Abstract
Objective:
To investigate the temporal pattern and relevant associations of CSF inflammatory measures after intraventricular hemorrhage (IVH).
Methods:
We analyzed prospectively collected CSF cell counts and protein and glucose levels from participants in the Clot Lysis Evaluation of Accelerated Resolution of IVH phase III (CLEAR III) trial. Corrected leukocyte count and cell index were calculated to adjust for CSF leukocytes attributable to circulating blood. Data were chronologically plotted. CSF inflammatory measures (daily, mean, median, maximum, and cases with highest quartile response) were correlated with initial IVH volume, IVH clearance rate, thrombolytic treatment, bacterial infection, and adjudicated clinical outcome at 30 and 180 days.
Results:
A total of 11,376 data points of CSF results from 464 trial participants were analyzed. Measures of CSF inflammatory response evolved during the resolution of IVH. This was significantly more pronounced with initial IVH volume exceeding 20 mL. Intraventricular alteplase was associated with a significantly augmented inflammatory response compared to saline, even after correcting for initial IVH volume. There was an association but nonpredictive correlation of CSF inflammation measures with culture-positive CSF bacterial infection. None of the CSF inflammatory measures, including cases with upper quartile inflammatory response, was associated with a significant detrimental effect on 30 or 180 days functional outcome or mortality after multivariate adjustment for measures of disease severity.
Conclusions:
Aseptic CSF inflammation after IVH is primarily dependent on the volume of initial bleed. Thrombolysis intensifies the inflammatory response, with no apparent detrimental effect on clinical outcome.
Clinicaltrials.gov identifier:
Intraventricular hemorrhage (IVH) commonly results in mortality and poor functional outcome.1–6 Experimental and clinical studies have demonstrated a CSF inflammatory response in association with IVH, reflected by changes in cell counts and protein and glucose levels.7–10 The expected time course of these changes and their relationship to the volume of hemorrhage are not known, nor is how IVH may influence CSF inflammatory measures in the setting of bacterial infection.11 With the advent of intraventricular thrombolysis aimed at enhancing clearance of IVH, there have been conflicting reports about the effect of this therapy on inflammatory response.7,9,12–18 These studies were limited by cohort size, patient selection, or follow-up, and none controlled for IVH volume or considered the potential effect of CSF inflammatory response on clinical outcome.
The recently concluded Clot Lysis: Evaluation of Accelerated Resolution in IVH phase III trial (CLEAR III)19 provided a unique opportunity to study the classic measures of CSF inflammatory response in the largest prospectively enrolled patient series with IVH assembled to date. We hypothesized that intraventricular CSF cell counts and protein and glucose levels reflect an inflammatory response in the days following IVH, that this response is greater in cases with higher volumes of initial IVH (iIVH), and that this may correlate with thrombolytic treatment, IVH clearance, or bacterial infection. We examined any potential effect of CSF inflammatory response on mortality and functional outcome.
METHODS
Standard protocol approvals, registrations, and patient consents.
This is a prospective observational cohort study of collected CSF laboratory results from patients enrolled in the CLEAR III trial (clinicaltrials.gov registration identifier NCT00784134). The respective participating sites had approval from their institutional review boards and the data handling was conducted in compliance with the Health Insurance Portability and Accountability Act.
Participants, treatment rendered, and CSF data collection and processing.
Treatment rendered, demographic characteristics, and disease severity measures of trial participants were reported with the primary results of CLEAR III,19 and are summarized in table e-1 at Neurology.org.
Enrolled participants had an IVH with obstruction of the third or fourth ventricle and associated intracerebral hemorrhage (ICH) volume less than 30 mL pragmatically treated with external ventricular drain (EVD) per local practice guidelines. Patients with underlying vascular etiology or uncorrected coagulopathy were excluded. As per protocol, enrolled patients had daily CSF samples collected from the EVD, and were analyzed for cell count, protein, and glucose during at least the first 7 consecutive days following randomization. Gram staining and cultures were added if bacterial infection was clinically suspected. CSF white blood cell (WBC) and red blood cell (RBC) counts (cells/µL), protein and glucose levels (mg/µL), and culture results were extracted from source records uploaded prospectively through the trial's secure electronic data capture system, along with peripheral blood WBC and RBC counts (103 cells/µL and 106 cells/µL, respectively).
Missing data points were not estimated except for peripheral RBCs and WBCs, where the value from an adjacent day was used if available. Participants with no recorded CSF results throughout days 0–10 were excluded (n = 36 of 500 enrolled participants). Hence laboratory CSF and peripheral blood results from 464 IVH participants in CLEAR III were studied.
Two additional measures, corrected WBCs (cWBC) and cell index (CI), were calculated based on the relative abundance of RBCs and WBCs in peripheral blood samples of the same (or adjacent) day.20–24 The 2 inflammatory surrogates were calculated per previously reported formulae.
where observed = CSF WBCs, predicted = CSF RBCs × peripheral WBCs × 103/peripheral RBCs × 106. Negative cWBC data points (observed < predicted), denoting weak or absent inflammatory response, were counted as 0 for analysis. A CI > 1 indicated relatively higher WBCs in CSF than in the peripheral blood, and <1 indicated the converse.
