Abstract
Objective:
To conduct a meta-analysis that investigates sex differences in the prevalence of mutations in the 3 most common genes that cause amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD)—chromosome 9 open reading frame 72 (C9orf72), progranulin (GRN), or microtubule-associated protein tau (MAPT)—in patients clinically diagnosed with these conditions.
Methods:
MEDLINE, EMBASE, and PsycINFO databases were searched (inception to June 30, 2016). Studies of patients with FTD or ALS that reported the number of men and women with and without mutations of interest were selected. Female to male pooled risk ratios (RR) and 95% confidence intervals (CI) for each mutation were calculated using random-effects models.
Results:
Thirty-two articles reporting 12,784 patients with ALS (including 1,244 C9orf72 mutation carriers) revealed a higher prevalence of female patients with C9orf72-related ALS (RR 1.16, 95% CI 1.04–1.29). Twenty-three articles reporting 5,320 patients with FTD (including 488 C9orf72 mutation carriers) revealed no sex differences in C9orf72-related FTD (RR 0.95, 95% CI 0.81–1.12). Thirty-six articles reporting 3,857 patients with FTD (including 369 GRN mutation carriers) revealed a higher prevalence of female patients with GRN-related FTD (RR 1.33, 95% CI 1.09–1.62). Finally, 21 articles reporting 2,377 patients with FTD (including 215 MAPT mutation carriers) revealed no sex difference in MAPT-related FTD (RR 1.21, 95% CI 0.95–1.55).
Conclusions:
Higher female prevalence of C9orf72 hexanucleotide repeat expansions in ALS and GRN mutations in FTD suggest that sex-related risk factors might moderate C9orf72 and GRN-mediated phenotypic expression.
Frontotemporal dementia (FTD) affects 15–22/100,000 Americans,1 and is the second leading cause of early-onset dementia, characterized by severe behavior and language disturbances due to frontal and temporal neuronal death.2 Amyotrophic lateral sclerosis (ALS), which affects approximately 5/100,000 Americans,3 lies on a clinicopathologic continuum with FTD,4 and causes severe motor impairment due to neuronal loss in the spinal cord and motor cortex.5 These incurable disorders have progressive, short disease courses, spanning approximately 3 years for ALS and 3–10 years for FTD.6,7 Sex differences in clinical prevalence have been identified in ALS,8 and while some studies report sex differences in FTD,9,10 others do not.11,12
Causal genetic mutations account for up to 10% of ALS13 and 10%–20% of FTD cases.14 Our aim was to conduct a meta-analysis examining differences between men and women in the prevalence of hexanucleotide repeat expansions (≥30 repeats) in the chromosome 9 open reading frame 72 (C9orf72) gene, the most common known genetic cause of both ALS and FTD. We also investigated sex differences in the 2 most common genes with mutations that cause FTD: microtubule-associated protein tau (MAPT) and progranulin (GRN). Because these mutations are located on autosomal genes and would be expected to be inherited similarly by men and women, if sex differences are found in the prevalence of mutations in those with ALS or FTD, this suggests there may be sex-specific risk factors (e.g., neurotoxic exposure) or biologic mechanisms (e.g., epigenetic modifiers such as hormonal changes, sex-mediated brain development) that moderate gene expression and alter the disease phenotype.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis15 recommendations.
Study selection and screening.
PsycINFO, EMBASE, and MEDLINE were systematically searched on June 30, 2016, with no timeframe restrictions. Relevant keywords and MeSH terms used were those describing or relating to clinical diagnoses of FTD or ALS, combined with keywords describing the genetic mutations of interest (see appendix e-1 at Neurology.org for complete search strategy). We restricted the search to human studies and English language articles. Reviews and meta-analyses were excluded, but reference lists were checked for additional relevant articles. All relevant hits were exported to Endnote reference management software (version X7.4).
Once duplicates were removed, abstracts were screened independently by 2 trained research assistants, RA1 and RA2. Full text screening of relevant articles was conducted by 2 independent teams of 3 raters (team 1: A.F.C., G.M., RA1; team 2: B.A., K.Z., RA2). Discrepancies were resolved through discussion with M.M., G.-Y.R.H., and M.C.T. Articles were included if study samples included patients with clinically diagnosed FTD or ALS using well-established diagnostic criteria,2,16–22 and if the study reported the total number of male and female patients with and without a mutation in the genes of interest (C9orf72, GRN, or MAPT) from the larger pool of patients with clinically diagnosed FTD or ALS. Consistency across articles was achieved by excluding those articles that diagnosed FTD or ALS solely based on neuropathology (e.g., CSF or postmortem diagnoses) without a previous clinical diagnosis of FTD or ALS. Articles containing duplicate samples already included in the meta-analysis were excluded. Articles that selected subgroups of patients from their larger pool of clinically diagnosed ALS and FTD cases based on criteria that could potentially introduce bias into our calculation of sex prevalence (e.g., included only a subgroup of mutation and noncarrier patients matched on age or sex, or included only patients whose sibling or other related family member also participated) were excluded.
