Abstract
Objective:
To determine whether and to what extent vitamin D deficiency is associated with multiple sclerosis (MS) risk.
Methods:
We conducted a prospective nested case-control study among women in the Finnish Maternity Cohort (FMC). The FMC had 1.8 million stored serum samples taken during the pregnancies of over 800,000 women at the time of this study. Through linkages with hospital and prescription registries, we identified 1,092 women with MS diagnosed between 1983 and 2009 with at least 1 serum sample collected prior to date of MS diagnosis; ≥2 serum samples were available for 511 cases. Cases were matched to up to 3 controls (n = 2,123) on date of birth (±2 years) and area of residence. 25-Hydroxyvitamin D (25[OH]D) levels were measured using a chemiluminescence assay. We used conditional logistic regression adjusted for year of sample collection, gravidity, and parity to estimate relative risks (RRs) and 95% confidence intervals (CIs).
Results:
A 50 nmol/L increase in 25(OH)D was associated with a 39% reduced risk of MS (RR 0.61, 95% CI 0.44–0.85), p = 0.003. Women with 25(OH)D levels <30 nmol/L had a 43% higher MS risk (RR 1.43, 95% CI 1.02–1.99, p = 0.04) as compared to women with levels ≥50 nmol/L. In women with ≥2 samples, MS risk was 2-fold higher in women with 25(OH)D <30 nmol/L as compared to women with 25(OH)D ≥50 nmol/L (RR 2.02, 95% CI 1.18–3.45, p = 0.01).
Conclusions:
These results directly support vitamin D deficiency as a risk factor for MS and strengthen the rationale for broad public health interventions to improve vitamin D levels.
While there is much evidence supporting a role for adequate vitamin D nutrition in reducing multiple sclerosis (MS) risk,1 there are currently only 2 prospective studies examining whether 25-hydroxyvitamin D (25[OH]D) levels in healthy individuals predict future MS risk.2,3 While both studies found that elevated levels of 25(OH)D (either ≥75 nmol/L3 or ≥100 nmol/L2) among healthy young adults were associated with a ∼60% decreased risk of later developing MS, both studies included fewer than 200 non-Hispanic Caucasian patients with MS, and one was not able to directly examine whether levels of insufficient or deficient 25(OH)D were associated with an increased risk of MS as only 5% of individuals had 25(OH)D levels below 50 nmol/L,2 and the other reported no associations with 25(OH)D lower than 75 nmol/L.3
Therefore, we sought to examine the association between 25(OH)D and MS risk in a large cohort of Finnish women, a population with historically low 25(OH)D levels,4 and specifically examine whether deficient 25(OH)D levels are associated with an increased MS risk.
METHODS
Study population.
The Finnish Maternity Cohort (FMC) began in 1983 and comprises over 800,000 women who gave a blood sample for routine prenatal testing. The majority of blood samples were collected between approximately 10–14 weeks gestation and the leftover serum has been stored at −25°C by the Finnish National Institute for Health and Welfare in Oulu, Finland. There are over 1.8 million serum samples stored in the biorepository, covering over 95% of all pregnancies in Finland since 1983.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the data protection authorities at the National Institute for Health and Welfare and by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District and by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health. Since 2002, informed consent has been collected from mothers to store the samples for research purposes; use of samples collected prior to 2002 for research purposes is allowed under Finnish law.
Case ascertainment and control selection.