Sequential daily CSF values for each participant were tracked in relation to the date of symptom onset of hemorrhagic stroke, designated as day 0. Results were available from day 0 to day 10 from ictus in varying frequencies. With least missing values on day 1 through day 9, these were selected for analysis. With more than half of participants missing CSF WBC differential, neutrophil/monocyte ratio was therefore not considered in our analyses.
For each laboratory measure, the daily mean and SD were calculated across the whole sample to distinguish outlier values exceeding ±2.5 SDs. Each statistical outlier was individually evaluated against the participant's own values at the days immediately preceding and following. Statistical outliers that were out of trend for the participant (42 data points) were deemed erroneous readings and were excluded. Table e-2 summarizes the 11,376 data points ultimately included in our analysis. Cases with many missing CSF values (<5 readings over days 0–10, n = 203) had no difference in demographic and disease measures in comparison to those with more complete CSF datasets (table e-3).
Assessment of IVH volume, IVH clearance, bacterial infection, and outcome.
To optimize accuracy and minimize bias, all IVH volumes were centrally measured from CT scans during the course of the trial at the trial's core image reading center using semiautomated segmentation and Hounsfield thresholds.19 Initial iIVH was determined at the last CT scan prior to randomization. Cases were categorized into iIVH strata <20, 20–50, and >50 mL as prespecified in the CLEAR III data analysis plan. End-of-treatment IVH volume (eotIVH) was measured 24 hours after the last dose of study agent (alteplase or saline). This was used to estimate IVH clearance, calculated as (iIVH − eotIVH/iIVH). Treatment received was defined as placebo (saline injections 3 times daily, n = 251) or thrombolytic agent (alteplase at similar frequency, n = 249) administered through the EVD for up to 12 doses, until a protocol-specified endpoint of third or fourth ventricular clearance, or >80% clearance of IVH volume. Bacterial infection was strictly defined, for the purpose of this study, as a positive culture from CSF during the sampling protocol or within the first month from enrollment, as reported by the site and verified in the source documents. Outcome assessments by modified Rankin Scale (mRS) at 30 and 180 days after symptom onset were independently adjudicated by the trial's outcomes center at the University of Glasgow, Scotland. Functional outcome was dichotomized as good (mRS = 0–3), poor (mRS = 4–6), or mortality (mRS = 6), as prespecified in the trial.
Statistical analyses.
Pearson correlation coefficients were calculated to evaluate the association between iIVH and IVH clearance rates and the CSF inflammatory measures on each day, and with mean, median, and maximum values for each measure. General linear models (GLMs) were used for assessments on the CSF inflammatory variables on a day-by-day analysis and overall period of 9 days. Mean, median, and maximum values of the variables were also modeled based upon the GLM. Bonferroni correction was used for adjustment of p value in analysis of the 3 iIVH volume strata.
Modeling the functional outcome utilized the generalized estimating equation (GEE) method for the repeated measurements of WBC, cWBC, CI, protein, and glucose. The model considered effect of iIVH volume (3 groups: <20, 20–50, and ≥50 mL), treatment rendered (alteplase and saline), as well as bacterial infection (yes and no). A variance–covariance structure of exchangeable correlation for repeated measures was used to account for the within-participant correlations. The same analysis was carried out for correlation between daily, mean, median, and maximum values of respective measures and poor functional outcome or mortality, adjusted for clinical variables (iIVH volume, IVH clearance, ICH volume, ICH location, and Glasgow Coma Scale [GCS]).
For analysis of the highest inflammatory response, participants were subdivided into 2 groups using the highest quartile of each CSF inflammation marker (WBC, cWBC, and CI). Multivariate GEE models were then conducted to compare the functional outcome (good vs poor) and mortality between the participants with highest inflammatory response (25% of all relevant participants) and the rest of the participants. Descriptive statistics such as mean, 95% confidence interval of the mean, median, interquartile range, and percentages were calculated to characterize the outcome measures. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC). Graphs were plotted by GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA). p Values were 2-sided and considered statistically significant at p < 0.05.
RESULTS
The overall temporal trends (figure 1) show an early, modest elevation in CSF WBC at days 1–3 parallel to a similar increase of CSF RBCs. After the third day, RBC counts decrease with a slower rate of WBC decline. This gap between the 2 slopes of RBC and WBC declines starting on the third day and coincides with a delayed surge in cWBC and a rise in CI. CSF protein levels fall steeply from onset to day 2, with a more gradual decrease thereafter, and CSF glucose shows very little fluctuation between 70 and 80 mg/dL throughout days 1–9 (figure e-1A).
Figure 1. General patterns.
