Abstract
Goat’s milk, considered a substitute for cow’s milk, has a high nutritional value. However, goat’s milk contains various allergens, predominantly β-lactoglobulin (BLG). In this study, we employed the CRISPR/Cas9 system to target the BLG locus in goat fibroblasts for sgRNA optimization and generate BLG knock-out goats through co-injection of Cas9 mRNA and small guide RNAs (sgRNAs) into goat embryos at the one-cell stage. We firstly tested sgRNA editing efficiencies in goat fibroblast cells, and approximately 8.00%–9.09% of the cells were modified in single sgRNA-guided targeting experiment. Among the kids, the genome-targeting efficiencies of single sgRNA were 12.5% (10 ng/μL sg1) and 0% (10 ng/μL sg2) and efficiencies of dual sgRNAs were 25.0% (25 ng/μL sg2+sg3 group) and 28.6% (50 ng/μL sg2+sg3 group). Relative expression of BLG in BLG knock-out goat mammary glands significantly (p < 0.01) decreased as well as other milk protein coding genes, such as CSN1S1, CSN1S2, CSN2, CSN3 and LALBA (p < 0.05). As expected, BLG protein had been abolished in the milk of the BLG knock-out goat. In addition, most of the targeted kids were chimeric (3/4), and their various body tissues were edited simultaneously. Our study thus provides a basis for optimizing the quality of goat milk, which can be applied to biomedical and agricultural research.
Introduction
The goat (Capra hirus) is one of the most important livestock species, providing products such as meat, hides, and milk. Goat’s milk and its byproducts, such as yogurt, cheese, and powder, are important components of the daily human diet in many countries [1]. Furthermore, goats are also used as mammary gland bioreactors in biomedical studies [2].
Goat’s milk has similar nutritional value to cow’s milk, with high percentages of fats and proteins. β-lactoglobulin (BLG) is a major whey protein allergen in goat and other ruminants’ milk [3]. Hydrolysis and heat do not suppress the allergenicity of BLG, and fermentation byproducts increases its immuno-reactivity [4, 5].
Zinc finger nuclease (ZFN) and transcription activator–like effector nucleases (TALENs) are recently developed genomic engineering tools. However, the complexity of protein design and synthesis of ZFN and TALENs had hampered their application in medical and biological research [6, 7]. Natronobacterium gregoryi argonaute (NgAgo) is a newly developed DNA guide endonuclease; however, it has been reported that Ago cannot cut the genomic DNA but can knockdown the gene expression[8, 9]. Clustered regularly interspaced short palindromic repeats (CRISPR) are short segments of prokaryotic DNA containing repetitive base sequences; CRISPR functions as an adaptive immune system in prokaryotes and has been adapted for genome editing in eukaryotes [10]. Small guide RNAs (sgRNAs) are used to guide Cas9 protein to specifically cleave DNA strands, causing double-strand breaks that are subsequently repaired through either non-homologous end joining or homology-directed repair mechanisms [11, 12]. Editing of the BLG gene was achieved in goat fibroblasts by using Cas9 [13] and TALENs[14], and BLG knock-out (KO) cattle has been generated by using ZFN [15]. CRISPR/Cas9 has also been used in knocking out Myostatin and FGF5 in goat [16, 17] and Myostatin in sheep [18] via injection of Cas9 mRNA and sgRNA. Thus, to generate BLG KO goats for use in our research, we employed the CRISPR/Cas9 system cytoplasmic injection method. We then characterized the changes in the genotype and phenotype during lactation in BLG KO goats. These results provided valuable insight into the BLG gene in goats and methods of goat milk quality improvement.
Materials & methods
Animals
Healthy goats (2 to 3 years old) were selected and housed at the Haimen Goat Research & Development Center in Jiangsu. All protocols involving the use of animals were performed in accordance with the approved Guidelines for Animal Experiments of Nanjing Agricultural University, which were approved by the Animal Care and Use Committee of Nanjing Agricultural University (Approval ID: SYXK2011-0036).
sgRNA design
The pX330 plasmid was donated by Libin Cui PhD in the US. The sgRNA was designed using the MIT CRISPR design tool website (http://crispr.mit.edu/). Then sgRNAs were screened by Cas-Offinder and sgRNAs with fewer mismatches were chosen. Three sgRNAs targeting exon 1 of the goat BLG gene (GenBank: Z33881) were chosen (Fig 1A). Two of the sgRNAs (sg1, sg3) were on the sense strand, and the third (sg2) was on the antisense strand; a guanine (G) was added at the 5`end of the guide sequence without a guanine at the beginning of the 5`end (S1 Table). The sgRNAs were cloned into pX330 to form the final vectors, Cas9-sg1, Cas9-sg2, and Cas9-sg3.
Fig 1. CRISPR/Cas9-mediated modification of the BLG locus in fibroblasts.
