Abstract
The present study was carried out to evaluate analgesic and anti-inflammatory activities in Inula cuspidata stem and root extracts along with heavy metals estimation in stem and root powder.
Stem and roots were extracted with chloroform (ICSCE, ICRCE) and methanol (ICSME, ICRME). Acute oral toxicity of all extracts was determined by OECD guidelines 425. Analgesic activity was investigated by using hot plate and acetic acid induced writhing models. Anti-inflammatory activity (acute) of all extracts was evaluated by carrageenan induced paw edema model. In addition, root and stem powder was screened for heavy metals (As, Pb, Cd, Hg) estimation using atomic absorption spectroscopy. In acute toxicity study no mortality was observed when each extract was orally administered with 2.0 g/kg. At the doses (100 and 200 mg/kg) ICRME followed by ICSME showed significant and dose dependent analgesic and anti-inflammatory effects compared with chloroform extracts. The heavy metals concentration in stem and root powders was found to be within the permissible limits as recommended by WHO for herbal raw materials. The findings of the present study validated the folkloric use of Inula cuspidata as analgesic and anti-inflammatory. In addition, the results intimate that heavy metals present in raw material were found to be within the defined limits, and it exhibits that the therapeutic efficacy of plant may not be effected, which can be otherwise possibly effected if the plant sequester high concentration of heavy metals from the polluted environment as well as from the soil rich in pesticides and sewage sludge etc.
Keywords: Inula cuspidata, Analgesic, Anti-inflammatory, Heavy metals, Eddy hot plate
Graphical abstract
1. Introduction
Pain according to the International Association for the Study of Pain (IASP) is unpleasant, sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.1
Inflammation is a protective mechanism by the body to remove the harmful stimuli such as pathogens, damaged cells or irritants and initiate the healing process in the body. Acute inflammation is the initial response of the body which begins within seconds to minutes after a tissue injury caused by the harmful stimuli. Acute inflammation will turn chronic if injurious foreign substances persist for longer time and cannot be removed by the body.2
Nonsteroidal anti-inflammatory drugs are generally used to treat inflammation but these drugs are associated with harmful side effects like GI irritation, ulceration, bleeding etc, in the same manner opioids which are used as powerful analgesics are accompanied with side effects such as addiction and dependence. As a result, researchers interest have been increased towards herbal medicines which can be more safe and efficacious then the conventional analgesics and NSAIDS. The plant derived natural products such as flavonoids, sterols, polyphenols, alkaloids, tannins and terpenes have gained importance in recent years due to their wide range of pharmacological activities. Now a days researchers are engaged in evaluation of biological activities possessed by these plant derived natural products.3 The identification of such natural products which possess lesser side effects and no addictive potential like opioids could play an important role in treatment of pain disorders and inflammation.
Trace elements present in medicinal plants play a key role in the synthesis and therapeutic activities of active primary and secondary metabolites but their available excess quantities to the permissible limits may contribute to various toxicities. Heavy metal content in the plants mainly depends upon the environmental conditions (atmospheric dusts, automobile and industrial exhausts) and nature of soil like pH, pesticides/fertilizers content and application of sewage sludge and waste water to the agricultural areas. The transmission rate of heavy metals from soil to plant depends upon the transfer coefficients of metals. Metals having higher transfer coefficient are readily taken up by the plants and vice versa. Consumption of herbal drugs with high heavy metal content may cause deleterious effects to the human health, so it is requisite to examine the level of heavy metals in herbal raw materials as well as in the finished products. The quantitative estimation of heavy metals is an essential step in quality control according to WHO guidelines.4, 5, 6, 7 So, in the present study concentration of four heavy metals (As, Cd, Pb and Hg) was estimated in the stem and root powders of Inula cuspidata using atomic absorption spectroscopy.
The genus Inula, a variable perennial herb distributed in Asian, African and European continents comprises of more than hundred species of the Compositae (Asteraceae) family belonging to the tribe Inuleae.8, 9 Inula species possess medicinal properties and used in folk medicines as tonic, stomachic, anti-inflammatory, bactericidal, diuretic, diaphoretic, hepatoprotective, antitumor and carminative.10 Previous chemical investigations revealed the presence of monoterpenoids, sesquiterpenoids, flavonoids and glycosides in the plant.11, 12, 13 In the previous studies different extracts of leaves have been studied for anti-inflammatory activity.14 The aqueous extracts of stem and whole plant have been recently investigated for anti-inflammatory and hepatoprotective activities.15, 16 However, to the best of our knowledge there is yet no published data regarding analgesic activity and heavy metal study of Inula cuspidata. Based on the above evidences the present study was designed to scientifically validate analgesic and anti-inflammatory activities of chloroform and methanol extracts of stem and roots of Inula cuspidata as well as to determine the concentration of four heavy metals (As, Pb, Cd and Hg) in stem and roots powders using atomic absorption spectroscopy.
