Abstract
Objective
To examine the clinical and economic value of point-of-care CD4 (POC-CD4) or viral load (VL) monitoring compared to current practices in Mozambique, a country representative of the diverse resource limitations encountered by HIV treatment programs in sub-Saharan Africa.
Design/Methods
We use the CEPAC-I model to examine the clinical impact, cost (2014 US$), and incremental cost-effectiveness ratio (ICER, $/year of life saved [YLS]) of ART monitoring strategies in Mozambique. We compare: 1) monitoring for clinical disease progression (CLIN) vs. annual POC-CD4 in rural settings without laboratory services and 2) biannual laboratory CD4 (LAB-CD4), biannual POC-CD4, and annual VL in urban settings with laboratory services. We examine the impact of a range of values in sensitivity analyses, using Mozambique’s 2014 per capita GDP ($620) as a benchmark cost-effectiveness threshold.
Results
In rural settings, annual POC-CD4 compared to CLIN improves life expectancy by 2.8 years, reduces time on failed ART by 0.6 years, and yields an ICER of $480/YLS. In urban settings, biannual POC-CD4 is more expensive and less effective than VL. Compared to biannual LAB-CD4, VL improves life expectancy by 0.6 years, reduces time on failed ART by 1.0 year, and is cost-effective ($440/YLS).
Conclusions
In rural settings, annual POC-CD4 improves clinical outcomes and is cost-effective compared to CLIN. In urban settings, VL has the greatest clinical benefit and is cost-effective compared to biannual POC-CD4 or LAB-CD4. Tailoring ART monitoring strategies to specific settings with different available resources can improve clinical outcomes while remaining economically efficient.
Keywords: HIV, point-of-care, viral load, Africa south of the Sahara, Antiretroviral Therapy, Highly Active/economics, CD4, ART, cost-effectiveness analysis
INTRODUCTION
For the millions worldwide on antiretroviral therapy (ART), the World Health Organization (WHO) recommends monitoring for ART failure [1]. ART failure results from poor ART adherence and/or virologic resistance, which can emerge in the setting of partially effective ART [2]. Three strategies are used to evaluate for ART failure: clinical, immunologic (CD4), or virologic (VL) monitoring. When access to laboratory testing is unavailable or unreliable, clinicians still depend on clinical monitoring alone for disease progression [3]. Immunologic monitoring with CD4 tests is used in settings where laboratory services are available, but virologic testing is not. Virologic monitoring, used in all developed countries, is preferred given its high sensitivity and specificity to diagnose ART failure, but its use has been restricted in resource-limited settings due to lack of available infrastructure, equipment, technical expertise, and cost [1].
POC-CD4 tests are now available and most often deployed in settings with insufficient access to laboratory infrastructure; they are in use in 30 countries throughout sub-Saharan Africa [4]. In Mozambique, such technology is already available in rural settings to determine ART-eligibility for newly diagnosed people living with HIV (PLWH) [5] and has been shown to be cost-effective [6]. Given available POC-CD4 in rural clinics, it is logical to consider extending its use for ART monitoring.
The value of POC-CD4 is less clear in settings with access to laboratory services. POC-CD4 could provide additional benefit in ART monitoring because it expedites clinical decision-making by reducing the turnaround time for test results and the number of lost tests [7]. However, in comparison to VL, which is more accurate and increasingly available, POC-CD4 might not be worth additional investment.
Using a modeling approach, we investigate whether POC-CD4 for ART monitoring could improve clinical outcomes and be economically efficient in rural or urban settings compared to current standards of care in Mozambique and compared to VL in urban settings.
METHODS
Analytic Overview
We use the Cost-Effectiveness of Preventing AIDS Complications–International (CEPAC-I) model to examine the clinical impact, cost, and cost-effectiveness of monitoring for ART failure in PLWH in Mozambique. These monitoring strategies differ in terms of the performance characteristics of the tests used to detect ART failure (i.e., bias and random error), time delay from observed ART failure until clinical decision-making, test frequency, and costs.
We specifically consider the following implementation strategies in two different settings. In the rural setting, we assume no laboratory infrastructure exists, so clinical monitoring (CLIN) is the standard of care; we incrementally compare the addition of annual POC-CD4 to CLIN, assuming a single platform Alere® Pima POC-CD4 technology is in place and available. We next examine an urban setting with established access to centralized laboratory services; here, biannual laboratory CD4 (LAB-CD4) is the standard of care, and annual VL is the proposed goal [8]. We investigate the potential benefits of replacing LAB-CD4 with either biannual POC-CD4 or annual VL. Because monitoring frequency differs by setting, a subscript indicates test frequency (e.g., POC-CD412 denotes monitoring every 12 months with POC-CD4).
We project the clinical (life expectancy [LE], time on failed ART) and economic outcomes (per person lifetime costs [2014 US$]) for these strategies, from which we calculate incremental cost-effectiveness ratios (ICERs, Δ$/ΔLE) with 3% annual discounting. We use the modified societal perspective, including all direct medical costs incurred by different funders but excluding indirect costs, such as lost economic productivity [9]. We consider monitoring strategies to be “cost-effective” if their ICERs are ≤$620/YLS [10], or less than the Mozambique 2014 annual per capita gross domestic product (GDP) [11].
Model Structure
CEPAC-I is a previously published Monte Carlo simulation model of HIV disease and treatment [6,12]. Simulated patients draw from initial distributions of age, sex, CD4, and VL populated from clinical trials and cohort data representative of a Mozambique population initiating ART [13]. In the first month of simulation, a hypothetical cohort of ART-eligible PLWH enters HIV care to initiate ART. Patients can die from acute HIV-associated events, chronic HIV disease, or non-HIV-associated causes.