Permissions were obtained to link the FMC database to the Finnish Hospital Discharge Register (HILMO) to identify women in the FMC who have received a diagnostic code for MS or a related disease (ICD-10 code G35, G36, H46; ICD-9 and ICD-8 codes 340, 341, 367, 377) between 1983 and 2009. HILMO includes both inpatient (since 1967) and outpatient (since 1998) neurologic visits, as well as patients in longer-term care institutions (since 1994). In Finland, the majority of patients with MS are diagnosed via visit to an inpatient clinic where they are evaluated by neurologists. However, to capture MS cases that may not be listed in HILMO, we also linked the FMC database with the registry of the Social Insurance Institution of Finland (Kela), which contains information on prescription drug reimbursements, including those for MS disease-modifying therapies. In order to be eligible for an MS disease-modifying drug prescription reimbursement, the patient must have a certificate from a doctor confirming MS diagnosis. We identified 1,264 women in the FMC who were listed in HILMO or Kela as having been diagnosed with MS between 1983 and 2009. For 1,252, we had either a medical record or Kela confirmation of the diagnosis. Medical records were available and reviewed for 612 women; all but 5 were clinically confirmed after review. Cases occurring prior to 2001 were confirmed using the Poser criteria5 and those in 2001 or later were confirmed using the McDonald criteria.6 For the remaining 640 women, medical records were not available, but the Kela registry listed them as having received disease-modifying therapy for MS. The date of MS diagnosis was set as the earliest diagnosis date recorded in HILMO, Kela, or the medical records. Date of MS onset was only available for the cases confirmed via medical record review and this date was used as the index date to identify serum samples in the FMC collected prior to onset. For Kela-confirmed cases, the date of diagnosis was used.
Each patient with MS was matched to up to 3 controls on birth date (±2 years) and area of residence (postal code: <10000, 10000 to <30000, 30000 to <50000, 50000 to 91000, >91000). At least 1, and up to 3, serum samples were available from pregnancies occurring prior to MS diagnosis. There were a total of 6,200 serum samples available from 1,092 cases and 2,123 controls. Two or more serum samples were available from 511 cases and 831 matched controls.
25(OH)D measurement.
Serum 25(OH)D levels were measured in all prediagnostic case and control samples using a chemiluminescence microparticle immunoassay and an Architect i2000SR automatic analyzer (Abbott Diagnostics, Abbott Park, IL). The overall assay coefficient of variation calculated from duplicate samples with repeated measures of 25(OH)D was 2.5%. The serum samples were assayed in 2 batches (n = 3,354 and n = 2,846) about 1 year apart. As such, 320 calibration samples in which 25(OH)D was measured in the first batch were also included in the second batch. The repeated measures of 25(OH)D level in these samples were highly correlated (r = 0.98). We calibrated the 25(OH)D levels in the study samples by regressing the 25(OH)D levels measured in the second assay on those in the first assay in the calibration samples. We used the resulting linear regression equation to then calibrate the 25(OH)D measured in the first batch to those in the second. We created age- and seasonally adjusted 25(OH)D levels by regressing the measured levels on the periodic function (−sin[2πX/12] − cos[2πX/12]), where X is the month of sample collection, and age at sample collection, as previously described.2
Statistical analysis.
The main analyses included all 1,092 MS cases and 2,123 age- and residence area–matched controls. For women with more than one 25(OH)D measurement, we averaged their levels across samples as an estimate of their longer-term 25(OH)D exposure. 25(OH)D exposure was modeled in 3 ways: (1) continuous; (2) in a priori defined categories of <30 nmol/L, 30 to <50 nmol/L, and >50 nmol/L, each representing deficient, insufficient, and sufficient 25(OH)D levels, respectively; and (3) quintiles that were derived from the distribution of 25(OH)D among the controls. Conditional logistic regression models were used to estimate the relative risks and 95% confidence intervals (CIs). In addition to the matching factors, multivariate models were adjusted for time of sample collection (number of samples collected during or after the 2004 recommendation that pregnant women use a vitamin D supplement),4 total gravidity (1, 2, ≥3), and parity (0, 1, ≥2). The missing indicator method was used to model gravidity and parity for women missing information on these covariates in order to retain all observations in the analyses. Tests for linear trends across the a priori defined categories and quintiles were conducted by assigning the median 25(OH)D value for each category/quintile to all cases/controls in that category and modeling the median 25(OH)D as a linear variable. In sensitivity analyses, we restricted to MS cases with clinical confirmation (via medical record review) of MS (n = 604) and to MS cases with more than one 25(OH)D measurement from multiple pregnancies prior to diagnosis (n = 511) and their matched controls (figure 1). To evaluate whether the associations between 25(OH)D and MS varied by season, samples were categorized by month of collection into months of high ultraviolet (UV) light (May through October, n = 800 cases/1,740 controls) or low UV light (November through April, n = 764 cases/1,677 controls). Women with samples collected in both high and low UV months will be in both analyses and we averaged 25(OH)D levels for women with more than one sample collected within the high or the low UV month period. Unconditional logistic regression adjusting for the age and geographic location, as well as the other factors listed above, was used to estimate relative risks (RRs) and 95% CIs associated with 50 nmol/L increases in 25(OH)D in the UV-specific analysis.
