Abstract
Objective
The objective of this study was to analyze the antifungal activity of citral, selected by screening natural products, against Candida albicans isolates from subjects who use dental prostheses.
Methodology
Screening of essential oils, including those from Mentha piperita L. (Briq), Origanum vulgare, and Zingiber officinale L., and the phytoconstituents citral and limonene, to select an appropriate natural product. Citral, which mediated the best antifungal response, was selected for biological assays. The minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) for citral and nystatin were determined by the microdilution method. Micromorphological analyses, time-kill curve, and modulation tests were performed.
Results
The MIC and MFC of citral were established as 32 μg/mL, consistent with fungicidal activity. The clinical strains were resistant to nystatin. Citral caused micromorphological alteration in the strains. In the time-kill curve, the growth of the clinical strain was reduction in growth equal to 3 log10 colony-forming units per milliliter after exposure to the MIC and MIC × 2 of citral for 2 h. Citral did not modulate the resistance of the studied strains to nystatin.
Conclusion
This study revealed the potential of citral as a fungicidal agent and highlighted the resistance of clinical strains of C. albicans to nystatin.
1. Introduction
Denture stomatitis is a prevalent inflammatory reaction in patients using dental prostheses and Candida infection is its main etiological factor [1–3]. Candida albicans is the most well-known fungal species associated with the clinical manifestation of this pathology. In addition to C. albicans, other less common Candida spp., such as C. tropicalis, C. glabrata, C. parapsilosis, and C. krusei, are pathogens present in the oral microbiota that can become pathogenic [4, 5].
Denture stomatitis associated with Candida spp. is difficult to treat and frequently presents with relapse [1]. Candida infections have become a serious health problem, as fungi grow increasingly resistant to the available drugs, resulting in high relapse rates; new drugs must be researched and evaluated for their effectiveness in antifungal treatment [6–8].
In dentistry, herbal medicine has been used successfully for many years. Herbal medicines are a promising source of therapeutics in the pharmacological field [9–11]. Citral is a phytoconstituent present in several commonly used essential oils, such as the oil from Cymbopogon citratus [10]. Some pharmacological properties of citral have been reported in the literature, including antitumor [12], bronchodilatory [13], antiprotozoal [14], and antimicrobial [2, 3, 7, 15, 16] effects.
However, there are few studies on its mode of antifungal action and the effects of citral in combination with licensed drugs against clinical strains of C. albicans of prosthetic origin. In this context, the present study analyzed the antifungal activity of the phytoconstituent citral, selected from screening of 5 natural products, on strains of C. albicans isolated from subjects who use dental prostheses.
2. Materials and Methods
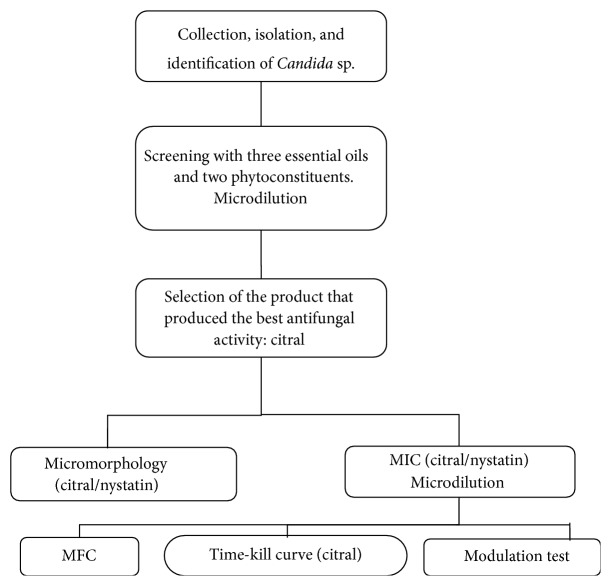
The present study was approved by the Research Ethics Committee of the Health Sciences Center, Federal University of Paraíba, under protocol number 0395/16, CAAE: 57435016.4.0000.5188. The biological material was collected in the Recanto do Poço community, Cabedelo, PB, Brazil. Laboratory tests were performed at the Mycology Laboratory, Federal University of Paraíba (UFPB). The methodological sequence used in the study is shown in Figure 1.
Figure 1.
Flowchart of the experimental plane of the antifungal tests performed.
2.1. Collection, Isolation, and Identification of Biological Material
The study included 11 adult participants, 18 years of age and older, of both sexes, who used dental prostheses (total or partial, with or without a metal structure). The convenience sample consisted of 21 strains of C. albicans.
The strains of C. albicans were collected from the oral cavity and the prosthesis base of each research participant from August to November 2016. We isolated and identified the species according to the criteria established by Lodder [17], Hoog and Guarro [18], Kurtzmann and Fell [19], and Sidrim and Rocha [20].