Data extraction.
Data were independently extracted and entered into separate databases by the 2 teams of raters. Disagreements were resolved by discussion and consensus. To characterize the dataset, we extracted available data describing study location (continent), method of patient recruitment (e.g., clinic-based referrals, population-based sampling), initial clinical diagnosis of patients (FTD or ALS), FTD subtype (e.g., behavioral variant23 or primary progressive aphasia24), diagnostic criteria, mean age at disease onset, mean disease duration, and whether the study sample contained unrelated patients. Given that ALS and FTD may occur together,25 we also extracted information regarding comorbid cases (e.g., patients with ALS who developed FTD or patients with FTD who developed ALS). We also recorded the number of male and female participants with and without mutations in the genes of interest. In addition, we recorded whether studies excluded patients with specific FTD or ALS mutations, and identified these excluded mutations. Finally, we recorded the type of genetic sequencing conducted (whole genome sequencing [WGS], whole exome sequencing [WES], targeted gene sequencing), and whether genetic testing was diagnostic grade (e.g., Clinical Laboratory Improvement Amendment certified) or research grade.
Data analysis.
Interrater agreement of articles included in the meta-analysis was assessed using the Cohen kappa statistic. Interrater agreement of data extraction was computed as percentage of agreement. We conducted separate meta-analyses for mutations in each gene (C9orf72, GRN, or MAPT) and for each clinical diagnosis (FTD or ALS), resulting in 4 separate analyses: (1) C9orf72-related ALS, (2) C9orf72-related FTD, (3) GRN-related FTD, and (4) MAPT-related FTD. For each article, we first calculated the proportion of women and men with each of the 3 mutations of interest relative to the total sample with and without that mutation, as follows: proportion of female carriers = no. of women with the mutation of interest ÷ no. of women with and without the mutation of interest; proportion of male carriers = no. of men with the mutation of interest ÷ no. of men with and without the mutation of interest.
Denominators included patients with no known mutation identified as well as patients with identified mutations other than the one of interest. Effect size was measured by calculating the female to male risk ratio (RR) of the above 2 proportions for each of the 3 mutations of interest. Corresponding 95% confidence intervals (CIs) of the RR were also computed. Risk estimates greater than 1.0 indicate higher prevalence in women, whereas estimates less than 1.0 indicate higher prevalence in men. RRs were considered statistically significant if the 95% CI did not include the neutral value of 1.0. For each analysis, we employed random-effects models to estimate the pooled RR across studies, as this is a more conservative approach and minimizes the effect of between-study heterogeneity.26
Given the potential influence of sex differences in patients with other known mutations that were included in the denominator of our prevalence estimate,27 we recorded whether studies excluded patients with other known mutations, or did not screen for or exclude any other known mutations (table e-1). Studies were grouped according to mutations excluded (e.g., none, all other known mutations, GRN, GRN and MAPT) and subgroup analyses were conducted to assess whether there were between-group differences in RR, using the Q-between statistic (Qb). We also assessed the effect of patient relatedness on any observed sex differences by conducting subgroup analyses that compared the RR of studies that included related patients to the RR of those that did not include related patients (using Qb). Finally, we conducted subgroup analyses to investigate whether RRs differed by method of patient recruitment or continent.
Heterogeneity was examined using the inconsistency index (I2). Publication bias was assessed using the Egger regression intercept test. p Values less than 0.05 were considered statistically significant; however, I2 values of 30% or higher were investigated further for sources of potential heterogeneity.28 Statistical analysis was conducted using Comprehensive Meta-Analysis version 3.
RESULTS
Literature search and screening.