Clinical care
Simulated PLWH attend clinic and are prescribed ART, which can be effective (i.e., leading to virologic suppression [<50 copies/mL] and rising CD4 counts) or not (i.e., detectable VL and declining CD4 counts). Patients can be lost to follow-up and subsequently return to care (Appendix; Table SDC1).
ART failure and monitoring
The model distinguishes between true and observed ART failure. “True” ART failure occurs when a patient’s VL rises despite being prescribed ART. This modeled biologic truth is only clinically actionable if there is also “observed” ART failure, in which a test and/or documented clinical event detects ART failure.
We define ART monitoring as any strategy used to detect observed ART failure [1]. CLIN detects ART failure if patients develop an opportunistic infection (OI), usually due to CD4 decline. Immunologic monitoring (i.e., LAB-CD4 or POC-CD4) detects true ART failure only after sufficient CD4 decline following virologic rebound. Because CD4 tests are subject to bias and random error, the observed CD4 test result differs from the true in vivo CD4 count (Appendix; Table SDC1) [6]. Immunologic monitoring and CLIN therefore detect both false positives (i.e., observed ART failure without true ART failure) and false negatives (i.e., true ART failure that is not observed). VL provides the earliest and most accurate diagnosis after true ART failure because it directly detects the virus.
Clinical management of ART failure
Upon observed 1st-line ART failure, patients undergo adherence counseling with an opportunity for 1st-line “re-suppression.” If observed failure is detected again, patients switch to 2nd-line ART. To account for real-life differences in test result availability and access to 2nd-line ART, a strategy-specific time delay occurs between the procurement of the test sample diagnosing ART failure and clinical decision-making. Patients with observed failure on 2nd-line ART despite another adherence intervention continue on 2nd-line ART until death without additional monitoring.
Input Parameters
Cohort characteristics and clinical care
Simulated patients have a median CD4 of 166/μL (IQR 78–226/μL) [13], and 79% achieve virologic suppression at 6 months of treatment (Table 1; Table SDC1) [14]. We incorporate loss to follow-up (LTFU) rates from sub-Saharan Africa (Appendix; Table SDC1).
Table 1.
Base case input parameters for an analysis of ART monitoring in Mozambique.
| Parameter | Base Case Value | ||
|---|---|---|---|
| Cohort characteristics [13] | |||
| Mean age, years (SD) | 30 (10) | ||
| Median CD4,/μL (IQR) | 166 (78–226) | ||
| Female, % | 69 | ||
|
| |||
| ART efficacy | |||
| Initial suppression, % [14] | 79 | ||
| Re-suppression after adherence intervention, % [38] | 54 | ||
|
| |||
| Annual costs (2014 US$) [16] | |||
| Clinical care | |||
| CD4 >200/μL | 36 | ||
| CD4 ≤200/μL | 53 | ||
| ART regimen costs | |||
| 1st-line (tenofovir/lamivudine/efavirenz) | 148 | ||
| 2nd-line (zidovudine/lamivudine/ritonavir/lopinavir) | 389 | ||
| Co-trimoxazole prophylaxis | 28 | ||
|
| |||
| ART monitoring strategies | |||
| Criteria for observed ART failure [8] | |||
| All strategies | WHO stage III or IV opportunistic infections* | ||
| Strategy-specific | LAB-CD4 | POC-CD4 | VL |
|
|
|||
| 50% decrease in CD4 CD4 < pre-ART nadir CD4 CD4 <100/μL |
VL >3,000 copies/mL | ||
| Characteristics of diagnostic tests** | |||
| Bias, % | 0 | − 4.1% | 0 |
| Random error, % | 15.8% | 19.1% | 0 |
| Test costs (2014 US$) [17, 18] | 11 | 13 | 20 |
| Time delay to clinical decision-making, months† | |||
| Adherence intervention | 2 | 0 | 2 |
| Switch to 2nd-line ART | 14 | 11 | 14 |
SD, standard deviation; IQR, interquartile range; ART, antiretroviral therapy; WHO, World Health Organization; LAB-CD4, laboratory CD4 ART monitoring strategy; POC-CD4, point-of-care CD4 ART monitoring strategy; VL, HIV RNA ART monitoring strategy.
When opportunistic infections occur in patients monitored with POC-CD412, LAB-CD46, or POC-CD46, ART failure is confirmed with a CD4 test; when opportunistic infections occur in patients monitored with VL12, ART failure is confirmed with an HIV RNA test.
Adapted from Scott et al [15]; details in Appendix; Table SDC2.
Adapted from Keiser et al [20].
ART failure and monitoring
Definition of observed ART failure
Observed ART failure is not diagnosed during the first year of ART [8]. CLIN detects observed ART failure in patients who experience a WHO stage III or IV OI. Immunologic and virologic monitoring detect observed ART failure according to Mozambique national guidelines; if OIs occur, patients are then tested by the strategy-specific tests to confirm ART failure (Table 1) [8].
Test characteristics and confirmatory tests
We derive bias and random error for both types of CD4 test from the published literature (Table SDC2) [15]. We consider VL to have no bias or random error (Table 1). If the first strategy-specific test meets criteria for observed ART failure, a second confirmatory test is performed the following month (CD4) or three months later (VL); observed ART failure is diagnosed only when both test results meet ART failure criteria [8].