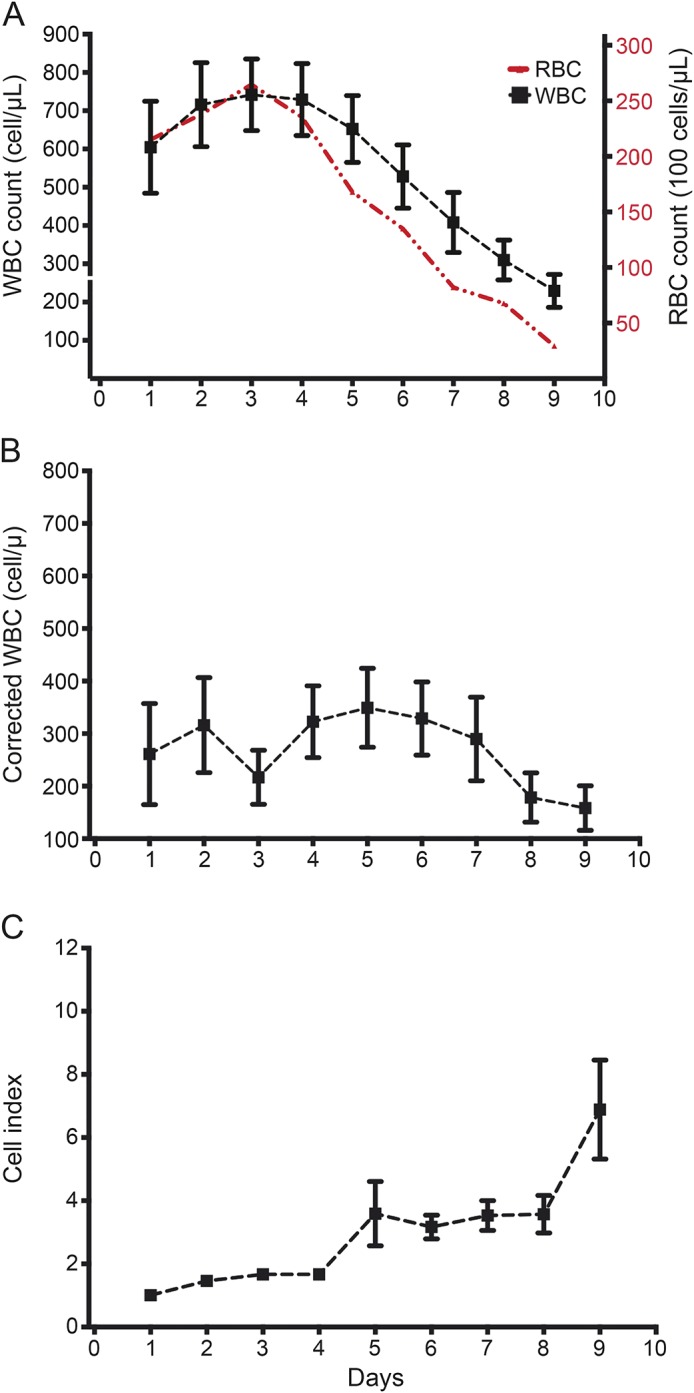
Overall patterns (mean ± SE) for the trends of (A) white blood cells (WBC), (B) corrected WBC, and (C) cell index during the resolution of IVH over days 1–9 postictus. RBC = red blood cell.
Effect of iIVH volume and IVH clearance.
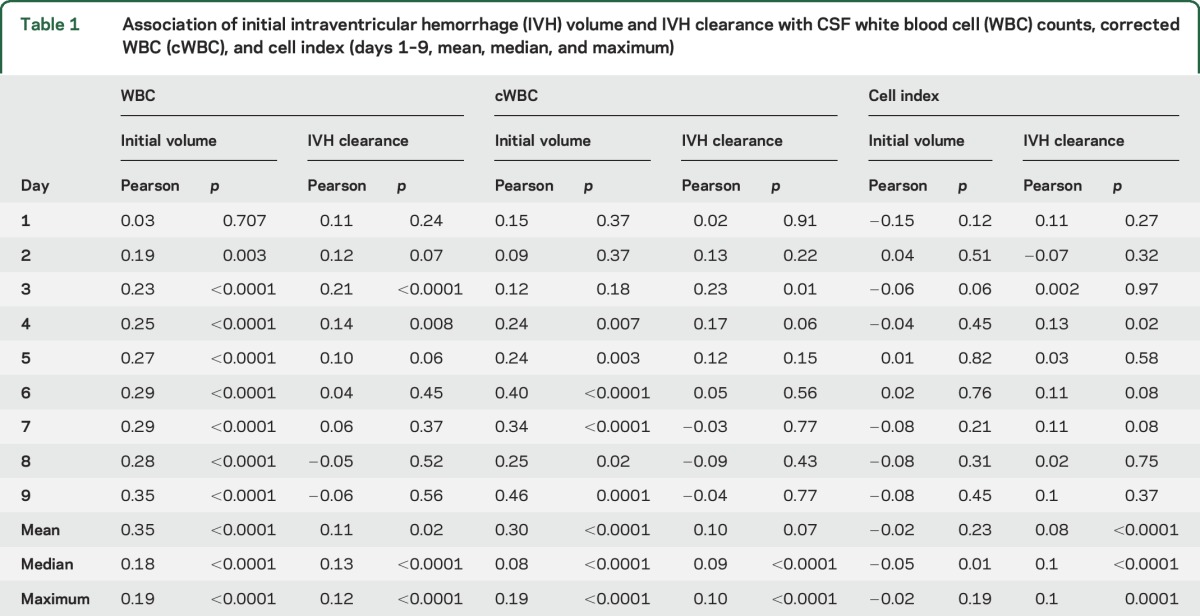
There were greater CSF WBC and protein in cases with higher iIVH (figures 2 and e-1B). A higher CSF WBC response was appreciated in cases with iIVH exceeding 20 mL (p < 0.0001); however, the difference between the 2 higher volume strata (20–50 and >50 mL) was less evident (p = 0.06). A similar pattern was noted with cWBC, while CI exhibited a similar trend but no differences in the 3 volume strata (figure 2). CSF glucose showed a similar but inverse correlation with iIVH, with levels lower in cases with iIVH volumes exceeding 20 mL (figure e-1B). iIVH and IVH clearance, considered as respective continuous variables, showed positive correlations with CSF WBC counts on days 2–9. Similar correlations were seen with cWBC but not CI. In per-patient analysis, iIVH demonstrated a strong positive correlation with mean, median, and maximum WBC and cWBC (p < 0.0001). CI, on the other hand, showed no relationship with iIVH but correlated positively with IVH clearance (on day 4, and mean, median, and maximum per participant) (table 1).
