Abstract
Objective:
Whereas Alzheimer disease (AD) is associated with inner retina thinning visualized by spectral-domain optical coherence tomography (SD-OCT), we sought to determine if the retina has a distinguishing biomarker for frontotemporal degeneration (FTD).
Methods:
Using a cross-sectional design, we examined retinal structure in 38 consecutively enrolled patients with FTD and 44 controls using a standard SD-OCT protocol. Retinal layers were segmented with the Iowa Reference Algorithm. Subgroups of highly predictive molecular pathology (tauopathy, TAR DNA–binding protein 43, unknown) were determined by clinical criteria, genetic markers, and a CSF biomarker (total tau: β-amyloid) to exclude presumed AD. We excluded eyes with poor image quality or confounding diseases. SD-OCT measures of patients (n = 46 eyes) and controls (n = 69 eyes) were compared using a generalized linear model accounting for intereye correlation, and correlations between retinal layer thicknesses and Mini-Mental State Examination (MMSE) were evaluated.
Results:
Adjusting for age, sex, and race, patients with FTD had a thinner outer retina than controls (132 vs 142 μm, p = 0.004). Patients with FTD also had a thinner outer nuclear layer (ONL) (88.5 vs 97.9 μm, p = 0.003) and ellipsoid zone (EZ) (14.5 vs 15.1 μm, p = 0.009) than controls, but had similar thicknesses for inner retinal layers. The outer retina thickness of patients correlated with MMSE (Spearman r = 0.44, p = 0.03). The highly predictive tauopathy subgroup (n = 31 eyes) also had a thinner ONL (88.7 vs 97.4 μm, p = 0.01) and EZ (14.4 vs 15.1 μm, p = 0.01) than controls.
Conclusions:
FTD is associated with outer retina thinning, and this thinning correlates with disease severity.
Frontotemporal degeneration (FTD) is an underinvestigated neurodegenerative condition as common as Alzheimer disease (AD) in people under age 65.1 FTD-associated neuropathology consists of 2 main proteinopathies: FTLD-tau, related to inclusions formed from the microtubule-associated protein tau; and FTLD-TDP, related to TAR DNA–binding protein 43 (TDP-43) containing inclusions.1 Despite these neuropathologic links, FTD actually involves a heterogeneous group of syndromes with only modest correlation between clinical presentation and neuropathology. Furthermore, it can be difficult to clinically differentiate FTD from AD; up to 30% of patients clinically diagnosed with FTD later have a neuropathologic diagnosis of AD.2–4 As therapies develop, biomarkers for the underlying pathology are critically important to assess clinical trial eligibility.
Spectral-domain optical coherence tomography (SD-OCT) enables high-resolution retinal imaging.5 Ophthalmology clinics routinely use SD-OCT, and image measurements have high reproducibility.6 SD-OCT has great biomarker potential as it is acquired in minutes, is inexpensive, is noninvasive, and has no known safety risks. SD-OCT studies have identified thinning of the inner retinal layers (retinal nerve fiber layer [RNFL] or ganglion cell layer [GCL]) in several neurodegenerative diseases including AD and amyotrophic lateral sclerosis (ALS), which is a TDP-43 proteinopathy related to FTD.7–10 Histopathology of optic nerves and retinas has corroborated the thinning associated with AD.11
We have found progressive outer retina (photoreceptor layer) thinning by SD-OCT in mice caused by a mutation in Rp1, a microtubule-associated protein.12 Thus we hypothesized that SD-OCT may show photoreceptor abnormalities in patients with FTD tauopathy since tau is also a microtubule-associated protein. In this study, we evaluated retinal layer thicknesses using SD-OCT in a large well-characterized cohort of patients with FTD compared to controls.
METHODS
Participants.
Thirty-eight consecutive patients diagnosed with FTD clinical syndromes (i.e., primary progressive aphasia [PPA], corticobasal syndrome [CBS], progressive supranuclear palsy [PSP], behavioral variant of FTD [bvFTD]) at the Penn Frontotemporal Degeneration Center of the University of Pennsylvania were prospectively enrolled (August 2014–January 2016). Neurologists prospectively reviewed all patients in consensus conference and diagnosed FTD according to published clinical criteria.1,13 All patients with PSP met criteria for probable PSP.14 Patients underwent a routine lumbar puncture and CSF was collected according to published standard operating procedures.15,16 CSF was analyzed for the total tau:β-amyloid (Aβ) 1–42 ratio (Luminex; Austin, TX), an autopsy validated marker for AD.2,15,16 Within 12 months of enrollment, patients underwent a Mini-Mental State Examination (MMSE) by neurologists masked to SD-OCT data.
Since autopsy became available for only 1 patient, we used a combination of previously validated CSF, genetic, and clinical data to define subgroups (tauopathy, TDP-43, and unknown pathology) of highly predictive pathologic subtypes. First, patients with a total tau:Aβ ratio >0.34 were considered to have presumed AD and excluded from analyses.2,15,16 Next, patients were genotyped according to risk of hereditary disease for pathogenic mutations in MAPT (OMIM:157140), progranulin (GRN) (OMIM:138945), C9orf72 (OMIM: 61426), and TARDBP p.I383V (OMIM: 605078; p.N39OS).17 Finally, patients meeting criteria for clinical phenotypes of PSP, nonfluent PPA, and CBS were categorized as FTLD-tau, while those meeting criteria for the semantic variant of PPA were categorized as FTLD-TDP.2,14,18–20
A group of 44 consecutive healthy controls without diabetes and intended for several different SD-OCT studies were prospectively enrolled (February 2015–April 2016) for retinal evaluation at the Scheie Eye Institute. Controls did not undergo cognitive testing but had no history of any neurodegenerative disease.