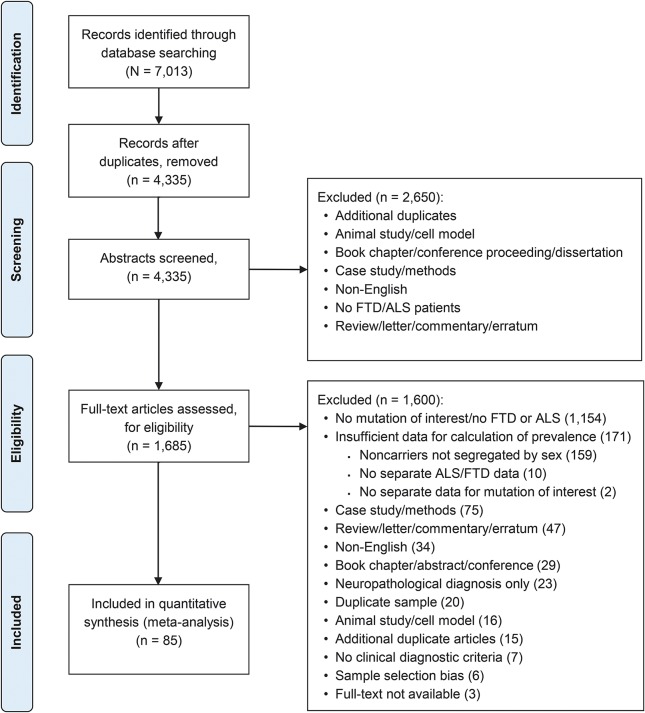
We retrieved 7,013 records in the initial database search and 1,685 articles were identified for full text review. Eighty-five articles were included in the meta-analysis (figure 1 and table e-1). A total of 159 articles could not be included in the meta-analysis because they did not provide sex-segregated data for mutation and nonmutation carriers, required to calculate sex-specific prevalence. To determine the success rate of obtaining sex-segregated data from authors, a subset of authors from these articles was contacted to provide this information, but the response rate was only 36%. Given this low response rate, and the potential for author response bias on our results, we did not pursue further author contact and only retained those articles that provided sex-segregated information in their publication. Interrater agreement between the 2 teams of raters for article inclusion in the meta-analysis was very high (κ = 0.87). For data extraction, the overall agreement rate was 96%. The majority of items had 100% agreement and the worst agreement was for the count of male and female mutation carriers, with 92% agreement. Discrepancies were discussed and 100% agreement was achieved.
Figure 1. Flowchart of included articles.
ALS = amyotrophic lateral sclerosis; FTD = frontotemporal dementia.
Study characteristics.
Descriptive information on each article included in the meta-analysis (and corresponding reference list) is provided in table e-1. Thirty-two articles reporting 12,784 patients with ALS (57% male, 9.7% C9orf72 expansion carriers) and 23 articles reporting 5,320 patients with FTD (53% male, 9.2% C9orf72 expansion carriers) provided relevant information on C9orf72 mutations. Thirty-six articles reporting 3,857 patients with FTD (51% male, 9.6% GRN mutation carriers) provided relevant information on GRN mutations. Twenty-one articles reporting 2,377 patients with FTD (53% male, 9.0% MAPT mutation carriers) provided relevant information on MAPT mutations. Although some articles reported cases where patients with ALS also developed FTD, or patients with FTD developed ALS, sex-segregated information for these comorbid cases were only provided in 16% of relevant articles, therefore data extraction of clinical diagnoses were restricted to the initial diagnoses of ALS or FTD. FTD sex-segregated subtype information was provided in only 24% (κ = 13) of relevant articles, and thus could not be analyzed further due to lack of sample representation. Sex-segregated age at onset data were only provided in 42% (κ = 36) of articles, so we recorded the mean age at onset of the entire FTD and ALS sample. Sex-segregated disease duration was only provided in 15% (κ = 13) of articles, so this was not analyzed further. Only one study29 recruited patients via random population sampling (ALS disease registry). The remaining studies recruited patients from specialty clinics or research databases of specialty clinic referrals or did not report their recruitment methods. No studies provided information on whether the genetic testing was diagnostic or research grade. No studies conducted WGS and 2 studies conducted WES, one of which reported using the gold standard Sanger sequencing validation method. The remaining 83 studies conducted targeted gene sequencing: 54 (65%) used Sanger sequencing, 10 (12%) used non-Sanger sequencing methods, and 19 (23%) did not describe their sequencing methods.
Meta-analysis.
C9orf72-related ALS.
