Abstract
Anacardium occidentale L. (Anacardiaceae) is used in South Cameroon as well as in other tropical countries by traditional practitioners as a folk remedy for treatment of diabetes mellitus. We demonstrated the antidiabetic potential of the plant extracts in n-streptozotocin diabetic rats. The aim of the current study was to investigate the antidiabetic effects of ethanol extract of leaves of A. occidentale on neonatal streptozotocin diabetic rats.
Two day old neonates were injected with 100 mg/kg of streptozotocin. At the end of the experimental period of 30 days, reduction in the fasting blood glucose levels, serum insulin, glycated hemoglobin levels, serum lipid parameters, and renal function biomarkers were estimated in the control and treated rats. Histopathological examination of liver, kidney and pancreas were also carried out.
On administration of 100 mg/kg of plant extract, blood glucose levels of the rats showed 8.01% and 19.25% decrease in the fasting blood glucose levels on day 15 and day 30, respectively. The administration of extract showed that the effects of extract treatment are comparable to treatment with the standard drug Pioglitazone.
These results demonstrate significant antidiabetic potential of the ethanol extract of leaves of A. occidentale, justifying the use of plant in the indigenous system of medicine. Further studies for investigating the specific compound(s) responsible for such beneficial role in diabetes would open new outlook in the therapy of type 2 diabetes.
Keywords: Anacardium occidentale, Neonatal-induced diabetic rats, Type 2 diabetes, Streptozotocin, Leaves
Graphical abstract
1. Introduction
Since past few decades type 2 diabetes has become a global health problem. As estimated by the World Health Organization more than 176 million people are suffering from this disease globally.1 The common cause of chronic morbidity and disability among the working population is the complications which are caused due to diabetes. Type 2 diabetes mellitus begins with a period of insulin resistance with increased pancreatic insulin secretion. As the disease advances, pancreatic functions are decreased and are no longer able to meet peripheral requirements. Thus, insulin levels fail to sustain with the body requirements.2 Experimental animal models of diabetes mellitus (DM) have been useful in gaining insights of the complex pathogenesis of DM. Streptozotocin (STZ) when injected in neonates of rats leads to the key features depicted in diabetic patients in a small period. Diabetes mellitus is a result of low insulin sensibility and dysfunction of the pancreatic beta-cell. Before the development of the disease, the condition is characterized by a symptomless pre-diabetic phase. Practitioners of traditional system of medicine in South Cameroon use Anacardium occidentale L. (Anacardiaceae) as a folk remedy for treating diabetes mellitus. Hence, we made an attempt to study the validity of this folk remedy by investigating the effects of ethanol extract of leaves in neonatal STZ diabetic rats.
The Cashew tree, known by the Latin name A. occidentale, is a member of the Anacardiaceae family. A. occidentale is grown widely in tropical countries like Malaysia, India and Brazil and occurs widely in Senegal and is known as Darkassou.3 Practitioners of Traditional system of medicine in South Cameroon and other tropical countries use A. occidentale L. (Anacardiaceae) as a folk remedy for treating diabetes mellitus.4, 5
A. occidentale commonly known as Cashew is also ethno pharmacologically known to be used in treatment of diarrhea, skin diseases, and various inflammatory conditions such as arthritis.6, 7, 8, 9, 10 It is also used for treatment of fevers, aches and pains.11, 12, 13 Literature reports reveal that the studies of acute, subacute toxicity and genotoxic effect and hypoglycemic effect of Cashew in mice and rats (A. occidentale L.) have been reported.14, 15, 16 There are numerous reports on the phytochemical studies of A. occidentale and they reveal the presence of various compounds, such as glucosides and glucose and flavonoids.17, 18, 19
The n-STZ rat model is adequate for testing type 2 diabetes.20 The (n5-STZ) rat model show signs of a clear basal hyperglycemia with glucose intolerance, a strong reduction of pancreatic insulin stores, high HbA1c values, a lack of plasma insulin response to glucose and a decreased (50%) basal plasma insulin level. A single dose of STZ when given to neonates of rats induces injury of beta-cell which is then followed by limited regeneration (short-term normalization of glycemia), at about 6–15 weeks period, significant beta-cell secretory dysfunction (type 2 diabetes) and an impaired glucose disposal rate is observed.20
2. Materials and methods
2.1. Plant material
Cashew leaves were collected from Tungareshwar forests of Vasai Taluka, Dist. Thane in the state of Maharashtra, India. The plant specimen was authenticated at the Botanical Survey of India, Pune; (M.S), India. A herbarium of the plant specimen (specimen voucher number no. YOGA1/No.BSI/WC/Tech/2008/69) was submitted at the Botany Department of BSI, Pune, M.S., India.