On the day of SD-OCT imaging, all patients and controls had a dilated eye examination to identify undiagnosed ophthalmic disease. Prior to analyses, we excluded participants (or eyes) with macular disease, diabetic retinopathy, hypertensive retinopathy, retinal vascular disease, glaucoma or optic nerve disease, high refractive error (±6.00 D spherical equivalent), substantial ocular media opacity, intraocular surgery within 90 days of enrollment, poor image quality, or incomplete image scans (figure e-1 at Neurology.org). A few patient and control eyes had incidental peripheral retina examination findings not affecting the macula (table e-1). Two patients had a history of diabetes without retinopathy on examination.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of Pennsylvania institutional review board. All participants gave written informed consent (with caregivers when appropriate).
Imaging protocol and image analysis.
All participants underwent SD-OCT imaging with the Heidelberg Spectralis (Heidelberg Engineering, Carlsbad, CA) with a standard macular volume scan protocol of 20° images, 25 high-resolution raster scans, and automated real time averaging of 25. An analyst masked to clinical information segmented individual retinal layers using the highly validated Iowa Reference Algorithm (IRA) (v3.6), and algorithm segmentation errors were manually corrected.21–23 This algorithm segmented 11 optical interfaces (10 layers) and provided thickness readings for the 9 regions of the standard Early Treatment of Diabetic Retinopathy Study (ETDRS) grid centered at the fovea (figure e-2). We selected the central circle at the fovea (diameter 1 mm), the average of the central 5 regions (diameter 3 mm), and the average of all 9 regions (diameter 6 mm) for analyses. Total retina thickness was defined as the distance from the RNFL's inner boundary to the interdigitation zone's outer boundary. The outer retina thickness was defined as the distance from the outer nuclear layer's (ONL) inner boundary to the interdigitation zone's outer boundary. With this definition, the outer retina thickness focuses on the photoreceptor layers.
Statistical analyses.