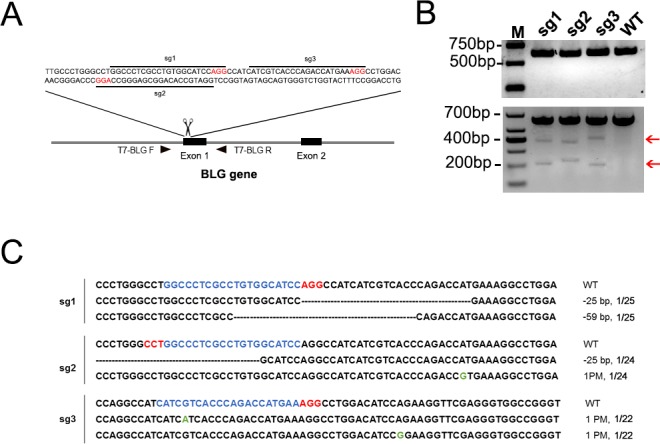
(A) Schematic diagram of sgRNA design for the goat BLG locus. Primers labeled as “BLG-T7-F” and “BLG-T7-R” were used for the T7E1 cleavage assay at the sg1, sg2, and sg3 target sites. (B) Targeting BLG loci using single sgRNA by electroporation. Top panel: PCR products of the target region of BLG from fibroblasts transfected with a single Cas9-sgRNA plasmid. Bottom panel: T7E1 assay of products shown in the top panel. M, marker; WT, wild-type cells without treatment with Cas9 plasmid. Red arrows indicate the expected cleaved products after T7E1 cleavage assay. (C) Sequencing results of sgRNAs targeting BLG.
Cell culture and transfection
Goat fetal fibroblasts were isolated as described previously [19] and cultured in Dulbecco’s modified Eagle’s medium (Gibco, USA) supplemented with 15% fetal bovine serum (FBS; Gibco) and 1% glutamine (Gibco) in T25 flasks or 6-well plates until reaching 80–90% confluence on the day of transfection. Cells were transfected with either single Cas9-sgRNA plasmid (1 μg/105 cells) or eGFP plasmid (1 μg/105 cells) using a Neon transfection system (Life Technologies, USA) or using Lipofectamine 2000 (Invitrogen, USA), according to the manufacturer’s protocols. Cells were collected for DNA extraction 72 h after transfection.
In vitro transcription
The in vitro transcription templates for Cas9 and the sgRNAs were amplified using the T7 promotor–appended primers listed in S3 Table and gel-purified using QiaQuick spin columns (Qiagen, Germany). The Cas9 template was transcribed in vitro using a T7 Ultra kit (Ambion, USA), and the sgRNA templates were transcribed in vitro using a MEGA shortscript kit (Ambion). The resulting Cas9 mRNA and sgRNAs were then purified using a MEGAclear kit (Ambion).
Preparation and injection of one-cell embryos
Goats were subjected to a superovulation protocol, as previously described [17]. Briefly, a progesterone sponge was implanted in the vagina for 11 days; when the sponge was removed, the animal was administered 100 IU of prostaglandin (Sansheng, China). The donors received a total of 200 IU of follicle-stimulating hormone (Sansheng) twice daily in a decreasing dose over 3 days (50/50, 25/25, and 15/15 IU), starting 48 h before sponge removal. Then donors were mated at 36 and 48 h after sponge removal. The recipients received 100 IU of pregnant mare serum gonadotropin (Sansheng) 24 h prior to sponge removal. One-cell-stage embryos were flushed from the donor oviducts 72 h after sponge removal. Collected one-cell-stage embryos were then injected with a mixture of Cas9 mRNA and sgRNAs (Table 1). Injected zygotes were then cultured in M2 medium containing 10% FBS (Gibco) at 37°C and 5% CO2. Cleaved two-cell to blastocyst stage embryos were transferred into estrous-synchronized recipient goats. Early pregnancy was confirmed by observing the estrous cycling 28–30 days after the embryo transfer.
Table 1. Summary of generation of gene-modified goats through micro-injection.
| sgRNA(ng/μL) | Cas9 mRNA (ng/μL) | Injected embryos/ Transferred embryos |
Recipients/ Pregnancies | Newborns | Targeted (%) |
|---|---|---|---|---|---|
| sg1(10) | 20 | 36/27 | 10/5 | 8 | 1 (12.5%) |
| sg2(10) | 48/29 | 10/5 | 7 | 0 (0%) | |
| sg2+sg3 (25+25) | 100 | 59/15 | 15/3 | 4 | 1 (25.0%) |
| sg2+sg3* (50+50) | 200 | 50/32 | 32/5 | 7 | 2 (28.6%) |
*One recipient died after transfer of zygotes. One kid was stillborn, and three died within 1 month after delivery.
T7 endonuclease 1 (T7E1) cleavage assay and Sanger sequencing of the PCR amplicons
Ear genomic DNA and cell DNA were extracted using a DNA extraction kit (Tiangen, China). The genomic regions surrounding each target site were amplified using PrimerStar HS DNA polymerase (Takara, China) with the primers listed in S3 Table and then purified using a QiaQuick spin column (Qiagen), following the manufacturer’s protocol. A total of 300 ng of the purified PCR product was mixed with NEB buffer 2 and subjected to the re-annealing process to enable heteroduplex formation: 95°C for 10 min, ramping from 95 to 85°C at −2°C/s, 85 to 25°C at −0.25°C/s, and holding at 25°C for 1 min. Next, 0.5 μL of T7E1 (NEB, USA) was added to the products, which were incubated for 30 min and then resolved on a 2.5% agarose gel. The PCR products with mutations detected using the T7E1 cleavage assay were then sub-cloned into the pMD-19T vector (Takara). For each sample, 12 to 25 random colonies were picked for Sanger sequencing.