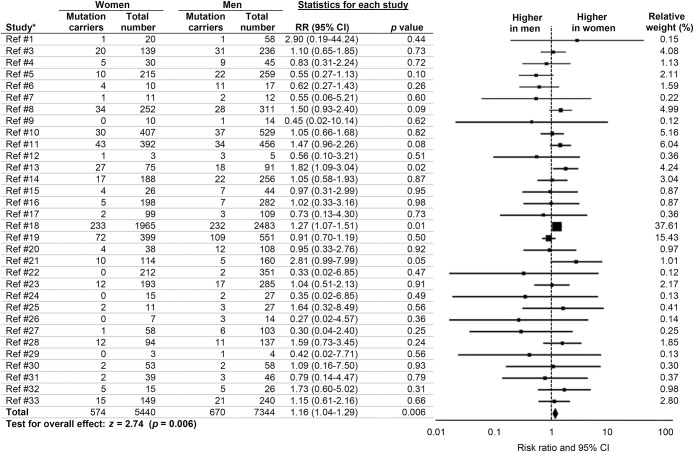
We observed a higher prevalence of female C9orf72 carriers with ALS (RR 1.16, 95% CI 1.04–1.29, p = 0.006) (figure 2). There was no heterogeneity (I2 = 0.0%, p = 0.64) and no publication bias (p = 0.07). Because the above analysis combined in the denominator both the studies that included all patients without the C9orf72 mutation, i.e., those with ALS mutations in genes other than C9orf72, as well as those with no known ALS mutations, we conducted subgroup analysis to compare effect sizes between studies that excluded other identified mutation carriers and those that did not screen for or exclude any other mutation carriers. There were no RR differences between studies that excluded patients with other non-C9orf72 ALS mutations and those that did not (Qb = 0.65, p = 0.72). There were also no RR differences between studies that included related patients in their samples and studies that did not (Qb = 2.05, p = 0.15). Effect sizes did not differ by continent (Qb = 5.34, p = 0.50) or by method of patient recruitment (Qb = 0.35, p = 0.95).
Figure 2. Sex differences in prevalence of C9orf72 hexanucleotide expansions in patients with amyotrophic lateral sclerosis.
Forest plot displays random-effects meta-analysis results for female:male risk ratios (RRs). The sizes of the squares are proportional to relative study weights. CI = confidence interval. *Study numbers correspond to the e-references.
C9orf72-related FTD.
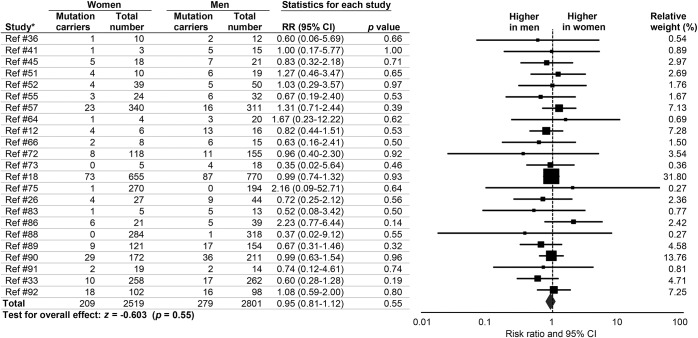
There were no sex differences in the prevalence of FTD C9orf72 carriers (RR 0.95, 95% CI 0.81–1.12, p = 0.55) (figure 3). There was no heterogeneity (I2 = 0.0%, p = 0.99) and no publication bias (p = 0.32). There were no RR differences between studies that excluded patients with other non-C9orf72 FTD mutations and those that did not (Qb = 2.75, p = 0.25). There were no differences between studies with related vs unrelated patients (Qb = 0.48, p = 0.49). Effect sizes did not differ by continent (Qb = 1.95, p = 0.74) or by method of patient recruitment (Qb = 0.87, p = 0.65).
Figure 3. Sex differences in prevalence of C9orf72 hexanucleotide expansions in patients with frontotemporal dementia.
Forest plot displays random-effects meta-analysis results for female:male risk ratios (RRs). The sizes of the squares are proportional to relative study weights. CI = confidence interval. *Study numbers correspond to the e-references.
GRN-related FTD.
