectiveness acceptability curves (CEACs) using the coefficient estimates of the treatment (T) variable and p-values obtained from the net benefit regression model [39–42]. A CEAC shows the probability that an intervention is cost-effective compared with the alternative, over a range of threshold values that decision makers may be willing-to-pay for an additional unit of LY or QALY [35].
Sensitivity analysis
Lower and upper bounds health state utilities of HCC by stage (±25%) were used for cost-effectiveness sensitivity analyses (S1 Table). In addition, pooled mean health state utilities by liver disease stage (S2–S5 Tables) [43–51], health state utilities for incurable HCC (mean 0.40) [52] or after disease progression (0.68) [53] from published literature were used for cost-effectiveness sensitivity analyses. In addition, multiple imputation was used to impute values for variables with a significant portion of missing data. Variables which were imputed were income quintile (n = 6, 0.5%), birth country (n = 98, 8.4%), Charlson-Deyo comorbidity index (n = 261, 22.3%), and cancer stage at HCC diagnosis (n = 510, 43.5%). The observed important covariates considered were age, sex, index year of HCC diagnosis, and ultrasound screening. Five independent draws from an imputation model were used to create five completed data sets and results were combined to obtain one imputation inference. Multiple Imputation procedure by logistic regression was used in a sequential process to generate monotone patterns (PROC MI with LOGISTIC in the MONOTONE statement) [54].
Analyses were performed using the SAS (version 9.4: SAS Institute, Cary, NC, USA) and the STATA (version 12.0: Stata Corporation, College Station, TX) statistical software applications.
Ethics approval
Ethics approval for the study was granted by the University of Toronto Health Sciences Research Ethics Board. Informed consent was not obtained because this secondary analysis accessed existing de-identified data; consent was therefore deemed to be neither feasible nor necessary by the Research Ethics Board.
Results
Description of cohort
Overall, 2,012 patients were identified as having a primary diagnosis of HCC from the OCR between 2007 and 2010. A representative flow chart of the study population can be found in S1 Fig. The final study cohort comprised 1,172 patients diagnosed with HCC after excluding patients who had curative treatments (radiofrequency ablation, surgical resection, liver transplantation, and percutaneous ethanol injection; n = 784) and relatively small number of non-curative palliative treatments (i.e. sorafenib+chemotherapy, TACE+chemotherapy, and TACE+sorafenib+chemotherapy; n = 56). The median and mean follow-up time of patients diagnosed with HCC were 152.5 days and 304 (standard deviation 377) days, respectively. Overall baseline characteristics for this cohort are summarized in S6 Table and those stratified by treatment are summarized in Table 1. Fifty three (4.5%) patients diagnosed with HCC received TACE alone (n = 38) or TACE+sorafenib (n = 15), 93 (7.9%) patients received sorafenib alone, and 66 (5.6%) patients received non-sorafenib chemotherapy alone during the study period; however, 960 (81.9%) patients received BSC or did not receive any treatment. With regard to TACE+sorafenib dual treatments, TACE was the first-line treatment (100%) anywhere from 100 to 1605 days before receiving sorafenib. Of 1,172 patients, 5.7% were stage I, 9.0% stage II, 28.5% stage III, 13.2% stage IV, and 43.5% unknown stage at diagnosis (S6 Table). No cirrhosis or cirrhosis and cancer stage were associated with receipt of all types of treatments (P < 0.05); Urban/rural residence and comorbidity were associated with TACE alone or TACE+sorafenib treatment and chemotherapy alone (P < 0.05). Birth country and ultrasound screening were associated with TACE alone or TACE+sorafenib treatment (P < 0.001). Additionally, age group and year of HCC diagnosis were associated with sorafenib treatment (P < 0.01). Patients with unknown stage were less likely to have received non-curative palliative treatments.
Table 1. Baseline characteristics of patients with hepatocellular carcinoma by type of treatment, 2007–2010.
| No treatment or BSC |
TACE alone or TACE + Sorafenib |
Sorafenib alone | Non-sorafenib chemotherapy alone |
|
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Overall | 960 (81.9) | 53 (4.5) | 93 (7.9) | 66 (5.6) |
| Age group (years) | ||||
| <60 | 269 (28.0) | 13 (24.5) | 16 (17.2) | 25 (37.9) |
| 60–69 | 235 (24.5) | 14 (26.4) | 24 (25.8) | 18 (27.3) |