2.2. Preparation of the extract
Fully matured shade dried leaves of Cashew were collected, cleansed and ground to coarse powder form. The samples were extracted by using Soxhlet extractor, with ethanol with a mass to volume ratio of 1:6 (g/mL). The ethanol extract was evaporated to dryness on the rotary evaporator and the residue stored in a refrigerator at 2–8 °C for use in subsequent experiments.
2.3. Acute oral toxicity studies
Animals were procured from Haffkine's Research Institute, Mumbai, India and acclimatized with free access to food and water for at least 1 week. Female Albino mice (Wistar strain) were selected for the study. Healthy young animals, 2 months old and 220–250 g weight range of commonly used laboratory strains were employed in the study. Females used were nulliparous and nonpregnant. The study group used 6 animals in each group.21 Animals were observed individually after dosing at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h and daily thereafter, for a total of 14 days. The body weights and food intake of animals were recorded. All observations were systematically recorded with individual records being maintained for each animal. Additional conditions like that of tremors, convulsions, salivation, diarrhea, lethargy, sleep, coma and lethality were observed.
2.4. Animals and induction of experimental diabetes
Two day old neonates were injected with optimized dose of 100 mg/kg of Streptozotocin (Sigma, no. 242-646-8) in acetate buffer 0.1 M, pH 4.5. At 4 weeks of age, rats were separated from their mothers and acclimatized with free access to food and water in an air-conditioned room (23 °C with 55% humidity) under a 12 h light: dark cycle. The animals were fed with standard rat pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. When animals were 12 weeks old, an oral glucose tolerance test was performed. All the animals were fasted overnight before experiments. Animals which were intolerant to glucose by OGTT and fasting blood glucose levels >120 mg/dL were selected for the study with ethanol extract of leaves. The study protocol was approved by Institutional Animal Ethics Committee of C. U. Shah College of Pharmacy, Mumbai, India (Approval No: CUSCP/IAEC/29/09-10). The administration of the drugs was done orally for 30 days. At the end of the experimental period, the rats were fasted overnight and blood samples were withdrawn from the retro orbital plexus. Serum samples were used for the various biochemical estimations.
2.5. Experimental groups
The rats were divided into 4 groups for study of neonatal streptozotocin-induced (n-STZ) model containing six animals each. Group 1 non-diabetic control received 1.5 ml of physiological NaCl-solution (Vehicle), group 2 was treated with the standard oral hypoglycemic agent Pioglitazone (2 mg/kg) in the same vehicle, group 3 diabetic control, treated with streptozotocin (100 mg/kg i.p) also received 1.5 ml of physiological NaCl-solution (Vehicle), group 4 received ethanol extract of cashew leaves 100 mg/kg. The extract was redissolved in 1.5 ml of physiological NaCl-solution and administered orally by a canule.
2.6. Collection of blood and determination of blood parameters
The effects of administration of ethanol extract of Cashew leaves to normal and diabetic rats were determined by measuring fasting plasma glucose levels, serum insulin levels, serum lipid profiles, liver glycogen levels (Nicholas, 1956), glycated hemoglobin levels and initial and final changes in body weight. Fasting plasma glucose, serum triglycerides, total cholesterol, were estimated on days 1, 15, and 30 of extract administration. Body weights were determined on day 1, 10, 20 and 30 of extract administration. All other biochemical parameters were determined on day 30 after the animals were sacrificed by decapitation. Serum insulin levels were estimated using a radio immunoassay kit issued by the Board of Radiation and Isotope Research, Bhaba Atomic Research Centre (BARC), Mumbai, India. On day 30, when the animals were sacrificed, the pancreas, liver and kidney of one animal from each group was excised and stored in 10% formalin after washing with normal saline and histopathological parameters were studied. The tissue was washed, dehydrated with alcohol, cleared with xylene and paraffin blocks were made. Serial sections of 5 μm thickness were cut using a rotary microtome. The sections were de-paraffinised with xylene and hydrated in descending grades of alcohol. The slides were then transferred to hematoxylin for 10 min, followed by rinsing with water. These were examined and later counter stained with eosin, rinsed with water, dehydrated with ascending grades of alcohol, cleared with xylene and mounted.