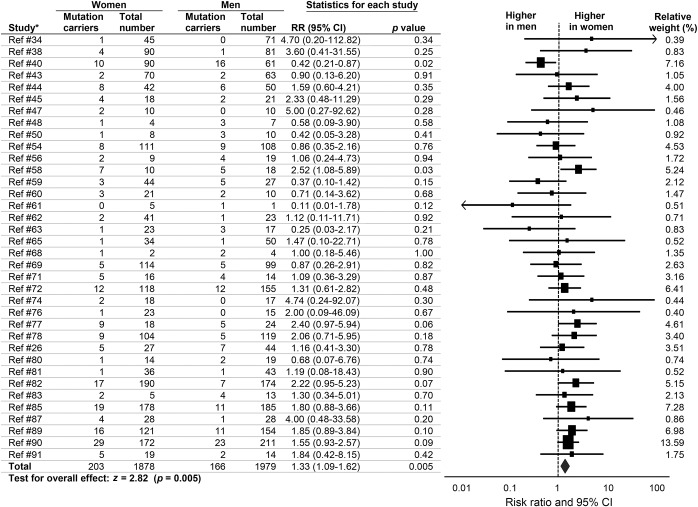
We found a higher prevalence of women with FTD carrying GRN mutations (RR 1.33, 95% CI 1.09–1.62, p = 0.005) (figure 4). There was no heterogeneity (I2 = 2.12%, p = 0.43) and no publication bias (p = 0.50). There were no RR differences between studies that excluded patients with other non-GRN mutations and those that did not (Qb = 5.90, p = 0.21). There were no differences between studies with related vs unrelated patients (Qb = 0.38, p = 0.54). Effect sizes did not differ by continent (Qb = 0.91, p = 0.63) or by method of patient recruitment (Qb = 0.24, p = 0.89).
Figure 4. Sex differences in prevalence of GRN mutations in patients with frontotemporal dementia.
Forest plot displays random-effects meta-analysis results for female:male risk ratios (RRs). The sizes of the squares are proportional to relative study weights. CI = confidence interval. *Study numbers correspond to the e-references.
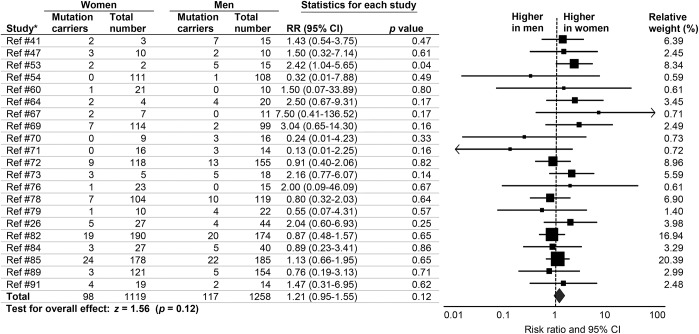
MAPT-related FTD.
We found no sex differences in prevalence of FTD MAPT carriers (RR 1.21, 95% CI 0.95–1.55, p = 0.12) (figure 5). There was no heterogeneity (I2 = 0.0%, p = 0.67) and no publication bias (p = 0.98). There were no RR differences between studies that excluded patients with other non-MAPT mutations and those that did not (Qb = 0.09 p = 0.96). There were also no differences between studies with related vs unrelated patients (Qb = 3.89, p = 0.05). Effect sizes did not differ by continent (Qb = 0.71, p = 0.87) or by type of patient recruitment (Qb = 0.09, p = 0.95).
Figure 5. Sex differences in prevalence of MAPT mutations in patients with frontotemporal dementia.
Forest plot displays random-effects meta-analysis results for female:male risk ratios (RRs). The sizes of the squares are proportional to relative study weights. CI = confidence interval. *Study numbers correspond to the e-references.
DISCUSSION
We conducted a meta-analysis to determine whether there are sex differences in the prevalence of mutation carriers in the most common gene that causes FTD and ALS (C9orf72) and the other 2 most common genes that cause FTD (GRN and MAPT).
C9orf72-related ALS.
While men are known to have a higher prevalence of ALS overall (combination of sporadic and mutation carriers),8 we found that women had a 16% higher prevalence of C9orf72-related ALS. Current explanations for higher male prevalence of sporadic ALS include their greater exposure to environmental risk factors (e.g., pesticides, greater amounts of physical activity, and occupations in the armed forces).30,31 There is no reason to assume that men with C9orf72-related ALS have less exposure to these risk factors. We propose a number of possible explanations for our findings. First, there may be unknown sex-related risk factors that moderate C9orf72 ALS pathogenic mechanisms that override the exogenous risk factors to explain this higher prevalence of women with C9orf72-mediated ALS. Thus, we suggest that future research should explore potential sex differences in the proposed C9orf72 expansion molecular mechanisms, such as haploinsufficiency of the C9orf72 protein,32 gain of neurotoxic protein (RNA or dipeptide repeat) aggregates,33 increased cortical excitability that disrupts the function of protective astrocytes in the motor cortex,34 or disruption of immune system homeostasis.35
Second, the sex effect in C9orf72-related ALS might be due to longer survival of women with this mutation. Given that women live on average longer than men,36 and penetrance of C9orf72 expansions increases with age,37 women are more likely to reach the age of complete penetrance and expression of the ALS phenotype. Compounding this sex difference in life expectancy, men with C9orf72 mutations have a shorter disease course.38 Because age at onset was rarely provided separately for men and women, we could not covary its effect on the observed sex difference. Longitudinal incidence studies are the only manner in which sex differences in survival and disease course can be accounted for when examining sex differences in C9orf72-related ALS.