| 70–79 | 271 (28.2) | 19 (35.9) | 41 (44.1) | 17 (25.8) |
| 80+ | 185 (19.3) | 7 (13.2) | 12 (12.9) | 6 (9.1) |
| Sex | ||||
| Female | 207 (21.6) | 13 (24.5) | 15 (16.1) | 13 (19.7) |
| Male | 753 (78.4) | 40 (75.5) | 78 (83.9) | 53 (80.3) |
| Income quintile | ||||
| Q1 (lowest) | 273 (28.4) | 8 (15.1) | 22 (23.7) | 15 (22.7) |
| Q2 | 224 (23.3) | 10 (18.9) | 22 (23.7) | 18 (27.3) |
| Q3 | 145 (15.1) | 15 (28.3) | 11 (11.8) | 12 (18.2) |
| Q4 | 150 (15.6) | 9 (17.0) | 18 (19.4) | 11 (16.7) |
| Q5 (highest) | 163 (17.0) | 11 (20.8) | 20 (21.5) | 9 (13.6) |
| Missing | - | 0 | 0 | - |
| Residence | ||||
| Urban | 861 (89.7) | 50 (94.3) | 84 (90.3) | 52 (78.8) |
| Rural | 98 (10.2) | 3 (5.7) | 9 (9.7) | 14 (21.2) |
| Missing | - | 0 | 0 | 0 |
| Birth country | ||||
| Canada | 476 (49.6) | 14 (26.4) | 34 (36.6) | 40 (60.6) |
| Other | 413 (43.0) | 28 (52.8) | 48 (51.6) | 21 (31.8) |
| Unknown/Missing | 71 (7.4) | 11 (20.8) | 11 (11.8) | - |
| Charlson-Deyo comorbidity index | ||||
| 0 | 315 (32.8) | 22 (41.5) | 37 (39.8) | 27 (40.9) |
| 1 | 239 (24.9) | 21 (39.6) | 29 (31.2) | 15 (22.7) |
| 2 | 102 (10.6) | 7 (13.2) | 7 (7.5) | 14 (21.2) |
| 3+ | 62 (6.5) | - | 6 (6.5) | - |
| No hospitalization record | 242 (25.2) | 0 | 14 (15.1) | - |
| Diabetes diagnosis | 475 (49.5) | 21 (39.6) | 50 (53.8) | 27 (40.9) |
| HIV | 15 (1.6) | - | - | - |
| Indicators of liver disease stage | ||||
| Viral hepatitis | 29 (3.0) | - | - | - |
| No cirrhosis | 245 (25.5) | - | 37 (39.8) | 27 (40.9) |
| Cirrhosis | 159 (16.6) | 17 (32.1) | 14 (15.1) | - |
| ALD + Cirrhosis | 29 (3.0) | - | - | - |
| Viral hepatitis + Cirrhosis | 29 (3.0) | - | - | - |
| Decompensated cirrhosis | 245 (25.5) | 17 (32.1) | 22 (23.7) | 14 (21.2) |
| ALD + Decompensated cirrhosis | 137 (14.3) | - | - | 7 (10.6) |
| Viral hepatitis + Decompensated cirrhosis | 28 (2.9) | - | - | - |
| ALD + Viral Hepatitis + Decompensated cirrhosis | 28 (2.9) | - | 0 | 0 |
| Ultrasound screening 2 years before HCC diagnosis | ||||
| ≥1 screens annually | 57 (5.9) | 11 (20.8) | - | 7 (10.6) |
| Inconsistent screening | 351 (36.6) | 21 (39.6) | 30 (32.3) | 23 (34.9) |
| No screening | 552 (57.5) | 21 (39.6) | 58 (62.4) | 36 (54.6) |
| Stage at HCC diagnosis | ||||
| Early (stage I) | 51 (5.3) | 12 (22.6) | - | - |
| Intermediate (stage II) | 69 (7.2) | 14 (26.4) | 10 (10.8) | 13 (19.7) |
| Advanced (stage III-IV) | 372 (38.8) | 20 (37.7) | 64 (68.8) | 33 (50.0) |
| Unknown | 468 (48.8) | 7 (13.2) | 17 (18.3) | 18 (27.3) |
| Year of HCC diagnosis | ||||
| 2007 | 248 (25.8) | 13 (24.5) | 8 (8.6) | 14 (21.2) |
| 2008 | 210 (21.9) | 14 (26.4) | 17 (18.3) | 16 (24.2) |
| 2009 | 233 (24.3) | 13 (24.5) | 35 (37.6) | 23 (34.9) |
| 2010 | 269 (28.0) | 13 (24.5) | 33 (35.5) | 13 (19.7) |
n = 1,172.
‘‘-“, counts less than six have been suppressed.
TACE, transarterial chemoembolization; BSC, best supportive care (formal palliative care); HCC, hepatocellular carcinoma.
Health care effects and costs
Effects and costs after diagnosis of HCC stratified by treatment strategies are summarized in Table 2. The lowest QALYL was among those who received sorafenib alone (9.77, 95% CI: 9.01–10.53) and the highest QALYL was among those who received non-sorafenib chemotherapy alone (11.57, 95% CI: 10.58–12.57). The lowest costs were among those who did not receive treatment or BSC ($36,415, 95% CI: $33,782-$39,048), followed by those who received TACE alone or TACE+sorafenib ($45,638, 95% CI: $39,180-$52,096); and the highest cost was among those who received sorafenib alone ($53,198, 95% CI: $44,941-$61,456), followed by those who received chemotherapy alone ($51,657, 95% CI: $38,913-$64,402).
Table 2. Health care effects and costs after diagnosis of hepatocellular carcinoma by treatment strategies, 2007–2010.
| Treatment strategies | Effects (mean, 95% CI) | Costs* (mean, 95% CI) | |
|---|---|---|---|
| PYLL | QALYL | ||
| No Treatment or BSC (n = 960) | 11.5710 (11.2764–11.8655) | 10.6226 (10.3531–10.8921) | $36,415 ($33,782-$39,048) |
| TACE alone or TACE + Sorafenib (n = 53) | 10.7860 (9.5982–11.9739) | 10.0879 (9.0078–11.1680) | $45,638 ($39,180-$52,096) |
| Non-sorafenib chemotherapy alone (n = 66) | 12.4255 (11.3347–13.5163) | 11.5722 (10.5770–12.5675) | $51,657 ($38,913-$64,402) |
| Sorafenib alone (n = 93) | 10.4988 (9.6655–11.3320) | 9.7664 (9.0062–10.5266) | $53,198 ($44,941-$61,456) |
*All costs reflect 2013 US$ per person.
BSC, best supportive care; TACE, transarterial chemoembolization; CI, confidence intervals; PYLL, potential years of life lost (a measure of premature mortality); QALYL, quality-adjusted life years lost.