We used generalized linear models for comparing the thickness measures of retinal layers between patients and controls without and with adjustment of participant demographics (age, sex, and race). The intereye correlation of thickness for participants contributing 2 eyes was accounted for by using generalized estimating equations.24,25 Similar analyses were made for comparisons across subgroups of patients and each subgroup of patients vs controls using generalized linear models. The thickness calculated from these linear regression models (with and without adjustment for demographic characteristics) were summarized as mean and standard error. We calculated the area under the receiver operating characteristic (ROC) curve using logistic regression models to evaluate the ability of outer retinal thickness to discriminate patients diagnosed with FTD or tauopathy subgroup patients from controls. We evaluated the Spearman correlation of MMSE with outer retina thickness, ONL thickness, and ellipsoid zone (EZ) thickness. For the ROC analysis and correlation analysis, the average thickness of 2 eyes was used for participants contributing 2 eyes. Our study of 27 patients (46 eyes) and 44 controls (69 eyes) provides at least 80% power to detect an effect size of 0.55 or more for the comparison of thickness measures between patients and controls. All the statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and 2-sided p < 0.05 was considered statistically significant.
RESULTS
Demographics.
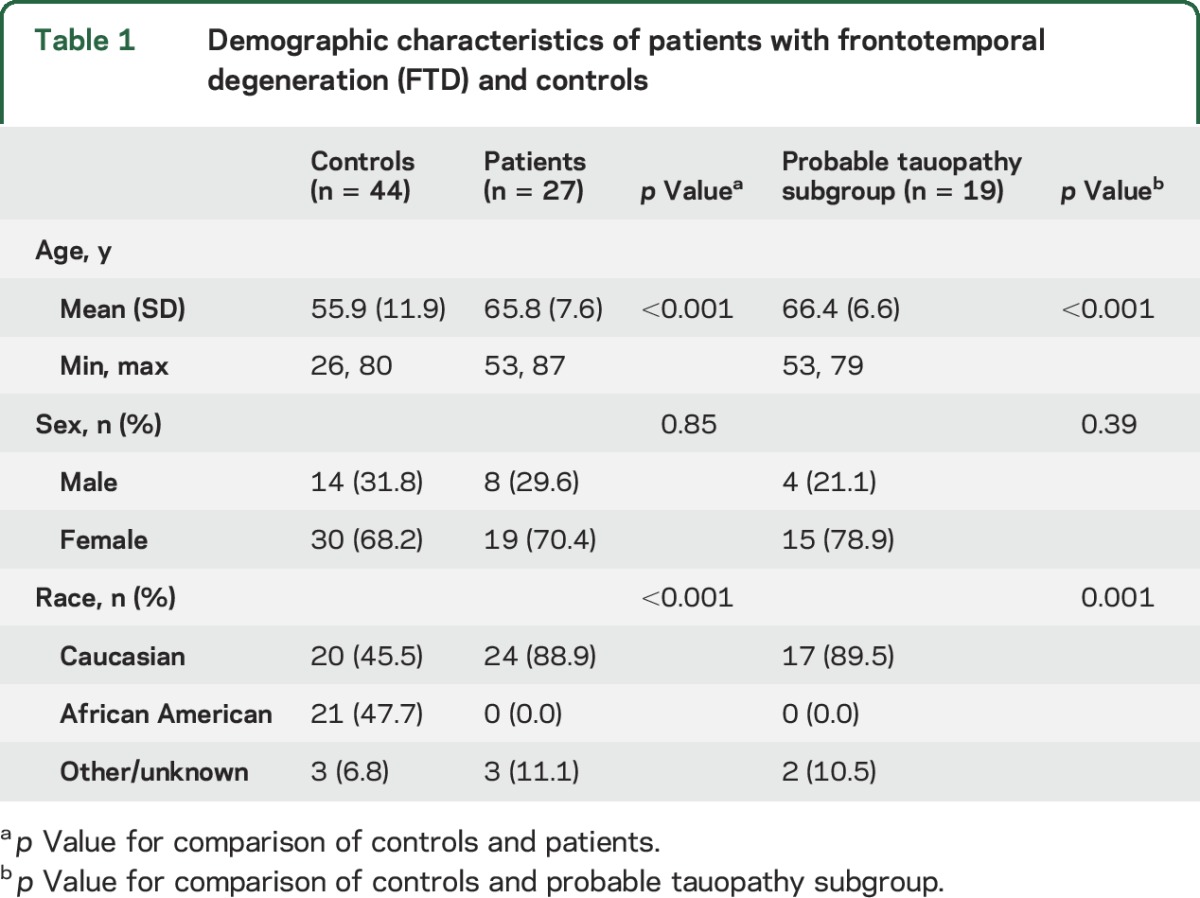
The reasons for prespecified exclusions are shown in figure e-1. This included 5 patients with presumed AD based on CSF biomarker analysis, 3 of whom were already excluded for poor image quality or confounding eye disease. After these exclusions, 27 patients (46 eyes) with a mean age of 66 years and 44 controls (69 eyes) with a mean age of 56 years were analyzed. The percent of male participants was similar (table 1). There were no African American patients, but 21 of 44 (48%) controls were African American (p < 0.001).
Table 1.
Demographic characteristics of patients with frontotemporal degeneration (FTD) and controls
Comparison of retinal layer thicknesses between patients with FTD and controls.
The retinal thicknesses of patients was compared to that of controls for the average of the 5 central ETDRS subfields (table 2). While total retinal thickness was not different between patients and controls, patients had a thicker outer plexiform layer (OPL) and thinner outer retinal layers (table 2).
Table 2.
Comparisons of retinal layer thicknesses (μm) between patients and normal controls
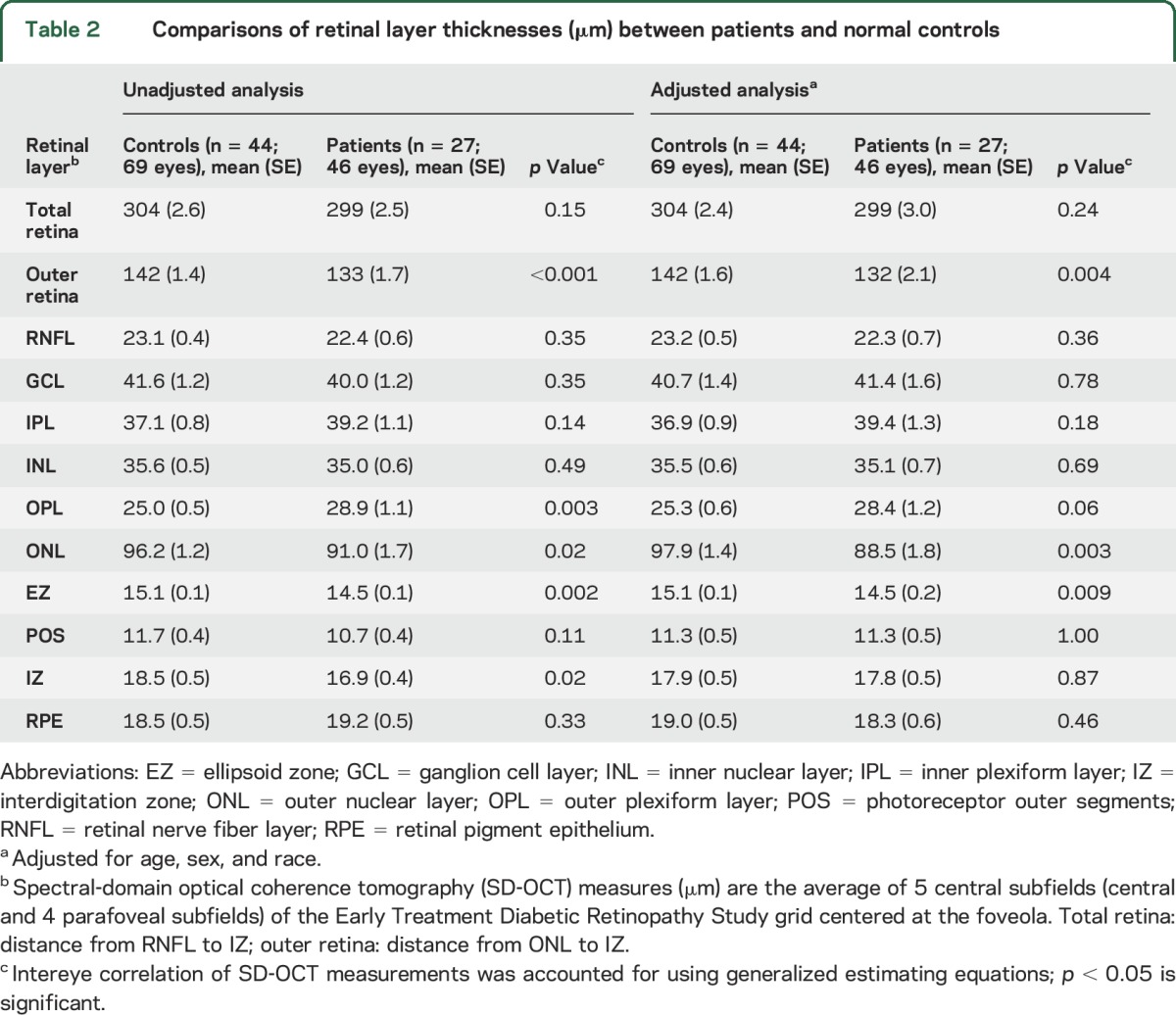