2.7. Statistical analysis
All statistical analyses were made using the software InStat for windows. All results were expressed as mean ± SEM. Post hoc Dunnett's test was used to determine statistical significance. The values were considered statistically significant when p < 0.05.
3. Results and discussion
Acute oral toxicity studies are performed in animals to evaluate the safety of plant based products and other formulations for humans. The safe doses in animals can be extrapolated to human doses. After clinical trials and other regulatory controls are completed for the product, the dosage for humans for a particular plant based product can be determined. It is reported that the pathophysiological observations for gastrointestinal and hematological disorders in animals have a close relativeness with those observed in humans.22 Although, doses can be extrapolated for safe use, it is difficult to ascertain the replication of same pharmacokinetic behavior of plant based products in humans compared to animals. Prior to any specific kind of pharmacological models, acute oral toxicity studies are recommended to be performed. In phyto-pharmacological studies, acute oral toxicity studies can provide the researcher an estimate of the various toxic effects of a single dose of any plant based product and the effects of each dose and each extract can vary depending on its source.23
In this study, the non-toxic nature of the ethanol extract of Cashew leaves was ascertained by carrying out acute oral toxicity studies. At the dose level of 100 mg/kg of ethanol extract of Cashew leaves, no lethality or any toxic reactions were found at any of the doses selected until the end of the study period. Changes in the bodyweight are used as a measure of side effects of drugs or health supplements on the normal physiological functions of the body. Upto a period of 15 days and 30 days, there was no significant difference observed in the body weights of the treatment group and the diabetic control group at p < 0.05 (Table 1). These results thus indicate that the tested ethanol extract of Cashew leaves did not have any detrimental effects on the normal pathophysiological functions or growth of the experimental animals at the selected dose level.24
Table 1.
Effect of plant extract on body weight in rats.
| Treatment | Body weight (g) |
|||
|---|---|---|---|---|
| On day 1 | Day 10 | Day 20 | Day 30 | |
| Normal control | 229.17 ± 7.4 | 238.17 ± 6.9 | 245.83 ± 6.1 | 249.50 ± 5.1 |
| Diabetic control | 206.83 ± 11.7 | 218.67 ± 12.2 | 210.83 ± 12.1 | 209.33 ± 13.3 |
| Pioglitazone (2 mg/kg) | 222.67 ± 8.8 | 230.33 ± 10.1 | 236.17 ± 8.82 | 239.67 ± 8.42 |
| Ethanol extract of leaves (100 mg/kg) | 213.67 ± 13.5 | 215.33 ± 18.0 | 223.67 ± 14.0 | 229.83 ± 15.0 |
Each of the values are expressed as mean ± S.E.M., n = 6.
It is known that in STZ induced diabetes model, the drug STZ reaches the beta cells through a glucose transporter mechanism. STZ is reported to cause alkylation of DNA by liberating high levels of nitric oxide and nitrosourea, resulting into inhibition of aconitase.25 In animal models of STZ the occurrence of insulin resistance is dependent on several factors like dose of STZ, the age, and the strain of animals. Report published earlier report that an i.p. injection of STZ on the second day of birth at dose level 90 mg kg−1, developed insulin resistance.26 In the present study, the extracts showed statistically significant reduction in the blood glucose levels at p < 0.05 on day 30 (Table 2). A statistically significant decrease in the glycated hemoglobin levels was observed at p < 0.05. Ethanol extract of leaves decreased the fasting insulin resistance index (FIRI) and it was comparable to standard – pioglitazone. A decrease in serum insulin levels was also observed as compared to the diabetic control, but it was not found to be statistically significant at p < 0.05 (Table 3).
Table 2.