2. Material and methods
2.1. Collection and authentication of plant material
Inula cuspidata stem and roots were procured from Nainital, Uttarakhand, India in the months of August and September 2013 and were authenticated (Voucher number 114758) at Botanical Survey of India, Dehradun. The stems and roots were shade dried, coarsely powdered and stored in an air tight container for further use.
2.2. Chemicals, drugs and instruments
The chemicals and drugs used in the study were carrageenan, tween 80 (Himedia, Mumbai, India). Sodium carboxy methyl cellulose, aspirin (Merck, Mumbai, India), indomethacin (Chemimpex International, Kolkata, India), tramadol (Abbott Health Care Pvt Ltd, Mumbai, India), lead, cadmium, arsenic, mercury standard samples (Merck, Mumbai, India) and all the other chemicals and reagents used in the study were of analytical grade. Rotary evaporator was procured from Heidolph, Schwabach, Germany. Hot plate analgesiometer was purchased from the Quality Apparatus, Ambala Cantt India. Digital plethysmometer was procured from IITC Life Sciences, USA. Atomic absorption spectrophotometer was procured from Electronic Corporation India Ltd., India.
2.3. Extraction of plant material
The dried and powdered plant material of both the parts was successively macerated with n-hexane, chloroform and methanol and concentrated under vacuum using rotary evaporator.17 Further chloroform and methanol extracts of stem and roots were evaluated for analgesic and anti-inflammatory activities.
2.4. Phyto chemical screening
The preliminary phytochemical screening was performed for identifying the phyto constituents present in chloroform and methanol extracts of stem and roots of Inula cuspidata.18
2.5. Animals
Male Swiss Albino mice (Mus musculus) weighing 25–35 g and Wistar albino rats (Rattus norvegicus) weighing 150–200 gm were obtained from Haryana Agricultural University, Hisar (India) and were kept in the animal house of Pharmacy Department, Banasthali University (India) for the experimental purpose. All the animals were housed in clean polypropylene cages and were maintained at standard conditions of temperature (22 ± 1 °C) and 12:12 h light/dark cycles. They were fed with standard pellet diet (Hindustan Lever Ltd, India) and had free access to water ad libitum. All the experimental procedures and protocols used in the study were approved by the Institutional Animal Ethics Committee (Ref No. BU/BT/628/14-15) and were in accordance with the guidelines of the CPCSEA.
2.6. Acute toxicity assay
Acute toxicity assay was performed in accordance to OECD guidelines 425 (limit test).19, 20 Swiss albino mice were randomly selected and divided into different groups, comprised of five animals per group. A single dose of each test extract (2000 mg/kg) was administered orally to their assigned groups. The control group received (mixture of 0.1% tween 80 and 0.5% sodium carboxy methyl cellulose) vehicle orally. The mice were observed continuously for the first 4 h and then periodically up to 24 h for toxic symptoms and mortality if any.
2.7. Analgesic activity
2.7.1. Hot plate method
The central analgesic activity of different extracts of Inula cuspidata roots and stem was evaluated by Eddy's hot plate method.21 The mice (n = 6) were divided into ten groups. Group I assigned as control group received vehicle orally. Group II designated as reference group received the standard drug tramadol 10 mg/kg i.p. Groups III,IV and V,VI which were served as test groups for chloroform (ICSCE) and methanol (ICSME) extracts of stem part were administered orally at the dose rate of 100 and 200 mg/kg. Similarly Groups VII,VIII and IX,X were designated as test groups for chloroform (ICRCE) and methanol (ICRME) extracts of root part, were administered orally at the dose rate of 100 and 200 mg/kg. Each animal was placed individually on the hot plate maintained at temperature 55 ± 2 °C. The reaction time (paw licking or jumping) was recorded for each mice at time intervals of 30 min, 60 min and 90 min after administration of drug or vehicle with cutoff time 15 sec to prevent tissue damage.22 The increase in reaction time in plant extracts and standard treated groups was compared with that of the control group.
