Abstract
Purpose
To compare the effectiveness and safety of combined intravenous and topical tranexamic acid with intravenous use alone in THA.
Methods
The electronic databases MEDLINE, EMBASE, BIOSIS, Cochrane central, and further adapted for Google and Google Scholar internet, last updated on Dec 30, 2016, were searched. Evaluated outcomes included total blood loss, transfusion rate, maximum postoperative Hb drop, and incidence of thromboembolic complications. The standard mean difference (SMD) or the relative risk (RR) was calculated for continuous or dichotomous data respectively. The quality of the trial was assessed, and meta-analyses were performed with the Cochrane Collaboration’s RevMan 5.0 software.
Results
Five RCTs with 457 patients were included. Combined TXA administration reduced blood loss (SMD, 1.39; 95%CI, 0.55 to 2.23; P<0.00001, I2 = 94%), hemoglobin decline (SMD, 0.84; 95%CI, 0.13 to 1.54; P = 0.01, I2 = 83%) and the need for transfusion (RR, 2.58; 95%CI, 1.59 to 4.18; P = 0.65, I2 = 0%) without increasing the rate of thromboembolic complications significantly (RR, 0.83; 95%CI, 0.27 to 2.54; P = 0.81, I2 = 0%).
Conclusion
The present study has emphasized that combined TXA administration can effectively reduce blood loss, hemoglobin decline and the need for transfusion without increasing the rate of thromboembolic complications.
Introduction
Total hip arthroplasty (THA) is one of the most common treatments in orthopedic surgery, associated with large amount of postoperative blood loss and significant rate of blood transfusion. It was estimated that the average blood loss during primary THA can be between 1000 and 2000 ml, and up to 37% of patients undergoing primary THA require blood transfusion for postoperative anemia[1]. Postoperative anemia may impede functional ability and increase the length of hospital stay and the costs. To reduce the need for blood transfusion, a variety of techniques have been used, including blood salvage, controlled hypotension, stimulation of erythropoiesis and the use of pharmacologic agents, such as Tranexamic acid[2].
Tranexamic acid (TXA), an antifibrinolytic drug which blocks the lysine blinding sites on plasminogen molecules and inhibits the formation of plasmin, has been widely used currently[3]. A large amount of evidences provided by prospective randomized controlled trials[4, 5] and meta-analysis studies[6, 7] have shown that TXA, applied either intravenously or locally, can reduce blood loss and subsequent transfusion without risking a high complication rate. Theoretically, a topical application of TXA may limit systemic absorption and decrease the risk of postoperative thromboembolic complications. It is proposed that topical application provides a maximum therapeutic concentration of TXA at the bleeding site, and induces partial microvascular hemostasis[8]. Another method which is the combined application of IV-TXA and topical TXA is deemed to be more effective than either regimen alone according to the above findings[9]. Recently, more and more researches have focused on the issue that whether combination application of intravenous and topical tranexamic acid has bigger value in THA when compared with only intravenous tranexamic acid[10–12].
Accordingly, we systematically reviewed the current evidences to investigate the effectiveness and safety associated with use of TXA by comparing combined intravenous and topical tranexamic acid with intravenous use alone. A Meta-analysis method was used to allow us to provide better evidence-based advice to researchers in this area.
Materials and methods
This systematic review and meta-analysis was performed according to PRISMA Statement Criteria[13].
Study selection and data extraction
The electronic databases MEDLINE, EMBASE, BIOSIS, Cochrane central, and further adapted for Google and Google Scholar internet, last updated on Dec 30, 2016, were searched. We reviewed the bibliographies of original trials, meta-analysis, and review articles identified for potential eligible articles. Moreover, gray literatures were also selected from the reference list of included studies. There were no restrictions as to the language and date. The search terms including ‘total hip arthroplasty’, ‘total hip replacement’, ‘THA’, ‘THR’, ‘tranexamic acid’ and ‘TXA’ were retrieved in the title, abstracts, and Medical Subject Headings. The search strategy was designed and refined, and two reviewers applied the search strategy to select references. In case of disagreement, it was discussed and consulted by a senior reviewer.
Two reviewers independently extracted all relevant data. Disagreement were resolved by discussion and, when necessary, with adjudication by a third reviewer. Effective data on the following study characteristics included: patient demographics, publication year, type of surgery, sample size, dose and administration of TXA, prophylactic antithrombotic therapy, surgical approach, blood transfusion trigger, total blood loss, Max Hb drop, transfusion rate, and incidence of thromboembolic complications. When necessary, details were sought from the authors of the primary studies.