We used 2 sterile swabs for each participant (Inlab Confiança, Brazil). The first, moistened in sterile physiological solution, was applied to the hard palate of the buccal cavity of the participant, swiped back and forth for 30 s, and then inserted in a test tube containing Sabouraud Dextrose Broth (SDA, Difco Laboratories, USA/France) for transport to the laboratory. The second swab was rubbed under the base of the prosthesis and immersed in a separate tube. The collected biological material was inoculated into 15 × 90 mm disposable Petri dishes (Inlab Confiança, Brazil) containing SDA (Difco Laboratories, USA/France), in the presence of 100 μg/mL chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA). After 24 to 48 h in a bacteriological oven at 35°C ± 2°C, we isolated the colonies with yeast-like fungi in CHROMagar™ Candida (Difco Laboratories, USA/France); after plaque growth, we evaluated the color and morphotypes of the colonies.
We identified the yeasts based on macromorphology, micromorphology, and physiological and biochemical tests. We analyzed germ tube production, yeast micromorphology on rice agar with TWEEN® 80, fermentation, and the assimilation of carbohydrates and performed molecular tests [20].
2.2. Fungal Strains and Inoculum Preparation
We used 20 clinical strains of C. albicans isolated from dental prosthesis users: LM-3B, LM-3P, LM-4B, LM-4P, LM-5B, LM-5P, LM-7B, LM-7P, LM-8B, LM-8P, LM-9B, LM-9P, LM-10B, LM-10P, LM-11B, LM-11P, LM-12B, LM-12P, LM-13B, and LM-13P. The species belonged to the Laboratory of Mycology, Department of Pharmaceutical Sciences, UFPB. The strains were maintained in SDA at 35°C ± 2°C.
Suspensions of the microorganisms were prepared in tubes containing 5 mL of sterile 0.9% saline solution (Farmax, Amaral, Divinopolis, MG, Brazil). The suspensions were shaken for 2 min with the aid of a vortex apparatus (Fanem, Guarulhos, SP, Brazil).
After shaking, the turbidity of each suspension was assessed and adjusted to that of a 0.5 McFarland barium sulfate suspension, which corresponds to an inoculum of approximately 106 colony-forming units (CFU)/mL. This suspension was then diluted 1 : 10 with distilled water, resulting in an inoculum containing approximately 105 CFU/mL, for use in the assays [21–24]. The final concentration obtained was approximately 1–5 × 105 CFU/mL. Confirmation of the final concentration was achieved by counting the microorganisms in a Neubauer chamber.
2.3. Culture Media
RPMI 1640 (bicarbonate-free) (Sigma-Aldrich, Steinheim, Germany) and SDA (Difco Laboratories, USA/France) were used for the antifungal activity assays. The culture media were prepared according to the manufacturers' instructions.
2.4. Essential Oils, Antifungal Standards, and Preparation of Products
The natural products used in the screen and the biological assays are listed in Table 1. The product that produced the best antifungal response in the screen was selected for the other biological assays. Therefore, the test groups consisted of citral and the licensed drug nystatin (Sigma-Aldrich, São Paulo, SP, Brazil).
Table 1.
Screening results for evaluation of the antifungal response of the products. Microdilution technique (1024 μg/ml to 128 μg/ml).
| Essential oil | |||||
|---|---|---|---|---|---|
| Scientific name | Family | Popular name | Batch | Fabricator | MIC (µg/mL) |
| Origanum vulgare L. | Lamiaceae | Oregano | 0149/05209/F | Sigma-Aldrich, São Paulo, SP, Brazil | 256 |
| Zingiber officinale | Zingiberaceae | Ginger | 0717/05209/F | Quinari Fragrâncias e Cosméticos Ltda., Ponta Grossa, PR, Brazil | + |
| Mentha piperita L. | Lamiaceae | Peppermint | 0809/05209/F | Sigma-Aldrich, São Paulo, SP, Brazil | 256 |
|
| |||||
| Phytoconstituent | |||||
| Name | Molecular formula | Batch | Fabricator | MIC (µg/mL) | |
|
| |||||
| Citral | C10H16O | STBC5273V | Sigma-Aldrich, São Paulo, SP, Brazil | 128 | |
| Limonene | C10H16 | 58296DK | Sigma-Aldrich, São Paulo, SP, Brazil | 256 | |
+: growth of microorganism.
The natural products were solubilized in up to 10% dimethyl sulfoxide with 0.02% TWEEN 80 (Diadema, SP, Brazil). They were then emulsified with 3 mL sterile distilled water to obtain an initial concentration of 1,024 μg/mL.