2.7.2. Acetic acid induced writhing method
For this study acetic acid induced writhing method was acquired. Mice (n = 6) were divided into two groups for each extract of root and stem of Inula cuspidata, in addition to that two more groups (n = 6) of mice were used for control and standard study. Control group received vehicle, standard group was treated with acetyl salicylic acid 100 mg/kg, p.o. Groups assigned for extracts were treated with 100 and 200 mg/kg, p.o. After 30 min, writhings were induced in mice by intra-peritoneal injection of 0.6 % v/v acetic acid. The number of writhing was counted over a period of 30 min. The percent inhibition of writhing count of the treated group was calculated from the mean writhing count of the control group by applying the formula.23, 24
2.8. Anti-inflammatory activity
2.8.1. Carrageenan induced paw edema
In the rat paw edema method, acute inflammation was produced in male wistar rats by injecting 0.1 ml of freshly prepared carrageenan solution (1% w/v) in normal saline into the sub-plantar region of the rat's paw. Animals were divided into 2 groups (n = 6) for each extract of root and stem, along with two more groups (n = 6) for control and standard. Control group received vehicle, standard group received indomethacin 10 mg/kg p.o and groups assigned for extracts received 100 and 200 mg/kg p.o before 60 min of carrageenan injection. Paw volume was measured with digital plethysmometer at the time intervals of 1, 2 and 3 h after carrageenan injection.25 The percentage inhibition was calculated by formula
2.9. Determination of heavy metal content
2.9.1. Preparation of working standard solutions
The standard stock solutions (10 ppm) each of lead (Pb), mercury (Hg), cadmium (Cd) and arsenic (As) were prepared in 0.1 M nitric acid. Further the working standard solutions ranging from 0.05–0.5 ppm each of Cd, Hg, As and 0.5–4 ppm of lead were prepared from their relevant standard stock solutions.
2.9.2. Sample preparation
0.5 gm coarse powder of each stem and root of Inula cuspidata, was weighed and transferred separately into casparian flasks. 10 ml of nitric acid-perchloric acid (4:1) mixture was added in respective flasks, followed by mixing. Small hoppers were fixed on each flask-top, and these flasks were kept for overnight maceration. Both solutions were heated on an electric hotplate until they became clean and transparent. After cooling the solutions were transferred to 25 mL volumetric flask and diluted with 2 % nitric acid solution to the desired volume. The blank solution was devoid of sample and taken as control.26 All the experiments were performed in triplicate for achieving more accurate results.
2.10. Statistical analysis
Data was represented as mean ± S.E.M. for analgesic and anti-inflammatory activity and mean ± S.D for heavy metal estimation. Statistical analysis for pharmacological activity was carried out by one way ANOVA followed by Dunnett test by using Graph-Pad prism software version 5.03. p value was regarded as significant, when p < 0.05.
3. Results
3.1. Acute toxicity
Oral administration of highest dose 2000 mg/kg of both stem and root extracts of Inula cuspidata did not produce any acute toxic symptoms. Moreover no mice mortality was occurred during the observation period of 24 h. The extracts were found to be safe at the highest dose of 2000 mg/kg; ten and twenty fold dilutions of the highest dose were selected for the analgesic and anti-inflammatory activities.