Inclusion and exclusion criteria
Inclusion criteria included (1) Randomized controlled trials (RCTs); (2) skeletally mature patients, aged 18 or older who received THA treatment regardless of the type or size of prosthesis implanted; (3) the intervention included intravenous and combined administration of TXA; (4) outcomes included total blood loss, transfusion rate, maximum postoperative Hb drop, and incidence of thromboembolic complications; (5) >10 patients in each group; (6) the primary outcome measures included transfusion rate, total blood loss, and maximum postoperative Hb drop; and the secondary outcome was the incidence of thromboembolic complications. Exclusion criteria included (1) patients with bleeding disorders or were on anticoagulant treatment; (2) animal models.
Statistical analysis
Study data were pooled together and analyzed by the Cochrane Collaboration’s RevMan 5.0 software. For each study, relative risks (RR) and 95% confidence intervals (CIs) were calculated for dichotomous outcomes, and standard mean differences (SMD) and 95% confidence intervals (CIs) were calculated for continuous outcomes. A fixed-effect model with the inverse-variance test was used for continuous variables and the Mantel-Haenszel test for dichotomous variables when a lower heterogeneity was found; and a random-effect model was used when the heterogeneity was significant. The presence of statistical heterogeneity was assessed through Chi-square tests (with P < 0.05 representing heterogeneity) and I2 statistic (with I2 > 50% indicating high heterogeneity). Subgroup analysis was conducted to find the source if possible under significant heterogeneity. A sensitivity analysis was also performed by investigating the effect of each individual study on the pooled effect size.
Assessment of methodological quality and publication bias
The methodological quality of these trials was evaluated in our meta-analysis. For RCTs, we assessed the risk of bias with the Cochrane Collaboration’s tool[14]. Assessments of five main fields included sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. The modified Jadad scale was applied to assess the quality of RCTs, and a score ≥ 4 was considered a high quality article[15].
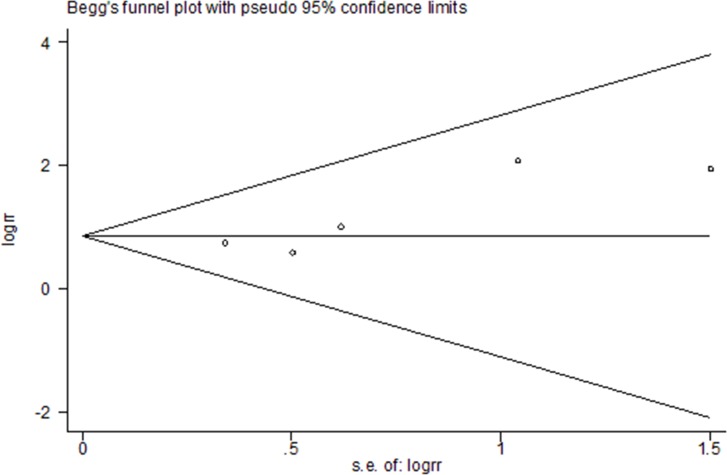
Possible publication bias regarding to primary outcome transfusion rate was evaluated by the Begg’s rank correlation test and the Egger’s regression test. Moreover, funnel plot for blood transfusion rate was also generated to evaluate potential publication bias among studies. Both analyses are performed using STATA 10.0 software. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
Results
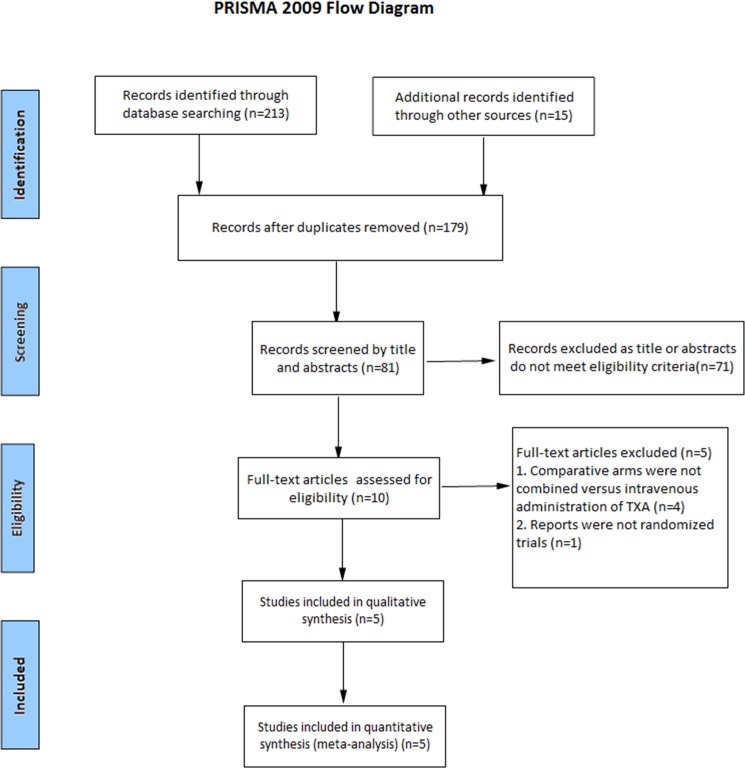
A total of 228 potentially relevant articles were identified. After reference to titles, abstracts and even full texts, five RCTs eventually matched the inclusion and exclusion standard for analysis[10, 12, 16–18]. The search process was shown in Fig 1. Three studies were published in English[10, 12, 17], and two were published in Chinese[16, 18]. Table 1 showed the basal line of patients' characteristics. The sample size ranged from 65 to 140. Clinical outcomes of the included studies were documented in Table 2.