Figure 2. Association of initial intraventricular hemorrhage (iIVH) volume.
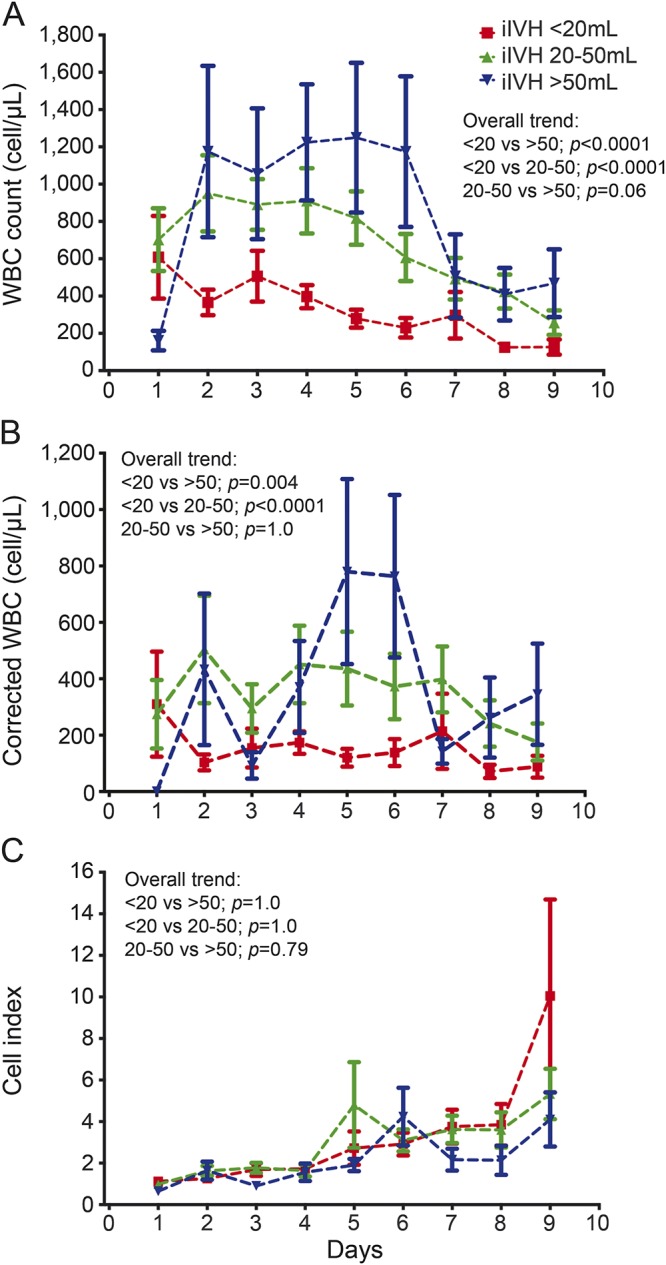
Temporal trend of white blood cells (WBC), corrected WBC, and cell index in the 3 prearticulated iIVH volume strata (<20 mL, n = 201; 20–50 mL, n = 120; and >50 mL, n = 54) over days 1–9 postictus. The p values listed with each panel refer to the difference in overall trend for the respective measure.
Table 1.
Association of initial intraventricular hemorrhage (IVH) volume and IVH clearance with CSF white blood cell (WBC) counts, corrected WBC (cWBC), and cell index (days 1–9, mean, median, and maximum)
Effect of thrombolytic treatment.
Participants receiving the alteplase group had consistently higher CSF WBC (p < 0.0001), cWBC (p = 0.009), CI (p = 0.002), and protein (p < 0.0001), and lower CSF glucose (p = 0.0003), compared to participants receiving saline (figures 3 and e-1C). We queried the effect of alteplase using a general linear model adjusting for iIVH volumes. Compared to saline, alteplase was associated with greater CSF inflammatory response as demonstrated by mean, median, and maximum CSF WBC, cWBC, and CI, independent of iIVH volume (tables e-4–e-6).
Figure 3. Association of thrombolytic treatment.