3.2. Analgesic activity
3.2.1. Hot plate method
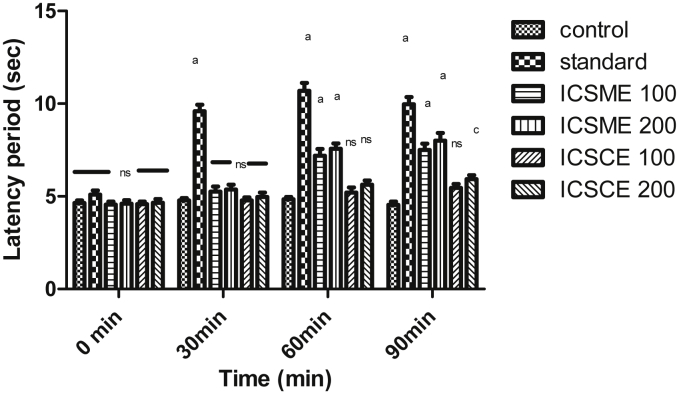
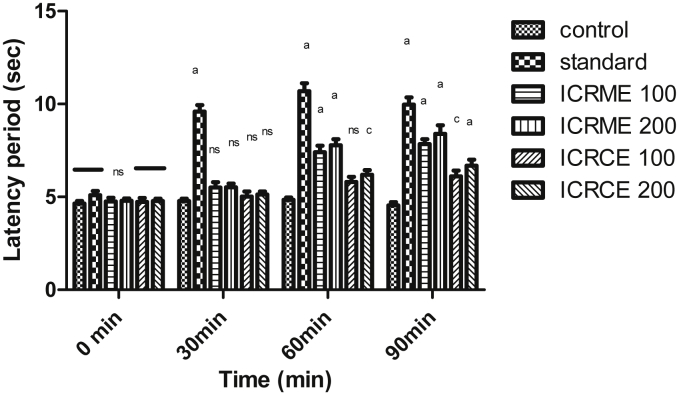
All the test extracts of both stem and roots of Inula cuspidata exhibited dose-dependent analgesic activity. The results for hot plate method are represented in Fig. 1, Fig. 2. The methanol extracts (ICSME, ICRME) at both the doses 100, 200 mg/kg exhibited a significant effect (p < 0.001) at 60 and 90 min readings as compared to control. Tramadol showed a significant effect (p < 0.001) at the dose of 10 mg/kg, i.p. The chloroform extracts (ICSCE, ICRCE) at the dose of 200 mg/kg exhibited significant effect (p < 0.05, p < 0.001) at 90 min reading as compared to control. The methanol extracts ICSME, ICRME with 100 and 200 mg/kg significantly increased the latency period by 7.5, 7.85, 8.01 and 8.39 s at 90 min, respectively, whereas tramadol (10 mg/kg, i.p.) significantly increased the latency period (10.69 s) at 60 min after that gradually decreased (9.97 s) at 90 min.
Fig. 1.
Effect of different extracts of Inula cuspidata stem on hot plate induced pain in mice. Data are presented as mean ± SEM, n = 6, a = p < 0.001, b = p < 0.01, c = p < 0.05, ns = non-significant compared to control.
Fig. 2.
Effect of different extracts of Inula cuspidata root on hot plate induced pain in mice. Data are presented as mean ± SEM, n = 6, a = p < 0.001, b = p < 0.01, c = p < 0.05, ns = non-significant compared to control.
3.2.2. Acetic acid induced writhing method
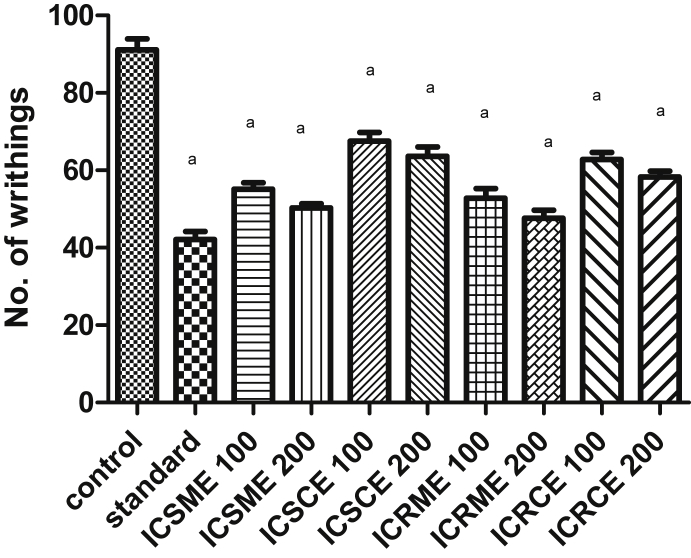
In the acetic acid induced writhing method, all the tested extracts of stem and roots significantly (p < 0.001) exhibited dose dependent reduction in number of writhes within the 30 min of injection of acetic acid (0.6%v/v), when compared to control. The results for the acetic acid induced writhing method are depicted in Fig. 3. The methanol extracts (ICSME, ICRME) of plant exhibited marked reduction in number of writhes at both the doses 100 and 200 mg/kg. The maximum inhibition of writhings was observed at 200 mg/kg for both the methanol extracts ICSME, ICRME (44.79%, 47.72%), which was comparable with standard acetyl salicylic acid at 100 mg/kg p.o (53.75%). The chloroform extracts (ICSCE, ICRCE) showed maximum writhing inhibition (30.17%, 36.01%) at dose of 200 mg/kg.
Fig. 3.
Effect of different extracts of Inula cuspidata stem and roots in acetic acid induced writhing in mice. Data are presented as mean ± SEM, n = 6, a = p < 0.001 compared to control.