Costs
CLIN adds no additional costs because the costs of detecting and treating OIs are included in HIV clinical care [16]. LAB-CD4, POC-CD4, and HIV RNA tests cost US$11, US$13, and US$20/test, respectively; we incorporate start-up costs for laboratory infrastructure and personnel training for HIV RNA tests as these are new technologies in Mozambique (Appendix; Table SDC3) [17,18]. All costs are from 2014 (Appendix).
Clinical management of ART failure
Adherence intervention
When CLIN detects ART failure, patients immediately receive an adherence intervention. In the other strategies, a confirmatory test is needed to finalize the ART failure diagnosis. When POC-CD4 is used, adherence counseling occurs when the confirmatory test is performed; there is a delay with LAB-CD4 or VL due to transport, processing time, and the potential for lost samples/results (Table 1).
Switch to 2nd line ART
The time delay before switching ART represents the time to receive test results, achieve centralized committee approval, and transport 2nd-line ART to the clinic for dispensing [19]. Estimates from sub-Saharan Africa range from 5–20 months [20]. Because laboratory-based strategies require additional time for specimen transport, we included a three month longer time delay for LAB-CD4 and VL (14 months) than for CLIN and POC-CD4 (11 months).
Performance characteristics of ART monitoring strategies
We use model output to quantify the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each ART monitoring strategy to detect ART failure. For instance, the sensitivity of each ART monitoring strategy is the number of patients correctly detected with observed ART failure (i.e., true positives) among all patients with true ART failure (Fig. SDC1).
Sensitivity Analyses
We examine the impact of plausible ranges of key parameters in one- and multi-way deterministic sensitivity analyses. POC-CD4 test costs are sensitive to the numbers of tests performed per machine. We evaluate the impact of POC-CD4 test costs ranging from $9.72/test (20 tests/day) to $210/test (16 tests/year); these machine volumes are consistent with current use in Mozambique. Longer time delays can result from downtime of POC-CD4 machines, stockouts of consumable, or when the number of daily tests exceeds the machine’s capacity (i.e., >20 tests/daily). We perform two-way sensitivity analysis on POC-CD4 test cost and time delay to investigate the impact of POC-CD4 capacity (i.e., decreased per test costs due to more tests/day and increased time delay when the number of daily tests exceeds the machine’s capacity). To examine the impact of improved transport to laboratory diagnostic hubs, we perform two-way sensitivity analysis on decreased time delay and increased cost for LAB-CD4 and VL. We also perform probabilistic sensitivity analysis to investigate the impact of uncertainty surrounding data estimates of the five monitoring-specific input parameters (Table SDC4).
Budget Impact Analysis
To investigate the affordability of ART monitoring strategies in Mozambique to its government and donors, we examine the costs associated with implementing POC-CD412 in a rural setting and POC-CD46 or VL12 in an urban setting. In Mozambique, 668,100 people are diagnosed with HIV and on ART in 2015, of whom 1% are on 2nd-line ART; we assume 20% live in a rural setting without access to laboratory services, and 80% live in a setting with laboratory services. We anticipate that 980,000 patients will initiate ART by 2025 to achieve 80% coverage as per PEPFAR projections (Table SDC5) [21]. We include strategy-specific monitoring costs, as well as ART and routine care costs associated with guideline-concordant care in Mozambique [8]. We examine undiscounted costs over a 10-year time horizon.
RESULTS
Rural Setting
Base case
The sensitivity to detect ART failure is 1.4% with CLIN, increasing to 34.6% with POC-CD412 (Table 2, top left). The PPV and NPV of CLIN are 70.7% and 69.5%, increasing to 93.8% and 83.5% for POC-CD412 (Fig. SDC1).
Table 2.
Base case results for an analysis of ART monitoring in Mozambique.
| Performance Characteristics | Projected Clinical and Economic Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Years on Failed ART | Undiscounted Life Years | Discounted Life Years* | Discounted Costs* (2014 US$) | ICER ($/YLS) | |
| Rural Setting | |||||||||
| CLIN | 1.4 | 99.7 | 70.7 | 69.5 | 4.4 | 17.1 | 11.5 | 2,360 | - |
| POC-CD412 | 34.6 | 99.3 | 93.8 | 83.5 | 3.8 | 19.9 | 12.8 | 3,000 | 490 |
|
| |||||||||
| Urban Setting | |||||||||
| LAB-CD46 | 23.7 | 99.5 | 92.2 | 83.9 | 3.9 | 19.8 | 12.7 | 3,120 | - |
| VL12 | 89.0 | 100.0 | 100.0 | 98.4 | 2.9 | 20.4 | 13.0 | 3,250 | 440 |
| POC-CD46 | 24.9 | 99.0 | 85.8 | 84.3 | 4.1 | 19.8 | 12.8 | 3,380 | DOMINATED |
CLIN, clinical ART monitoring strategy; POC-CD4, point-of-care CD4 ART monitoring strategy; LAB-CD4, laboratory CD4 ART monitoring strategy; VL, HIV RNA ART monitoring strategy; ICER, incremental cost-effectiveness ratio; YLS, year of life saved; DOMINATED indicates that a strategy is more expensive and confers less clinical benefit than an alternative strategy. Subscripts denote number of months between tests in a given strategy.
Discounted at 3%
For PLWH initiating ART, the undiscounted (discounted) life expectancy monitored with CLIN is 17.1 (11.5) years, which increases to 19.9 (12.8) years with POC-CD412 (Table 2, top right). Discounted lifetime costs are US$2,360 for CLIN and increase to US$3,000 with POC-CD412, resulting in a cost-effective ICER, US$480/YLS.