C9orf72-related FTD.
Unlike the results for clinically diagnosed ALS cases, we did not observe a sex difference in C9orf72 carriers with FTD. It is possible that diagnostic errors, which are more common in women relative to men with FTD,39 may have resulted in missed female C9orf72 carriers in the included FTD study samples. However, it is also plausible that sex-related risk factors might selectively interact with ALS and FTD disease mechanisms and epigenomic factors could be contributing to the difference in phenotype expression in women with C9orf72 mutations.
GRN-related FTD.
Similar to C9orf72-related ALS, we observed a 33% higher female prevalence of GRN-related FTD. GRN expression during embryogenesis plays a role in determining whether an individual develops a male or female brain.40 Therefore it is possible that differences in sex hormones, possibly including changes that occur at certain stages of life (e.g., menopause), change the risk for developing GRN-mediated disease in a sex-dependent fashion.41 We encourage future studies to provide sex-segregated age at onset data as well as menopausal status of women in order to further investigate this possibility. Moreover, given that there are over 50 known mutations in GRN that cause haploinsufficiency of the GRN protein, leading to neurodegeneration and expression of the FTD phenotype,42 we suggest that future research should examine how sex-related risk factors might moderate mutation-specific pathogenic mechanisms, such as disruption of neuroprotection and anti-inflammatory responses,43 or buildup of TAR-DNA binding protein aggregates.44
MAPT-related FTD.
Given that MAPT mutations lead to neurodegeneration by either loss of tau protein function or an accumulation of defective hyperphosphorylated tau protein,45 our findings of no sex differences in prevalence of MAPT-related FTD suggest that men and women with MAPT-related FTD may not differ in susceptibility to mutated tau.
Strengths and limitations.
The main strength of our meta-analysis is that the included studies comprised a large pooled sample size of 21,028 patients, despite the exclusion of 159 studies that did not provide relevant sex-segregated information. The large sample size improves the generalizability of our evidence of sex differences in the prevalence of certain ALS and FTD mutations.
One limitation of the studies included in this meta-analysis is the reliance on recruitment through specialty clinic referrals rather than population or community-based sampling, which could reduce generalizability of the findings. For example, some clinic studies might be prone to participant selection bias because patients were recruited based on their relationships with each other or index cases. Representativeness of clinic samples can also be affected because patients referred to clinics are often younger and present with a more rapidly progressive onset of neurodegeneration.46 While these selection biases remain important limitations, we attempted to reduce their potential effects by excluding studies that selected patients based solely on family relatedness (e.g., included only index patients and their siblings). Moreover, we directly examined whether there were differences in effect sizes between studies that included related and unrelated patients, and here we found no differences. It is also important to note that the proportion of men and women diagnosed with the 2 disorders in the studies included in this meta-analysis, which was based predominately on specialty clinic sampling, is consistent with reports identified in population-based studies,9–12,46 thus making it less likely that our observed prevalence findings were affected by selection bias. In addition, due to the low population prevalence of ALS3 and FTD,1 and the need and expense for genetic testing, clinic-based studies are a reasonable approach to doing research in the area.
Given that included studies did not describe the grade of genetic testing, and that most studies conducted targeted gene sequencing, we cannot rule out the possibility of false-negative results in the reported mutation data. However, given that any testing procedure used in a study would be applied to both male and female patients, it is not clear how this bias in genetic testing would affect sex-based prevalence calculations.
As previously mentioned, we were unable to examine the effect of age at onset and disease duration on sex differences due to a lack of sex-segregated reporting. This underlies the need for longitudinal incidence-based studies that further explore how gene-mediated ALS and FTD disease courses might differ for men and women. However, given the challenges to conducting longitudinal studies with these relatively rare diseases, a more feasible next step might be to utilize large-scale multicenter cross-sectional biomarker collection studies such as the ANSWER ALS initiative (answerals.org) and Genetic FTD Initiative (genfi.org.uk/index.html) to further examine sex differences in pathogenic mutations.