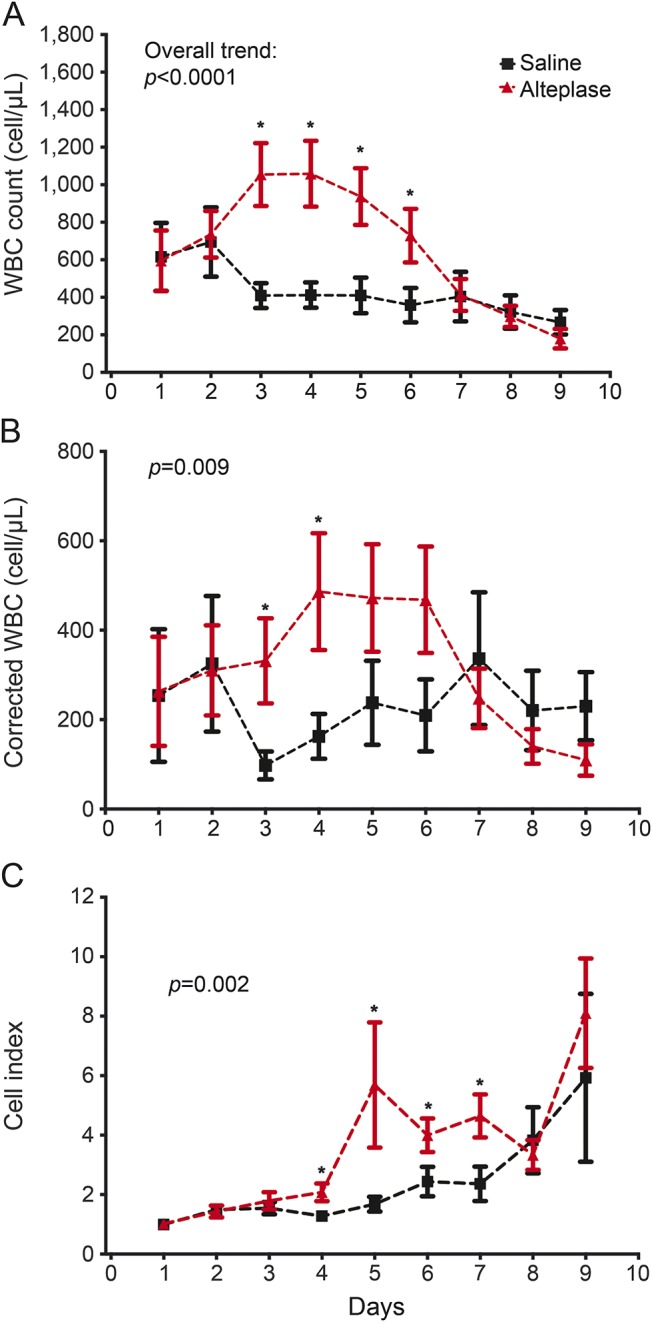
Temporal trend CSF white blood cells (WBC), corrected WBC, and cell index in cases with alteplase vs saline administered via external ventricular drain over days 1–9 postictus. The p value listed with each panel refers to the difference in overall trend for the respective measure. *Refers to significantly different daily values.
Effect of bacterial infection.
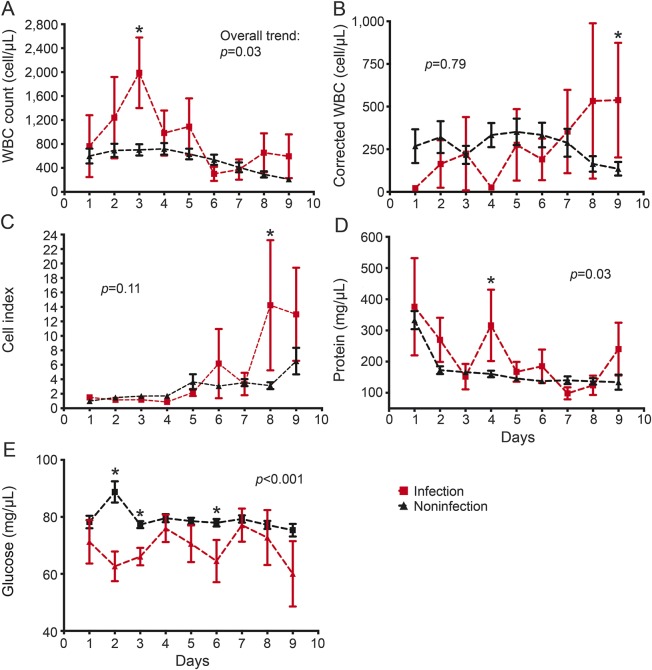
Participants with positive CSF bacterial culture during the sampling protocol (days 1–10, n = 21) were analyzed in comparison to the remaining cohort (figure 4). The overall trends of CSF measures confirmed that even in the context of IVH, cases with CSF infection have a higher CSF WBC (p = 0.03) and protein (p = 0.03) and lower glucose levels (p < 0.001). This distinction was lost when correcting for blood in CSF using cWBC (p = 0.79) and CI (p = 0.11). Per-patient analysis of CSF WBC expressed as mean, median, or maximum values showed a strong association with bacterial infection (p = 0.002, p < 0.001, and p = 0.003, respectively). A similar correlation was evident with CI but not cWBC (table e-7). There was no predictive trend of CSF inflammatory response preceding the time of sample with first positive culture (figure e-2). Extending the analysis to cases with CSF-positive culture within 30 days (n = 31) showed a weaker correlation with CSF inflammatory response assessed in days 1–9 (figure e-3).
Figure 4. Association of bacterial infection.
Temporal trend of CSF (A) white blood cells (WBC), (B) corrected WBC, (C) cell index, (D) protein, and (E) glucose during days 1–9 postictus in cases with and without bacterial infection manifested during the study period (within 10 days postictus, n = 21). The p value listed with each panel refers to the difference in overall trend for the respective measure. *Refers to significantly different daily values − daily value p < 0.05.