Detection of off-target activity
Potential off-targets (OTs) of the sgRNAs were computationally predicted using Cas-Offinder [20], according to the goat genome assembly v2.0 [21]. We selected OTs containing “NGG” or “NAG” protospacer adjacent motifs and fewer than five mismatched nucleotides (S2 Table). The potential OTs of BLG-modified goat were screened using the methods described above (S3 Table).
Gene expression analysis
Total RNA was extracted from the mammary glands of hormonally induced lactating BLG KO goat and wild-type (WT) goats two weeks after hormone-induced lactation using an RNA extraction kit (Tiangen). The concentration of extracted RNA was determined using a NanoDrop spectrophotometer (Thermo, 2000C). First-strand cDNAs were generated through reverse transcription using 1 μg of total RNA and oligo-dT primers. The sequences and GenBank accession numbers of the primer sets used to amplify the target genes are listed in S3 Table. qPCR assessment was performed using an Applied Biosystems StepOneTM Real-Time PCR Systems (Applied BioSystems, USA), and fluorescence was detected using SYBR Green (Roche, Germany) in a reaction volume of 20 μL. The quantity of each measured cDNA sample was normalized to the reference gene, GAPDH. Target-gene relative expression was determined using the ΔΔCT method. For ease of comparison, the average expression level of each gene from the control goats was set at 1.00. The primers used were described by Eleni et al [22].
Induction of lactation and isolation of whey protein in goat’s milk
Lactation was hormonally induced in 8-month-old goats according to a previously described protocol [23]. Briefly, the animals were given estradiol (0.25 mg/kg, intramuscularly [IM]; Sansheng) and progesterone (0.75 mg/kg, IM; Sansheng) every other day from the 1st day to the 13th day; prednisolone (0.4 mg/kg, IM; Energy Chemical, China) was given from the 14th day to the 16th day. Daily mammary massage was carried out since the 5th day. Milk components were analyzed using a Julie Z9 milk analyzer (MilkoScope, Bulgaria). The isolation of whey protein in goat’s milk was performed as described previously [14].
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Coomassie blue staining, and western blot
Whey protein in the milk serum was analyzed by SDS-PAGE with Coomassie blue staining. The proteins in the whey were isolated as described above. Samples were denatured in SDS gel-loading buffer at 98°C for 10 min. Total proteins (1 μL milk serum) were separated by 12% SDS-PAGE and stained with Coomassie blue or electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, USA). After incubation in blocking buffer (5% BSA in Tris-buffered saline containing 0.1% Tween 20) for 1 h at room temperature, the membrane was incubated overnight at 4°C with a Rabbit anti-BLG primary antibody (Bioss, China, bs-2065R, 1:500 dilution). After washing, the membrane was incubated with a goat anti-rabbit IgG (H + L) secondary antibody (Abcam, USA, ab97051, 1: 5,000 dilution) for 1 h at RT. After washing, the signal was detected using an ECL western blot detection system (Fujifilm, Tokyo, Japan).
Data analysis
The data were analyzed using one-way analysis of variance, followed by Duncan’s’ multiple comparison test using SPSS software (SPSS Inc., USA). Data were derived from at least three independent experiments. Differences in the relative expression of mammary gland genes were analyzed by Student’s t-test. A value of p < 0.05 was considered significant.
Results
Efficiency of the CRISPR/Cas9 system in fibroblasts
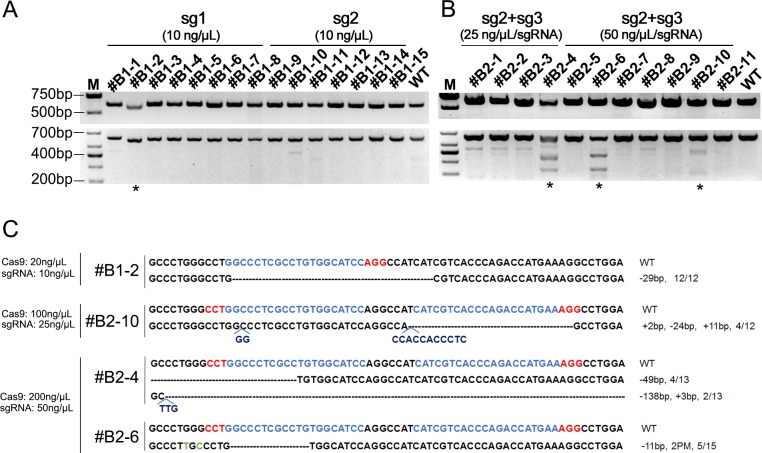
Three sgRNAs targeting the goat BLG locus were designed. To test the genome editing efficiencies of different transfection methods, a 9-kb plasmid containing enhanced green fluorescent protein was transfected into goat fibroblasts using lipofection or electroporation method. Flow cytometry was performed after 48 h, and the result revealed that about 1.4% of the cells were green fluorescence–positive in the lipofection group, and 16.5% of cells were green fluorescence–positive in the electroporation group (S1 Fig). Therefore, we chose the electroporation method for subsequent experiments. To test the editing efficiency of the three sgRNAs, each of the three Cas9-sgRNA plasmids was transfected into goat fibroblasts, and genomic DNA was extracted 72 h after transfection. The target region was PCR amplified and subjected to the T7E1 cleavage assay. Additional bands with expected sizes (sg1: ~396 bp and ~226 bp; sg2: ~382 bp and ~240 bp; sg3: ~425 bp and 197 bp) in the T7E1 cleavage assay indicated that the BLG loci were modified in the three populations of transfected cells (Fig 1B).