Fig 1. Flow chart.
Table 1. Characteristics of included studies.
| Studies | Surgery | TXA intervention | No. | Age | Sex ratio (M/F) | Prophylactic antithrombotic therapy | Surgical approach | Blood transfusion trigger | The modified Jadad scale |
|---|---|---|---|---|---|---|---|---|---|
| Xie 2015 | Primary THA | I: 1.5g IV-TXA | 70 | 59.5 ± 11.5 | 20/50 | Enoxaparin | Posterolateral | Hb < 70g/L or Hb 70–100g/L with symptomatic anemia | 6.5 |
| C: 1g IV-TXA+2g topical TXA | 70 | 60.5 ± 11.0 | 22/28 | ||||||
| Wu 2016 | Revision THA | I: 15mg/kg IV-TXA | 42 | 59.5 ± 11.3 | 21/21 | LMWH | Posterolateral | Hb < 80g/L or had symptoms of anemia |
5 |
| C: 15mg/kg IV-TXA+3g topical TXA | 42 | 60.1 ± 10.4 | 23/19 | ||||||
| Zeng 2016 | Primary THA | I: 15mg/kg IV-TXA | 50 | 54 ± 12.6 | 24/26 | LMWH | Posterolateral | Hb < 70g/L or any anemia-related organ dysfunction | 7 |
| C: 15mg/kg IV-TXA+1g topical TXA | 50 | 53.6 ± 14.8 | 29/21 | ||||||
| Zhang 2015 | Primary THA | I: 1g IV-TXA | 34 | 63.4 ± 6.8 | 23/11 | Enoxaparin | Anterolateral | Hb < 70g/L or Hb < 90g/L with anemia-related organ dysfunction | 4 |
| C: 1g IV-TXA+100mg topical TXA | 34 | 64.7 ± 8.1 | 24/10 | ||||||
| Sun 2016 | Primary THA | I: 1g IV-TXA | 34 | 66.2 ± 7.4 | 13/21 | LMWH | Posterolateral | Hb < 70g/L | 4 |
| C: 15mg/kg IV-TXA+1g topical TXA | 31 | 64.8 ± 8.3 | 11/20 |
I: Intravenous group; C: Combined group; LMWH: low-molecular-weight heparin.
Table 2. Outcomes of included studies.
| Studies | TXA intervention | Blood loss (ml) | Max Hb drop | Transfusion rate | Incidence of thromboembolic complications |
|---|---|---|---|---|---|
| Xie 2015 | IV-TXA | 878.0±210 | 3.4±0.8 | 3(4.3%) | 1 |
| Combined TXA | 776.8±189.0 | 3.0±0.8 | 0(0%) | 2 | |
| Wu 2016 | IV-TXA | 1794.6 ± 162.6 | 4.5±0.7 | 19(45.2%) | 0 |
| Combined TXA | 1326.9 ±143.2 | 3.7±0.6 | 9(21.4%) | 1 | |
| Zeng 2016 | IV-TXA | 1002.6 ± 366.8 | NR | 8(16%) | 2 |
| Combined TXA | 835.5 ± 343.5 | NR | 1(2%) | 2 | |
| Zhang 2015 | IV-TXA | 1019 ± 137 | NR | 9(26.5%) | 0 |
| Combined TXA | 787 ± 110 | NR | 5(14.7%) | 0 | |
| Sun 2016 | IV-TXA | 1150 ± 310 | NR | 9(26.5%) | 2 |
| Combined TXA | 780 ± 270 | NR | 3(9.7%) | 1 |
NR: Not report; I: Intravenous group; T: Topical group
Five studies examined were evaluated using the modified Jadad scale (Table 1). The modified Jadad score ranged from 4 to 7. Methodological quality of the included studies was detailed in Table 3. Two studies[10, 12] did not report the method of sequence generation, while three studies[12, 16, 18] did not report the blinding. Two studies[10, 12] used sealed envelopes for allocation concealment. All included studies reported outcomes of more than 95% of participants. Besides, all the results of selective outcome reporting were unclear.