CSF inflammatory response and clinical outcome.
No correlation was found between the CSF inflammatory measures (WBC, cWBC, and CI) and poor functional outcome (mRS 4–6) or mortality at 30 or 180 days (tables e-8 and e-9). General linear model of 30- and 180-day outcomes confirmed that no measure of CSF inflammation (mean, median, or maximum CSF WBC counts, cWBC, or CI) had a significant association with poor outcome or mortality after correction for prognostic indicators in the CLEAR III trial (iIVH volume, IVH clearance, ICH volume, thalamic/nonthalamic ICH location, and GCS at presentation) (tables e-10 and e-11). There was a marginal correlation (p = 0.05) of median CI during days 1–9 with mRS 4–6 at 30 but not 180 days.
IVH participants with highest inflammatory response.
The upper quartile of participants based on CSF WBC, representing the group with strongest inflammation, did not exhibit any notable difference in baseline demographic features or medical history compared to the remaining CLEAR III participants, except for slightly higher female preponderance. The clinical management in the 2 groups was effectively similar in terms of timing of EVD insertion, initiation, duration, and frequency of dosing. However, patients with higher inflammatory response had lower GCS at presentation (p = 0.02); this group also had higher iIVH volumes (p = 0.0006). A greater fraction of patients in the high inflammation cohort received alteplase (67.2% vs 44.8%, odds ratio 2.4, confidence interval 1.63–3.93; p = 0.0067) and achieved higher IVH clearance (79% vs 40.2%, odds ratio 5.9, confidence interval 3.59–9.83; p < 0.0001). Participants with highest inflammatory response also required longer EVD time and intensive care unit stay. Despite the greater disease severity, no difference in 30- or 180-day functional outcome or mortality was seen in this group (table e-12). Similar results were noted when considering cWBC and CI upper quartiles for definition of the high inflammation group (tables e-13 and e-14).
DISCUSSION
We investigated classic CSF measures for the time course of inflammatory response and relevant associations in participants in the CLEAR III trial. This represents the largest prospectively enrolled cohort with IVH compiled to date, with systematic sampling of CSF and adjudicated measurements of IVH volume and clinical outcome. Our results demonstrate that an aseptic CSF inflammation is a normal response in the days following IVH. Greater extent of inflammatory response was present in cases with higher iIVH volume, most evidenced by early elevated WBC at days 2–3, slightly later cWBC elevation at days 4–5, and a lesser extent of elevated CI, accompanied by decreased CSF glucose and gradually decreasing CSF protein. The CSF inflammatory response was more pronounced in cases with higher IVH clearance, mostly illustrated by uncorrected WBC counts. This could be partly due to the release of hemorrhage-driven leukocytes trapped in the clot or to proinflammatory effects of clot degradation products. It is also possible that cases with lower IVH clearance had a more delayed inflammation that was not observed in days 1–9 of CSF sampling in our study.
Intraventricular thrombolytic therapy was associated with a surge in CSF pleocytosis that was independent of IVH volume and clearance rate. All prior studies to date of CSF inflammatory measures after hemorrhagic stroke represented single site case series, with potential selection biases, and few examined IVH specifically (table e-15). While the general trend of CSF inflammatory measures is noted in several reports, none was powered to examine effects of IVH volume or clearance rates, and none presented any meaningful correlations with clinical outcome. Only one study examined the effect of thrombolysis with appropriate randomized controls, but it included only 12 participants.14 This study, along with another one,14,15 suggested proinflammatory properties of thrombolytic drug consistent with our results.
The CSF pleocytosis did not independently affect mortality or functional outcome. No measure of CSF inflammation (daily, mean, median, or maximum sampled values) correlated with functional outcome or mortality with or without controlling for iIVH volume, IVH clearance, or thrombolytic treatment. Despite their greater disease severity (larger iIVH and lower GCS score), no difference in long-term functional outcome or mortality was seen in patients with the most severe cases of CSF inflammation. This suggests a compensatory clinical benefit of greater clearance rates and thrombolytic therapy. Such benefit of thrombolysis and greater IVH clearance was indeed documented in the trial in the subgroup of cases with large volume IVH.19 These results do not exclude more subtle sequelae of inflammatory response not measured by mRS at 30 and 180 days. It remains possible that modulation of the inflammatory response, including the use of steroids or nonsteroidal anti-inflammatory agents, could potentially further enhance the benefits of thrombolytic therapy. Our study lacked information on CSF neutrophils vs monocyte pleocytosis, and did not examine cytokines or other measures that could be more sensitive markers of harmful or beneficial impact. Other reports have indeed correlated peripheral blood monocyte counts with untoward outcome after hemorrhagic stroke,25–27 but we are unaware of specific studies addressing IVH or examining CSF cell types.