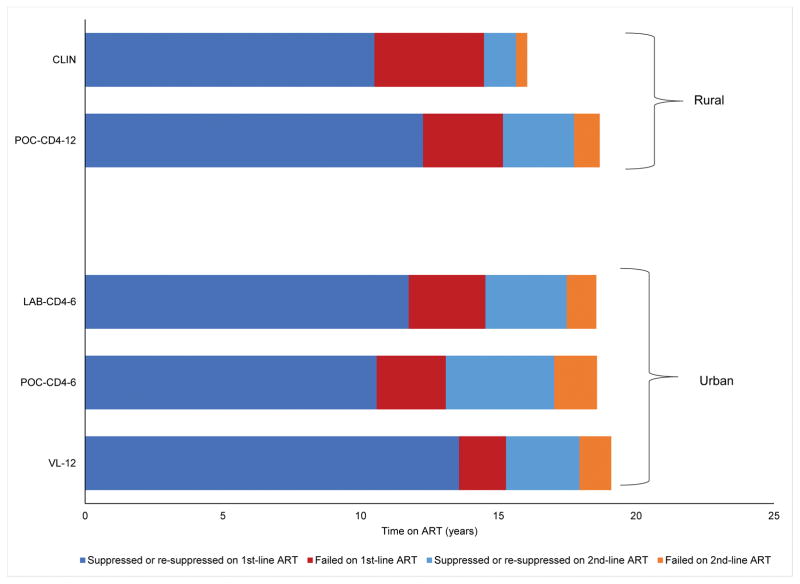
In CLIN, PLWH spend 10.5 years suppressed on 1st-line ART, which increases to 12.3 years with POC-CD412 (Fig. 1, top). PLWH spend 4.0 years taking failed 1st-line ART in CLIN, which is reduced by 1.1 years with POC-CD412. More patients (29.4%) are transitioned to 2nd-line ART using POC-CD412 monitoring than with CLIN (12.0%).
Fig. 1. Mean time spent on suppressed and failed ART.
Mean per person years spent suppressed (dark blue) and failed (red) on 1st-line ART, and suppressed (light blue) and failed (orange) 2nd-line ART for the rural setting (CLIN and POC-CD412, top) and urban setting (LAB-CD46, POC-CD46, and VL12, bottom). We include the time initially suppressed and failed, as well as time re-suppressed and failed after an adherence intervention. Strategies do not sum to total life expectancy since time spent lost to follow-up is not included. The subscript indicates test frequency (e.g., POC-CD412 denotes monitoring every 12 months with POC-CD4). CLIN, clinical ART monitoring strategy; POC-CD4, point-of-care CD4 ART monitoring strategy; LAB, laboratory CD4 ART monitoring strategy; VL, HIV RNA ART monitoring strategy; ART, antiretroviral therapy.
One-way sensitivity analyses
POC-CD412 remains clinically preferred but no longer cost-effective if test bias is <−30% (>6× base case) or random error is >28% (>1.4× base case). When operating at capacity (i.e., 20 tests/day at $9.72/test), POC-CD412 is cost-effective; only when POC-CD4 costs exceed $27/test (i.e., 20 tests/month) is POC-CD412 no longer cost-effective. When time delays are prolonged prior to adherence intervention and/or ART switch to 2nd-line, the cost-effectiveness of POC-CD412 compared to CLIN is minimally affected (ICERs, $470–480/YLS). Without a confirmatory CD4 test, the ICER of POC-CD412 rises compared to CLIN ($860/YLS). With more frequent testing, POC-CD4 is less economically efficient (e.g., ICER, $750/YLS with POC-CD46).
Multi-way and probabilistic sensitivity analysis
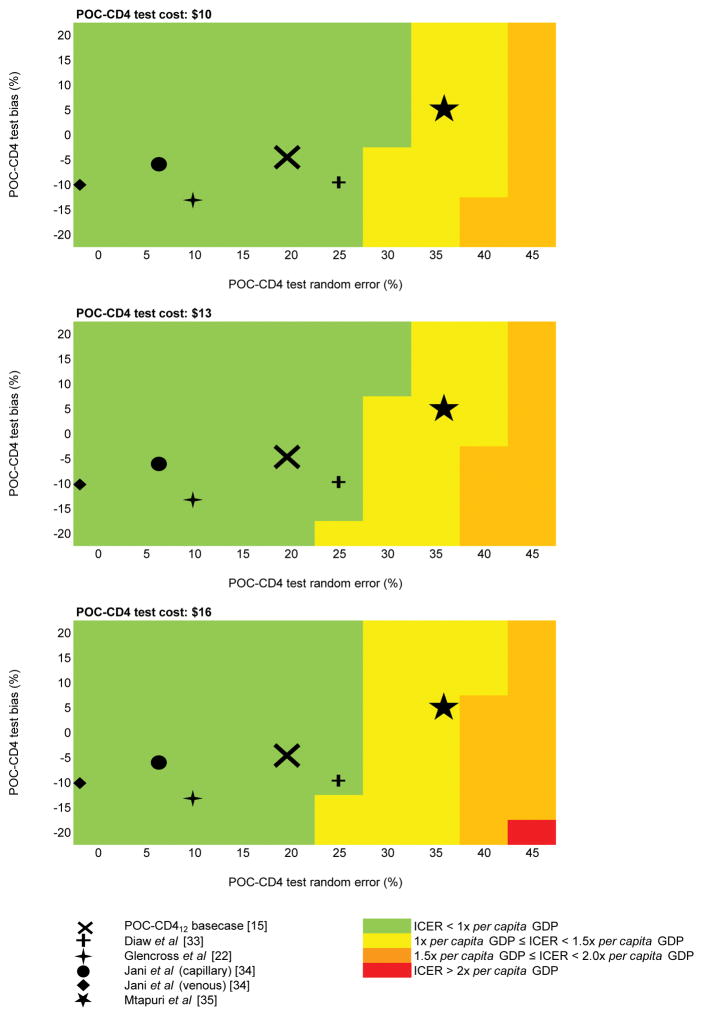
When we simultaneously vary POC-CD412 bias, random error, and cost, POC-CD412 remains cost-effective compared to CLIN for the POC-CD4 random error and bias reported in all but one published study (Fig. 2). In sensitivity analysis regarding POC-CD4 capacity, POC-CD412 is most cost-effective when used at maximum capacity (ICER, $440/YLS) but remains cost-effective even at modest capacity (i.e., 180 tests/year). When test volumes overwhelm machine capacity, POC-CD412 remains cost-effective compared to CLIN but with reduced clinical benefit. In PSA, POC-CD412 is cost-effective in 86.1% of simulations at a willingness to pay threshold (WTP) of $620/YLS (Fig. SDC2A).