Sequencing of T-clones showed that the three target sites were edited with indels: 25- and 59-bp deletions at the sg1 target site; a 1-bp point mutation and a 25-bp deletion around the sg2 target site; two 1-bp point mutations near the sg3 target site (Fig 1C). The sequencing results showed short deletions (4 to 59 bp) and point mutations caused by single sgRNA-guided Cas9 editing, however, no insertion was detected in our cell-targeting experiments (Fig 1C). The single sgRNA-targeting efficiencies are 8.00% (2/25), 8.33% (2/24), and 9.09% (2/22) for sg1, sg2, and sg3, respectively.
Generation of genome-modified goats
To generate BLG KO goats, we collected a total of 193 embryos; one-cell embryos were then co-injected with sgRNAs and different concentrations of Cas9 mRNA by cytoplasmic injection. A total of 103 zygotes were transferred into 67 pseudo-pregnant female goats (1.54 embryos per recipient). Among the 67 recipients, 18 pregnancies were established, as determined by estrous cycle observation. After approximately 150 days of gestation, 26 kids were delivered, one (#B1-8 injected with sg1) of which was stillborn and three (#B2-4 injected with 50 ng/μL sg2+sg3, #B2-8 and #B2-10 injected with 100 ng/μL sg2+sg3) of which died within 1 month after birth (Table 1).
Genomic DNA was isolated from the ear of the dead fetus and infant goats for T7E1 assay and Sanger sequencing. The results showed that 15.38% (4/26) of the infants were edited at the BLG locus. A lower-molecular-weight band was observed in the PCR product of #B1-2, which indicated a homozygous deletion at the BLG locus (Fig 2A). In addition, 12.5% (1/8) of infants in the group co-injected with sg1(10 ng/μL) and Cas9 mRNA (100 ng/μL) exhibited BLG modifications at target sites, whereas 0% of infants in the group co-injected with sg2 (10 ng/μL) and Cas9 mRNA (100 ng/μL) were BLG-modified. Furthermore, 25.0% of the infants (1/4) were modified by co-injection of sg2 and sg3 (25 ng/μL per sgRNA) with 100 ng/μL Cas9 mRNA, and 28.6% (2/7) were modified in the group co-injected with sg2 and sg3 (50 ng/μL per sgRNA) and 200 ng/μL Cas9 mRNA (Table 1 and Fig 2). Different indel types were observed in our targeted goats, such as more insertions and point mutations in the dual sgRNA-targeted goats and a longer fragment deletion in #B2-4 (Fig 2C). These indels could cause frame shift mutations and changes of protein sequence (S2 Fig).
Fig 2. CRISPR/Cas9-mediated one-step generation of BLG KO goats.
(A) Top panel: PCR products of the target region of BLG from newborn goats micro-injected with Cas9 and single sgRNA. Bottom panel: Results of T7E1 assay of products shown in the top panel. #B1-1 to #B1-8 were injected with sg1, and #B1-9 to #B1-15 were injected with sg2. (B) Top panel: PCR products of the target region of BLG from newborn goats micro-injected with Cas9 and dual sgRNAs. Bottom panel: Results of T7E1 assay of products shown in the top panel. #B2-1 to #B2-4 were injected with sg2+sg3 (25 ng/μL/sgRNA), and #B2-5 to #B2-11 were injected with sg2+sg3 (50 ng/μL/sgRNA). (C) Sequencing results of BLG-modified kids injected with different concentrations. WT, wild-type. PM, point mutation.
The bands of unexpected size were observed in the lanes of #B1-1, #B1-10, #B1-11, #B2-1 to #B2-3, #B2-8, and #B2-9 in the T7E1 assay, and resulted from heterozygous genotypes at the BLG locus (Z33881 g. 1866 C > T, 2015 G > T, 2391 A > C, S3 Fig).