Next, retinal thicknesses were compared between patients and controls by adjusting for age, sex, and race. These results were similar and showed that patients as compared to controls had thinning of the outer retina, ONL, and EZ (table 2). When the OPL and ONL were combined and analyzed as a single layer, patients still showed thinning compared to controls (117 vs 123 μm, p = 0.02). We then excluded controls age ≤55 years, providing a group of 23 controls (34 eyes) and average age of 65 years. Unadjusted analysis still showed outer retina thinning of patients compared to these controls (133 vs 141 μm, p = 0.01). Adjusted analysis also showed outer retina thinning of patients compared to these controls (131 vs 144 μm, p = 0.003). When adjusted analysis was performed without African American participants, the outer retina of patients was again thinner than in controls (133 vs 145 μm, p = 0.002).
Similar findings were found for analyses of the ETDRS central foveal circle and the average of all ETDRS subfields (table e-2).
The ROC analysis for discriminating patients from controls found that the area under the curve (AUC) was 0.72 (95% confidence interval [CI] 0.60–0.84) for the outer retina thickness and 0.81 (95% CI 0.71–0.91) for outer retina thickness and age.
Presumed molecular pathology subgroup categorization and comparison.
Of the 27 patients, all had CSF biomarker analysis except 6 who were unable to undergo CSF collection for logistical reasons. These 6 patients met clinical criteria for PSP, which is highly associated with a tauopathy neuropathologically.14,18,19 Two patients had familial forms of FTD; one patient had a MAPT E10+16 C>T mutation and one patient had a hexanucleotide expansion in C9orf72. No patient had a TARDBP mutation or GRN mutation. One patient who died during the study had a clinical diagnosis of PSP and a neuropathologic diagnosis of corticobasal degeneration, a 4-repeat tauopathy.
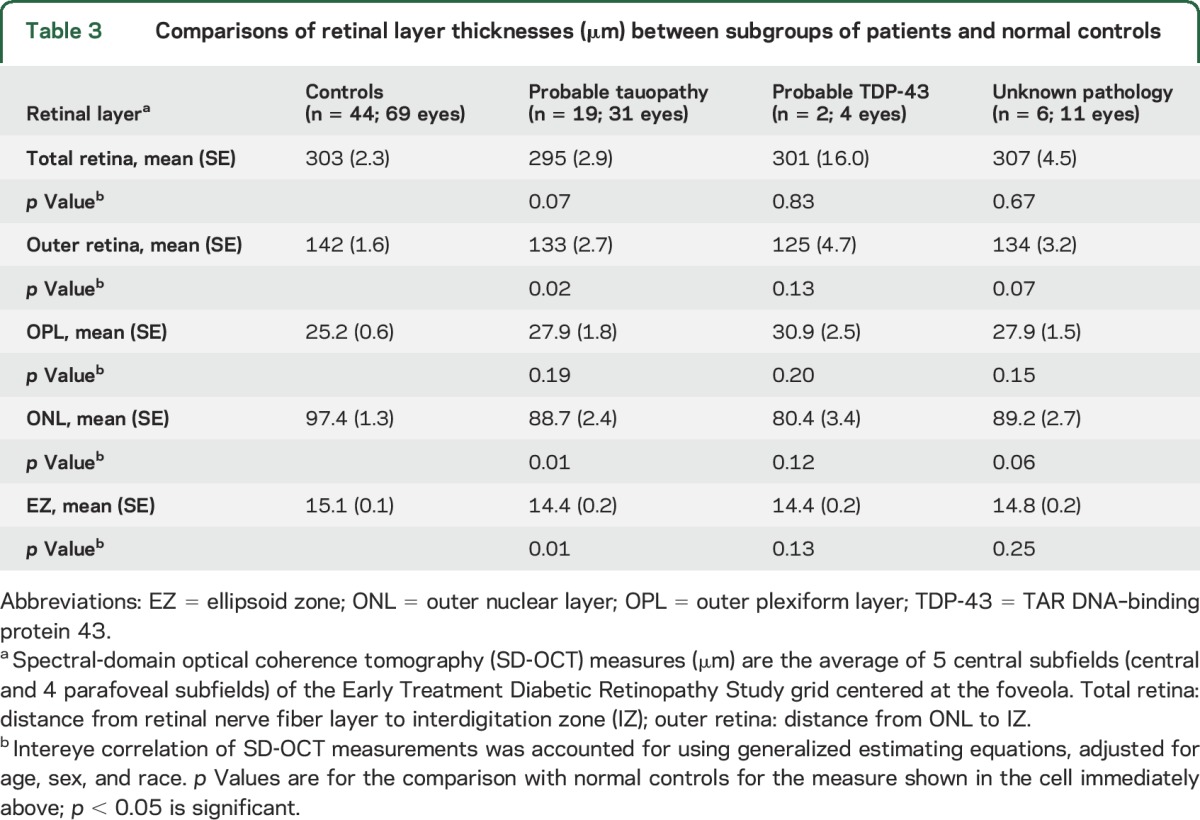
The tauopathy subgroup demographics are shown in table 1. This included the following: PSP (12), CBS (4), nonfluent PPA (2), and the patient with a pathogenic mutation in MAPT predictive of FTLD-tau. Adjusted analysis showed that the tauopathy subgroup as compared to controls had thinning of the outer retina, ONL, and EZ. All other subgroups, although too small for useful statistical analysis, showed ONL thinning that was not different from that in controls (table 3).