Fig. 2. Heat maps of the ICER of POC-CD412 relative to CLIN.
Heat maps of multi-way sensitivity analysis in the rural setting display the ICER of POC-CD412 relative to CLIN. Three panels are displayed, each showing results using different costs for POC-CD4 tests. On each panel, POC-CD412 random error increases left to right along the horizontal axes, and POC-CD412 bias becomes more negative down the vertical axes. The POC-CD412 base case value (from Scott et al [15], a POC-CD4 meta-analysis) is marked with an X. Other published estimates of POC-CD4 test bias and random error are marked with a cross (Diaw et al [35]), a four-pointed star (Glencross et al [22]), a circle (Jani et al, capillary [36]), a diamond (Jani et al, venous [36]), and a five-pointed star (Mtapuri et al [37]). LAB, laboratory; POC, point-of-care; ICER, incremental cost-effectiveness ratio; GDP, per capita gross domestic product.
Urban setting
Base case
In the urban setting, the sensitivity to detect ART failure is: 23.7% (LAB-CD46), 24.9% (POC-CD46), and 89.0% (VL12) (Table 2, bottom left). The PPV of each ART monitoring strategy is: 92.2% (LAB-CD46), 85.8% (POC-CD46), and 100.0% (VL12). The NPV is: 83.9% (LAB-CD46), 84.3% (POC-CD46), and 98.4% (VL12).
We project a life expectancy of 19.8 years for PLWH monitored with LAB-CD46 or POC-CD46, which increases to 20.4 years with VL12 (Table 2, bottom right). Discounted life expectancies for LAB-CD46, POC-CD46, and VL12 are 12.7, 12.8, and 13.0 years. Discounted per person lifetime costs increase from US$3,120 (LAB-CD46) to US$3,250 (VL12) to US$3,380 (POC-CD46). POC-CD46 confers fewer life years and higher costs compared to VL12; VL12 is cost-effective compared to LAB-CD46 (ICER, US$440/YLS).
In LAB-CD46, PLWH spend 11.7 years suppressed and 2.8 years failing on 1st-line ART, which decreases to 10.6 years and 2.5 years with POC-CD46, respectively (Fig. 1, bottom). PLWH monitored with VL12 spend 13.6 years suppressed on 1st-line ART and only 1.7 years on failed 1st-line ART. Initiation of 2nd-line ART varies from 32.1% (LAB-CD46) to 41.2% (POC-CD46) and is lowest when VL12 is used for ART monitoring (30.9%).
One-way sensitivity analyses
POC-CD46 remains dominated (i.e., less effective, higher costs) by VL12 across a wide range of parameters (Appendix). When LAB-CD4 test random error is reduced or when monitoring is less frequent, clinical outcomes improve in LAB-CD4 so that VL12 is less cost-effective in comparison (ICERs, US$500–960/YLS). When more patients suppress with ART or re-suppress after adherence interventions, VL12 monitoring provides fewer clinical benefits in comparison to LAB-CD412. Among populations with higher CD4 counts at ART-initiation (≥350/μL), the clinical benefits of VL12 are greater and the ICER is lower ($370/YLS). VL12 is no longer cost-effective when VL costs >$24/test.
Multi-way and probabilistic sensitivity analysis
We simultaneously vary the time delay for VL12 and the probability of re-suppression for all ART monitoring strategies; we then compare VL12 to LAB-CD46 because POC-CD46 is dominated. Increased VL12 time delays reduces its clinical benefit (i.e., more months spent on failing ART); clinical benefits of VL12 in comparison to LAB-CD46 wane as re-suppression efficacy rises in both strategies (Fig. SDC3). Reducing transport time for laboratory-based strategies could improve clinical outcomes and be cost-effective; POC-CD46 is only preferred when operating at capacity (i.e., $9.72/test) and laboratory-based strategies are twice their current test costs (Table SDC7). In PSA, VL12 is the preferred strategy 68.9% of the time at the WTP threshold of $620/YLS (Fig. SDC2b).