3.3. Anti-inflammatory activity
3.3.1. Carrageenan induced paw edema
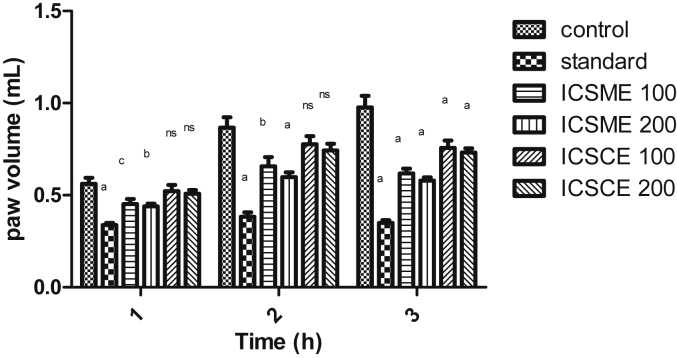
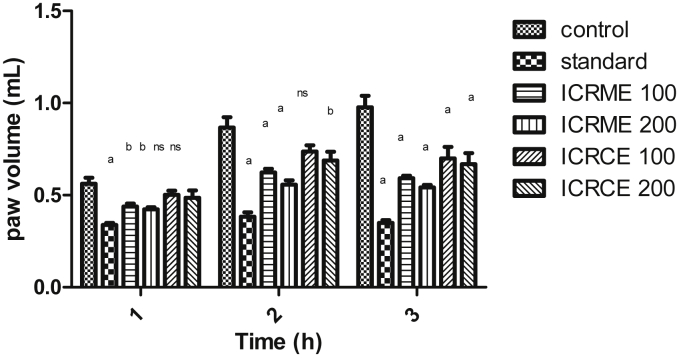
The results of anti-inflammatory activity for all the tested extracts of stem and roots of plant are depicted in Fig. 4, Fig. 5. All the extracts exhibited dose dependent reduction in paw edema volume at different doses of 100 and 200 mg/kg. The methanol extract (ICRME) at both doses 100 and 200 mg/kg, as well as ICSME at dose of 200 mg/kg significantly (p < 0.001) inhibited carrageenan induced paw edema volume at 2 h and 3 h as compared to control, while the methanol extract ICSME (100 mg/kg) significantly (p < 0.001) inhibited carrageenan induced paw edema volume at 3 h. The chloroform extracts (ICSCE, ICRCE) of both the parts at the dose of 100 and 200 mg/kg showed significant (p < 0.001) reduction in paw volume only at 3 h. The methanol extracts (ICSME, ICRME) exhibited a significant reduction in paw edema volume (40.64%, 44.52%) at the dose of 200 mg/kg at 3 h. The standard indomethacin showed significant (p < 0.001) inhibition (64.18%) of paw edema volume at 3 h at the dose of 10 mg/kg,p.o.
Fig. 4.
Effect of different extracts of Inula cuspidata stem on carrageenan induced paw edema in rats. Data are presented as mean ± SEM, n = 6, a = p < 0.001, b = p < 0.01, c = p < 0.05, ns = non-significant compared to control.
Fig. 5.
Effect of different extracts of Inula cuspidata root on carrageenan induced paw edema in rats. Data are presented as mean ± SEM, n = 6, a = p < 0.001, b = p < 0.01, ns = non-significant compared to control.
3.4. Estimation of heavy metal content
The results of quantitative estimation of heavy metal analysis in stem and root powders are depicted in Table 1. Accumulations of four heavy metals As, Cd, Pb and Hg in both the powders were found to be within the defined limits as mentioned in WHO guidelines for raw herbal materials. As and Hg content was higher in stem (0.22 ppm, 0.25 ppm) to that of root powder (0.15 ppm, 0.12 ppm) whereas Cd and Pb content detected were in higher quantities in root (0.1 ppm, 3.4 ppm) as compared to stem powder which were found to be 0.07 ppm and 1.61 ppm, respectively.
Table 1.
Heavy metal content in stem and root powders of Inula cuspidata.
| Heavy metals | Standard value (ppm) | Observed value (ppm) in I. Cuspidata |
|
|---|---|---|---|
| Stem powder | Root powder | ||
| As | 3 | 0.22 ± 0.021 | 0.15 ± 0.02 |
| Cd | 0.3 | 0.07 ± 0.015 | 0.1 ± 0.018 |
| Pb | 10 | 1.61 ± 0.072 | 3.4 ± 0.062 |
| Hg | 1 | 0.25 ± 0.05 | 0.12 ± 0.04 |
Values are expressed as mean ± SD, n = 3.