Table 3. Assessments of risk of bias of the randomized controlled trials.
| Studies | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|
| Xie 2015 | Unclear | Opaque sealed envelopes | Yes | Yes | Unclear |
| Wu 2016 | Unclear | Sealed envelopes | Unclear | Yes | Unclear |
| Zeng 2016 | A computer-generated randomization list | Unclear | Yes | Yes | Unclear |
| Zhang 2015 | A random number table | Unclear | Unclear | Yes | Unclear |
| Sun 2016 | A random number table | Unclear | Unclear | Yes | Unclear |
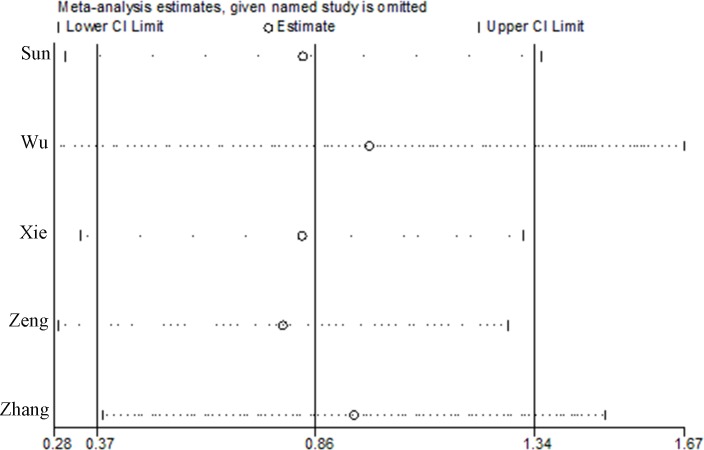
Sensitivity analyses were conducted by excluding each study one by one to explore whether one study markedly affected the results or highly contributed to the heterogeneity. It was indicated that the overall results were not substantially influenced by any single study and the data in this systematic review, and meta-analysis were in a relatively robust situation (Fig 2). There was little evidence of publication bias regarding to transfusion rate in relation to risk of intervention, as indicated by the Begg’s test (P = 0.221) and Egger’s test (P = 0.493). Fig 3 represented funnel plots examining for potential publication bias between studies.
Fig 2. Sensitivity analysis of the meta-analysis.
Fig 3. Publication bias analysis of the meta-analysis.
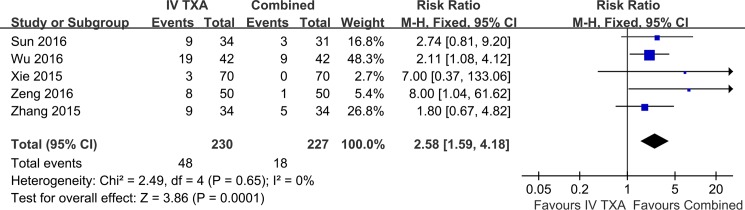
Five studies provided data on the effect of IV-TXA comparing combined TXA on blood transfusion in THA (Fig 4). The risk ratio for attaining blood transfusion was 2.58 (95%CI, 1.59 to 4.18) with applying the fixed-effects model. Results gave a pooled rate of 20.9% (48 of 230) in the IV-TXA group and of 7.9% (18 of 227) in the combined TXA group. IV-TXA administration led to a higher transfusion rate significantly. The studies consistently suggested benefit with little evidence of heterogeneity between findings (P = 0.65, I2 = 0%).
Fig 4. Meta-analysis of transfusion rate on IV-TXA versus combined TXA administration.
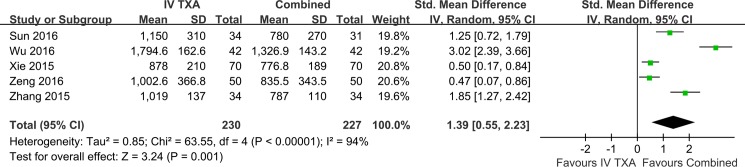
Five studies reported on the amount of blood loss in THA (Fig 5). There was significant statistical heterogeneity between studies (P<0.00001, I2 = 94%) and random-effects was used. Combined TXA administration significantly reduced total blood loss in THA (SMD, 1.39; 95%CI, 0.55 to 2.23) compared with IV-TXA administration. Subgroup analysis was carried out for the amount of blood loss (Table 4). Perhaps the difference between primary and revision hip Arthroplasty was one of the reasons contributing to significant heterogeneity.