Figure 1. Finnish Maternity Cohort (FMC) multiple sclerosis (MS) study population.

RESULTS
25(OH)D levels exhibited the expected seasonal distribution (figure 2A), but the yearly 25(OH)D averages were remarkably stable between 1983 and 2004, the year when Finland formally recommended pregnant women take vitamin D supplements (figure 2B).
Figure 2. Variation in 25-hydroxyvitamin D (25[OH]D) levels by month and year of collection.
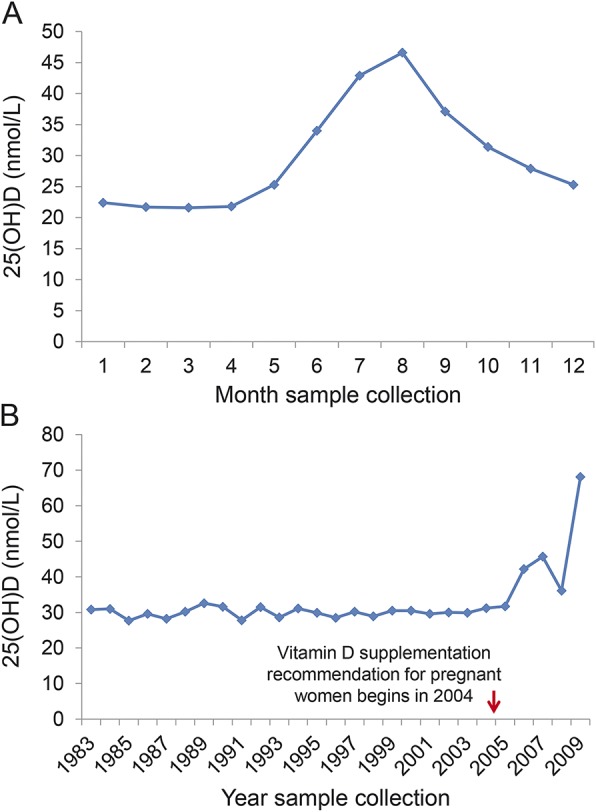
(A) Variation in 25(OH)D levels by month of serum sample collection. (B) Average 25(OH)D levels by calendar year of sample collection. *Only 1 sample collected in 2009.
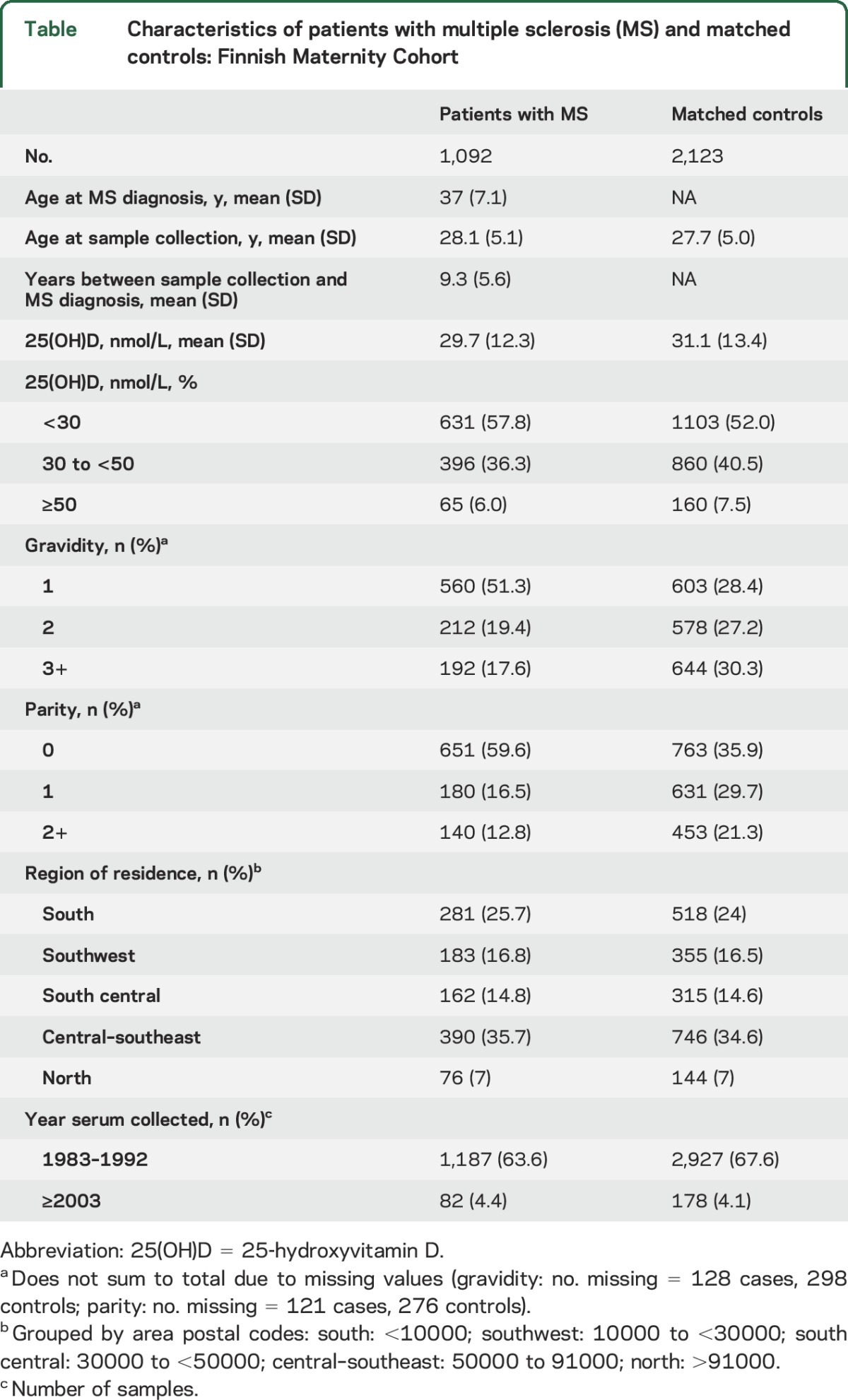
Women who developed MS had average prediagnostic serum 25(OH)D levels 1.3 nmol/L lower than controls; over half of patients and controls had deficient levels of 25(OH)D (<30 nmol/L), and over one-third of patients and controls had insufficient levels (30 to <50 nmol/L) (table). Only 6 patients and 9 controls had 25(OH)D levels ≥75 nmol/L, and of these, only 1 patient and 2 controls had levels ≥100 nmol/L. Among the 604 patients with MS with medical record confirmation, there was an average of 9.5 years between first sample collection and recorded date of MS diagnosis.