Net benefit regression
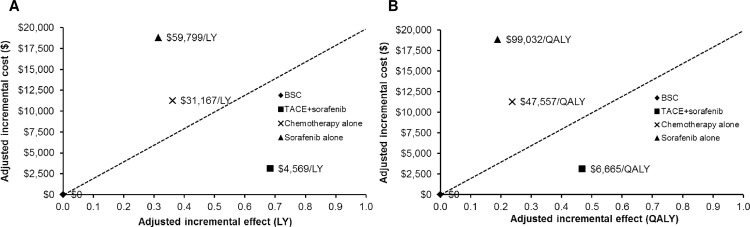
Compared with no treatment or BSC (adjusted for important covariates), TACE alone or TACE+sorafenib was estimated to yield the highest incremental QALYs (incremental QALYs = 0.47), followed by chemotherapy alone (0.24) and sorafenib alone (0.19). Among treatments, TACE alone or TACE+sorafenib was more effective and slightly more costly than no treatment or BSC (Table 3). Fig 1A and 1B demonstrate plots of incremental effects (LYs and QALYs) and incremental costs of treatments relative to the lowest cost scenario (no treatment or BSC), TACE alone or TACE+sorafenib treatments below the line (dotted diagonal line representing the ceiling ratio) appeared to be acceptable.
Table 3. Adjusted incremental effects, incremental costs, and incremental cost-effectiveness ratios of non-curative palliative treatment strategies for hepatocellular carcinoma compared with no treatment or best supportive care, 2007–2010: net benefit regression.
| Treatment Strategies | Mean LYs | Mean QALYs | Mean Total Effect (PYLL) | Mean Total Effect (QALYL) | MeanTotal Cost ($) | Adjusted Incremental Effect* (LYs) | Adjusted Incremental Effect* (QALYs) | Adjusted Incremental Cost ($)† | Adjusted ICER ($/LY gained) | Adjusted ICER ($/QALY gained) |
|---|---|---|---|---|---|---|---|---|---|---|
| No treatment or BSC | 0.7034 | 0.5422 | 11.5710 | 10.6226 | $36,415 | |||||
| TACE alone or TACE + Sorafenib | 1.6715 | 1.2828 | 10.7860 | 10.0879 | $45,638 | 0.68283 | 0.46815 | $3,120 | $4,569 | $6,665 |
| Non-sorafenib chemotherapy alone | 1.3314 | 0.9628 | 12.4255 | 11.5722 | $51,657 | 0.36137 | 0.23683 | $11,263 | $31,167 | $47,557 |
| Sorafenib alone | 1.3370 | 0.9474 | 10.4988 | 9.7664 | $53,198 | 0.31474 | 0.19005 | $18,821 | $59,799 | $99,032 |
*Incremental effect is calculated as treatment effect minus no treatment or BSC effect, adjusted for relevant covariates (dummy variables), including age, sex, income quintile, urban/rural residence, birth country, Charlson-Deyo comorbidity index, diabetes, HIV, indicators of liver disease stage, ultrasound screening, stage at HCC diagnosis, and year of HCC diagnosis. Positive value indicates increase in the effect relative to “no treatment or BSC”.
†Incremental cost is calculated as treatment cost minus no treatment or BSC cost, adjusted for aforementioned covariates. Positive value indicates increase in cost relative to “no treatment or BSC”. Values are expressed as the mean. All costs reflect 2013 US$ per person.
BSC, best supportive care (formal palliative care); TACE, transarterial chemoembolization; PYLL, potential years of life lost; QALYL, quality-adjusted life years lost; LY, life year; QALY, quality-adjusted life years.
Fig 1.
A and B. Efficiency frontier: plot of incremental (A) life years (LYs) and (B) quality-adjusted life years (QALYs) and costs of non-curative palliative treatments: i) transarterial chemoembolization (TACE) alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or best supportive care [BSC]). The dotted diagonal line represents the ceiling ratio. If an intervention lies above the line, it will not be acceptable on cost-effectiveness grounds.
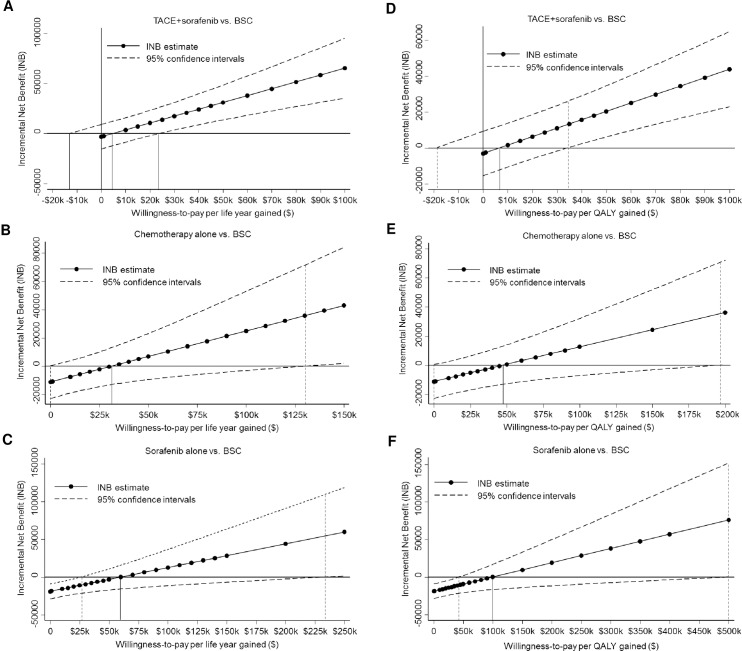
Fig 2A–2C (LYs) and Fig 2D–2F (QALYs) show estimates of INB (i.e. ICER estimate) and its 95% CIs as a function of willingness-to-pay threshold. The lowest ICER estimates for TACE alone or TACE+sorafenib were $6,665/QALY gained (95% CI: -$18,800-$34,500/QALY). Alternative ICER estimates in order were: for chemotherapy, $47,557/QALY (95% CI: $0-$196,500/QALY); and sorafenib alone, $99,032/QALY (95% CI: $42,500-$500,000/QALY).
Fig 2.