2.5. Screening and Determination of the Minimum Inhibitory Concentration
The screening of the natural products and determination of the minimum inhibitory concentration (MIC) of the selected product were performed using the microdilution technique, performed in triplicate, in sterile, U-bottom, 96-well microplates (Kasvi, Italy) [21, 23–26].
We added 100 μL of 2× RPMI (Sigma-Aldrich, São Paulo, SP, Brazil) to each well of a plate. Subsequently, we dispensed 100 μL of the 2× products into the wells of the first row of the plate; the products were serially diluted by the withdrawal of a 100 μL aliquot from the concentrated well into a successor well, resulting in doubling dilutions from 1,024 μg/mL to 128 μg/mL for the screen and from 1,024 μg/mL to 2 μg/mL for the MIC determination. Finally, 10 μL of the yeast inoculum was added to each well, such that each plate column contained a particular fungal strain.
Viability controls were performed on the fungal strains in the liquid medium under the same assay conditions. The plates were sealed and incubated at 35°C ± 2°C for 24 to 48 h. The MICs of the products used in the biological assays were defined as the lowest concentrations capable of visually inhibiting the fungal growth in the wells, as compared to the growth under control conditions.
2.6. Minimum Fungicidal Concentration
After determination of the MIC, 10 μl aliquots of the supernatant from the wells corresponding to the MIC and the 2 immediately more concentrated concentrations (MIC, MIC × 2, and MIC × 4) were subcultured in a SDA containing plate, which was then incubated at 37°C for 24–48 h. The MFC was the lowest drug concentration that showed either no growth or fewer than three colonies. The assays were performed in triplicate, and the geometric mean values were calculated [27]. The MFC/MIC ratio was calculated to determine if the substance had fungistatic (MFC/MIC ≥ 4) or fungicidal (MFC/MIC < 4) activity [28].
2.7. Micromorphology Analysis
To study possible alterations in the micromorphology of C. albicans cells exposed to citral, we microcultured the samples on a slide in a Petri dish (camera wet) [29]. The molar agar-fubah-TWEEN 80 culture medium was fractionated into sterile tubes containing the MICs of the test products. A tube with the culture medium alone was used as the control. After homogenization, each culture medium was spread on a glass slide.
C. albicans in SDA were seeded on the slides and incubated at 35°C ± 2°C for 24 to 48 h. The slides were analyzed by light microscopy, at a magnification of 400×, to observe the formation of characteristic structures, such as blastoconidia, pseudohyphae, and chlamydoconidia.
2.8. Time-Kill Curve
We assessed the effects of the test product on the time-kill curves of the fungal strains using the methodology described by Klepser et al. [30], with some improvements.
For the analysis of microbial death kinetics, we selected 2 strains: an American Type Culture Collection standard (ATCC 76645) and a clinical strain, LM-8B, which demonstrated sensitivity to citral. In this assay, we observed the behavior of the selected yeast strains over 24 h in the presence of the MICs of citral.
Initially, 100 μL RPMI 1640 (Sigma-Aldrich, São Paulo, SP, Brazil) was added to each well of a 96-well, U-bottom microplate with 10 μL of the supernatants with various citral concentrations (MIC, MIC × 2, and MIC × 4) and incubated for 24 to 48 h at 35°C ± 2°C.
The inoculum was plated on a Petri dish (Alamar, Diadema, SP, Brazil) containing SDA (Difco Laboratories, Detroit, MI, USA) culture medium. A 10 μL aliquot of the inoculum was removed from the microplate with a calibrated bacteriological loop (Inlab Confiança, Brazil) and then uniformly streaked along the surface of the SDA culture medium in the Petri dish at 0, 2, 4, 8, 12, and 24 h. The inoculated dishes were incubated at 35°C ± 2°C for 48 h.
The experiment was carried out in triplicate. The curves were plotted as the colony count (log10 CFU/mL) as a function of time (h) with GraphPad Prism 5.0 (GraphPad for Windows, San Diego, CA, USA). Fungicidal activity was classified as a reduction in growth ≥3 log10 (≥99.9%) and fungistatic activity as a reduction in growth of <3 log10 (<99.9%) CFU/mL, compared with the initial inoculum [30].
2.9. Modulation Test
To evaluate the modulatory action of citral on the resistance of C. albicans strains to nystatin, we determined the MIC of nystatin, using the microdilution technique in a sterile, 96-well, U-bottom plate (Kasvi, Italy), in the presence of a sub-MIC of citral (MIC/8) [31, 32].
The citral was emulsified at 4 μg/mL in RPMI 1640 medium for a final 100 μg/mL solution. This solution was added to each well of the microplate. Subsequently, 100 μg/mL of nystatin was dispensed into the wells of the first row followed by microdilution, in order to obtain concentrations of 1,024 μg/mL to 4 μg/mL. Finally, 10 μL of the yeast inoculum was added to the wells, such that each column contained 1 particular strain.