A final limitation of the included studies is the lack of sex-segregated information regarding FTD subtype. To further understand how sex affects susceptibility to neural pathology and FTD phenotypic expression, it would be interesting to examine whether sex differences in mutation prevalence differ across FTD subtypes. Moreover, given that up to 40% of patients initially diagnosed with ALS can develop comorbid FTD, and up to 10%–15% of patients initially diagnosed with FTD can develop ALS,25 it would be interesting to explore whether sex differences are observed across comorbid phenotypes. While the included studies did not provide sufficient sex-segregated information for comorbid cases, this remains an area for future exploration.
Our findings support the consideration of sex in the understanding of pathogenic mechanisms of C9orf72-related ALS and GRN-related FTD. Ultimately, this information could increase our understanding of ALS and FTD etiology, which could be important for treatment and management of the disorders, and could also be factored into genetic counseling recommendations.
Supplementary Material
ACKNOWLEDGMENT
Dinat Khan (Sunnybrook Health Sciences Center, Toronto, Canada) assisted with abstract screening and data extraction. Amir Sepehry (University of British Columbia, Vancouver, Canada) assisted with abstract screening. Henry Lam (Sunnybrook Health Sciences Center) assisted with the systematic literature search.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- C9orf72
chromosome 9 open reading frame 72 gene
- CI
confidence interval
- FTD
frontotemporal dementia
- Q b
Q-between statistic
- RR
risk ratio
- WES
whole exome sequencing
- WGS
whole genome sequencing
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ashley F. Curtis: study concept and design, acquisition of data, data analysis, interpretation of data, drafting of manuscript. Mario Masellis: study concept and design, interpretation of data, critical revision of manuscript for intellectual content. Ging-Yuek Robin Hsiung: study concept and design, interpretation of data, critical revision of manuscript for intellectual content. Rahim Moineddin: data analysis, critical revision of manuscript for intellectual content. Kathy Zhang: acquisition of data, critical revision of manuscript for intellectual content. Bonnie Au: acquisition of data, critical revision of manuscript for intellectual content. Geneva Millett: acquisition of data, critical revision of manuscript for intellectual content. Mary C. Tierney: study concept and design, acquisition of data, data analysis, interpretation of data, drafting of manuscript, study supervision.
STUDY FUNDING
Funding for the project was provided by several sources. A.F.C. was funded through a Postdoctoral Fellowship award from the Canadian Consortium on Neurodegeneration in Aging (CCNA). The CCNA is supported by a grant from the Canadian Institute of Health Research (CIHR; CAN 137794) with funding from several partners. G.-Y.R.H. was supported by a CIHR Clinical Genetics Investigatorship and the Ralph Fisher Alzheimer Society of British Columbia Professorship in Alzheimer's disease. M.C.T. was supported by a Clinician Scientist Award from the Department of Family and Community Medicine, University of Toronto. Indirect funding related to the current work included the CIHR CCNA grant (CAN 137794) to M.M., G.-Y.R.H., E.R., I.M., and M.C.T.; and a grant to E.K. and I.M. from ALS Canada (Brain Canada Hudson Grant), to M.M. from CIHR (MOP137116), and to I.M. from CIHR (179009 and 74580). The funders were not involved in any aspect of study conception, design, data collection, analysis, manuscript preparation, or submission of manuscript for publication.