Table.
Characteristics of patients with multiple sclerosis (MS) and matched controls: Finnish Maternity Cohort
In multivariate-adjusted analyses including all cases and controls, a 50 nmol/L increase in 25(OH)D was associated with a 39% reduced risk of MS (adj RR 0.61, 95% CI 0.44–0.85, p = 0.003). Compared to women with 25(OH)D levels ≥50 nmol/L, women with levels <30 nmol/L (deficient) had a 43% increased risk of MS (figure 3A), and compared to women with insufficient levels (30 to <50 nmol/L), 25(OH)D-deficient women had a 27% increased MS risk (adj RR 1.27, 95% CI 1.07–1.50, p = 0.005). In quintile analyses, women with extreme deficiency (bottom 2 quintiles 25[OH]D <26.8 nmol/L) had a 53%–66% increased risk of MS as compared to women in the top quintile (25[OH]D ≥41 nmol/L), and the overall trend of increasing MS risk with decreasing 25(OH)D was statistically significant (figure 4). Similar inverse associations between serum 25(OH)D and MS risk were observed in analyses stratified by season of blood collection. The RR associated with a 50 nmol/L increase was 0.71, 95% CI 0.52–0.95, p = 0.02, using serum samples collected in high UV months (May through October) and RR 0.65, 95% CI 0.43–0.98, p = 0.04, in low UV months (November through April).