The MIC values of the standard antifungal were then compared in the absence and presence of the MIC/8 of citral. The plates were incubated for 24 to 48 h at 35°C ± 2°C for the evaluation of fungal growth. The tests were performed in triplicate.
3. Results and Discussion
Citral mediated the best antifungal response against C. albicans of the 5 screened products, which had been selected based on the ethnobotanical literature [33–35]; it inhibited the fungal strains at a concentration of 128 μg/mL (Table 1).
All of the yeast strains were resistant to ginger essential oil. Sharifzadeh and Shokri [36] found that the MIC of ginger essential oil was 2,500 μg/mL for C. albicans strains that were resistant or sensitive to fluconazole. Aghazadeh et al. [37] determined the MIC of ginger extracts as 5 mg/mL. We found that oregano oil had a MIC of 256 μg/mL, in agreement with the study by Sharifzadeh and Shokri [36], which determined the MIC as 300 μg/mL. Mint essential oil and the phytoconstituent limonene both had MICs of 256 μg/mL.
As shown in Table 2, with the exception of its MIC against C. albicans LM-3B, the antifungal activity of citral was determined as 32 μg/mL (MIC 90%). This MIC was similar to that (64 μg/mL) determined by Leite et al. [15] against strains of C. albicans isolated from the blood. Lima et al. [7] found that 512 μg/mL citral inhibited all C. albicans yeasts isolated from the blood, urine, respiratory tract, and vaginal secretions, suggesting higher resistance in strains isolated from those sites rather than from the oral cavity.
Table 2.
Results of MIC and MFC evaluation of citral and nystatin and MIC/MFC ratio of citral on C. albicans.
| C. albicans | Citral (μg/mL) | Nystatin (μg/mL) | Control of strains | Control of the culture medium | |||
|---|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC | Activity antifungal | MIC | |||
| 3B | 64 | 64 | 1 | Fungicide | >1024 | + | − |
| 3P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 4B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 4P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 5B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 5P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 7B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 7P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 8B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 8P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 9B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 9P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 10B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 10P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 11B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 11P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 12B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 12P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 13B | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| 13P | 32 | 32 | 1 | Fungicide | >1024 | + | − |
| ATCC 76645 | 32 | 32 | 1 | Fungicide | 256 | + | − |
+: growth of microorganism; −: without microorganism growth.
The citral MFC coincided with its MIC in the samples tested. According to Siddiqui et al. [28], a substance has fungistatic activity when the MFC/MIC ratio is ≥4 and fungicidal activity when the MFC/MIC ratio is <4. Hafidh et al. [38] characterized an MFC/MIC ratio between 1 : 1 and 2 : 1 as fungicidal activity, whereas they ascribed fungistatic activity to a ratio greater than 2 : 1. According to these methodologies, we observed fungicidal activity by citral. This finding corroborates the findings of Leite et al. [15] that the MFC of citral corresponded to its MIC (64 μg/mL) for all the strains analyzed. Sousa et al. [16] observed MFC values between 256 μg/mL and 1,024 μg/mL (MFC50 and MFC90, resp.) for citral against C. tropicalis isolated from the blood, indicative of fungicidal activity against the tested yeasts.
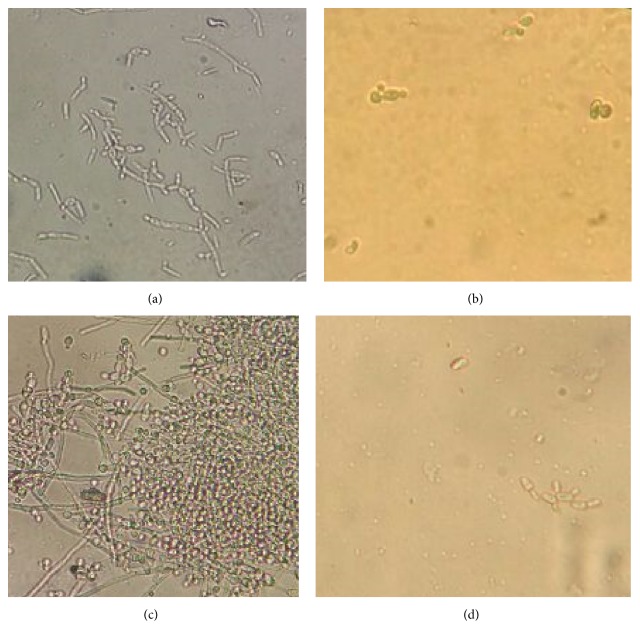
The micromorphological evaluations (Figure 2) of C. albicans ATCC 76645 and LM-11P under an optical microscope revealed fungal growth, characterized by the formation of pseudohyphae and blastoconidia. In the presence of citral, we observed few growth-related structures, with more blastoconidia than pseudohyphae, indicating that citral altered the morphology of the strains. According to Alves et al. [39], specific morphological changes are associated with the pathogenicity of microorganisms, as local environmental factors can alter the status of commensal fungi, thereby making them infectious. The yeast-hyphal morphological transition is relevant for the virulence of fungal infections. Leite et al. [15] also observed that C. albicans strains had few blastoconidia and lacked pseudohyphae in the presence of citral. Thus, these results are relevant to the development of a novel antifungal agent.