A-F. Estimates of incremental net benefit (i.e. incremental cost-effectiveness ratio, ICER) and its 95% confidence intervals as a function of willingness-to-pay threshold for an additional life year: (A) transarterial chemoembolization (TACE) alone or TACE+sorafenib vs. no treatment or best supportive care (BSC); (B) non-sorafenib chemotherapy alone vs. BSC; and (C) sorafenib alone vs. BSC; and for an additional quality-adjusted life year (QALY): (D) TACE alone or TACE+sorafenib vs. BSC; (E) non-sorafenib chemotherapy alone vs. BSC; and (F) sorafenib alone vs. BSC.
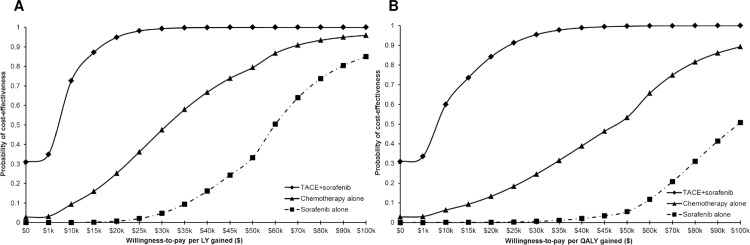
Fig 3A and 3B show CEACs which plot the probability that each treatment strategy is cost-effective compared with no treatment or BSC as a function of willingness-to-pay threshold for an additional LY and QALY, respectively (S7 Table and Table 4). The results showed that if with a threshold of $50,000/QALY gained, TACE alone or TACE+sorafenib treatments would have a cost-effectiveness probability of 99.7%; chemotherapy and sorafenib treatments would have a cost-effectiveness probability of 53.4% and 5.5%, respectively (Fig 3B and Table 4). If a threshold of $100,000/QALY gained was to be chosen, TACE alone or TACE+sorafenib treatments would have a cost-effectiveness probability of 100%, and chemotherapy and sorafenib alone would have a cost-effectiveness probability of 89.3% and 50.9%, respectively (Fig 3B and Table 4).
Fig 3.
A and B. Cost-effectiveness acceptability curves showing the probability that each non-curative palliative treatment strategy: i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; or iii) sorafenib alone is cost-effective compared with no treatment or BSC for a given willingness-to-pay threshold for an additional (A) life year (LY); and (B) quality adjusted life year (QALY).
Table 4. Estimates of incremental net benefit and probability of cost-effectiveness of non-curative palliative treatment strategies for hepatocellular carcinoma compared with no treatment or best supportive care as a function of willingness-to-pay threshold per additional QALY over the study period 2007–2010.
| λ thresholds | TACE alone or TACE + Sorafenib | Sorafenib alone | Non-sorafenib chemotherapy alone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| INB estimate (SE) | P-value* | Probability of cost-effectiveness | INB estimate (SE) | P-value* | Probability of cost-effectiveness | INB estimate (SE) | P-value* | Probability of cost-effectiveness | |
| $0 | -3120 (6284) | 0.310 | 31.0% | -18821 (5049) | <0.001 | 0% | -11263 (5898) | 0.028 | 2.8% |
| $1,000 | -2652 (6267) | 0.336 | 33.6% | -18631 (5035) | <0.001 | 0% | -11026 (5883) | 0.031 | 3.1% |
| $10,000 | 1561 (6183) | 0.400 | 60.0% | -16920 (4968) | <0.001 | 0% | -8895 (5804) | 0.063 | 6.3% |
| $20,000 | 6243 (6236) | 0.159 | 84.1% | -15020 (5010) | 0.001 | 0.2% | -6526 (5853) | 0.133 | 13.3% |
| $30,000 | 10924 (6438) | 0.045 | 95.5% | -13119 (5172) | 0.006 | 0.6% | -4158 (6043) | 0.246 | 24.6% |
| $40,000 | 15606 (6776) | 0.011 | 98.9% | -11219 (5444) | 0.020 | 2.0% | -1790 (6361) | 0.389 | 38.9% |
| $50,000 | 20288 (7232) | 0.003 | 99.7% | -9318 (5810) | 0.055 | 5.5% | 579 (6788) | 0.466 | 53.4% |
| $60,000 | 24969 (7784) | 0.001 | 99.9% | -7418 (6254) | 0.118 | 11.8% | 2947 (7306) | 0.343 | 65.7% |
| $70,000 | 29651 (8413) | <0.001 | 100% | -5517 (6760) | 0.207 | 20.7% | 5315 (7897) | 0.251 | 74.9% |
| $80,000 | 34332 (9104) | <0.001 | 100% | -3617 (7315) | 0.311 | 31.1% | 7683 (8546) | 0.185 | 81.6% |
| $90,000 | 39014 (9844) | <0.001 | 100% | -1716 (7909) | 0.414 | 41.4% | 10052 (9241) | 0.139 | 86.1% |
| $100,000 | 43695 (10622) | <0.001 | 100% | 184 (8534) | 0.491 | 50.9% | 12420 (9971) | 0.107 | 89.3% |
*one-sided P-value.
TACE, transarterial chemoembolization; BSC, best supportive care (formal palliative care); QALY, quality-adjusted life year; λ, willingness-to-pay; INB, incremental net benefit; SE, standard error.