Fig 5. Meta-analysis of total blood loss on IV-TXA versus combined TXA administration.
Table 4. Subgroup analysis relating total blood loss.
| Group | No. | P Heterogeneity | I2 | SMD | 95%CI | P | P Interaction |
|---|---|---|---|---|---|---|---|
| Total | 5 | <0.00001 | 94% | 1.39 | 0.55–2.23 | 0.001 | |
| Surgery | |||||||
| Primary THA | 4 | <0.0001 | 86% | 0.98 | 0.39–1.58 | 0.001 | <0.00001 |
| Revision THA | 1 | - | - | 3.02 | 2.39–3.66 | <0.00001 | |
| Surgical approach | |||||||
| Posterolateral | 4 | <0.00001 | 95% | 1.28 | 0.31–2.25 | 0.009 | 0.33 |
| Anterolateral | 1 | - | - | 1.85 | 1.27–2.42 | <0.00001 | |
| Antithrombotic therapy | |||||||
| LMWH | 3 | <0.00001 | 96% | 1.56 | 0.16–2.97 | 0.03 | 0.68 |
| Enoxaparin | 2 | <0.0001 | 94% | 1.15 | -0.16–2.47 | 0.09 |
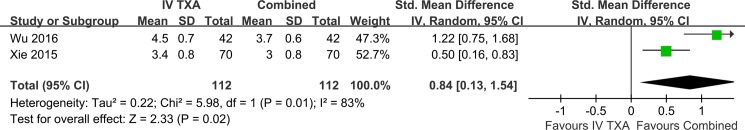
Two studies with 224 patients presented data on maximum postoperative Hb drop after surgery (Fig 6). There was significant statistical heterogeneity between studies (P = 0.01, I2 = 83%), and random-effects was used. Pooled results revealed higher maximum postoperative Hb drop in the IV-TXA group compared with combined group (SMD, 0.84; 95%CI, 0.13 to 1.54).
Fig 6. Meta-analysis of maximum postoperative Hb drop on IV-TXA versus combined TXA administration.
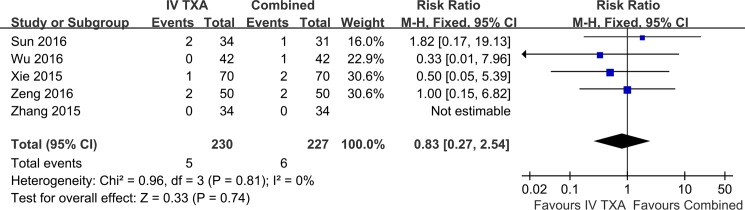
The data of thromboembolic complications were available in all five studies (Fig 7). It included intramuscular venous thrombosis, deep vein thrombosis and pulmonary embolism. There were 5 of 230 in the IV-TXA group and 6 of 227 in the combined TXA group. No significant difference was found between IV-TXA group and combined group (RR, 0.83; 95%CI, 0.27 to 2.54). There was no evidence of heterogeneity between studies (P = 0.81, I2 = 0%).
Fig 7. Meta-analysis of thromboembolic complications on IV-TXA versus combined TXA administration.
Discussion
Five RCTs involving the application of TXA in THA fulfilled the inclusion criteria of this meta-analysis. Combined intravenous and topical TXA administration reduced blood loss, hemoglobin decline and the need for transfusion without increasing the rate of thromboembolic complications significantly.
Five RCTs met the inclusion criteria of meta-analysis and have all been published since 2015. The quality of each study was graded and scored from 4 to 7 according to the modified Jadad scoring system. Two studies[10, 12] did not report the method of sequence generation, while three studies[12, 16, 18] did not report the blinding. Two studies[10, 12] used sealed envelopes for allocation concealment. All five studies about selective outcome reporting were unclear. These issues may have resulted in low modified Jadad scores and information bias. Higher quality RCTs is needed for better assessing the effect of TXA administration methods. Pooled results showed significant heterogeneity when analyzing the amount of blood loss, so subgroup analysis was performed. The difference between primary and revision hip Arthroplasty was one of the reasons contributing to significant heterogeneity. It may also result from variations in methodology, different types of prosthesis, or different doses of TXA, etc. However, there were insufficient data to support these subgroup analyses. In addition, we performed a sensitivity analysis by investigating the effect of each individual study on the pooled effect size, and it was indicated by the results that no article substantially influenced the results and the current meta-analyses were comparatively reliable. Although our results showed that combined TXA group was effective in reducing blood loss, pooling data from a range of surgical operations resulted in substantial statistical heterogeneity and uncertain clinical interpretation. In short, due to apparent heterogeneity across studies and a paucity of included studies, the findings from our study should be dealt with some caution.