Table 3.
Comparisons of retinal layer thicknesses (μm) between subgroups of patients and normal controls
The ROC analysis for discriminating the tauopathy subgroup and controls found that the AUC was 0.70 (95% CI 0.56–0.84) for outer retina thickness and 0.81 (95% CI 0.70–0.92) for outer retina thickness and age.
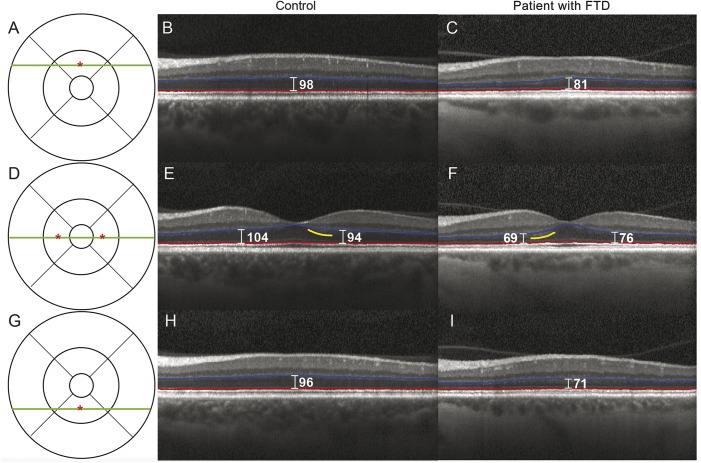
Figure 1 demonstrates a tauopathy patient with ONL thinning compared to a control matched for age, sex, and race. This patient has a MAPT E10+16 C>T mutation and was clinically diagnosed with bvFTD with features of the semantic variant of PPA.
Figure 1. Frontotemporal degeneration (FTD) patient with outer retina thinning.
Representative spectral-domain optical coherence tomography (SD-OCT) images from a control and a patient with FTD show outer nuclear layer (ONL) thinning. The control is a 61-year-old woman. The patient is a 60-year-old woman who was clinically diagnosed with the behavioral variant of FTD with features of the semantic variant of primary progressive aphasia. This patient with FTD has a presumed tauopathy as she has a MAPT E10+16 C>T mutation. The Early Treatment Diabetic Retinopathy Study grid is shown in A, D, and G, with the green line indicating the location of the SD-OCT scan and the red asterisk indicating the location of a point measurement. The control SD-OCT images are shown in B, E, and H, and the patient images are shown in C, F, and I. The segmentation lines from the Iowa Reference Algorithm for the ONL are shown in red and blue. Yellow lines indicate locations of hyperreflectivity related to the Henle fiber layer.29 Point measurements of the ONL thickness were made using the caliper function of the Heidelberg Spectralis (Heidelberg Engineering, Carlsbad, CA) and labeled in white (μm).