Sensitivity analysis
Plots of incremental effects (QALYs) and incremental costs of treatments relative to the lowest cost scenario (no treatment or BSC) according to the lower (-25%) and upper bound (+25%) health state utilities of cancer stage showed that TACE alone or TACE+sorafenib treatments appeared to be acceptable, similar to base case (S2 Fig). Similarly, when using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression, TACE alone or TACE+sorafenib was found to be the lowest ICER estimates $4,152/QALY gained (95% CI: -$11,050-$20,900/QALY), followed by chemotherapy alone, $28,291/QALY (95% CI: $0-$70,000/QALY) and sorafenib alone, $50,569/QALY (95% CI: $23,800-$96,800/QALY) (S3–S5 Figs).After multiple imputation for variables of missing data such as income quintile, birth country, Charlson-Deyo comorbidity index, and cancer stage at EAC diagnosis and adjusting for confounding covariates, plots of incremental effects and costs of treatments relative to no treatment or BSC, TACE alone or TACE+sorafenib treatments appeared to be acceptable (S6 Fig). Additionally, ICER estimates in order were for: TACE alone or TACE+sorafenib, $16,206/QALY gained (95% CI: -$5,800-$45,000/QALY); non-sorafenib chemotherapy alone, $47,881/QALY (95% CI: $12,500-$153,000/QALY); and sorafenib alone, $75,128/QALY (95% CI: $37,500-$205,500/QALY) (S7 Fig). The CEACs showed that if a threshold of $50,000/QALY gained was to be chosen, TACE alone or TACE+sorafenib treatments would have a cost-effectiveness probability of 98.7%; non-sorafenib chemotherapy and sorafenib alone would have a cost-effectiveness probability of 53.8% and 12.3%, respectively. If a threshold of $100,000/QALY gained was to be chosen, TACE alone or TACE+sorafenib treatments would have a cost-effectiveness probability of 100%, and chemotherapy and sorafenib alone would have a cost-effectiveness probability of 92.3% and 76.5%, respectively (S8 Fig).
After multiple imputation, when TACE alone was used as a separate treatment, TACE+sorafenib was estimated to yield the highest adjusted incremental QALYs (0.80), followed by TACE alone (0.37), non-sorafenib chemotherapy (0.29), and sorafenib alone (0.26) compared with no treatment or BSC. In contrast, TACE alone was estimated to yield the lowest adjusted incremental cost ($1,494), followed by chemotherapy ($13,825), sorafenib alone ($19,706), and TACE+sorafenib ($24,420). Finally, TACE alone was found to be the most cost-effective strategy (ICER: $4,053/QALY, 95% CI -$30,000-$50,000/QALY) followed by TACE+sorafenib (ICER: $30,622/QALY, 95% CI $4,700-$71,000/QALY), chemotherapy (ICER: $47,911/QALY, 95% CI $13,000-$150,000/QALY), and sorafenib alone (ICER: $74,941/QALY, 95% CI $37,500-$204,000). See S8 Table, S9 Fig, S10 Fig and S11 Fig.
Discussion
This study evaluated the real-world cost-effectiveness of non-curative palliative oncologic treatments such as TACE alone or TACE plus sorafenib, sorafenib alone, and non-sorafenib chemotherapy as compared to no treatment or BSC among patients diagnosed with HCC. Our results suggest that sorafenib treatment is the most widely used palliative treatment in advanced-stage HCC patients (68.8%), followed by chemotherapy (50%), and TACE alone or TACE plus sorafenib (37.7%). Compared with no treatment or BSC, the adjusted incremental benefit of TACE alone or TACE+sorafenib has been estimated to yield more units of LYs and QALYs and less cost than other non-curative palliative treatment options. The ICER of TACE alone or TACE+sorafenib treatment develops cost-effectiveness at a threshold of $6,665/QALY (95% CI: -$18,800-$34,500/QALY) which is below the commonly cited threshold per QALY of $50,000 [55]. The CEACs show that if a threshold of $50,000/QALY gained is to be chosen, TACE alone or TACE plus sorafenib would have a cost-effectiveness probability of 99.7%. Our cost-effectiveness results provide evidence that TACE alone or TACE plus sorafenib treatment appears to be acceptable treatment for patients with potential intermediate- or advanced-stage HCC in Ontario.
Non-curative palliative treatments are provided with a hope of providing HCC patients prolonged survival and improved quality of life. Unfortunately, advances have been modest. Although drug eluting beads theoretically could improve the efficacy and safety of TACE, a recent clinical and economic impact of drug eluting beads in TACE was unable to show improved prognosis in patients with unresectable HCC [56]. A recent meta-analysis evaluating the efficacy and safety of the combination therapy of TACE plus sorafenib in patients with intermediate- or advanced-stage of HCC suggests improved overall survival and time to progression, with tolerable toxicity compared to TACE alone [9]. Additionally, there is some evidence that TACE with adjuvant sorafenib is superior to sorafenib alone with respect to time to progression in patients with advanced-stage HCC [57]. A recent randomised controlled trial showed that regorafenib, an oral multikinase inhibitor, is the only systemic treatment shown to provide survival benefit in HCC patients progressing on first-line sorafenib treatment. This finding is associated with an increase in median survival from 7.8 months to 10.6 months [58].
HCC associated with chronic viral hepatitis has attendant increased rates of disease recurrence and poor survival. Control of hepatitic viral replication is an important prognostic intervention for infected patients, especially given recent advances in novel antiviral therapies [59]. Optimal outcomes in the cost effectiveness of HCC treatment necessitate patients at risk of HCC be diagnosed early and referred for treatment in a timely manner, leading to a better prognosis with multidisciplinary involvement.
The advantage of using net benefit framework in our study is that influential covariates can be adjusted for in the regression model to obtain a more accurate INB [35]. This NBR found several covariates associated with INB (P < 0.05), including age group and sex (from λ $10,000 to λ $100,000), Charlson-Deyo comorbidity index (from λ $0 to λ $100,000), and intermediate and advanced cancer stage (from λ $60,000 to λ $100,000).
A sensitivity analysis of multiple imputation for variables of missing data and adjusting for confounding covariates appeared robust to the base case relating to the incremental effects and costs and ICER of non-curative palliative treatments relative to no treatment or BSC. The effect and cost estimates through multiple imputation would be well suited to analyses of administrative claims data in which some covariates are missing.