In THA treatment, it may cause significant perioperative bleeding because of the large exposed surface of cancellous bone and activation of local fibrinolysis. Patients, with elder age, were often affected by postoperative anemia and associated with increasing mortality and morbidity. TXA was introduced with the aim of reducing blood loss and transfusion requirements.
Intravenous administration of TXA in THA has been well established in the literature. Theoretically, intravenous TXA was hypothesized to be distributed in the whole blood cycle, thereby reducing its therapeutic concentration at the bleeding site, while topically applied TXA was predominantly distributed in the bleeding site and supplied a higher concentration[19]. It was considered to have little or no systemic absorption of TXA when applied topically, with the advantage of preventing the potential thromboembolic complications[20]. Chang reported a smaller decrease in hemoglobin (1.87 g/dL) and a lower transfusion rate (17% versus 35%) in the topical TXA group, and no increase in major complications was identified in patients managed with topical tranexamic acid[21]. Previous systematic reviews and meta-analyses have discussed the efficacy of topical and intravenous regimen of tranexamic acid in primary total hip arthroplasty[22–24]. The topical administration of TXA in THA carried similar hemostasis effects compared with intravenous use without an increased risk of thrombotic complications[22]. However, the optimal route for TXA application remains unknown. Recently, another mode of TXA administration which combined the use of intravenous and topical TXA has been introduced in THA. It was deemed to be more effective than either regimen alone. Nielsen et al[25] reported a persistent and sustained reduction in blood loss of 37% for combined IV and IA TXA compared with IV-TXA alone, at 24 hours postoperatively and on postoperative day 2. As related studies were too lacking, the effect of combined intravenous and topical TXA administration was unclear, when compared with intravenous or topical administration alone.
In 2016, two meta-analyses[26, 27] comparing combined use of intravenous and topical versus intravenous tranexamic acid in primary total joint arthroplasty was reported. However, both total knee arthroplasty and total hip arthroplasty were included in these studies. Of note, a feature specific to TKA was the common use of a tourniquet, which would help reducing local bleeding before TXA administration and is likely to impair TXA delivery to the surgical site intra-operatively[28]. This change may contribute to the increasing heterogeneity between two procedures. In our meta-analysis, this was the first time comparing IV-TXA with combined TXA administration in THA. We reviewed the clinical data currently available and tried to answer the question of the most effective regimen for TXA administration. In this study, the pooled rate of transfusion in the combined TXA group was 7.9% compared with 20.9% in the IV-TXA group, suggesting that combined TXA group significantly reduced the risk of transfusion. In addition, total blood loss and maximum Hb drop in the combined TXA group were significantly decreased when comparing with intravenous TXA studies. Therefore, combined TXA administration was clinically superior to IV-TXA in reducing blood loss, hemoglobin decline and the need for transfusion.
Zeng et al[17] reviewed previously reported results of IV and topical administration of TXA in THA. Most surgeons preferred a 10–30 mg/kg or 1–2g dose of TXA for IV use and 1 or 3 g for topical use. Ueno[29] found that a lower amount of TXA with IV administration can be used to achieve appropriate outcomes in THA than topical TXA administration. However, it was recommended for a single-TXA-use strategy (intravenous or topical), and the dose for combined administration was still unclear[17]. In our meta-analysis, all studies reported the dose for combined TXA administration. It was a 15mg/kg or 1g dose of TXA for IV use and a 0.1g-3g dose for topical use. Except Wu 2016 for revision THA, it was feasible and safety, with a total dose of TXA of approximately 3 g or less for combined administration. Rajesparan et al[30] reported a negative correlation between the dose per kg of TXA and total blood loss in women. However, the number of patients in the study was limited. The maximum dose of TXA was approximately 3 g for primary THA in some literatures[28].
In our meta-analysis, IV-TXA was administered 5–15 minutes before skin incision, and topical TXA was applied at the acetabulum and the femoral canal after acetabulum and femur preparation and the joint space after fascial closure. When combined TXA was administrated, the duration of fibrinolysis activation would be longer than intravenous use alone. In terms of equal total dose, it would maintain a high plasma concentration with combined TXA administration than the single intravenous use[10], which might be the reason that we found less blood loss in combined group.