Budget Impact Analysis
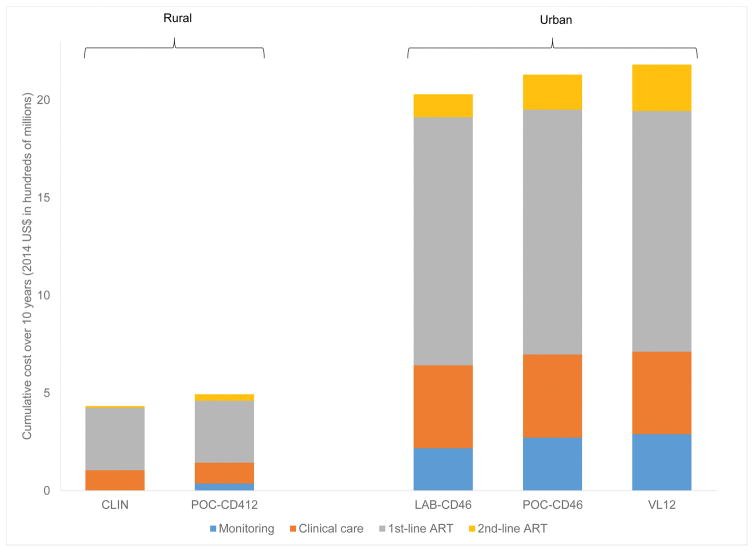
Using model output, we project costs of $153.4 million/year for guideline-concordant HIV care in 2015; PEPFAR estimates costs of $159.7 million/year, comprising approximately 90% from international donors and ~10% from the Mozambique Ministry of Health [21]. We estimate costs of guideline-concordant HIV care with CLIN are $433.7 million over 10 years; POC-CD412 would cost $60.1 million more (13.8% of CLIN budget). In settings with laboratory services, we project that guideline-concordant care with LAB-CD46 will cost $2.0 billion over 10 years; VL12 would cost $151.6 million more (7.5% of LAB-CD46 budget). In both settings, improving ART monitoring decreases 1st-line ART costs but increases 2nd-line ART costs and adds monitoring costs (Fig. 3).
Fig. 3. Budget impact analysis over 10 years for rural and urban settings.
Budget impact analysis over a 10-year time horizon for the rural (CLIN and POC-CD412) and urban (LAB-CD46, POC-CD46, and VL12) settings. Cumulative costs (2014 US$, millions) are on the vertical axis and include: clinical care (gray), 1st-line ART (gold), 2nd-line ART (blue), and monitoring costs (orange). Projected life expectancy for each strategy are shown in life years above each column. US$, US dollars; CLIN, clinical; POC, point-of-care; LAB, laboratory; VL, viral load; ART, antiretroviral therapy.
DISCUSSION
Using POC-CD4 to monitor for ART failure could improve clinical outcomes and be cost-effective in settings without access to laboratory services. Where laboratory services are already available, however, VL offers the greatest clinical benefits and is cost-effective compared to LAB-CD4, given recently reduced costs for HIV RNA tests in Mozambique.
In rural settings without laboratory services, POC-CD4 monitoring improves outcomes at good value. Over 10 years, POC-CD412 would cost an additional 14.9% of the CLIN budget, in return for adding 2.8 years of life (16.6% of CLIN life expectancy). With POC-CD4, fewer PLWH are inappropriately moved to 2nd-line ART when they are not failing 1st-line ART, and fewer PLWH truly failing 1st-line ART are maintained on it. POC-CD412 remains economically efficient even with less favorable operating characteristics or higher test costs, which can occur when point-of-care technology is used by less well-trained staff [22] or less frequently [23]. More frequent monitoring offers minimal additional clinical benefit; its lower PPV results in more patients incorrectly diagnosed with ART failure and unnecessarily started on more expensive 2nd-line ART.
In settings with existing laboratory services, POC-CD4 for ART monitoring is not beneficial, especially when opportunities exist for further investment in viral load. When compared to LAB-CD4, the more expensive POC-CD4 results in more false positive results and more unnecessary switches to costly 2nd-line ART. Although POC-CD4 allows clinicians to receive more rapid test results, the impact of expedited clinical decision-making regarding ART failure has less clinical benefit than might be anticipated. In contrast to newly diagnosed PLWH who frequently do not link to care if they do not learn the results of their CD4 test [13,24], PLWH in care and on ART can be retested at the next clinical visit if laboratory-based test results have been lost, unless they become lost to follow-up.
ART monitoring provides value only if it improves clinical care. Our findings support other modeling studies, underscoring that investments in ART monitoring strategies can offer good value in very resource-limited settings, if opportunities are available to implement adherence interventions or 2nd-line ART. With severely constrained budgets, however, expanding access to ART is a more efficient use of funds [16,25]. If ART suppression or re-suppression rates are high (i.e., ART failure is less common), the value of more accurate but more costly monitoring strategies, such as VL, is reduced [26]. In Mozambique, 19–24% of patients on 1st-line ART have evidence of virologic failure [27,28], similar to other settings in sub-Saharan Africa [29,30]; while scale-up of ART coverage continues, investment in longitudinal care is essential to maintain virologic suppression, gain long-term benefits of ART, and reduce transmissions and deaths.
Findings from our budget impact analysis highlight the cost tradeoffs with different ART monitoring strategies. At 10 years, we project VL12 would add $72.5 million in monitoring costs but would save $37.9 million in costs of 1st-line ART prescribed to patients failing it. The $151.7 million total increase in costs is largely due to an increase of $119.8 million in 2nd-line ART costs for patients failing 1st-line ART despite an adherence intervention. If 2nd-line ART costs decline, VL will become more affordable. With improved ART monitoring and access to suppressive 2nd-line ART, these patients would no longer be left on failing 1st-line ART, which can contribute to the development of increased viral resistance and more HIV transmissions [2].