Analysis of mammary glands and milk from BLG-targeted goats
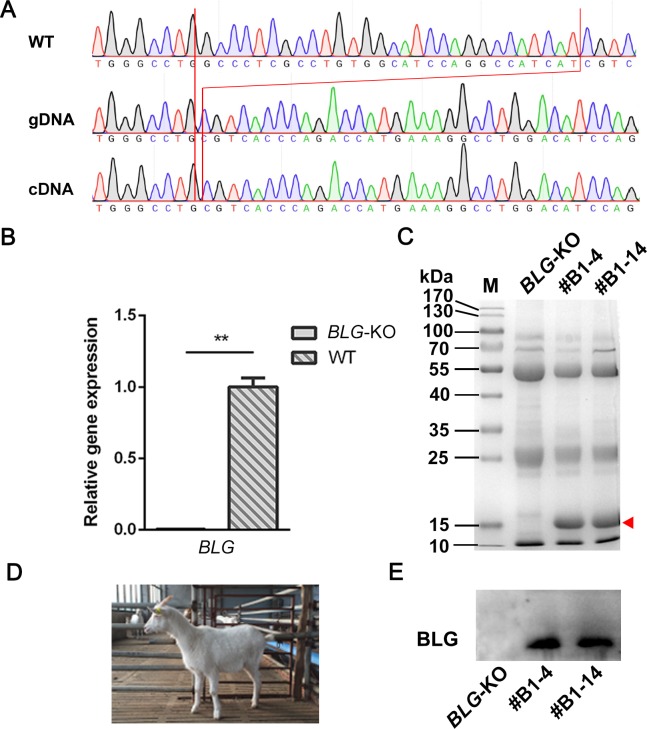
To investigate the phenotype change, the BLG KO goat #B1-2 and WT goats were hormonally induced for lactation. We then collected the milk and mammary gland tissues. Genomic DNA was extracted from the mammary gland tissues for PCR amplification, and the mRNA was extracted and used for RT-PCR amplification. The genomic BLG locus and CDS region of BLG mRNA were amplified and sequenced. The results revealed a 29-bp deletion in #B1-2 BLG exon 1, which perfectly matched the previous sequencing results of ear tissue (Fig 3A). Quantitative PCR results showed that BLG gene expression in BLG KO goats was significantly (p < 0.01) lower than in WT goats. (Fig 3C). To investigate the effect of knocking out BLG gene on other main milk protein coding genes’ expression level during lactation, the mRNA levels of alpha-S1-casein (CSN1S1), alpha-S2-casein (CSN1S2), beta-casein (CSN2), kappa-casein (CSN3), and lactalbumin (LALBA) were also detected. The result showed that the mRNA level of CSN1S1, CSN1S2, CSN2, CSN3, and LALBA significantly (p < 0.01) dropped (S4 Fig) in the BLG KO goat’s mammary glands.
Fig 3. Analysis of genotype and BLG expression in milk and mammary gland.
(A) Comparison of the BLG target region of gDNA in mammary glands of the BLG KO (#B1-2) and WT goats, and cDNA of the BLG KO goat. Red lines indicate a 29-bp deletion at the BLG locus. (B) Analysis of BLG expression in mammary glands. Values with two asterisks are significantly different (p < 0.01). Three assay replicates were performed for each tissue sample. (C) Analysis of whey protein from hormonally induced goat’s milk. Analysis of whey proteins by Coomassie blue staining after separation by SDS-PAGE. Equal amounts of milk samples were loaded. The red triangle indicates WT BLG bands. (D) A photo of a live targeted goat. (E) Detecting the BLG protein in whey by western blot. BLG-KO, the biallelic BLG KO goat; #B1-4 and #B1-14, wild-type goats.
To investigate the expression of BLG protein in kids’ milk, we performed SDS-PAGE with Coomassie blue staining. The Coomassie blue staining results of #B1-2 sample showed two unexpected bands indicating unknown proteins with a lower or a higher molecular weight, respectively (Fig 3C). Furthermore, western blot results showed that the BLG protein had been abolished in the milk of the BLG KO goat (Fig 3E).
In comparison with WT goats, fat, protein, lactose, and solid not fat in the milk of the BLG KO goat decreased by 7.68,7.97, and 7.71%, respectively (Table 2).
Table 2. Comparison of the components of milk from the bi-allelic BLG KO goat versus WT goats.
| Animal | Fat (%) | Protein (%) | Lactose (%) | SNF (%) |
|---|---|---|---|---|
| BLG KO | 4.65 | 4.45 | 3.35 | 8.51 |
| WT | 4.92±0.20 | 4.82±0.06 | 3.64±0.04 | 9.22±0.11 |
SNF, solid not fat.
Chimerism analysis
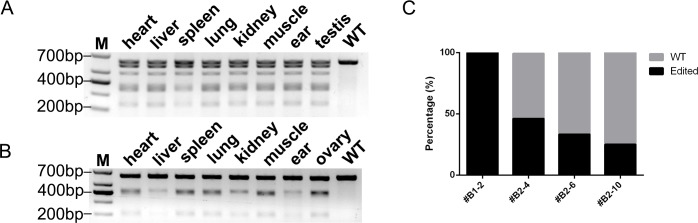
We counted the proportion of edited clones in targeted goat T-clone sequences and found that the edited clone proportion was less than 50%, except for #B1-2 (Fig 4C), suggesting that the modifications in the targeted kids are not mono-allelic but chimeric. To determine whether organs and tissues from CRISPR/Cas9 modified goats were edited simultaneously, we collected organs and tissues from dead targeted kids (#B2-4 and #B2-10) and extracted the genomic DNA. The target regions were amplified and subjected to the T7E1 assay. The results showed that all target sites in the different organs from two targeted kids were modified simultaneously, indicating that Cas9 editing occurred during early embryogenesis. (Fig 4A and 4B).
Fig 4. Chimerism analysis.