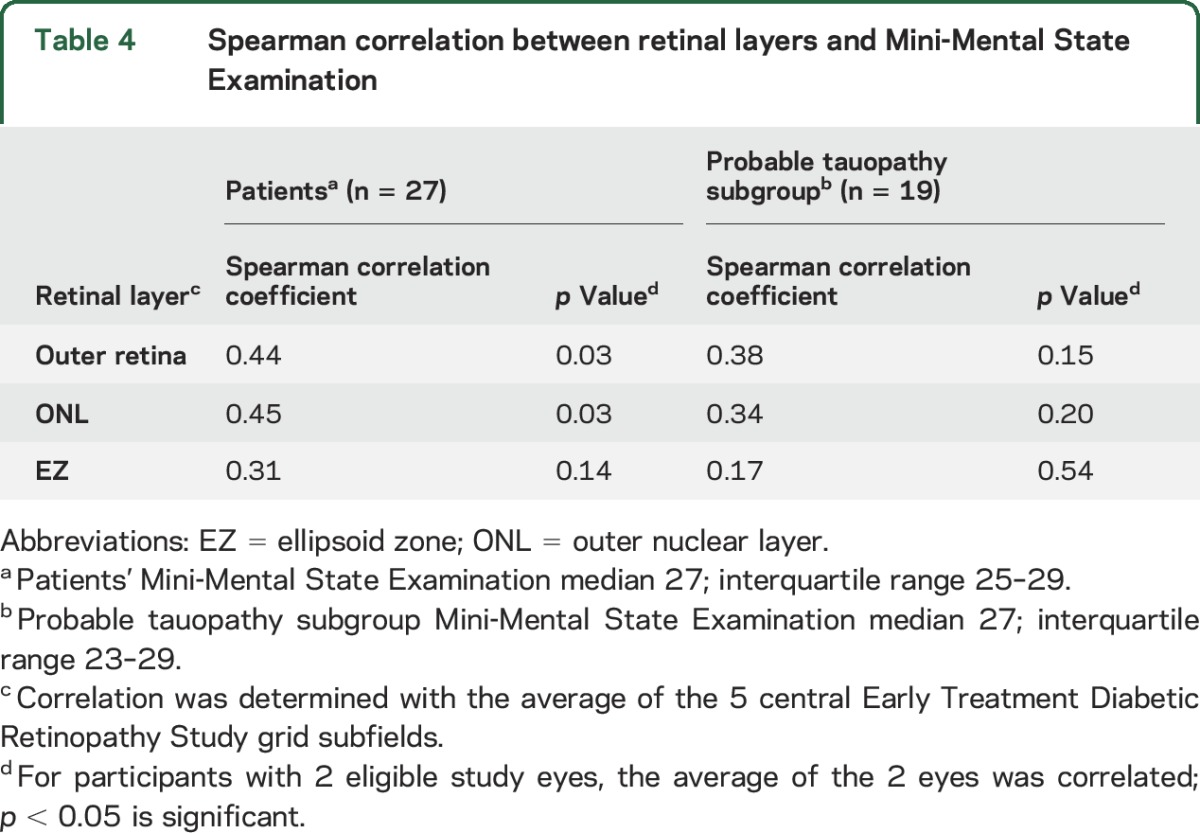
Correlation of retinal layers with MMSE.
Among patients with FTD, the outer retina and ONL thicknesses were positively correlated with MMSE (table 4). The tauopathy subgroup did not have correlations with MMSE.
Table 4.
Spearman correlation between retinal layers and Mini-Mental State Examination
DISCUSSION
While AD is associated with inner retina thinning,7–9 our study revealed that FTD is associated with outer retina thinning. The FTD and tauopathy subgroup had thinning of both the ONL and EZ, and the outer retina thinning was driven by the ONL thinning. The ONL was, on average, about 10% thinner in patients with FTD compared to controls. FTD has distinct neuropathology compared to AD, and our finding that FTD is predominantly associated with photoreceptor layer (outer retina) thinning suggests that FTD also has retinal changes that appear to be distinct from AD.
As potential mechanism-based therapeutics emerge,26,27 biomarkers predictive of pathology for FTD are critically important to more easily separate FTD from AD, and to separate FTLD-tau from FTLD-TDP.1 While CSF biomarkers have utility, they require an invasive procedure, and CSF biomarkers differentiating FTLD-tau from FTLD-TDP require additional validation.2 As a biomarker test, SD-OCT image acquisition has ideal features as it is quick, noninvasive, and low cost. The outer retina thickness had an AUC of 0.81 when combined with age, suggesting that an automated segmentation algorithm enables SD-OCT of the retina to have biomarker potential for FTD. Given ongoing biomarker studies in neuroimaging and CSF, it is possible that SD-OCT would best be used within a multiple biomarker model to predict underlying pathology.2,28
The Henle nerve fiber layer usually is isoreflective with the ONL but is known to appear hyperreflective at some locations depending on the orientation of SD-OCT light in relation to the retina.29 Therefore, some argue that it is difficult to accurately measure the ONL in relation to the OPL. Our ONL measurement includes the Henle layer, and there are several reasons to give confidence to our finding of ONL thinning in FTD. First, the IRA has previously shown the capability to accurately segment the OPL and ONL,30 as also shown in figure 1. Second, the analyst using the IRA was masked. Third, even when we combined the OPL and ONL thicknesses, we still found that patients had statistically significant thinning compared to controls.29 Fourth, artifacts from the Henle layer would not affect EZ measurements. Our finding of EZ thinning in FTD further supports ONL thinning as 2 distinct and adjacent photoreceptor layers were abnormally thin.
Photoreceptors are sensitive to microtubule-associated defects, as microtubules are critical to the structure and function of photoreceptor cilia.31 We previously found that SD-OCT detects ONL thinning and EZ abnormalities in mice with an Rp1 mutation.12 Therefore, we hypothesized that patients with FTD tauopathy may have similar findings, as Rp1 and tau are both microtubule-associated proteins within the proteome of photoreceptor cilia.32 Our analysis of highly predictive molecular pathology found that the tauopathy subgroup, our largest group, also showed thinning of the ONL and EZ. Although immunohistochemistry studies of tau expression in the human retina have variable results, tau aggregates have been identified within photoreceptors.33 Another report demonstrated phosphorylated tau predominantly in the inner nuclear layer (INL) of a patient with PSP, but did not include detailed analyses of retinal morphology.34 Disease-specific p62 inclusions were also found within the INL of a patient with ALS with a C9orf72 mutation.10 However, the same investigators found no inclusions and RNFL thinning in a different patient with ALS with no known mutation.10 These varying studies alone do not exclude the possibility that a pathologic mechanism involving tau or TDP-43 could cause photoreceptor layer thinning. Additional immunohistochemistry studies with morphologic analyses and SD-OCT correlation in well-characterized patients with FTD are needed. Interestingly, our data also showed ONL thinning and EZ thinning in the probable TDP-43 pathology subgroup, but it was not significantly different from controls. The number of patients in this subgroup was small, making it difficult to draw any definite conclusions.