There are a number of limitations in this study that should be noted. First, there is possibility of confounding by indication involving factors that are not observed, measured, or captured in routinely collected data in administrative health databases. Utilization of non-curative palliative treatment for HCC such as TACE, sorafenib, and chemotherapy seem low. The management of HCC is complex due to the underlying conditions which needs to be performed in a multidisciplinary approach [60,61]. Second, our analysis included a “sorafenib alone” sub-group based on the information available to us in our databases. While it is true that sorafenib is not publicly funded for those under 65 years of age, there is no recorded data for this sub-population within our databases and thus further investigation on this group would not be possible without significant speculation and assumption. The authors feel that our current analysis of patients receiving sorafenib alone, despite being limited by the aforementioned factors, remain generalizable to other populations globally as it provides a cost-effectiveness assessment of patients receiving sorafenib with large numbers in each subgroup. Third, our analysis considered only one course of each palliative monotherapy among patients receiving multiple courses. A recent study in North America found that Medicare expenditures doubled between receiving one and four or more TACE treatments, but expenses were distributed over more than an additional year of life [62]. Fourth, in our study, the majority of patients did not receive ultrasound screening (56.9%), especially those receiving sorafenib (62.4%), chemotherapy (54.6%), or BSC (57.5%). The lack of screening may have represented a missed opportunity for more curative treatment options. Lastly, this analysis was not able to include patients who may also have undergone combined or sequential treatment modalities (e.g. curative and non-curative palliative treatments) which are effective in improving the outcome of patients with HCC.
Conclusion
In summary, our data shows that compared with no treatment or BSC, TACE alone or TACE and sorafenib combination treatment has the largest incremental benefit and is cost-effective if a threshold of $50,000/QALY gained is to be chosen, making it the preferred strategy for patients with intermediate- or advanced-stage HCC. Further research into new combination treatment strategies that afford the best tumor response and cost-effectiveness analysis of such new treatments are needed to dictate policy for this difficult to manage disease.
Supporting information
(TIF)
i) transarterial chemoembolization (TACE) alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or best supportive care [BSC]): Sensitivity analysis according to (A) lower bound (-25%) and (B) upper bound (+25%) of mean health state utilities of disease stage according to published literature and assumption. The dotted diagonal line represents the ceiling ratio. If an intervention lies above the line, it will not be acceptable on cost-effectiveness grounds.
(TIF)
Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or BSC). Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
Efficiency frontier: plot of incremental (A) life years (LYs) and (B) quality-adjusted life years (QALYs) and costs of non-curative palliative treatments: i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or BSC): Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(A) TACE alone or TACE+sorafenib vs. no treatment or BSC; (B) non-sorafenib chemotherapy alone vs. BSC; and (C) sorafenib alone vs. BSC; and for an additional QALY: (D) TACE alone or TACE+sorafenib vs. BSC; (E) non-sorafenib chemotherapy alone vs. BSC; and (F) sorafenib alone vs. BSC. Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; or iii) sorafenib alone is cost-effective compared with no treatment or BSC for a given willingness-to-pay threshold for an additional (A) life year (LY); and (B) quality adjusted life year (QALY). Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
Efficiency frontier: plot of incremental (A) life years (LYs) and (B) quality-adjusted life years (QALYs) and costs of non-curative palliative treatments: i) TACE alone; ii) TACE+sorafenib; iii) non-sorafenib chemotherapy alone; and iv) sorafenib alone relative to lowest cost scenario (no treatment or BSC): Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(A) TACE alone vs. no treatment or BSC; (B) TACE+sorafenib vs. no treatment or BSC; (C) non-sorafenib chemotherapy alone vs. BSC; and (D) sorafenib alone vs. BSC. Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
i) TACE alone; ii) TACE+sorafenib; iii) non-sorafenib chemotherapy alone; or iv) sorafenib alone is cost-effective compared with no treatment or BSC for a given willingness-to-pay threshold for an additional (A) life year (LY); and (B) quality adjusted life year (QALY). Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank Matthew Kumar, Nelson Chong, and Refik Saskin from the Institute for Clinical and Evaluative Sciences for data linkage. The authors are also grateful to Katrina Chan from the Ontario Institute for Cancer Research and Cancer Care Ontario for assistance in data acquisition.
Abbreviations
- HCC
hepatocellular carcinoma
- TACE
transarterial chemoembolization
- BSC
best supportive care
- ICER
incremental cost-effectiveness ratio
- LY
life year
- QALY
quality-adjusted life year
- INB
incremental net benefit
- OCR
Ontario Cancer Registry
- ICD-9
International Statistical Classification of Disease and Related Health Problems, 9th Revision
- OHIP
Ontario Health Insurance Plan
- ODB
Ontario Drug Benefit
- ALD
alcoholic liver disease
- PYLL
potential years of life lost
- QALYL
quality-adjusted life years lost
- CEAC
cost-effectiveness acceptability curve
- CI
confidence interval
Data Availability
The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). The data are administrative claims from Ontario's public health care system. The policies around access are put in place to comply with provincial privacy legislation and the Privacy Commissioner of Ontario. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan is available from the authors (attached as Supporting Information).
