Abstract
Herein, we report a 47-year-old woman with ischemic cardiomyopathy who underwent ablation therapy due to an electrical storm without any triggers. The voltage mapping in sinus rhythm with the Rhythmia system and Orion catheter displayed several LAVAs in and around the anteroapical scar area. Although the patient did not tolerate the induced clinical ventricular tachycardia, which was reproductively induced, 35-second-mapping in the scar zone with the Orion catheter demonstrated the VT circuit with the critical isthmus. This report shows the possibility of the new ultra-high density mapping system in a specific ischemic VT patient.
Keywords: Ablation, Ventricular tachycardia, Ischemic cardiomyopathy, High-resolution mapping, Multi electrodes
1. Case report
Twelve years prior to presentation, a now 47-year-old woman suffered a large left ventricular anterior myocardial infarction. Two years previously she developed recurrent ventricular tachycardia (VT) requiring external defibrillation and received an implantable cardioverter defibrillator (ICD) for secondary prevention. Despite medical therapy, the patient endured several and repeated appropriate ICD shocks without other identifiable causes. The incidence of tachycardia and ICD shocks escalated, and the patients was brought to the electrophysiology laboratory for emergent catheter ablation.
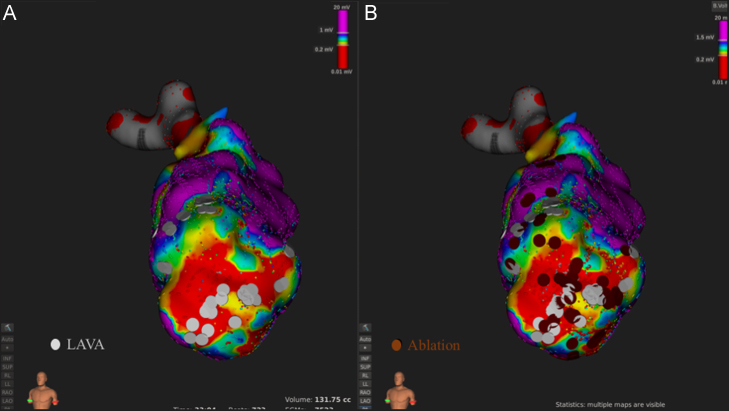
Mapping points (n = 9790) were obtained from the Orion basket catheter (64 electrodes of 0.4 mm2 area; 2.5 mm spacing) using continuous (automated) acquisition over 33 min in sinus rhythm. Cardiac beats were automatically accepted by the mapping system based on (i) 12-lead ECG morphology (>75% similarity to the reference ECG morphology), (ii) respiration gating (expiration phase), (iii) catheter motion stability (<2 mm catheter movement during each acquisition time), and (iv) catheter tracking quality (<3, nominal value). A prior surface 12-lead electrocardiogram of the clinical tachycardia suggested that the exit site of VT might be the anteroapical region of the left ventricle. This region was closely studied and local abnormal ventricular activities (LAVAs) were identified at the margins and within myocardial scar delineated by bipolar voltage mapping (Fig. 1A).
Fig. 1.
LAVAs were identified inside and around the anterior scar (A). Complete LAVA elimination was achieved with 32 RF applications in 33 minutes. (B).