4. Discussion
The literature survey has revealed that the plant Inula cuspidata has been used in folk medicines for the treatment of ailments such as pain and inflammation. Thus the present investigation was designed to evaluate scientifically the traditional claims for the above mentioned uses of Inula cuspidata. The preliminary phytochemical analysis of Inula cuspidata stem and roots showed presence of alkaloids, flavonoids, triterpenoids, steroids, tannins and phenolic compounds.
Sesquiterpene lactones are the major group of secondary metabolites present in genus Inula based on literature survey. The bioactive compounds isolated from various Inula species belongs to different classes of sesquiterpene lactones such as eudesmanolides, guaianolides, pseudoguaianolides, germacranolides and xanthanolides. The results from several authors indicate that sesquiterpene lactones possess a wide array of biological activities namely anticancer, anti-inflammatory, antibacterial and many more. The earlier studies suggest that Inula compounds exhibit anti-inflammatory activity by the inhibition of phospholipase A2, cyclooxygenase and lipoxygenase enzymes which are responsible for production of arachidonic acid, prostaglandins and leukotrienes, the crucial mediators of inflammation. Additionally, these compounds also inhibit nitric oxide synthase, responsible for production of nitric oxide which also acts as one of the mediator in inflammation.8, 9
The present study depicts the analgesic activity of different extracts of stems and roots of Inula cuspidata in both the methods centrally acting hot plate model and peripherally acting acetic acid induced writhing model. All the extracts protected mice against both hot and chemical induced noxious stimuli. Intra-peritoneally administered acetic acid, caused elevated levels of prostaglandins PGE2 and PG-F2α in peritoneal fluid. The abdominal constriction in mice was associated with sensitization of peritoneal nociceptors by the prostaglandins.27, 28 The analgesic effects exhibited by the extracts may be associated with the inhibition of prostaglandins level.
The hot plate model has been employed extensively for the screening of compounds exhibiting analgesia by central mechanism, where elevation in pain threshold of mice towards heat is determined. It is well known fact that, the response (paw licking, jumping) by mice to noxious thermal stimuli in hot plate method is supra-spinally mediated response.29 The analgesic effect exhibited by the extracts in hot plate test could be due to their interaction with various receptors present in supra-spinal sites.
Carrageenan-induced edema is commonly used experimental model for evaluation of acute inflammation in animals. Carrageenan, when injected into the sub-plantar region of rat's paw, produces inflammatory reaction (edema) which is visible within 30 min. The presumptive mechanism of action of carrageenan-induced edema has been found to be biphasic. The early phase (1–2 h) is due to the liberation of serotonin, histamine and bradykinin, while the second phase is attributed to the release of prostaglandins.30, 31 All the extracts exhibited maximum inhibition in paw volume at 3 h, suggesting that the extracts could possibly have an inhibitory effect on the release of prostaglandins at the second phase.
Plants accumulate elements from the environment which are essential for their growth and at the same time they can accumulate the non-essential elements like heavy metals which possess no beneficial properties and are found to be exclusively toxic. The content of heavy metals in plants are affected by many factors like atmospheric pollution, nature of soil, climatic conditions, agriculture practices, storage of plants and many more factors.
The high levels of heavy metals induce toxicity by the generation of reactive oxygen species (ROS). The increased ROS level is responsible for oxidative stress which leads to severe life threatening diseases.32
Lead is non-essential toxic element which cause various deleterious effects on human body like chronic nephropathy, hypertension, brain damage and anemia due to defect in heme synthesis. Infants and children are more sensitive to lead toxicity. Due to high lead exposure children may face lead encephalopathy and low level of exposure leads to lack of concentration in children. Cadmium (Cd) toxicity can cause kidney damage like tubular dysfunction and chronic exposure leads to skeletal damage like osteomalacia and osteoporosis which is due to the excessive urinary loss of calcium. Chronic exposure of Cd also causes pulmonary damage. Mercury even in small amount is responsible for major health problems. The major risks associated with mercury toxicity are neurotoxicity, genotoxicity and also it cause malfunctioning of kidneys, muscles and nerves.33 Arsenic is another toxic element which when consumed in excess amount can lead to chronic arsenic poisoning. Long-term exposure to inorganic arsenic, mainly through drinking of contaminated water, eating of food prepared with this water and eating food irrigated with arsenic-rich water, can increase the risk of skin lesions and skin cancer.34 As all the four toxic elements were found to be within the permissible limits in both the plant parts and therefore these extracts can be considered safe for human consumption, and act as an important therapeutic agent in the treatment of pain and inflammation.