(A) Detection of Cas9 editing in different tissues. Dead goat #B2-4 tissues were analyzed by the T7E1 assay. (B) Detection of Cas9 editing in different tissues. Dead goat #B2-10 tissues were analyzed by the T7E1 assay. (C) Analysis of the percentage of edited genome in BLG KO goats. The percentage of editing positive clones among TA clones of #B1-2 (n = 12), #B2-4 (n = 13), #B2-6 (n = 15), and #B2-10 (n = 12).
OT analysis
OT effects are of concern to many researchers utilizing the CRISPR/Cas9 system [24]. We screened a total of nine potential OT sites, including each three for sg1, sg2, and sg3, to determine whether OT effects occurred (S2 Table). T7E1 cleavage assay results showed no detectable OT effects in our BLG-modified goats (S5 Fig).
Discussion
BLG protein presents in milk from cows and goats but not in human milk, and therefore is a major allergen in cow or goat milk [25]. It can cause an intestinal allergic reaction, especially diarrhea in infants [26]. However, neither heat processing nor fermentation could eliminate BLG protein in milk [5, 27, 28]. Therefore, the generation of BLG KO livestock is necessary to evaluate the biology of BLG-free milk. In this study, we achieved locus-specific modification in goat fibroblasts using CRISPR/Cas9. We report a convenient way to generate BLG KO goats by co-injection of Cas9 mRNA and sgRNAs in goat one-cell-stage embryos.
We first tested the efficiency of CRISPR/Cas9 system in goat fibroblasts. Sequencing results showed a low editing activity for single sgRNA-guided targeting (8.00%-9.09%, Fig 1), which may be due to the low transfection efficiency (S1 Fig). It is reported that a high cleavage activity (9–70%) was achieved in goat fibroblasts by electroporation [13]. Wang achieved approximately 50–75% editing efficiency for single sgRNA cleavage and 61.11% efficiency for dual sgRNA-guided cleavage using Blasticidin to eliminate cells that were transfected unsuccessfully [16]. Therefore, CRISPR/Cas9 can be successfully applied in goat cell genome editing.
To determine whether we could generate BLG KO goats through co-injection of Cas9 mRNA and sgRNAs into one-cell-stage embryos, we determined the injection concentration of Cas9 and sgRNA according to Huang’s group [29] at the first round of injection because the low concentration of Cas9 mRNA and sgRNA they used may reduce the off-target activity. As a result, poor single sgRNA-targeting efficiencies (12.5 and 0%) were obtained, and the low concentrations of Cas9 mRNA and sgRNA (Table 1) could have been the primary reason. However, some studies have reported that dual sgRNA targeting might increase the efficiency of modification and cause large fragment deletions [30]. Also, low toxicity has been reported for high concentrations of Cas9 mRNA and sgRNA in embryos [18, 31]. Therefore, we co-injected dual sgRNAs with Cas9 mRNA to improve the targeting efficiency. As summarized in Table 1, the targeting efficiencies of the 50 ng/μL sg2+sg3 group (25.0%) and 100 ng/μL sg2+sg3 group (28.6%) are similar to that reported in other studies using the injection method (15–59%) [16, 18]. Furthermore, some studies reported that the editing efficiency could even be 80%-100% in mice and rabbits [32, 33]. Thus, co-injection of embryos with sgRNAs and Cas9 mRNA is a convenient and less laborious method for generating genome-modified livestock.
In our study, a 29-bp deletion in BLG exon 1, including part of the signal peptide coding sequence, caused a frameshift mutation. The level of BLG expression dropped sharply (p < 0.01) in the BLG KO goats (Fig 3B), in agreement with reports from other transgenic goat studies [14]. As the Coomassie blue staining results showed, we hypothesized that a shorter peptide with mutations would be translated and that the expression of WT BLG protein in milk from the BLG KO goat would be abolished, which could explain the disappearance of 18-kDa WT BLG band in the BLG KO goat milk sample (Fig 3C). Furthermore, the western blot result confirmed the lack of WT BLG protein in the BLG KO goat milk (Fig 3E).
The specificity of the CRISPR/Cas9 system is of significant concern to researchers. Our OT detection showed no OT activity at the predicted loci. Recent in vitro studies suggested that mismatches beyond the seed sequence (8- to 12-bp nucleotides adjacent to protospacer adjacent motifs) can be tolerated [24, 34]. Although a few OT activities of Cas9 have been reported [35, 36], many other studies in livestock have shown a high fidelity of Cas9 [13, 16, 17, 32, 37, 38]. In addition, the use of Cas9 nickase (Cas9D10A or Cas9H840A) [39] or truncated sgRNAs [40] to reduce the occurrence of OT effects have been reported. An “enhanced specificity” SpCas9 (eSpCas9) with low OT activity has been developed, suggesting that Cas9 could be utilized for high-precision genome modification in animals and gene therapy [41].
In Table 2, we found a drop of total protein in the BLG KO goat’s milk. BLG accounts for about 50% of the whey protein in ruminants and nearly 10% of the gross protein in milk [42]. So, abolishment of BLG protein could possibly reduce the total protein in milk (Table 2, Fig 3B and 3E). It is reported that knocking out CSN2 in mice led to decrease of total protein and increase of whey protein, and BLG concentration was negatively related to casein ratio in goat milk, suggesting a compensation effect between casein and whey proteins [43, 44]. Therefore, this compensation effect could possibly explain why the percentage of protein dropped less than 10% and the enhanced bands of caseins and other whey proteins in the SDS-PAGE result (Fig 3C). However, it needs to be further investigated whether the drop of milk protein coding genes’ expression was related to the knock-out of BLG.