Figure 3. Association between 25-hydroxyvitamin D (25[OH]D) a priori categories and multiple sclerosis (MS) risk.
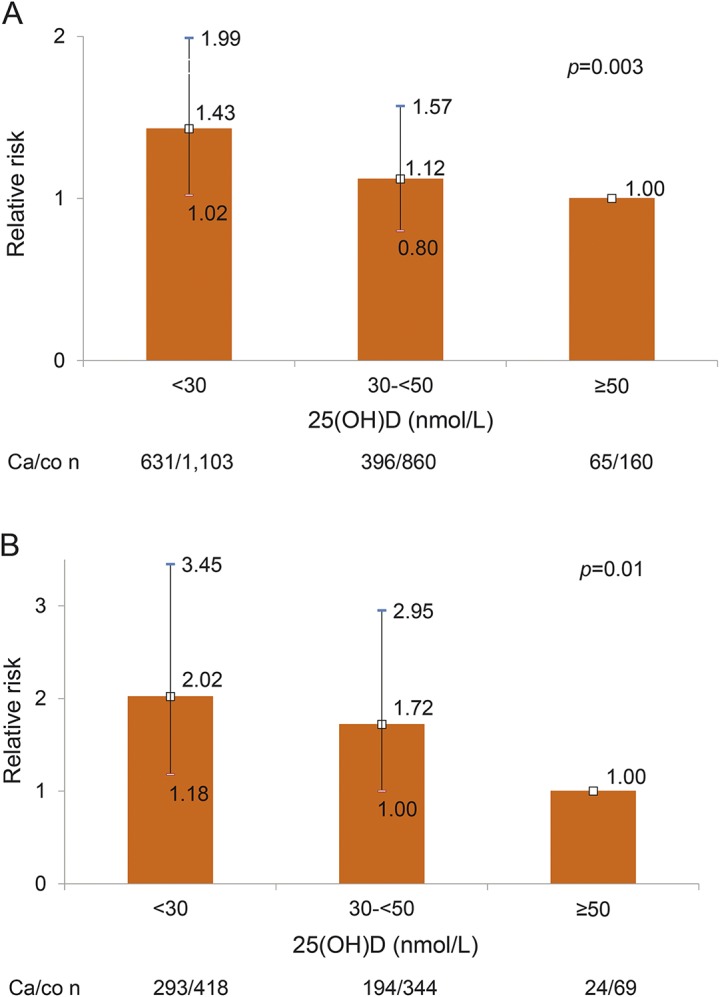
(A) Relative risk for MS in women in the Finnish Maternity Cohort (FMC) by a priori category of 25(OH)D in all cases and matched controls. (B) Relative risk for MS in women in the FMC by a priori category of 25(OH)D in all cases and matched controls with 2 or more samples. Error bars indicate 95% confidence intervals.
Figure 4. Association between 25-hydroxyvitamin D (25[OH]D) quintiles and multiple sclerosis (MS) risk.

Relative risk for MS in women in the Finnish Maternity Cohort by quintiles of 25(OH)D in all cases and matched controls. Error bars indicate 95% confidence intervals.
Risk estimates in analyses restricted to 511 MS cases and matched controls with 2 or more serum samples prior to diagnosis were stronger with a 50 nmol/L increase in 25(OH)D associated with a 41% reduced risk of MS (adj RR 0.59, 95% CI 0.34–1.03, p = 0.07) and 25(OH)D levels <30 nmol/L with a 2-fold increase in MS risk as compared to levels ≥ 50 nmol/L (figure 3B). In quintile analyses, women in the bottom 2 quintiles (<26.8 nmol/L) had a 37%–87% increased risk of MS as compared to women in the top quintile of 25(OH)D (≥41 nmol/L): Q1 vs Q5: RR 1.37, 95% CI 0.89–2.12; Q2 vs Q5: RR 1.87, 95% CI 1.25–2.79; p trend = 0.03). Results among women with MS confirmed by medical record review and their matched controls were similar (data not shown).