5. Conclusion
In conclusion, our results reveal that among all the extracts of roots and stem of Inula cuspidata, both methanol root and stem extracts exhibited significant analgesic and anti-inflammatory activities followed by chloroform extracts. These findings validated the claim for the traditional use of this plant in the treatment of pain and inflammatory ailments. Further research work is needed to segregate the active constituents from the active extract exhibiting significant analgesic and anti-inflammatory activities. In addition to this, research regarding the mechanism responsible for these activities is also required which will guarantee its clinical worth. The heavy metal content in stem and root powders was found to be within permissible limits as per WHO guidelines for herbal raw materials. This finding give support to the claim that the therapeutic efficacy of plant has not been effected, as content of heavy metals is present within the permissible limits. Further research work is required for the estimation of all the possible essential elements present in the plant, so that we can presume the total effectiveness of plant.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgement
Authors are thankful to the Vice Chancellor, Banasthali University for providing all the necessary facilities required for the study. The authors are also thankful to Department of Pharmacy, Banasthali University for their continuous support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Hassan F.I., Abdulkadir U.Z., Yaro A.H., Danmalam U.H. Analgesic, anti-inflammatory and antipyretic activities of the methanol leaf extract of Dalbergia saxatilis Hook.F in rats and mice. J Ethnopharmacol. 2015;166:74–78. doi: 10.1016/j.jep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Agli M.D., Lorenzo C., Badea M. Plant food supplements with anti-inflammatory properties: a systematic review (1) Crit Rev Food Sci Nutr. 2013;53(4):403–413. doi: 10.1080/10408398.2012.682123. [DOI] [PubMed] [Google Scholar]
- 3.Yanfen C., Shuhong T., Fanlin Z., Luwei X., Zhibin S. Antinociceptive and anti-inflammatory activities of Schefflera octophylla extracts. J Ethnopharmacol. 2015;171:42–50. doi: 10.1016/j.jep.2015.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Serife T. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012;134:2504–2508. doi: 10.1016/j.foodchem.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 5.Kulhari A., Sheorayan A., Bajar S., Sarkar S., Chaudhury A., Kalia R.K. Investigation of heavy metals in frequently utilized medicinal plants collected from environmentally diverse locations of north western India. SpringerPlus. 2013;2:676–684. doi: 10.1186/2193-1801-2-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou Kheir R., Shomar B., Greve M.B., Greve M.H. On the quantitative relationships between environmental parameters and heavy metals pollution in Mediterranean soils using GIS regression-trees: the case study of Lebanon. J Geochem Explor. 2014;147:250–259. [Google Scholar]
- 7.Gupta S., Pandotra P., Gupta A.P. Volatile (As and Hg) and non-volatile (Pb and Cd) toxic heavy metals analysis in rhizome of Zingiber officinale collected from different locations of North Western Himalayas by Atomic Absorption Spectroscopy. Food Chem Toxicol. 2010;48:2966–2971. doi: 10.1016/j.fct.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y.M., Zhang M.L., Shi Q.W., Kiyota H. Chemical constituents of plants from the genus Inula. Chem Biodivers. 2006;3:371–384. doi: 10.1002/cbdv.200690041. [DOI] [PubMed] [Google Scholar]
- 9.Seca A.M., Grigore A., Pinto D.C., Silva A.M. The genus Inula and their metabolites: from ethnopharmacological to medicinal uses. J Ethnopharmacol. 2014;154(2):286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Mathela C.S., Tiwari A., Padalia R.C., Chanotia C.S. Chemical composition of Inula cuspidata C.B. Clarke. Indian J Chem. 2008;47(B):1249–1253. [Google Scholar]
- 11.Bohlmann F., Singh P., Jakupovic J. Further ineupatorolide-like germacranolides from Inula cuspidata. Phytochemistry. 1982;21(1):157–160. [Google Scholar]
- 12.Rastogi R.P., Agarwal S.K., Sahai R. Chemical investigation of Inula cuspidata. Indian Drugs. 1981:100–101. [Google Scholar]