Changes in the CSF picture in association with bacterial infection have been well described in the diagnosis of meningitis/ventriculitis.11,28,29 Yet blood contamination of the CSF can also mask a true septic response. Cases with culture-positive CSF infection diagnosed during the course of CSF sampling had higher pleocytosis and protein and lower glucose. Adjustment using cWBC or CI at least partially eliminated the difference observed in uncorrected WBC. Our findings support the previous reports on the tendency of these measures (cWBC and CI) to overcorrect and thereby potentially mask a true infection.20,22 From a practical perspective, there were no inflammatory measures predictive of infection in the setting of IVH, hence there is no substitute to culturing CSF frequently in order to exclude infection, especially in cases with greater CSF pleocytosis or lower glucose levels.
Our study had several other limitations. There were missing data resulting from sites failing to report daily CSF results as per trial protocol. The effect of missing data was mitigated by comparison of cases with and without missing data, by randomized treatment assignment, and by adjudicated outcome assessment unrelated to the sampling of CSF measures. The absence of mandated daily CSF cultures and the frequent use of antibiotic prophylaxis may have confounded the detection of true infection and its correlation with classic CSF measures. The relationship of CSF inflammatory markers to fever and systemic and neurologic adverse events was not addressed herein, and is being examined in separate analyses beyond the scope of this report. Factors associated with permanent CSF shunting in the CLEAR III trial were addressed in another recent publication.30
Supplementary Material
GLOSSARY
- CI
cell index
- CLEAR III
Clot Lysis: Evaluation of Accelerated Resolution in IVH phase III trial
- cWBC
corrected white blood cells
- eotIVH
end-of-treatment intraventricular hemorrhage volume
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- GEE
generalized estimating equation
- GLM
general linear model
- ICH
intracerebral hemorrhage
- iIVH
initial intraventricular hemorrhage
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
- RBC
red blood cell
- WBC
white blood cell
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: CLEAR III Trial Investigators, Harold Adams, Opeolu Adeoye, Sachin Agarwal, Maria Aguilar, E. Francois Aldrich, Safdar Ansari, David Antezana, Agnieszka Ardelt, Fuat Arikan, Pal Barzo, Peter Bazso, Julian Bösel, Mel Boulton, Diederik Bulters, Ken Butcher, Paul Camarata, Jean-Louis Caron, Kevin Cockroft, Mauricio Concha, H. Mark Crabtree, Salvador Cruz-Flores, Laszlo Csiba, David Decker, Alberto Torres Díaz, Benjamin Emanuel, William Freeman, Michael Froehler, Latorre Julius Gen, Martin Gizzi, Nicole Gonzales, R. Scott Graham, David Greer, Christiana Hall, Sagi Harnof, Mark Harrigan, Ann Helms, Alan Hoffer, James Holsapple, Hagen Huttner, Michael Jacoby, Jack Jallo, Michael Luke James, Eric Juttler, Marcelo Kern, Thomas Kerz, Inam Kureshi, Christos Lazaridis, David LeDoux, Jody Leonardo, Alexandre Luiz Longo, George Lopez, Darren Lovick, Andreas Luft, Jorge Marconde de Souza, Nevo Margalit, Mason Markowski, Joan Marti-Fabregas, Sheila Cristina Ouriques Martins, A.D. Mendelow, Dominik Michalski, Thomas Mirsen, Asma Moheet, David Newell, George Newman, Christopher Ogilvy, Hiren Patel, Pedro Telles Cougo Pinto, Sven Poli, Michael Reinert, Guy Rosenthal, Fernando Santiago, Michael Schneck, David B. Seder, Qaisar A. Shah, Kavian Shahi, Gisele Sampaio Silva, David Sinclair, Laszlo Szapary, Ashis H. Tayal, Fernando Testai, Michel Torbey, Kristi Tucker, Stanley Tuhrim, Igor Ugorec, Panayiotis Varelas, Chitra Venkatasubramanian, Paul Vespa, Katja Wartenberg, Michael Weaver, Lawrence Wechsler, and Menashe Zaaroor
AUTHOR CONTRIBUTIONS
All authors were involved in the concept, design, drafting, and critical revision of the manuscript. D.H. and I.A.A. co-chaired the CLEAR trial and supervised patient screening and enrollment. M.D.F., J.K.E., S.J., D.F.H., W.Z., and I.A.A. were involved in study conceptualization, hypothesis, and study design. M.D.F., J.K.E., N.M., K.L., A.S., H.A.Z., and M.J. were involved in data collection, analysis, and interpretation of results. Y.C., M.W., R.E.T., and L.Z. contributed to the statistical analysis. M.D.F., H.A.Z., and J.K.E. were responsible for writing the manuscript with supervision by I.A.A. and for preparing tables, panels, and figures.
STUDY FUNDING
The CLEAR III trial was supported by grant 5U01NS062851 from the NIH, National Institute of Neurologic Disorders and Stroke. Issam A. Awad, Daniel Hanley, Agnieszka Stadnik, Nichol McBee, Karen Lane, Ying Cao, and Richard E. Thompson received salary support for this grant. Alteplase was donated by Genentech, Inc. (San Francisco, CA). The funding sponsors had no role in the design or reporting of this study.