DISCLOSURE
A. Curtis reports no disclosures relevant to the manuscript. M. Masellis: advisor to Bioscape Medical Imaging CRO, Novartis, and UCB; received honoraria from Novartis; received royalties from Henry Stewart Talks; received an investigator-initiated research grant from Teva; and received contract research support from Novartis and Axovant. G. Hsiung received honoraria as a consultant for Merck and Eli Lilly. He has also received support as a site investigator in clinical trials sponsored by Biogen, Eli Lilly, Genentech, Roche, and TauRx. R. Moineddin, K. Zhang, B. Au, G. Millett, I. Mackenzie, E. Rogaeva, and M. Tierney report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry 2013;25:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick's disease. Arch Neurol 2001;58:1803–1809. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P, Kaye W, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis: United States, 2012–2013. MMWR Surveill Summ 2016;65:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Itzcovich T, Xi Z, Martinetto H, et al. Analysis of C9orf72 in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Argentina. Neurobiol Aging 2016;40:192.e113–192.e115. [DOI] [PubMed] [Google Scholar]
- 5.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 2004;27:723–749. [DOI] [PubMed] [Google Scholar]
- 6.Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr 2012;24:1034–1045. [DOI] [PubMed] [Google Scholar]
- 7.Gordon PH, Salachas F, Lacomblez L, et al. Predicting survival of patients with amyotrophic lateral sclerosis at presentation: a 15-year experience. Neurodegenerative Dis 2012;12:81–90. [DOI] [PubMed] [Google Scholar]
- 8.Verde F, Ticozzi N. Amyotrophic Lateral Sclerosis: Epidemiology and Risk Factors: Acquired Neuromuscular Disorders. Berlin: Springer; 2016:219–230. [Google Scholar]
- 9.Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimer Dement 2017;13:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardi L, Frangipane F, Smirne N, et al. Epidemiology and genetics of frontotemporal dementia: a door-to-door survey in southern Italy. Neurobiol Aging 2012;33:2948.e1–2948.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosso SM, Kaat LD, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 2003;126:2016–2022. [DOI] [PubMed] [Google Scholar]
- 12.Borroni B, Alberici A, Grassi M, et al. Is frontotemporal lobar degeneration a rare disorder? Evidence from a preliminary study in Brescia county, Italy. J Alzheimers Dis 2010;19:111–116. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Sayana P, Zhang X, Le W. Genetics of amyotrophic lateral sclerosis: an update. Mol Neurodegener 2013;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippi M, Agosta F, Ferraro PM. Charting frontotemporal dementia: from genes to networks. J Neuroimaging 2016;26:16–27. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 16.Englund B, Brun A, Gustafson L, et al. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994;57:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam M. Primary progressive aphasia. Ann Neurol 2001;49:425–432. [PubMed] [Google Scholar]
- 19.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994;124:96–107. [DOI] [PubMed] [Google Scholar]
- 22.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 23.Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007;21:S14–S18. [DOI] [PubMed] [Google Scholar]
- 24.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari R, Kapogiannis D, Huey E, Momeni P. FTD and ALS: a tale of two diseases. Curr Alzheimer Res 2011;8:273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borenstein M, Hedges LV, Higgins J, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 27.Orr-Urtreger A, Shifrin C, Rozovski U, et al. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology 2007;69:1595–1602. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J, Green S. 9.5.2: Identifying and measuring heterogeneity, version 50. Cochrane Handbook for Systematic Reviews of Interventions. 2008:1. [Google Scholar]
- 29.Kenna KP, McLaughlin RL, Byrne S, et al. Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J Med Genet 2013;50:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 2013;9:617–628. [DOI] [PubMed] [Google Scholar]
- 31.Gallo V, Bueno-De-Mesquita HB, Vermeulen R, et al. Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol 2009;65:378–385. [DOI] [PubMed] [Google Scholar]
- 32.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belzil VV, Bauer PO, Prudencio M, et al. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol 2013;126:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams KL, Fifita JA, Vucic S, et al. Pathophysiological insights into ALS with C9ORF72 expansions. J Neurol Neurosurg Psychiatry 2013;84:931–935. [DOI] [PubMed] [Google Scholar]
- 35.Atanasio A, Decman V, White D, et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 2016;6:23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nationen V. World Population Prospects: The 2010 Revision. New York: UN Population Division, Department of Economic and Social Affairs; 2011. [Google Scholar]
- 37.Majounie E, Renton AE, Mok K, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 2012;11:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney J, McLaughlin R, Vajda A, et al. Novel gender selective survival effect of C9orf72 in European ALS cohorts (P5. 093). Neurology 2016;86:P5.093. [Google Scholar]
- 39.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 2011;72:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki M, Hwi-Cheul L, Kayasuga Y, et al. Roles of progranulin in sexual differentiation of the developing brain and adult neurogenesis. J Reprod Dev 2009;55:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiba S, Suzuki M, Yamanouchi K, Nishihara M. Involvement of granulin in estrogen-induced neurogenesis in the adult rat hippocampus. J Reprod Dev 2007;53:297–307. [DOI] [PubMed] [Google Scholar]
- 42.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916–919. [DOI] [PubMed] [Google Scholar]
- 43.Toh H, Chitramuthu BP, Bennett HP, Bateman A. Structure, function, and mechanism of progranulin: the brain and beyond. J Mol Neurosci 2011;45:538–548. [DOI] [PubMed] [Google Scholar]
- 44.Eriksen JL, Mackenzie IR. Progranulin: normal function and role in neurodegeneration. J Neurochem 2008;104:287–297. [DOI] [PubMed] [Google Scholar]
- 45.Rademakers R, Cruts M, Van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004;24:277–295. [DOI] [PubMed] [Google Scholar]
- 46.Logroscino G, Traynor B, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 2008;79:6–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.