The potential thromboembolic complications should also be concerned. So far, TXA has been applied in THA for more than ten years; a larger amount of clinical evidence had confirmed the safety, without increasing the incidence of both DVT and PE[31]. A retrospective study by Poeran et al[32] included more than 870, 000 patients receiving THA or TKA during a 6-year period, the results found no significant difference on postoperative DVT between TXA and control group (0.4 Vs 0.5%). In our meta-analysis, four studies except Zhang 2015 have reported that Doppler ultrasound was used to detect thromboembolic complications. All the studies used low molecular weight heparin (LMWH) or Enoxaparin as DVT prophylaxis. Total, five cases of thromboembolic complications (2.2%) was found in IV-TXA group and six cases (2.6%) were in the combined TXA group, which did not reach a significant statistical difference. It was indicated that there was no superiority in the rate of thromboembolic events in combined TXA group compared with intravenous TXA group in this study. Nonetheless, uncertainty still remains as the difference of intramuscular venous thrombosis, and DVT and PE screening and more evidence were required to assess the safety of TXA[8].
There are limitations to the current analysis. Firstly, only five reports were included, and the sample size of each trial was small, which would influence the results. Secondly, most of the available data would support the safety in healthy patients for TXA use rather than those with higher risks. It was underpowered, making it difficult to draw reliable conclusions regarding the use of TXA in high-risk patients[2]. Finally, the optimal dose of TXA administration was still unclear. The decision was sometimes based on the surgeon’s preference or the experience from previous literatures. Additional large studies were required to further refine the right dose of TXA administration.
Conclusion
The present study has emphasized that combined TXA administration can effectively reduce blood loss, hemoglobin decline and the need for transfusion without increasing the rate of thromboembolic complications when compared with IV-TXA use alone. It is the initial systematic review that evaluates the safety and efficiency of combined TXA administration versus intravenous use alone following THA. High-quality RCTs and well-designed trails are still needed to detect the therapeutic dose or other adverse effects in the future.
Supporting information
(PDF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Kim C, Park SS, Davey JR. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. Journal of Blood Medicine. 2015;6:239–44. doi: 10.2147/JBM.S61915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg. 2015;23(12):732–40. Epub 2015/10/24. doi: 10.5435/JAAOS-D-14-00223 . [DOI] [PubMed] [Google Scholar]
- 3.Mccormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585–617. doi: 10.2165/11209070-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452–6. Epub 2014/05/06. doi: 10.1016/j.arth.2014.03.032 . [DOI] [PubMed] [Google Scholar]
- 5.Barrachina B, Lopez-Picado A, Remon M, Fondarella A, Iriarte I, Bastida R, et al. Tranexamic Acid Compared with Placebo for Reducing Total Blood Loss in Hip Replacement Surgery: A Randomized Clinical Trial. Anesth Analg. 2016;122(4):986–95. Epub 2016/03/19. doi: 10.1213/ANE.0000000000001159 . [DOI] [PubMed] [Google Scholar]
- 6.Moskal JT, Capps SG. Meta-analysis of Intravenous Tranexamic Acid in Primary Total Hip Arthroplasty. Orthopedics. 2016:1–10. Epub 2016/06/02. doi: 10.3928/01477447-20160526-02 . [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Wu K, Kong G, Feng W, Deng Z, Wang H. The efficacy of topical tranexamic acid in total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord. 2016;17:81 Epub 2016/02/18. doi: 10.1186/s12891-016-0923-06-0923-0 ; PubMed Central PMCID: PMC4754977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. 2014;21(6):987–93. Epub 2014/12/03. doi: 10.1016/j.knee.2014.09.010 . [DOI] [PubMed] [Google Scholar]
- 9.Huang Z, Ma J, Shen B, Pei F. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(12):2342–6. Epub 2014/07/11. doi: 10.1016/j.arth.2014.05.026 . [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Ma J, Yue C, Kang P, Pei F. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip International the Journal of Clinical & Experimental Research on Hip Pathology & Therapy. 2015;26(1):481–92. [DOI] [PubMed] [Google Scholar]
- 11.Machin JT, Batta V, Soler JA, Sivagaganam K, Kalairajah Y. Comparison of intra-operative regimes of tranexamic acid administration in primary total hip replacement. Acta Orthop Belg. 2014;80(2):228–33. Epub 2014/08/06. . [PubMed] [Google Scholar]