DISCUSSION
This study of reproductive age Finnish women is the largest longitudinal investigation to date to directly assess whether levels of vitamin D in healthy individuals predict their risk of developing MS. In analyses based on women with 2 or more measurements of 25(OH)D levels, which are less affected by random variations than those based on a single measurement, we found a 2-fold increase in MS risk when comparing women who were vitamin D deficient (25[OH]D < 30 nmol/L) with those with adequate vitamin D levels ≥50 nmol/L. While we were not able to directly assess MS risk with elevated 25(OH)D levels given that only 15 individuals had 25(OH)D ≥75 nmol/L, our results are consistent with a linear association of 25(OH)D and MS risk with decreasing risk as levels of 25(OH)D rise. These results complement and expand those of 2 prior prospective studies of similar design, one conducted in the United States among 148 non-Hispanic white patients with MS and 296 controls in the US military,2 and the other among 192 patients and 384 controls residing in northern Sweden.3 In both studies, elevated levels of 25(OH)D (above 75 or 100 nmol/L) were associated with a 60% reduced risk of MS. However, neither study reported the effects of vitamin D insufficiency or deficiency. In the US study, too few individuals had insufficient or deficient 25(OH)D levels (only 5% <50 nmol/L) to directly assess this question (average 25[OH]D in non-Hispanic white participants was 75 nmol/L),2 and in the Swedish study,3 while overall mean 25(OH)D levels were lower (40 nmol/L), investigators reported not finding any associations between 25(OH)D levels and other “predefined 25(OH)D strata,” but they did not report specific findings.
Our findings suggest that correcting vitamin D deficiency among reproductive age women may reduce their future risk of developing MS. In a previous study in this cohort,7 we found that maternal vitamin D deficiency during pregnancy was associated with about a 2-fold increased risk of MS in the offspring, and a Danish study8 found low neonatal 25(OH)D levels were associated with an increased MS risk in adulthood, suggesting that correcting maternal vitamin D deficiency during pregnancy may also reduce the risk of MS in the offspring. What is less clear, however, is what, if any, specific recommendation with regards to timing of vitamin D supplementation can be made. It is possible that individuals with sufficient levels (≥50 nmol/L) of 25(OH)D had behavior practices, such as regularly using supplements, that they or their children followed for many years. More research on the benefits of timing and optimal dose of vitamin D supplementation on MS risk are needed, but striving to achieve vitamin D sufficiency over the life course will likely have multiple health benefits.
Strengths of our study include a large, nested case-control sample, with over 1,000 patients with MS and 2,000 controls, drawn from a well-defined population-based cohort of Finnish women, and the utilization of the national HILMO and Kela registries to identify MS cases, an approach that minimizes selection bias. Further, serum samples were collected on average 9.3 years prior to the MS diagnosis, reducing reverse causation as an explanation of our results. Serum samples were collected from the majority of mothers during the first trimester of pregnancy at ∼10–14 weeks gestation. While vitamin D metabolism changes to meet the vitamin D and calcium needs of both the mother and fetus,9 longitudinal studies of 25(OH)D in pregnant women and comparisons of 25(OH)D in pregnant and nonpregnant women suggest that 25(OH)D levels during the first trimester are reflective of nonpregnancy 25(OH)D levels.4,10 Some limitations of this study to consider include the inability to adjust for other MS risk factors such as body mass index in early life, Epstein-Barr virus infection, smoking, and human leukocyte antigen status, but previous work suggests these are probably not major confounders of the vitamin D–MS association.11–13 Further, the majority of the women in the FMC are Caucasian, and as such, our results may not be generalizable to women of other race groups. We do not have specific information on individual race/ethnicity and other demographic variables such as education level were not available. Previous findings of a decreased MS risk with increasing 25(OH)D levels has held for both men and women,2,3 so vitamin D deficiency may also increase risk of MS in men, but this requires separate study. Finally, it should be noted that although the assay that we used to assess serum 25(OH)D levels is highly reliable, and thus the ranking of women according to their vitamin D status is likely accurate, there are variations in absolute 25(OH)D levels across methods and within methods across different laboratories.14 These potential variations should be considered when comparing absolute 25(OH)D levels across different studies.