Funding Statement
This study was supported through provision of data by the Institute for Clinical Evaluative Sciences and Cancer Care Ontario and through funding support to Institute for Clinical Evaluative Sciences from an annual grant by the Ministry of Health and Long-Term Care, and from the Ontario Institute for Cancer Research. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by Institute for Clinical Evaluative Sciences, Cancer Care Ontario, Ontario Institute for Cancer Research or the Government of Ontario is intended or should be inferred. Hla-Hla Thein received a New Investigator Award IA-034 from the Ontario Institute for Cancer Research Health Services Research Program.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. Available at: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/canadian-cancer-statistics-2013-EN.pdf. Accessed May 1, 2015. [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127. doi: 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011; 53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nature reviews Disease primers. 2016; 2:16018 doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016; 150:835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 8.Fu QH, Zhang Q, Bai XL, Hu QD, Su W, Chen YW, et al. Sorafenib enhances effects of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014; 140:1429–1440. doi: 10.1007/s00432-014-1684-5 [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014; 9:e100305 doi: 10.1371/journal.pone.0100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 11.Peng S, Zhao Y, Xu F, Jia C, Xu Y, Dai C. An updated meta-analysis of randomized controlled trials assessing the effect of sorafenib in advanced hepatocellular carcinoma. PLoS One. 2014; 9:e112530 doi: 10.1371/journal.pone.0112530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih YT, Xu Y, Liu L, Smieliauskas F. Rising Prices of Targeted Oral Anticancer Medications and Associated Financial Burden on Medicare Beneficiaries. J Clin Oncol. 2017:JCO2017723742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010; 25:1739–1746. doi: 10.1111/j.1440-1746.2010.06404.x [DOI] [PubMed] [Google Scholar]
- 14.Muszbek N, Shah S, Carroll S, McDonald H, Dale P, Maroun J, et al. Economic evaluation of sorafenib in the treatment of hepatocellular carcinoma in Canada. Curr Med Res Opin. 2008; 24:3559–3569. doi: 10.1185/03007990802563706 [DOI] [PubMed] [Google Scholar]
- 15.Camma C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013; 57:1046–1054. doi: 10.1002/hep.26221 [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Yang Y, Wen F, He X, Tang R, Du Z, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015; 27:853–859. doi: 10.1097/MEG.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 17.Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991:246–257. [PubMed] [Google Scholar]
- 18.Jembere N, Campitelli MA, Sherman M, Feld JJ, Lou W, Peacock S, et al. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population; a population-based study, 1990–2009. PLoS One. 2012; 7:e40917 doi: 10.1371/journal.pone.0040917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thein HH, Isaranuwatchai W, Campitelli MA, Feld JJ, Yoshida E, Sherman M, et al. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatology. 2013; 58:1375–1384. doi: 10.1002/hep.26231 [DOI] [PubMed] [Google Scholar]
- 20.Thein HH, Campitelli MA, Yeung LT, Zaheen A, Yoshida EM, Earle CC. Improved Survival in Patients with Viral Hepatitis-Induced Hepatocellular Carcinoma Undergoing Recommended Abdominal Ultrasound Surveillance in Ontario: A Population-Based Retrospective Cohort Study. PLoS One. 2015; 10:e0138907 doi: 10.1371/journal.pone.0138907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thein HH, Qiao Y, Young SK, Zarin W, Yoshida EM, de Oliveira C, et al. Trends in health care utilization and costs attributable to hepatocellular carcinoma, 2002–2009: a population-based cohort study. Curr Oncol. 2016; 23:e196–220. doi: 10.3747/co.23.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 24.Fleming ID. AJCC/TNM cancer staging, present and future. J Surg Oncol. 2001; 77:233–236. [DOI] [PubMed] [Google Scholar]
- 25.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008; 372:293–299. doi: 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camma C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008; 28:62–75. doi: 10.1111/j.1365-2036.2008.03692.x [DOI] [PubMed] [Google Scholar]
- 27.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005; 41:707–716. doi: 10.1002/hep.20636 [DOI] [PubMed] [Google Scholar]
- 28.Life Tables, Canada, Provinces and Territories, 2000 to 2002. Statistics Canada—Catalogue No. 84-537-XIE. Minister of Industry, 2006. Available at: http://www.prdh.umontreal.ca/BDLC/data/pdfs/84-537-XIE_(00-02).pdf. Accessed January 25, 2015.
- 29.Jacobs P, Yim R. Using Canadian administrative databases to derive economic data for health technology assessments Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. Available at: https://www.cadth.ca/media/pdf/H0483_Canadian_Admin_Databases_mg_e.pdf. Accessed 19 January, 2017. [Google Scholar]
- 30.Pink GH, Bolley HB. Physicians in health care management: 3. Case Mix Groups and Resource Intensity Weights: an overview for physicians. CMAJ. 1994; 150:889–894. [PMC free article] [PubMed] [Google Scholar]
- 31.Pink GH, Bolley HB. Physicians in health care management: 4. Case Mix Groups and Resource Intensity Weights: physicians and hospital funding. CMAJ. 1994; 150:1255–1261. [PMC free article] [PubMed] [Google Scholar]
- 32.Statistics Canada, CANSIM, table 326–0021 and Catalogue nos. 62-001-X and 62-010-X. [Available online at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ09a-eng.htm; cited 29 January 2015].
- 33.Organization for Economic Co-Operation and Development. PPPs and exchange rates. 2015. Available from: http://stats.oecd.org/Index.aspx?DatasetCode=SNA_TABLE4. Accessed April 22, 2015.