Figure 2.
Micromorphology of C. albicans in absence (control) and presence of citral. (a) C. albicans ATCC 76645 in the absence of the product, demonstrating structures such as blastoconidia and pseudohyphae. (b) C. albicans ATCC 76645 in the presence of the phytoconstituent, rare yeasts, and pseudohyphae are observed. (c) C. albicans LM-11P in the absence of citral exhibiting large amounts of cell structures, blastoconidia, and pseudohyphae. (d) C. albicans LM-11P under the action of citrine MIC, with rare yeasts and pseudohyphae.
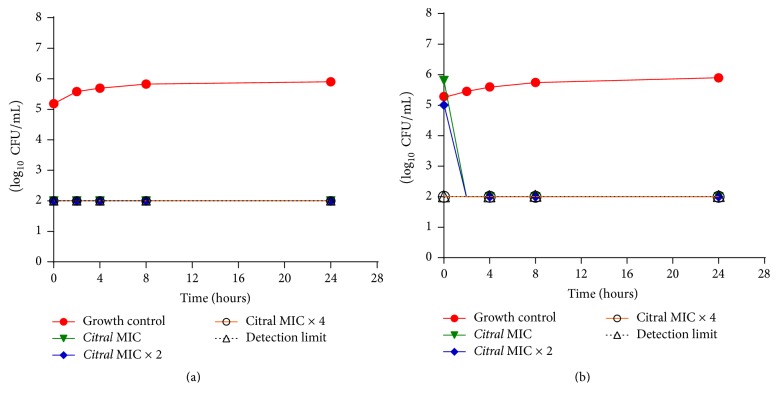
We analyzed yeast growth as a function of time in the presence of various citral concentrations. Two strains of C. albicans were subjected to microbial death kinetics analysis (Figure 3). We assessed the viable cell count over time to verify the fungicidal or fungistatic activity of the test product and to evaluate the interaction of the test product with the microorganism, in order to characterize the dynamic relationship between the product concentration and length of exposure [40]. We found a reduction in the growth of the clinical strain equal to 3 log10 CFU/mL after 2 h of exposure to the MIC and MIC × 2 of citral, classified as a fungicidal effect according to the criteria of Klepser et al. [40]. We did not observe growth of the clinical strain at any of the time points after exposure to the MIC × 4 of citral, or growth of the standard strain at any of the citral concentrations. These results revealed the concentration-dependence of the fungicidal activity of citral.
Figure 3.
(a) Time-death curve for C. albicans ATCC 76645 when exposed to various concentrations of citral. (b) Time-death curve for C. albicans LM-8B under different concentrations of citral.
Leite et al. [15] and Zore et al. [3] also observed the fungicidal effect of citral on C. albicans using microbial death kinetics analysis. In the first study [15], the time required for citral fungicidal activity was 4 h at the MIC (64 μg/mL) and 2 h at the MIC × 2. In the latter study, 640 μg/mL citral killed 99.9% of the inoculum in 2 h.
The possible mechanisms of action of citral in fungal cells have not yet been fully elucidated. Park et al. [41] observed that citral was able to cause hyphal degeneration, cell wall membrane separation, and mitochondrial disintegration in the fungal species Trichophyton mentagrophytes. According to Harris [42], citral acts predominantly on the fungal cell membrane, affecting its structure and causing cell death by inhibiting spore germination, proliferation, and cellular respiration.
Lima et al. [7] and Leite et al. [15] concluded that the MIC of citral was not altered by the presence of sorbitol, suggesting that it does not act by modifying the fungal cell wall or by binding to ergosterol, but likely affects another target.
Most of the strains were resistant to nystatin, so it was not possible to determine a MIC; the exception, C. albicans ATCC 76645, was sensitive to a concentration of 256 μg/mL citral.
The resistance of Candida spp. to nystatin has been documented in the literature; Sousa et al. [16] observed growth of all C. tropicalis yeasts isolated from the blood in the presence of fluconazole, except for C. tropicalis ATCC 13803. The authors emphasized that this drug is widely used in clinical practice because of its efficacy and low toxicity. Due to frequent exposure to antifungal drugs, Candida isolates and their species are regularly developing resistance.