DISCLOSURE
M. Fam, H. Zeineddine, J. Khader Eliyas, A. Stadnik, M. Jesselson, N. McBee, K. Lane, Y. Cao, M. Wu, L. Zhang, R. Thompson, S. John, and W. Ziai report no disclosures relevant to the manuscript. D. Hanley has testified in legal proceedings. I. Awad has testified in legal proceedings. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009;40:1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruscalleda J, Peiro A. Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology 1986;28:34–37. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 2006;59:767–773; discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 4.Mayfrank L, Hutter BO, Kohorst Y, et al. Influence of intraventricular hemorrhage on outcome after rupture of intracranial aneurysm. Neurosurg Rev 2001;24:185–191. [DOI] [PubMed] [Google Scholar]
- 5.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med 1999;27:617–621. [DOI] [PubMed] [Google Scholar]
- 6.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology 1990;40:616–619. [DOI] [PubMed] [Google Scholar]
- 7.Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: time course, magnitude and effect of t-PA. J Neurol Sci 2012;315:93–95. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res 2007;1180:140–154. [DOI] [PubMed] [Google Scholar]
- 9.Gaberel T, Montagne A, Lesept F, et al. Urokinase versus alteplase for intraventricular hemorrhage fibrinolysis. Neuropharmacology 2014;85:158–165. [DOI] [PubMed] [Google Scholar]
- 10.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg 2002;96:287–293. [DOI] [PubMed] [Google Scholar]
- 11.Fulkerson DH, Sivaganesan A, Hill JD, et al. Progression of cerebrospinal fluid cell count and differential over a treatment course of shunt infection. J Neurosurg Pediatr 2011;8:613–619. [DOI] [PubMed] [Google Scholar]
- 12.Gaberel T, Magheru C, Parienti JJ, Huttner HB, Vivien D, Emery E. Intraventricular fibrinolysis versus external ventricular drainage alone in intraventricular hemorrhage: a meta-analysis. Stroke 2011;42:2776–2781. [DOI] [PubMed] [Google Scholar]
- 13.Goto H, Fujisawa H, Oka F, et al. Neurotoxic effects of exogenous recombinant tissue-type plasminogen activator on the normal rat brain. J Neurotrauma 2007;24:745–752. [DOI] [PubMed] [Google Scholar]
- 14.Kramer AH, Jenne CN, Zygun DA, et al. Intraventricular fibrinolysis with tissue plasminogen activator is associated with transient cerebrospinal fluid inflammation: a randomized controlled trial. J Cereb Blood Flow Metab 2015;35:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ducruet AF, Hickman ZL, Zacharia BE, et al. Exacerbation of perihematomal edema and sterile meningitis with intraventricular administration of tissue plasminogen activator in patients with intracerebral hemorrhage. Neurosurgery 2010;66:648–655. [DOI] [PubMed] [Google Scholar]
- 16.Ziai W, Moullaali T, Nekoovaght-Tak S, et al. No exacerbation of perihematomal edema with intraventricular tissue plasminogen activator in patients with spontaneous intraventricular hemorrhage. Neurocrit Care 2013;18:354–361. [DOI] [PubMed] [Google Scholar]
- 17.Fountas KN, Kapsalaki EZ, Parish DC, et al. Intraventricular administration of rt-PA in patients with intraventricular hemorrhage. South Med J 2005;98:767–773. [DOI] [PubMed] [Google Scholar]
- 18.Volbers B, Wagner I, Willfarth W, Doerfler A, Schwab S, Staykov D. Intraventricular fibrinolysis does not increase perihemorrhagic edema after intracerebral hemorrhage. Stroke 2013;44:362–366. [DOI] [PubMed] [Google Scholar]
- 19.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017;389:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeer K, Siegmund R, Pfister W, Isenmann S, Deufel T. Correction of ventricular cerebrospinal fluid (CSF) samples for blood content does not increase sensitivity and specificity for the detection of CSF infection. Clin Chem Lab Med 2008;46:842–848. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg RG, Smith PB, Cotten CM, Moody MA, Clark RH, Benjamin DK Jr. Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count. Pediatr Infect Dis J 2008;27:1047–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonsu BK, Harper MB. Corrections for leukocytes and percent of neutrophils do not match observations in blood-contaminated cerebrospinal fluid and have no value over uncorrected cells for diagnosis. Pediatr Infect Dis J 2006;25:8–11. [DOI] [PubMed] [Google Scholar]
- 23.Mayefsky JH, Roghmann KJ. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med 1987;82:1175–1181. [DOI] [PubMed] [Google Scholar]
- 24.Pfausler B, Beer R, Engelhardt K, Kemmler G, Mohsenipour I, Schmutzhard E. Cell index: a new parameter for the early diagnosis of ventriculostomy (external ventricular drainage)-related ventriculitis in patients with intraventricular hemorrhage? Acta Neurochir 2004;146:477–481. [DOI] [PubMed] [Google Scholar]
- 25.Adeoye O, Knight WA, Khoury J, et al. A matched comparison of eptifibatide plus rt-PA versus rt-PA alone in acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:e313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morotti A, Phuah CL, Anderson CD, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke 2016;47:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke 2015;46:2302–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schade RP, Schinkel J, Roelandse FW, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg 2006;104:101–108. [DOI] [PubMed] [Google Scholar]
- 29.Pfisterer W, Muhlbauer M, Czech T, Reinprecht A. Early diagnosis of external ventricular drainage infection: results of a prospective study. J Neurol Neurosurg Psychiatry 2003;74:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy SB, Awad I, Harnof S, et al. Permanent cerebrospinal fluid shunting after intraventricular hemorrhage in the CLEAR III trial. Neurology 2017;89:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.