Our analysis includes several assumptions and limitations. We assume that the VL strategy includes no CD4 monitoring tests [31], which would increase costs and reduce its cost-effectiveness. While our analysis does not formally include HIV transmissions or the development of resistance, we assess time on failed ART as a proxy for these outcomes. Increased time on failed ART, as occurs with CLIN or CD4 compared to VL, will result in more transmissions, more virologic resistance, and further increases the value of VL compared to other strategies. We do not include additional start-up costs of POC-CD4 in urban settings where they are not currently in use; however, sensitivity analyses on POC-CD4 costs demonstrate the impact of a more costly POC-CD4 test. If POC-CD4 technology has greater throughput/capacity or is combined with additional tests relevant to HIV, then additional benefits or efficiencies could exist that our analysis would not capture. We did not include point-of-care VL in our analysis because it is not yet commercially available and its test characteristics and costs are not clearly described. Finally, we used the Mozambique per capita GDP as a benchmark for cost-effectiveness and as a familiar reference point. Concerns have been raised about the use of “demand-side” thresholds [32], although this benchmark is supported by theory and frequently cited [33]. Additionally, “supply-side” cost-effectiveness thresholds derived from current health spending profiles are not readily available in resource-limited settings. Criteria for resource allocation decisions are further complicated in settings like Mozambique, where international donors finance more than 90% of the HIV program [34].
National ART programs provide services in a diversity of settings, some with better access to laboratory infrastructure than others [5]. In rural communities which already have access to POC-CD4, our results support using POC-CD4 for ART monitoring with ongoing attention towards further scale-up of laboratory services, including VL. In settings where laboratory services are already available, POC-CD4 does not offer clinical or economic benefits compared to LAB-CD4 for ART monitoring. VL will improve clinical outcomes and be cost-effective and is worth further investment.
Supplementary Material
Acknowledgments
Conceptualization: EPH, IVJ, RAP
Data curation: EPH, IVJ, KLR, RW, BO, RAP, PM
Formal analysis: EPH, KLR, BO, SR, PPP, RAP, RPW
Funding acquisition: EPH, KAF, RPW
Investigation: EPH, IVJ, KLR, RW, BO, TP
Methodology: EPH, SR, PPP, KAF, RAP, RPW
Project administration: EPH, KLR, BO, RPW
Resources: PPP, KAF, RAP, RPW
Software: KLR, BO, PPP, RAP
Supervision: EPH, PPP, RAP, RPW
Validation: EPH, KLR, BO, SR, PPP, RAP
Visualization: EPH, BO, RPW
Writing original draft: EPHWriting reviewing & editing: EPH, IVJ, KLR, RW, BO, SR, PPP, PM, KAF, TP, RAP, RPW
Funding: This work was supported by the National Institutes of Health [R01AI058736; R37AI093269; K01HL123349, P30AI069354], UNITAID [2017-01-UCPOC2b], and by the Steve and Deborah Gorlin MGH Research Scholars Award (RPW). The content is solely the responsibility of the authors, and the study’s findings and conclusions do not necessarily represent the official views of the NIH.
Financial Disclosures: Dr. Hyle is a co-author at UpToDate.com.
Footnotes
Competing Interests: None.
Declaration of Interests: None
References
- 1.World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. 2016 [6/15/2017]. Available from: http://www.who.int/hiv/pub/guidelines/keypopulations-2016/en/ [PubMed]
- 2.TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016 Jan 28; doi: 10.1016/S1473-3099(15)00536-8. Epub 2016/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Mbougua JB, Boyer S, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis. 2011 Nov;11(11):825–33. doi: 10.1016/S1473-3099(11)70168-2. Epub 2011/08/13. [DOI] [PubMed] [Google Scholar]
- 4.Alere. Studies & Implementation. 2017 [6/14/2017]. Available from: http://www.alerehiv.com/ww/home/studies-and-implementation.html.
- 5.UNAIDS. Resposta global à SIDA relatório do progresso, 2016 Moçambique United Nations. 2016 [cited 6/15/2017]. Available from: http://www.unaids.org/sites/default/files/country/documents/MOZ_narrative_report_2016.pdf.
- 6.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J, et al. The clinical and economic impact of point-of-care CD4 testing in Mozambique and other resource-limited settings: a cost-effectiveness analysis. PLoS Med. 2014 Sep;11(9):e1001725. doi: 10.1371/journal.pmed.1001725. Epub 2014/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014 Mar;14(3):239–49. doi: 10.1016/S1473-3099(13)70250-0. Epub 2013/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozambique Ministry of Health. Guia de tratamento antiretroviral e infecções oportunistas no adulto, criança, adolescente e grávida. 2014 [6/14/2017]. Available from: https://aidsfree.usaid.gov/sites/default/files/4.25.16_mozambique_art_2014_rktagged.pdf.
- 9.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016 Sep 13;316(10):1093–103. doi: 10.1001/jama.2016.12195. Epub 2016/09/14. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. Macroeconomics and health: Investing in health for economic development. [cited 2014 June 10]. Available from: Available: http://whqlibdoc.who.int/publications/2001/924154550x.pdf. [Google Scholar]
- 11.The World Bank. 2017 [6/14/2017]. Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=MZ.