The relationship between exposure and resistance may also explain the results of this study; the frequent use of nystatin or other drugs prescribed as antifungals for denture stomatitis associated with Candida spp. has made C. albicans strains resistant to nystatin [6–8]. Microbial interactions in the oral cavity favor the proliferation of microorganisms, which facilitates the development of Candida drug resistance [43, 44].
The resistance of clinical strains of C. albicans isolated from prosthesis users to the antifungal standard nystatin, which is used for the treatment of Candida-associated denture stomatitis, demonstrates the importance of investigating the efficacy and mode of action of novel products on this clinically relevant pathogen. Such research will facilitate the discovery of new antifungal agents.
The standard yeast ATCC 76645 possesses fewer fungal structures, like pseudohyphae and blastoconidia, than strains of clinical origin (Figures 2(a)–2(c)). This strain has not interacted with other microorganisms, unlike clinical C. albicans isolates. This characteristic may account for the sensitivity of C. albicans ATCC 76645 to nystatin; by contrast, the clinical strains were unaffected by this antifungal, resulting in growth at the concentrations studied.
The addition of citral to the growth medium at a subinhibitory concentration of 4 μg/mL (MIC/8) did not alter the resistance of the clinical strains or the standard to nystatin, as assessed by proliferation. Furthermore, there are no studies in the literature investigating the ability of citral to modulate nystatin activity. The ability of unconventional compounds to increase the antimicrobial activity or reverse the resistance of microorganisms to drugs classifies the compounds as modifiers of antifungal activity [31]. Citral was not classified as a modifier of resistance to the antifungal nystatin in the studied strains.
This laboratory study has limitations. Given the increase in fungal infections and the high prevalence of denture stomatitis associated with Candida, the search for new, alternative treatments for these pathologies is necessary. Citral may be a promising candidate, as it mediated antifungal activity against clinical strains of C. albicans isolated from dental prosthesis users.
4. Conclusions
Among the natural products analyzed, citral produced the best antifungal response. Citral demonstrated fungicidal activity against C. albicans yeasts isolated from dental prosthesis users, as well as against the standard strain. The phytoconstituent caused morphological modifications to the yeasts studied.
Citral was distinguished by its promotion of microbial death in clinical C. albicans within 2 h of exposure, at both the MIC and MIC × 2. We verified the resistance of all of the clinical strains to nystatin. Citral did not alter the resistance of the strains to the licensed drug.
Citral is a promising phytoconstituent; future research should assess its mode of action against fungi and its cytotoxic potential, to facilitate the development of new antifungal agents against C. albicans spp.
Acknowledgments
The authors are grateful to the Postgraduate Program in Dentistry of the Federal University of Paraíba (UFPB).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Julliana Cariry Palhano Freire, José Klidenberg de Oliveira Júnior, Daniele de Figueredo Silva, Janiere Pereira de Sousa, Felipe Queiroga Sarmento Guerra, and Edeltrudes de Oliveira Lima are responsible for drafting the paper. Julliana Cariry Palhano Freire participated in the project design, collection and analysis of data, drafting the paper, and critical revision of the intellectual content. José Klidenberg de Oliveira Júnior, Daniele de Figueredo Silva, Janiere Pereira de Sousa, and Felipe Queiroga Sarmento Guerra participated in data collection, data analysis, and revision of the paper. Edeltrudes de Oliveira Lima guided all stages of the work and participated in both review and drafting of the project and the paper, including final approval of the version to be published.