- 13.Verma R.S., Padalia R.C., Chauhan A. Leaf essential composition of Inula cuspidata (Wall. Ex DC.) C.B. Clarke from India. J Essent Oil Res. 2014;26(4):233–237. [Google Scholar]
- 14.Thapliyal S., Goel K.K., Goel N. Anti-inflammatory activity of Inula cuspidata leaf extract. Asian J Chem. 2011;23(2):943–944. [Google Scholar]
- 15.Kaur A.K., Wahi A.K., Bhandari A., Kumar S., Gupta R. Evaluation of anti-inflammatory effects of Inula cuspidata whole plant, stem and flower extract against carageenan induced paw edema in rats. WJPPS. 2014;3(11):601–608. [Google Scholar]
- 16.Kaur A.K., Wahi A.K., Mehta N.M., Bhandari A., Kumar S., Gupta R. Hepatoprotective activity of Inula cuspidata flower, stem and whole plant extract against carbon tetrachloride induced toxicity in rats. Int J Pharm Sci Rev Res. 2014;27(1):25–30. [Google Scholar]
- 17.Quality Control Methods for Medicinal Plant Materials. WHO headquarters; Geneva: 1998. [Google Scholar]
- 18.Harborne J.B. Chapman and Hall Ltd; London: 1998. Phytochemical Methods. [Google Scholar]
- 19.OECD Guidelines for Testing of Chemicals: 425. 2008. Paris. [Google Scholar]
- 20.Bhandare A.M., Kshirsagar A.D., Vyawahare N.S., Hadambar A.A., Thorve V.S. Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food Chem Toxicol. 2010;48:3412–3417. doi: 10.1016/j.fct.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Eddy N.B., Leimback D. Synthetic analgesics II. Dithienylbutenyl and dithienyl butylamines. J Pharmacol Exp Ther. 1953;107:385–402. [PubMed] [Google Scholar]
- 22.Goyal M., Ghosh M., Nagori B.P., Sasmal D. Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi J Biol Sci. 2013;20:365–371. doi: 10.1016/j.sjbs.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q., Wang Y., Guo S., Shen Z., Wang Y., Yang L. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi. J Ethnopharmacol. 2014;152:540–545. doi: 10.1016/j.jep.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Arumugam P., Murugan M., Thangaraj N. Evaluation of anti-inflammatory and analgesic effects of aqueous extract obtained from root powder of Inula racemosa Hook. J Med Plants Res. 2012;6(14):2801–2806. [Google Scholar]
- 25.Badole S.L., Zanwar A.A., Ghule A.E., Ghosh P., Bodhankar S.L. Analgesic and anti-inflammatory activity of alcoholic extract of stem bark of Pongamia pinnata (L.) Pierre. Biomed Aging Pathol. 2012;2(1):19–23. [Google Scholar]
- 26.The Ayurvedic Pharmacopeia of India. Govt of India Ministry of Health & Welfare Dept of Ayush; NewDelhi: 2008. [Google Scholar]
- 27.Bose A., Mondal S., Gupta J.K., Ghosh T., Dash G.K., Si S. Analgesic, anti-inflammatory and antipyretic activities of the ethanolic extract and its fractions of Cleome rutidosperma. Fitoterapia. 2007;78:515–520. doi: 10.1016/j.fitote.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Sengar N., Joshi A., Prasad S.K., Hemalatha S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J Ethnopharmacol. 2015;160:140–148. doi: 10.1016/j.jep.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Wani T.A., Kumar D., Prasad R. Analgesic activity of the ethanolic extract of Shorea robusta resin in experimental animals. Indian J Pharmacol. 2012;44(4):493–499. doi: 10.4103/0253-7613.99322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Z.Z., Ma K.J., Ran X. Analgesic, anti-inflammatory and antipyretic activities of the petroleum ether fraction from the ethanol extract of Desmodium podocarpum. J Ethnopharmacol. 2011;133:1126–1131. doi: 10.1016/j.jep.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Yu C.H., Tang W.Z., Peng C. Diuretic, anti-inflammatory, and analgesic activities of the ethanol extract from Cynoglossum lanceolatum. J Ethnopharmacol. 2012;139:149–154. doi: 10.1016/j.jep.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Armenta M.M., Ruiz C.N., Valverde F.F., García A.S., Rios C. Histochemical changes in muscle of rats exposed subchronically to low doses of heavy metals. Environ Toxicol Pharmacol. 2011;32:107–112. doi: 10.1016/j.etap.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Trace Elements in Human Nutrition and Health. WHO; Geneva: 1996. [Google Scholar]
- 34.Flanagan S.V., Johnston R.B., Zheng Y. Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ. 2012;90:839–846. doi: 10.2471/BLT.11.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]