- 12.Wu YG, Zeng Y, Yang TM, Si HB, Cao F, Shen B. The Efficacy and Safety of Combination of Intravenous and Topical Tranexamic Acid in Revision Hip Arthroplasty: A Randomized, Controlled Trial. J Arthroplasty. 2016. Epub 2016/05/18. doi: 10.1016/j.arth.2016.03.059 . [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. Epub 2009/07/28. doi: 10.1016/j.jclinepi.2009.06.005 . [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 Epub 2011/10/20. doi: 10.1136/bmj.d5928 ; PubMed Central PMCID: PMC3196245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord. 2001;12(3):232–6. Epub 2001/03/13. . [DOI] [PubMed] [Google Scholar]
- 16.Sun SW, Yang L, Xie SA, Wang J, Xu RB. [Combined use of intraarticular and intravenous tranexamic acid in total hip arthroplasty.]. Chinese Journal of Tissue Engineering Research. 2016;20(48):7149–55. doi: 10.3969/j.issn.2095-4344.2016.48.001 [Google Scholar]
- 17.Yi Z, Bin S, Jing Y, Zongke Z, Pengde K, Fuxing P. Tranexamic Acid Administration in Primary Total Hip Arthroplasty: A Randomized Controlled Trial of Intravenous Combined with Topical Versus Single-Dose Intravenous Administration. J Bone Joint Surg Am. 2016;98(12):983–91. Epub 2016/06/17. doi: 10.2106/JBJS.15.00638 . [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wang DR, Zhao GP, Qin HX. [Clinical results of combined intravenous and topical application of tranexamic acid in total hip arthroplasty.]. The Journal of Practical Medicine. 2015;31(20):3358–60. doi: 10.3969/j.issn.1006-5725.2015.20.023 [Google Scholar]
- 19.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005–15. Epub 2014/08/03. doi: 10.1302/0301-620X.96B8.33745 . [DOI] [PubMed] [Google Scholar]
- 20.Alshryda S, Mason J, Sarda P, Nargol A, Cooke N, Ahmad H, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969–74. Epub 2013/11/08. doi: 10.2106/JBJS.L.00908 . [DOI] [PubMed] [Google Scholar]
- 21.Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552–7. Epub 2014/01/05. doi: 10.1007/s11999-013-3446-0 ; PubMed Central PMCID: PMC3971210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Dong Q, Zhang YG. Intravenous versus topical tranexamic acid in primary total hip replacement: A systemic review and meta-analysis. Int J Surg. 2016;32:10–8. Epub 2016/06/06. doi: 10.1016/j.ijsu.2016.05.064 . [DOI] [PubMed] [Google Scholar]
- 23.Hanna SA, Prasad A, Lee J, Achan P. Topical Versus Intravenous Administration of Tranexamic Acid in Primary Total Hip Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Orthop Rev (Pavia). 2016;8(3):6792 Epub 2016/10/21. doi: 10.4081/or.2016.6792 ; PubMed Central PMCID: PMC5066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Liang Y, Chen P, Fang Y, He J, Wang J. Intravenous versus topical tranexamic acid in primary total hip replacement: A meta-analysis. Medicine (Baltimore). 2016;95(50):e5573 Epub 2016/12/16. doi: 10.1097/MD.0000000000005573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen CS, Jans O, Orsnes T, Foss NB, Troelsen A, Husted H. Combined Intra-Articular and Intravenous Tranexamic Acid Reduces Blood Loss in Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial. J Bone Joint Surg Am. 2016;98(10):835–41. Epub 2016/05/20. doi: 10.2106/JBJS.15.00810 . [DOI] [PubMed] [Google Scholar]
- 26.Shang J, Wang H, Zheng B, Rui M, Wang Y. Combined intravenous and topical tranexamic acid versus intravenous use alone in primary total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. International Journal of Surgery. 2016;36:324–9. doi: 10.1016/j.ijsu.2016.11.033 [DOI] [PubMed] [Google Scholar]
- 27.Zhang XQ, Ni J, Ge WH. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total joint arthroplasty: a meta-analysis of randomized controlled trials. International Journal of Surgery. 2016;38:15–20. doi: 10.1016/j.ijsu.2016.11.136 [DOI] [PubMed] [Google Scholar]
- 28.Lin ZX, Woolf SK. Safety, Efficacy, and Cost-effectiveness of Tranexamic Acid in Orthopedic Surgery. Orthopedics. 2016;39(2):119–30. Epub 2016/03/05. doi: 10.3928/01477447-20160301-05 . [DOI] [PubMed] [Google Scholar]
- 29.Ueno M, Sonohata M, Fukumori N, Kawano S, Kitajima M, Mawatari M. Comparison between topical and intravenous administration of tranexamic acid in primary total hip arthroplasty. Journal of Orthopaedic Science. 2016;21(1):44–7. doi: 10.1016/j.jos.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 30.Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776–83. Epub 2009/06/02. doi: 10.1302/0301-620X.91B6.22393 . [DOI] [PubMed] [Google Scholar]
- 31.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. Bone & Joint Journal. 2011;93(1):39–46. [DOI] [PubMed] [Google Scholar]
- 32.Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. Bmj Clinical Research. 2014;349(aug12 8):g4829–g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.