References
- 1.Gendreau L., Loewy Z. G. Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics. 2011;20(4):251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan M. S. A., Malik A., Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Medical Mycology. 2012;50(1):33–42. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
- 3.Zore G. B., Thakre A. D., Jadhav S., Karuppayil S. M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18(13):1181–1190. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Leite D. P., Piva M. R., Martins-Filho P. R. Identificação das espécies de Candida em portadores de estomatite protética e avaliação da susceptibilidade ao miconazol e à terapia fotodinâmica. Revista de Odontologia da UNESP. 2015;44(1):12–17. doi: 10.1590/1807-2577.1027. [DOI] [Google Scholar]
- 5.Martins C. H., Pires R. H., Cunha A. O., et al. Candida/Candida biofilms. First description of dual-species Candida albicans/C. rugosa biofilm. Fungal Biology. 2016;120(4):530–537. doi: 10.1016/j.funbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Iosif L., Preoteasa C. T., Murariu-Maqureanu C., Preoteasa E. Clinical study on thermography, as modern investigation method for Candida-associated denture stomatitis. Romanian Journal of Morphology and Embryology. 2016;57(1):191–195. [PubMed] [Google Scholar]
- 7.Lima I. O., De Medeiros Nóbrega F., De Oliveira W. A., et al. Anti-Candida albicans effectiveness of citral and investigation of mode of action. Pharmaceutical Biology. 2012;50(12):1536–1541. doi: 10.3109/13880209.2012.694893. [DOI] [PubMed] [Google Scholar]
- 8.Marcos-Arias C., Eraso E., Madariaga L., Quindós G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complementary and Alternative Medicine. 2011;11, article 119:7. doi: 10.1186/1472-6882-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira F. Q., Gobira B., Guimarães C., Batista J., Barreto M., Souza M. Espécies vegetais indicadas na odontologia. Revista Brasileira de Farmacognosia. 2007;17(3):466–476. doi: 10.1590/S0102-695X2007000300022. [DOI] [Google Scholar]
- 10.da Silva C. D. B., Guterres S. S., Weisheimer V., Schapoval E. E. S. Antifungal activity of the lemongrass oil and citral against Candida spp. Brazilian Journal of Infectious Diseases. 2008;12(1):63–66. doi: 10.1590/s1413-86702008000100014. [DOI] [PubMed] [Google Scholar]
- 11.Silva M. A. S., Silva M. A., Higino J. S., Pereira M. S., Carvalho A. A. T. Atividade antimicrobiana e antiaderente in vitro do extrato de Rosmarinus officinalis Linn. sobre bactérias orais planctônicas. Revista Brasileira de Farmacognosia. 2008;18(2):236–240. doi: 10.1590/S0102-695X2008000200017. [DOI] [Google Scholar]
- 12.Xia H., Liang W., Song Q., Chen X., Chen X., Hong J. The in vitro study of apoptosis in NB4 cell induced by citral. Cytotechnology. 2013;65(1):49–57. doi: 10.1007/s10616-012-9453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangprayool T., Kupittayanant S., Chudapongse N. Participation of citral in the bronchodilatory effect of ginger oil and possible mechanism of action. Fitoterapia. 2013;89(1):68–73. doi: 10.1016/j.fitote.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso J., Soares M. J. In vitro effects of citral on Trypanosoma cruzi metacyclogenesis. Memorias do Instituto Oswaldo Cruz. 2010;105(8):1026–1032. doi: 10.1590/S0074-02762010000800012. [DOI] [PubMed] [Google Scholar]
- 15.Leite M. C. A., Bezerra A. P. B., Sousa J. P., Guerra F. Q., Lima E. d. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/378280.378280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa J., Costa A., Leite M., et al. Antifungal activity of citral by disruption of ergosterol biosynthesis in fluconazole resistant Candida tropicalis. International Journal of Tropical Disease Health. 2016;11(4):1–11. doi: 10.9734/IJTDH/2016/21423. [DOI] [Google Scholar]
- 17.Lodder I. The Yeast: A Taxonomic Study. Amsterdam, The Netherlands: Horth Helland Publishing; 1970. [Google Scholar]
- 18.Hoog G. S., Guarro J. Atlas of Clinical Fungi. Central Bureau Voorschimm el Cultures. Virgii: Universitant Rovira; 1995. [Google Scholar]
- 19.Kurtzmann C. P., Fell J. W. The Yeast: A Taxonomic Study. 4th. New York, NY, USA: Elsevier; 1998. [Google Scholar]
- 20.Sidrim J. J. C., Rocha M. F. G. Micologia Médica À Luz De Autores Contemporâneos. Rio de Janeiro, Brazil: Ed. Guanabara; 2004. [Google Scholar]
- 21.Cleeland R., Squires E. Evaluation of new antimicrobials in vitro and in experimental animal infections. In: Lorian V. M. D., editor. Antibiotics in Laboratory Medicine. Williams & Wilkins; 1991. pp. 739–788. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Protocol M27-A2. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 2nd. Wayne, Pa, USA: NCCLS; 2002. [Google Scholar]
- 23.Hadacek F., Greger H. Testing of antifungal natural products: methodologies, comparability of results and assay choice. Phytochemical Analysis. 2000;11(3):137–147. doi: 10.1002/(SICI)1099-1565(200005/06)11:3<137::AID-PCA514>3.0.CO;2-I. [DOI] [Google Scholar]