There is limited literature on the SD-OCT findings of FTD. Patients with clinically diagnosed PSP have been studied by 2 groups. Evaluating 15 patients with PSP, one group found thinning of both the GCL–inner plexiform layer and the ONL.35 With 16 patients with PSP, the findings of another group included thinning of the INL and nonsignificant thinning of the ONL.36 While both studies suggest that patients with PSP may have ONL thinning, inconsistencies between these studies and our study may stem from differences in segmentation methods or differences between the PSP groups and our presumed tauopathy subgroup. Recently, a different study found inner retina thinning in 17 patients with FTD.37 It is difficult to directly compare our data with this study, which did not test for the CSF total tau:Aβ biomarker to exclude AD and did not categorize patients by underlying molecular pathology. Their cohort could have a high proportion of patients with TDP-43 proteinopathy, and it is possible that these patients may have inner retina thinning compared to tauopathy patients. Indeed, inner retina thinning was reported in 12 patients with a progranulin mutation, a mutation associated with TDP-43 neuropathology.38 Further work is needed to determine if patients with TDP-43 pathology have inner or outer retina thinning and how these patients compare to tauopathy patients.
Among our patients with FTD, we also found a positive correlation of outer retina thinning with MMSE. The nonsignificant correlation in the tauopathy subgroup may be related to sample size. Others have reported that inner retina thinning in patients with AD may also correlate with disease severity.8 Our data suggest an additional utility of outer retina thickness measurements and the importance of larger, longitudinal studies to determine if outer retina thickness may estimate factors related to prognosis and survival.
The strengths of our study are twofold. First, we implemented a deep endophenotyping approach to patients with FTD based on highly predictive pathology. Our study carefully excluded confounding eye diseases with eye examinations and excluded patients with AD. Our notable number of excluded eyes and patients suggests the importance of this study design. We then uniquely grouped patients by presumed molecular pathology. FTD represents a heterogeneous group of clinical syndromes; this approach is important to reduce discrepancies between clinical diagnosis and molecular pathology. In the tauopathy subgroup, we demonstrated a direct relationship between outer retina thinning and a specific form of pathology. Second, we used the extensively validated IRA. Contrasting with other segmentation approaches, the IRA enables detailed outer retina segmentation, leading to our finding of EZ thinning in patients with FTD. Importantly, the IRA has demonstrated excellent capability of identifying inner retina thinning, but did not show inner retina thinning in our patients with FTD.39
One weakness of our data is the different demographics of controls and patients. While all patients were recruited consecutively, differences reflect the different populations of FTD vs controls recruited during a routine eye examination. Indeed, FTD is considerably less common in African Americans.40 To account for demographic differences, we adjusted for age, sex, and race. In addition, when we removed the African American participants from the control group or reached an age-matched control group by excluding those age ≤55 years, we still found that patients had significant outer retina thinning compared to controls. Another caveat of our data is the limited number of patients in the nontauopathy subgroups; this must be considered before generalizing our results to all patients with FTD.
Using the IRA and a deep endophenotyping approach to patients primarily composed of presumed tauopathy molecular pathology, our study revealed that FTD is predominantly associated with outer retina thinning, including thinning of the ONL and EZ. This contrasts with the inner retina thinning typically seen in AD. Furthermore, the outer retina thinning in FTD correlates with MMSE. Our study suggests that measurements of retinal thickness have the potential to serve as biomarkers for FTD and may relate to disease severity. Future work should focus on direct comparison of AD patients with FTD patients and comparison of the different subgroups of FTD using similar methods and longitudinal studies with autopsy confirmation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Kyungmoo Lee (University of Iowa) for assistance with the Iowa Reference Algorithm and Stacey Cesarano and Alicia Fleming (University of Pennsylvania) for contributing to the enrollment of controls.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- AUC
area under the curve
- bvFTD
behavioral variant of frontotemporal degeneration
- CBS
corticobasal syndrome
- CI
confidence interval
- ETDRS
Early Treatment of Diabetic Retinopathy Study
- EZ
ellipsoid zone
- FTD
frontotemporal degeneration
- GCL
ganglion cell layer
- INL
inner nuclear layer
- IRA
Iowa Reference Algorithm
- MMSE
Mini-Mental State Examination
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PPA
primary progressive aphasia
- PSP
progressive supranuclear palsy
- RNFL
retinal nerve fiber layer
- ROC
receiver operating characteristic
- SD-OCT
spectral-domain optical coherence tomography
- TDP-43
TAR DNA–binding protein 43
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Benjamin Kim: study concept and design, acquisition of data, writing of manuscript. David Irwin: acquisition of data, critical revision of manuscript for intellectual content. Delu Song: acquisition of data. Ebenezer Daniel: acquisition of data, critical revision of manuscript for content. Jennifer D. Leveque: acquisition of data. Aaishah R. Raquib: acquisition of data, critical revision of manuscript for content. Wei Pan: acquisition of data. Gui-Shuang Ying: acquisition of data, critical revision of manuscript for content. Tomas S. Aleman: critical revision of manuscript for intellectual content. Joshua L. Dunaief: study concept and design, critical revision of manuscript for intellectual content. Murray Grossman: study concept and design, critical revision of manuscript for intellectual content.