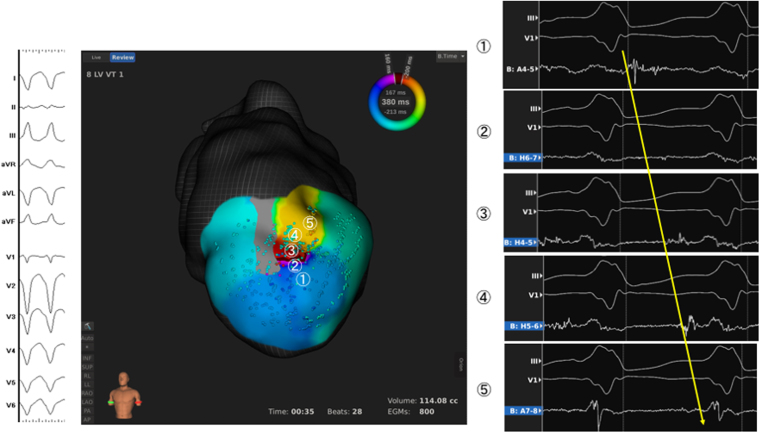
After creating the substrate map, the Orion catheter was placed on the sites where several LAVAs were identified. Although we reproducibly induced the clinical VT (CL 380 ms) with programmed ventricular pacing with extra-stimuli, the patient could not tolerate the tachycardia and showed a marked drop of her arterial blood pressure. However, within 35 s of tachycardia, the Rhythmia mapping system acquired >800 spatial points, delineating the circuit of the clinical VT: a circuit of figure of eight with the critical isthmus co-localizing with abnormal potentials mapped during sinus rhythm. During induced VT, identical to the clinical VT, the tachycardia isthmus was activated from the inferior to superior anterior wall of the scar, with mid-diastolic potentials sequentially mapped (Fig. 2).
Fig. 2.
Entire activation potentials in the critical isthmus were collected in 35 seconds; entrance (①), middle of the channel (②-④), and the exit (⑤).
Following mapping, the ablation was targeted at the area of diastolic potentials during VT under sinus rhythm. Afterward, the clinical VT could not be induced with the same pacing protocol as that before the RF energy applications, while before applications VT was readily inducible. However, additional applications were performed inside and at the border zone of the scar to extinguish residual LAVAs. Following the ablation, no VT was induced with programmed ventricular pacing with three extra-stimuli and burst pacing.
Entrainment and activation mapping of reentrant tachycardia incidences are classical and elegant methodologies to clarify the exact path and isthmus of the electrical circuit [1], [2], [3], [4]. However, due to tachycardia and hemodynamic instability, this is not always possible. With the high spatial resolution and spatial sampling of the Rhythmia mapping system, it is feasible to rapidly map the tachycardia circuit when time is limited. In addition, the smaller-spacing multipolar catheter may better obtain the tiny near-field potentials more distinctly in the scar area, reducing the far-field effect, than conventional catheters [5]. Since ablating single clinical VT may not be the best approach and substrate mapping and ablation [6] are recommended to eliminate the potential circuits of the other multiple VTs, we eliminated all residual LAVAs after ablating the critical site (Fig. 1B). Indeed, the same results would have been achieved with a pure substrate based ablation approach. However, this approach with the high spatial resolution mapping system may increase clarity of the VT mechanism.
Conflict of interest
Masateru Takigawa is a temporary advisor of the Rhythmia system for Boston scientific Japan.
Disclosure
Stefano Capellino is an employee of Boston Scientific.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2017.06.002.
Appendix A. Supplementary material
Video S1: Activation map during the ventricular tachycardia.
.
References
- 1.Waldo A.L. Atrial flutter: entrainment characteristics. J Cardiovasc Electrophysiol. 1997;8:337–352. doi: 10.1111/j.1540-8167.1997.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson W.G., Sager P.T., Friedman P.L. Entrainment techniques for mapping atrial and ventricular tachycardias. J Cardiovasc Electrophysiol. 1995;6:201–216. doi: 10.1111/j.1540-8167.1995.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 3.de Chillou C., Lacroix D., Klug D. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105:726–731. doi: 10.1161/hc0602.103675. [DOI] [PubMed] [Google Scholar]
- 4.Fukunaga M., Goya M., Hiroshima K. Impact of catheter ablation of ventricular tachycardia in patients with prior myocardial infarctions. J Arrhythm. 2016;32:462–467. doi: 10.1016/j.joa.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berte B., Relan J., Sacher F. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015 doi: 10.1111/jce.12761. [Jul 22] [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Di Biase L., Burkhardt J.D., Lakkireddy D. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66:2872–2882. doi: 10.1016/j.jacc.2015.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: Activation map during the ventricular tachycardia.