In conclusion, we developed a convenient method for generating BLG KO goats through micro-injection of Cas9 mRNA and sgRNAs. Deletion of the BLG coding sequence induces decreases in both BLG mRNA expression and BLG protein concentration in mammary glands and milk. In addition, our study provides a basis for generating BLG KO goats and obtaining humanized milk.
Supporting information
(PDF)
(PDF)
(PDF)
Expression levels of CSN1S1, CSN1S2, CSN2, CSN3, and LALBA.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Ruoxin Jia, Guomin Zhang and Jingang Wang for technical support and thank Yu Ji, Tiewei Ma, Aoxiang Zhu, Yuanhang Shen and Zifei Liu for animal care. We are also grateful to Dr. Libin Cui for providing pX330 plasmid, which was constructed by Feng Zhang’s laboratory.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is financially supported by the China National Program for Transgenic Animal (2014ZX08008-004) and the National Nature Science Foundation of China (No. 31272443). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haenlein GFW. Goat milk in human nutrition. Small Ruminant Research. 2004;51(2):155–63. [Google Scholar]
- 2.Maga EA, Shoemaker CF, Rowe JD, Bondurant RH, Anderson GB, Murray JD. Production and Processing of Milk from Transgenic Goats Expressing Human Lysozyme in the Mammary Gland. Journal of Dairy Science. 2006;89(89):518–24. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Kumar P, Betzel C, Singh TP. Structure and function of proteins involved in milk allergies. Journal of Chromatography B. 2001;756(2):183–7. [DOI] [PubMed] [Google Scholar]
- 4.Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998;53(supplement):102–5. [DOI] [PubMed] [Google Scholar]
- 5.Sélo I, Négroni L, Créminon C, Yvon M, Peltre G, Wal JM. Allergy to Bovine â–Lactoglobulin: Specificity of Human IgE Using Cyanogen Bromide–Derived Peptides. International Archives of Allergy & Immunology. 1998;117(1):20–8. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Chen Y, Jiao R. TALEN-mediated Drosophila genome editing: protocols and applications. Methods. 2014;69(1):22–31. doi: 10.1016/j.ymeth.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 7.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161(3):1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F, Shen XZ, Jiang F, Wu Y, Han C. DNA-guided genome editing using the Natronobacterium gregoryi Argonaute. Nat Biotechnol. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Qi J, Dong Z, Shi Y, Wang X, Qin Y, Wang Y, et al. NgAgo-based fabp11a gene knockdown causes eye developmental defects in zebrafish. Cell Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339(6121):819–23. doi: 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong Z, Zhu S, Xu Y, Fu X. Homologous recombination in human embryonic stem cells using CRISPR/Cas9 nickase and a long DNA donor template. Protein Cell. 2014;5(4):258–60. doi: 10.1007/s13238-014-0032-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JM, Kim D, Kim S, Kim JS. Genotyping with CRISPR-Cas-derived RNA-guided endonucleases. Nature communications. 2014;5:3157 doi: 10.1038/ncomms4157 [DOI] [PubMed] [Google Scholar]
- 13.Ni W, Qiao J, Hu S, Zhao X, Regouski M, Yang M, et al. Efficient gene knockout in goats using CRISPR/Cas9 system. PloS one. 2014;9(9):e106718 doi: 10.1371/journal.pone.0106718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui C, Song Y, Liu J, Ge H, Li Q, Huang H, et al. Gene targeting by TALEN-induced homologous recombination in goats directs production of beta-lactoglobulin-free, high-human lactoferrin milk. Scientific reports. 2015;5:10482 doi: 10.1038/srep10482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu S, Luo J, Song Z, Ding F, Dai Y, Li N. Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell Research. 2011;21(21):1638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Yu H, Lei A, Zhou J, Zeng W, Zhu H, et al. Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Scientific reports. 2015;5:13878 doi: 10.1038/srep13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo R, Wan Y, Xu D, Cui L, Deng M, Zhang G, et al. Generation and evaluation of Myostatin knock-out rabbits and goats using CRISPR/Cas9 system. Scientific reports. 2016;6:29855 doi: 10.1038/srep29855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F, Santos-Neto PCD, et al. Efficient Generation of Myostatin Knock-Out Sheep Using CRISPR/Cas9 Technology and Microinjection into Zygotes. PloS one. 2015;10(8):e0136690 doi: 10.1371/journal.pone.0136690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Y, Deng M, Zhang G, Ren C, Zhang H, Zhang Y, et al. Abnormal expression of DNA methyltransferases and genomic imprinting in cloned goat fibroblasts. Cell Biol Int. 2016;40(1):74–82. doi: 10.1002/cbin.10540 [DOI] [PubMed] [Google Scholar]