- 12.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013 Oct 31;369(18):1715–25. doi: 10.1056/NEJMsa1214720. Epub 2013/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: An observational cohort study. Lancet. 2011 Oct 29;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 14.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, Yapo V, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Côte d’Ivoire. J Acquir Immune Defic Syndr. 2011 Apr;56(4):356–64. doi: 10.1097/QAI.0b013e3182084b5a. Epub 2010/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott LE, Campbell J, Westerman L, Kestens L, Vojnov L, Kohastsu L, et al. A meta-analysis of the performance of the Pima CD4 for point of care testing. BMC Med. 2015;13:168. doi: 10.1186/s12916-015-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korenromp EL, Gobet B, Fazito E, Lara J, Bollinger L, Stover J. Impact and cost of the HIV/AIDS national strategic plan for Mozambique, 2015–2019--projections with the Spectrum/Goals model. PLoS One. 2015;10(11):e0142908. doi: 10.1371/journal.pone.0142908. Epub 2015/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche Molecular Diagnostics. Global Access Program. 2016 [6/15/2017]. Available from: https://molecular.roche.com/about/global-access-program/
- 18.Jani I, Sitoe N, Lehe J, Peter T, editors. Cost comparison of point-of-care and laboratory CD4 testing in resource-limited settings [abstract]. 6th International AIDS Society Conference on HIV Pathogenesis and Treatment; 2011; Rome, Italy. [Google Scholar]
- 19.Moon TD, Burlison JR, Sidat M, Pires P, Silva W, Solis M, et al. Lessons learned while implementing an HIV/AIDS care and treatment program in rural Mozambique. Retrovirology (Auckl) 2010 Apr 23;3:1–14. doi: 10.4137/RRT.S4613. Epub 2010/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, Brinkhof MW, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009 Sep 10;23(14):1867–74. doi: 10.1097/QAD.0b013e32832e05b2. Epub 2009/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozambique Country Operational Plan (COP) 2016 Strategic Direction summary. Washington, D.C: PEPFAR; 2016. [6/14/2017]. Available from: https://www.pepfar.gov/documents/organization/257637.pdf. [Google Scholar]
- 22.Glencross DK, Coetzee LM, Faal M, Masango M, Stevens WS, Venter WF, et al. Performance evaluation of the Pima point-of-care CD4 analyser using capillary blood sampling in field tests in South Africa. J Int AIDS Soc. 2012;15(1):3. doi: 10.1186/1758-2652-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson B, Schnippel K, Ndibongo B, Long L, Fox MP, Rosen S. How to estimate the cost of point-of-care CD4 testing in program settings: An example using the Alere Pima Analyzer in South Africa. PLoS One. 2012;7(4):e35444. doi: 10.1371/journal.pone.0035444. Epub 2012/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Initiating Antiretroviral Therapy for HIV at a Patient’s First Clinic Visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016 May;13(5):e1002015. doi: 10.1371/journal.pmed.1002015. Epub 2016/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keebler D, Revill P, Braithwaite S, Phillips A, Blaser N, Borquez A, et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Health. 2014 Jan;2(1):e35–43. doi: 10.1016/S2214-109X(13)70048-2. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528(7580):S68–S76. doi: 10.1038/nature16046. 12/03/print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruperez M, Pou C, Maculuve S, Cedeno S, Luis L, Rodriguez J, et al. Determinants of virological failure and antiretroviral drug resistance in Mozambique. J Antimicrob Chemother. 2015 Sep;70(9):2639–47. doi: 10.1093/jac/dkv143. Epub 2015/06/19. [DOI] [PubMed] [Google Scholar]
- 28.Tilghman M, Tsai D, Buene TP, Tomas M, Amade S, Gehlbach D, et al. Pooled nucleic acid testing to detect antiretroviral treatment failure in HIV-infected patients in Mozambique. J Acquir Immune Defic Syndr. 2015 Nov 01;70(3):256–61. doi: 10.1097/QAI.0000000000000724. Epub 2015/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolling DI, Goodall RL, Chirara M, Hakim J, Nkurunziza P, Munderi P, et al. The virological durability of first-line ART among HIV-positive adult patients in resource limited settings without virological monitoring: a retrospective analysis of DART trial data. BMC Infect Dis. 2017 Feb 21;17(1):160. doi: 10.1186/s12879-017-2266-3. Epub 2017/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castelnuovo B, Kiragga A, Musaazi J, Sempa J, Mubiru F, Wanyama J, et al. Outcomes in a cohort of patients started on antiretroviral treatment and followed up for a decade in an urban clinic in Uganda. PLoS One. 2015;10(12):e0142722. doi: 10.1371/journal.pone.0142722. Epub 2015/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford N, Stinson K, Gale H, Mills EJ, Stevens W, Perez Gonzalez M, et al. CD4 changes among virologically suppressed patients on antiretroviral therapy: a systematic review and meta-analysis. J Int AIDS Soc. 2015;18:20061. doi: 10.7448/IAS.18.1.20061. Epub 2015/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. 2015 Mar; doi: 10.1016/j.jval.2016.02.017. CHE Research Paper 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 2016 Jul 24; doi: 10.1093/heapol/czw096. Epub 2016/07/28. [DOI] [PubMed] [Google Scholar]
- 34.Resch S, Ryckman T, Hecht R. Funding AIDS programmes in the era of shared responsibility: an analysis of domestic spending in 12 low-income and middle-income countries. Lancet Glob Health. 2015 Jan;3(1):e52–61. doi: 10.1016/S2214-109X(14)70342-0. Epub 2014/12/30. [DOI] [PubMed] [Google Scholar]
- 35.Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M, et al. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr. 2011 Dec 1;58(4):e103–11. doi: 10.1097/QAI.0b013e318235b378. [DOI] [PubMed] [Google Scholar]
- 36.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, et al. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011 Mar 27;25(6):807–12. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 37.Mtapuri-Zinyowera S, Chideme M, Mangwanya D, Mugurungi O, Gudukeya S, Hatzold K, et al. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr. 2010 Sep;55(1):1–7. doi: 10.1097/QAI.0b013e3181e93071. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]
- 38.Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144. doi: 10.1371/journal.pone.0116144. Epub 2015/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.