Our results further support and extend those of previous prospective studies of 25(OH)D levels in young adults and risk of MS, and suggests that many individuals are exposed to an increased MS risk that could be reduced by broad population-based programs to prevent vitamin D deficiency.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Leslie Unger for technical assistance and Dr. Marja-Liisa Sumelahti, MD, PhD, and Professor Anne Remes, MD, PhD, for assistance with MS case ascertainment.
GLOSSARY
- 25(OH)D
25-hydroxyvitamin D
- CI
confidence interval
- FMC
Finnish Maternity Cohort
- HILMO
Finnish Hospital Discharge Register
- ICD
International Classification of Diseases
- MS
multiple sclerosis
- RR
relative risk
- UV
ultraviolet
Footnotes
Editorial, page 1538
AUTHOR CONTRIBUTIONS
K.L. Munger contributed to the design of the study, obtaining funding, statistical analysis, and writing the first draft of the manuscript. K. Hongell contributed to data collection and critical editing of the manuscript. J. Åivo contributed to data collection and critical editing of the manuscript. M. Soilu-Hänninen contributed to the design of the study, data collection, and critical editing of the manuscript. H.-M. Surcel contributed to the design of the study, obtaining funding, data collection, and critical editing of the manuscript. A. Ascherio contributed to the design of the study, obtaining funding, statistical analysis, and critical editing of the manuscript.
STUDY FUNDING
This study was funded by the National Institute of Neurological Disorders and Stroke (NIH R01 NS073633, PI: Ascherio). The funding agency had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
DISCLOSURE
K. Munger has received grant funding from the National Multiple Sclerosis Society. K. Hongell and J. Åivo report no disclosures relevant to the manuscript. M. Soilu-Hänninen has obtained honoraria for scientific presentations or advisory board memberships and attended conferences at the cost of pharmaceutical companies (Bayer, Biogen Idec Finland, Genzyme, Merck, Novartis, Roche, Sanofi, Orion, and Teva) and obtained research grants from Bayer, Biogen Idec Finland, and Novartis. H. Surcel reports no disclosures relevant to the manuscript. A. Ascherio reports grants from NIH, National Multiple Sclerosis Society, Department of Defense, Michael J. Fox Foundation, Accelerated Cure Project, and Chronic Fatigue Initiative, and honoraria for scientific presentations from Bayer HealthCare, Almirall, and Serono. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ascherio A, Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention: an update. Semin Neurol 2016;36:103–114. [DOI] [PubMed] [Google Scholar]
- 2.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 3.Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012;79:2140–2145. [DOI] [PubMed] [Google Scholar]
- 4.Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab 2010;95:1749–1757. [DOI] [PubMed] [Google Scholar]
- 5.Poser C, Paty D, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 6.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 7.Munger KL, Aivo J, Hongell K, Soilu-Hanninen M, Surcel HM, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol 2016;73:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen NM, Munger KL, Koch-Henriksen N, et al. Neonatal vitamin D status and risk of multiple sclerosis: a population-based case-control study. Neurology 2017;88:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiex NW, Kalkwarf HJ, Specker BL. Vitamin D metabolism in pregnancy and lactation. In: Feldman D, Pike J, Adams J, editors. Vitamin D, 3rd ed. Cambridge: Academic Press; 2011:679–694. [Google Scholar]
- 10.More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol 2003;106:209–213. [DOI] [PubMed] [Google Scholar]
- 11.Munger KL, Levin LI, O'Reilly EJ, Falk KI, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Mult Scler 2011;17:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokry LE, Ross S, Ahmad OS, et al. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med 2015;12:e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhead B, Baarnhielm M, Gianfrancesco M, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet 2016;2:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binkley N, Dawson-Hughes B, Durazo-Arvizu R, et al. Vitamin D measurement standardization: the way out of the chaos. J Steroid Biochem Mol Biol 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