- 20.Bae S, Park J, Kim JS. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30(10):1473–5. doi: 10.1093/bioinformatics/btu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Xie M, Jiang Y, Xiao N, Du X, Zhang W, et al. Sequencing and automated whole-genome optical mapping of the genome of a domestic goat (Capra hircus). Nat Biotechnol. 2013;31(2):135–41. doi: 10.1038/nbt.2478 [DOI] [PubMed] [Google Scholar]
- 22.Tsiplakou E, Flemetakis E, Kouri ED, Karalias G, Sotirakoglou K, Zervas G. The effect of long term under- and over-feeding on the expression of six major milk protein genes in the mammary tissue of sheep. J Dairy Res. 2015;82(3):257–64. doi: 10.1017/S0022029915000333 [DOI] [PubMed] [Google Scholar]
- 23.Cammuso C, Porter C, Nims S, Gaucher D, Melican D, Bombard S, et al. Hormonal induced lactation in transgenic goats. Anim Biotechnol. 2000;11(1):1–17. doi: 10.1080/10495390009525943 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Wang Y, Wu X, Wang J, Wang Y, Qiu Z, et al. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol. 2015;33(2):175–8. doi: 10.1038/nbt.3127 [DOI] [PubMed] [Google Scholar]
- 25.Wal JM. Cow's milk allergens. Allergy. 1998;53(11):1013–22. [DOI] [PubMed] [Google Scholar]
- 26.Gall H, Kalveram CM, Sick H, Sterry W. Allergy to the heat-labile proteins alpha-lactalbumin and beta-lactoglobulin in mare's milk. Journal of Allergy & Clinical Immunology. 1996;97(6):1304–7. [DOI] [PubMed] [Google Scholar]
- 27.Ehn Brittmarie, †, ‡, Ekstrand B, Ulf Bengtsson A, Ahlstedt S. Modification of IgE Binding during Heat Processing of the Cow's Milk Allergen β-Lactoglobulin. Journal of Agricultural & Food Chemistry. 2004;52(5):1398–403. [DOI] [PubMed] [Google Scholar]
- 28.Ehn BrittMarie, Allmere Toomas, Telemo Esbjörn, Ulf Bengtsson a, Ekstrand Bo. Modification of IgE Binding to β-Lactoglobulin by Fermentation and Proteolysis of Cow's Milk. Journal of Agricultural & Food Chemistry. 2005;53(9):3743–8. [DOI] [PubMed] [Google Scholar]
- 29.Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, et al. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Research. 2013;23(5):720 doi: 10.1038/cr.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Xu F, Zhu C, Ji J, Zhou X, Feng X, et al. Dual sgRNA-directed gene knockout using CRISPR/Cas9 technology in Caenorhabditis elegans. Scientific reports. 2014;4:7581 doi: 10.1038/srep07581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Yang H, Shivalila Chikdu S, Dawlaty Meelad M, Cheng Albert W, Zhang F, et al. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;153(4):910–8. doi: 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan L, Sui T, Chen M, Deng J, Huang Y, Zeng J, et al. CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Scientific reports. 2016;6:22024 doi: 10.1038/srep22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura H, Gurumurthy CB, Sato T, Sato M, Ohtsuka M. CRISPR/Cas9-based generation of knockdown mice by intronic insertion of artificial microRNA using longer single-stranded DNA. Scientific reports. 2015;5:12799 doi: 10.1038/srep12799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nature Biotechnology. 2014;32(7):677–83. doi: 10.1038/nbt.2916 [DOI] [PubMed] [Google Scholar]
- 35.Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31(9):839–43. doi: 10.1038/nbt.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Du Y, Shen B, Zhou X, Li J, Liu Y, et al. Efficient generation of gene-modified pigs via injection of zygote with Cas9/sgRNA. Scientific reports. 2015;5:8256 doi: 10.1038/srep08256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv Q, Yuan L, Deng J, Chen M, Wang Y, Zeng J, et al. Efficient Generation of Myostatin Gene Mutated Rabbit by CRISPR/Cas9. Scientific reports. 2016;6:25029 doi: 10.1038/srep25029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Y, Yuan L, Wang Y, Chen M, Deng J, Lv Q, et al. Efficient dual sgRNA-directed large gene deletion in rabbit with CRISPR/Cas9 system. Cellular and molecular life sciences: CMLS. 2016;73(15):2959–68. doi: 10.1007/s00018-016-2143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cong L, Zhang F. Genome Engineering Using CRISPR-Cas9 System: Springer New York; 2015. 197–217 p. [DOI] [PubMed] [Google Scholar]
- 40.Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32(3):279–84. doi: 10.1038/nbt.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaymaker IM, Gao LY, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351(6268):84–8. doi: 10.1126/science.aad5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creamer LK, Sawyer L. MILK PROTEINS | Beta-Lactoglobulin. Encyclopedia of Dairy Sciences. 2002:1932–9. [Google Scholar]
- 43.Hallén E. Coagulation properties of milk. Journal of Animal Breeding and Genetics 1990. Vol 107 No 6 pp 458–464. 2008;107(1–6):458–64. [Google Scholar]
- 44.Kumar S, Clarke AR, Hooper ML, Horne DS, Leaver J, Springbett A, et al. Milk Composition and Lactation of β-Casein-Deficient Mice. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(13):6138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Expression levels of CSN1S1, CSN1S2, CSN2, CSN3, and LALBA.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.