STUDY FUNDING
Supported by NIH (Bethesda, MD) grants including AG017586, NS053488, AG052943, 2-P30-EY01583-26, and K23NS088341 (to D.J.I.). Funding was also provided in the form of block grants for general research purposes to the Scheie Eye Institute by Research to Prevent Blindness (New York, NY) and the Paul and Evanina Mackall Foundation Trust (Chicago, IL).
DISCLOSURE
Benjamin Kim reports research funding relevant to this work from block grants to the Scheie Eye Institute from Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. D. Irwin reports grants from the NIH relevant to this work. D. Song, E. Daniel, J. Leveque, A. Raquib, W. Pan, G. Ying, T. Aleman, and J. Dunaief report no disclosures relevant to the manuscript. M. Grossman reports grants from the NIH relevant to this work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 2015;129:469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin DJ, Trojanowski JQ, Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005;128:1996–2005. [DOI] [PubMed] [Google Scholar]
- 4.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006;59:156–165. [DOI] [PubMed] [Google Scholar]
- 5.Adhi M, Duker JS. Optical coherence tomography: current and future applications. Curr Opin Ophthalmol 2013;24:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network Writing Committee, Bressler SB, Edwards AR, et al. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol 2014;132:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppola G, Di Renzo A, Ziccardi L, et al. Optical coherence tomography in Alzheimer's disease: a meta-analysis. PLoS One 2015;10:e0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Martin E, Bambo MP, Marques ML, et al. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer's disease. Acta Ophthalmol 2016;94:e454–e459. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Ramos T, Benito-Leon J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J Alzheimers Dis 2013;34:659–664. [DOI] [PubMed] [Google Scholar]
- 10.Volpe NJ, Simonett J, Fawzi AA, Siddique T. Ophthalmic manifestations of amyotrophic lateral sclerosis (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 2015;113:T12. [PMC free article] [PubMed] [Google Scholar]
- 11.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med 1986;315:485–487. [DOI] [PubMed] [Google Scholar]
- 12.Song D, Grieco S, Li Y, et al. A murine RP1 missense mutation causes protein mislocalization and slowly progressive photoreceptor degeneration. Am J Pathol 2014;184:2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DJ, McMillan CT, Toledo JB, et al. Comparison of cerebrospinal fluid levels of tau and Abeta 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol 2012;69:1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood EM, Falcone D, Suh E, et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol 2013;70:1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006;66:41–48. [DOI] [PubMed] [Google Scholar]
- 19.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017;81:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE Rev Biomed Eng 2010;3:169–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvin MK, Abramoff MD, Wu X, Russell SR, Burns TL, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging 2009;28:1436–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Wu X, Chen DZ, Sonka M. Optimal surface segmentation in volumetric images: a graph-theoretic approach. IEEE Trans Pattern Anal Mach Intell 2006;28:119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 25.Ying GS, Maguire MG, Glynn R, Rosner B. Tutorial on biostatistics: linear regression analysis of continuous correlated eye data. Ophthalmic Epidemiol 2017;24:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mis MS, Brajkovic S, Tafuri F, Bresolin N, Comi GP, Corti S. Development of therapeutics for C9ORF72 ALS/FTD-related disorders. Mol Neurobiol 2017;54:4466–4476. [DOI] [PubMed] [Google Scholar]
- 27.Panza F, Solfrizzi V, Seripa D, et al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer's disease. Biomed Res Int 2016;2016:3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitwell JL, Josephs KA. Neuroimaging in frontotemporal lobar degeneration: predicting molecular pathology. Nat Rev Neurol 2012;8:131–142. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang Y, Walsh AC, Keane PA, Heussen FM, Pappuru RK, Sadda SR. Different phenotypes of the appearance of the outer plexiform layer on optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2013;251:2311–2317. [DOI] [PubMed] [Google Scholar]
- 30.Demirkaya N, Wit FW, van Den Berg TJ, et al. HIV-associated neuroretinal disorder in patients with well-suppressed HIV-infection: a comparative cohort study. Invest Ophthalmol Vis Sci 2016;57:1388–1397. [DOI] [PubMed] [Google Scholar]
- 31.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia 2012;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Tan G, Levenkova N, et al. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 2007;6:1299–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leger F, Fernagut PO, Canron MH, et al. Protein aggregation in the aging retina. J Neuropathol Exp Neurol 2011;70:63–68. [DOI] [PubMed] [Google Scholar]
- 34.Schon C, Hoffmann NA, Ochs SM, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One 2012;7:e53547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht P, Muller AK, Sudmeyer M, et al. Optical coherence tomography in parkinsonian syndromes. PLoS One 2012;7:e34891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider M, Muller HP, Lauda F, et al. Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J Neural Transm 2014;121:41–47. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari L, Huang SC, Magnani G, Ambrosi A, Comi G, Leocani L. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer's disease. J Alzheimers Dis 2017;56:1101–1107. [DOI] [PubMed] [Google Scholar]
- 38.Ward ME, Taubes A, Chen R, et al. Early retinal neurodegeneration and impaired Ran-mediated nuclear import of TDP-43 in progranulin-deficient FTLD. J Exp Med 2014;211:1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci USA 2016;113:E2655–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou CE, Yaffe K, Perez-Stable EJ, Miller BL. Frequency of dementia etiologies in four ethnic groups. Dement Geriatr Cogn Disord 2006;22:42–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.