- 24.Sahin F., Güllüce M., Daferera D., et al. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004;15(7):549–557. doi: 10.1016/j.foodcont.2003.08.009. [DOI] [Google Scholar]
- 25.Eloff J. N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 1998;64(8):711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 26.Konemam E. W., Winn W. C., Allen S. D., et al. Diagnóstico Microbiológico. 5th. Rio de Janeiro, Brazil: MEDS; 2001. [Google Scholar]
- 27.Espinel-Ingroff A., Chaturvedi V., Fothergill A., Rinaldi M. G. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. Journal of Clinical Microbiology. 2002;40(10):3776–3781. doi: 10.1128/JCM.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui Z. N., Farooq F., Musthafa T. N. M., Ahmad A., Khan A. U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. Journal of Saudi Chemical Society. 2013;17(2):237–243. doi: 10.1016/j.jscs.2011.03.016. [DOI] [Google Scholar]
- 29.Dalmau L. M. Remarques sur la technique mycologique. Annales De Parasitologie Humaine Et Comparee. 1929;7:536–545. [Google Scholar]
- 30.Klepser M. E., Ernst E. J., Lewis R. E., Ernst M. E., Pfaller M. A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrobial Agents and Chemotherapy. 1998;42(5):1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutinho H. D. M., Costa J. G. M., Lima. E. O., Falcão-Silva V. S., Siqueira-Júnior J. P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy. 2008;54:328–330. doi: 10.1159/000151267. [DOI] [PubMed] [Google Scholar]
- 32.Eliopulos G. M., Moellering R. C. Antibiotics in Laboratory Medicine. Baltimore, Md, USA: Williams & Wilkins; 1991. Antimicrobial combinations; pp. 434–441. [Google Scholar]
- 33.Agra M. D. F., Silva K. N., Basílio I. J. L. D., De Freitas P. F., Barbosa-Filho J. M. Survey of medicinal plants used in the region Northeast of Brazil. Brazilian Journal of Pharmacognosy. 2008;18(3):472–508. doi: 10.1590/S0102-695X2008000300023. [DOI] [Google Scholar]
- 34.Cordeiro J., Félix L. Conhecimento botânico medicinal sobre espécies vegetais nativas da caatinga e plantas espontâneas no agreste da Paraíba, Brasil. Revista Brasileira de Plantas Medicinais. 2014;16(3 suppl 1):685–692. doi: 10.1590/1983-084x/13_077. [DOI] [Google Scholar]
- 35.Vieira D., Amaral F., Maciel M., Nascimento F. F., Libério A. Plantas e constituintes químicos empregados em Odontologia: revisão de estudos etnofarmacológicos e de avaliação da atividade antimicrobiana in vitro em patógenos orais. Revista Brasileira de Plantas Medicinais. 2014;16(1):135–167. doi: 10.1590/S1516-05722014000100020. [DOI] [Google Scholar]
- 36.Sharifzadeh A., Shokri H. Antifungal activity of essential oils from Iranian plants against fluconazole-resistant and fluconazole-susceptible Candida albicans. Avicenna Journal Phytomedicine. 2016;6(2):215–222. [PMC free article] [PubMed] [Google Scholar]
- 37.Aghazadeh M., Bialvaei A. Z., Aghazadeh M., et al. Survey of the antibiofilm and antimicrobial effects of Zingiber officinale (in vitro study) Jundishapur Journal of Microbiology. 2016;9(2) doi: 10.5812/jjm.30167.e30167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafidh R. R., Abdulamir A. S., Vern L. S., et al. Inhibition of growth of highly resistant bacterial and fungal pathogens by a natural product. Open Microbiology Journal. 2011;5:96–106. doi: 10.2174/1874285801105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves L. A., Freires I. A., Pereira T. M., Souza A., Lima E. O., Castro R. D. Effect of Schinus terebinthifolius on Candida albicans growth kinetics, cell wall formation and micromorphology. Acta Odontologica Scandinavica. 2013;71:965–971. doi: 10.3109/00016357.2012.741694. [DOI] [PubMed] [Google Scholar]
- 40.Klepser M. E., Wolfe E. J., Jones R. N., Nightingale C. H., Pfaller M. A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrobial Agents and Chemotherapy. 1997;41(6):1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M. J., Gwak K. S., Yang I., et al. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 2009;80(5):290–296. doi: 10.1016/j.fitote.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Harris R. Progress with superficial mycoses using essential oils. International Journal of Aromatherapy. 2002;12(2):83–91. doi: 10.1016/S0962-4562(02)00032-2. [DOI] [Google Scholar]
- 43.Vasilas A., Molina L., Hoffman M., Haidaris C. G. The influence of morphological variation on Candida albicans adhesion to denture acrylic in vitro. Archives of Oral Biology. 1992;37(8):613–622. doi: 10.1016/0003-9969(92)90123-P. [DOI] [PubMed] [Google Scholar]
- 44.Verran J., Motteram K. L. The effect of adherent oral streptococci on the subsequent adherence of Candida albicans to acrylic in vitro. Journal of Dentistry. 1987;15(2):73–76. doi: 10.1016/0300-5712(87)90003-0. [DOI] [PubMed] [Google Scholar]