- 34.Siegel JE, Torrance GW, Russell LB, Luce BR, Weinstein MC, Gold MR. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost Effectiveness in Health and Medicine. Pharmacoeconomics. 1997; 11:159–168. [DOI] [PubMed] [Google Scholar]
- 35.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002; 11:415–430. doi: 10.1002/hec.678 [DOI] [PubMed] [Google Scholar]
- 36.D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score J Am Stat Assoc 1984; 79:516–524. [Google Scholar]
- 38.O'Brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual Quant 2007; 41:673–690. [Google Scholar]
- 39.van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994; 3:309–319. [DOI] [PubMed] [Google Scholar]
- 40.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006; 6:52 doi: 10.1186/1472-6963-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves—facts, fallacies and frequently asked questions. Health Econ. 2004; 13:405–415. doi: 10.1002/hec.903 [DOI] [PubMed] [Google Scholar]
- 42.Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of "community acquired" syncope. BMC Health Serv Res. 2006; 6:68 doi: 10.1186/1472-6963-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003; 98:630–638. [DOI] [PubMed] [Google Scholar]
- 44.Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Arch Intern Med. 2004; 164:2377–2382. doi: 10.1001/archinte.164.21.2377 [DOI] [PubMed] [Google Scholar]
- 45.Siebert U, Sieberer R, Greiner W, et al. Patient-based health-related quality of life in different stages of chronic hepatitis C [Abstract]. Hepatology 2001;34(Pt 2): AB222A. [Google Scholar]
- 46.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008; 28:582–592. doi: 10.1177/0272989X08315240 [DOI] [PubMed] [Google Scholar]
- 47.Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001; 96:579–583. doi: 10.1111/j.1572-0241.2001.03537.x [DOI] [PubMed] [Google Scholar]
- 48.Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006; 10:1–113, iii. [DOI] [PubMed] [Google Scholar]
- 49.Hsu PC, Federico CA, Krajden M, Yoshida EM, Bremner KE, Anderson FH, et al. Health utilities and psychometric quality of life in patients with early- and late-stage hepatitis C virus infection. J Gastroenterol Hepatol. 2012; 27:149–157. doi: 10.1111/j.1440-1746.2011.06813.x [DOI] [PubMed] [Google Scholar]
- 50.Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008; 11:527–538. doi: 10.1111/j.1524-4733.2007.00297.x [DOI] [PubMed] [Google Scholar]
- 51.Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DK, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. 2012; 26:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013; 59:300–307. doi: 10.1016/j.jhep.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 53.NICE technology appraisal guidance 189—Sorafenib for the treatment of advanced hepatocellular carcinoma 2010. Available at: https://www.nice.org.uk/guidance/ta189. Accessed Jul7 17, 2017.
- 54.Kimberly Ault. Multiple Imputation for Ordinal Variables: A Comparison of SUDAAN PROC IMPUTE and SAS® PROC MI. SESUG 2012. Available at: http://analytics.ncsu.edu/sesug/2012/SD-12.pdf; Accessed May 9, 2016.
- 55.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014; 371:796–797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 56.Vadot L, Boulin M, Guiu B, Aho LS, Vourc'h M, Musat A, et al. Clinical and economic impact of drug eluting beads in transarterial chemoembolization for hepatocellular carcinoma. J Clin Pharm Ther. 2015; 40:83–90. doi: 10.1111/jcpt.12230 [DOI] [PubMed] [Google Scholar]
- 57.Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013; 269:603–611. doi: 10.1148/radiol.13130150 [DOI] [PubMed] [Google Scholar]
- 58.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 59.Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol. 2015; 7:673–687. doi: 10.4254/wjh.v7.i4.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barone C, Koeberle D, Metselaar H, Parisi G, Sansonno D, Spinzi G. Multidisciplinary approach for HCC patients: hepatology for the oncologists. Ann Oncol. 2013; 24 Suppl 2:ii15–23. [DOI] [PubMed] [Google Scholar]
- 61.Burak KW, Sherman M. Hepatocellular carcinoma: Consensus, controversies and future directions. A report from the Canadian Association for the Study of the Liver Hepatocellular Carcinoma Meeting. Can J Gastroenterol Hepatol. 2015; 29:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breunig IM, Shaya FT, Hanna N, Seal B, Chirikov VV, Daniel Mullins C. Transarterial chemoembolization treatment: association between multiple treatments, cumulative expenditures, and survival. Value Health. 2013; 16:760–768. doi: 10.1016/j.jval.2013.03.1630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
i) transarterial chemoembolization (TACE) alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or best supportive care [BSC]): Sensitivity analysis according to (A) lower bound (-25%) and (B) upper bound (+25%) of mean health state utilities of disease stage according to published literature and assumption. The dotted diagonal line represents the ceiling ratio. If an intervention lies above the line, it will not be acceptable on cost-effectiveness grounds.
(TIF)
Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or BSC). Sensitivity analyses using pooled mean health state utilities by liver disease stage, health state utilities for incurable HCC or after disease progression.
(TIF)
Efficiency frontier: plot of incremental (A) life years (LYs) and (B) quality-adjusted life years (QALYs) and costs of non-curative palliative treatments: i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; and iii) sorafenib alone relative to lowest cost scenario (no treatment or BSC): Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(A) TACE alone or TACE+sorafenib vs. no treatment or BSC; (B) non-sorafenib chemotherapy alone vs. BSC; and (C) sorafenib alone vs. BSC; and for an additional QALY: (D) TACE alone or TACE+sorafenib vs. BSC; (E) non-sorafenib chemotherapy alone vs. BSC; and (F) sorafenib alone vs. BSC. Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
i) TACE alone or TACE+sorafenib; ii) non-sorafenib chemotherapy alone; or iii) sorafenib alone is cost-effective compared with no treatment or BSC for a given willingness-to-pay threshold for an additional (A) life year (LY); and (B) quality adjusted life year (QALY). Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
Efficiency frontier: plot of incremental (A) life years (LYs) and (B) quality-adjusted life years (QALYs) and costs of non-curative palliative treatments: i) TACE alone; ii) TACE+sorafenib; iii) non-sorafenib chemotherapy alone; and iv) sorafenib alone relative to lowest cost scenario (no treatment or BSC): Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(A) TACE alone vs. no treatment or BSC; (B) TACE+sorafenib vs. no treatment or BSC; (C) non-sorafenib chemotherapy alone vs. BSC; and (D) sorafenib alone vs. BSC. Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
i) TACE alone; ii) TACE+sorafenib; iii) non-sorafenib chemotherapy alone; or iv) sorafenib alone is cost-effective compared with no treatment or BSC for a given willingness-to-pay threshold for an additional (A) life year (LY); and (B) quality adjusted life year (QALY). Sensitivity analysis according to multiple imputation for variables of missing data.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). The data are administrative claims from Ontario's public health care system. The policies around access are put in place to comply with provincial privacy legislation and the Privacy Commissioner of Ontario. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan is available from the authors (attached as Supporting Information).