Effect of plant extract on fasting blood glucose levels in rats.
| Treatment | Fasting blood glucose (mg/dl) ± SEM |
||
|---|---|---|---|
| On day 0 | Day 15 | Day 30 | |
| Normal control | 78.67 ± 0.84 | 76.5 ± 1.38 | 75.83 ± 1.82 |
| Diabetic control | 150.33 ± 6.92 | 149.33 ± 5.86 | 154.50 ± 5.40 |
| Pioglitazone (2 mg/kg) | 144.83 ± 7.24 | 129.50 ± 5.47a | 113.33 ± 3.30a |
| Ethanol extract of cashew leaves (100 mg/kg) | 147.67 ± 6.09 | 135.83 ± 4.40 | 123.83 ± 2.87a |
Each of the values are expressed as mean ± S.E.M., n = 6.
Significantly different from control, p < 0.05.
Table 3.
Effect of plant extract on serum insulin and glycated hemoglobin in rats.
| Treatment | Serum insulin on day 30 (Uiu/ml) | Glycated hemoglobin on day 30 (%) | FIRI on day 30 |
|---|---|---|---|
| Normal control | 16.99 ± 1.5 | 3.92 ± 0.08 | 51.71 ± 5.1 |
| Diabetic control | 16.52 ± 1.9 | 5.6 ± 0.27 | 103.37 ± 14.8 |
| Pioglitazone (2 mg/kg) | 13.43 ± 1.4 | 4.25 ± 0.18a | 60.44 ± 5.86a |
| Ethanol extract of cashew leaves (100 mg/kg) | 11.69 ± 0.93 | 4.92 ± 0.17a | 57.90 ± 4.69a |
Each of the values are expressed as mean ± S.E.M., n = 6.
FIRI denotes fasting insulin resistance index.
Significantly different from control, p < 0.05.
In diabetes, it is observed that an increase in levels of free fatty acids occurs. The circulating free fatty acids have deleterious effect on the endothelial functions by various pathways and mechanisms which include free radical production, protein kinase C activation and increase in severity of dyslipidemia.27 Oxidative stress is caused by an increase in free radical production by the body and which lead to micro and macro vascular complication in diabetes. Oxidative stress has a strong interlink to the complications and progress of diabetes and insulin resistance. With an increase in oxidative stress, coupled with increase in free fatty acids and blood glucose level, the insulin activity and secretion levels are adversely affected.28 In the animals treated with ethanol extract of leaves, the triglycerides levels on day 30 were found to be decreased and statistically significant as compared to diabetic control at p < 0.05 (Table 4). The lipid profiles for VLDL-C levels showed statistically significant results as compared with diabetic control at p < 0.05 (Table 5). However, significant reductions in LDL-C and HDL-C were not observed at p < 0.05 (Table 6). The renal markers were accessed to ascertain the effect of drug treatment in diabetic rats. However, there was no significant difference observed between the treatment group and diabetic control at p < 0.05 (Table 7).
Table 4.
Effect of plant extract on serum triglyceride levels in rats.
| Treatment | Serum triglyceride (mg/dl) ± SEM |
||
|---|---|---|---|
| On day 0 | Day 15 | Day 30 | |
| Normal control | 58.55 ± 3.65 | 61.21 ± 3.99 | 60.06 ± 4.13 |
| Diabetic control | 97.33 ± 8.12 | 98.71 ± 7.97 | 104.89 ± 7.55 |
| Pioglitazone (2 mg/kg) | 94.80 ± 3.05 | 84.59 ± 2.67 | 75.93 ± 2.52a |
| Ethanol extract of cashew leaves (100 mg/kg) | 96.63 ± 4.22 | 88.84 ± 4.16 | 81.69 ± 4.47a |
Each of the values are expressed as mean ± S.E.M., n = 6.
Significantly different from control, p < 0.05.
Table 5.
Effect of plant extract on lipid parameters in rats.
| Treatment | Serum Parameter on day 30 |
||
|---|---|---|---|
| HDL-C (mg/dl) | LDL-C (mg/dl) | VLDL-C (mg/dl) | |
| Normal control | 37.35 ± 1.81 | 28.73 ± 3.95 | 12.01 ± 0.82 |
| Diabetic control | 34.01 ± 2.01 | 49.38 ± 4.94 | 20.98 ± 1.51 |
| Pioglitazone (2 mg/kg) | 39.74 ± 2.28 | 29.31 ± 3.80a | 15.15 ± 0.78a |
| Ethanol extract of cashew leaves (100 mg/kg) | 36.19 ± 1.80 | 35.39 ± 3.03 | 16.34 ± 0.89a |
Each of the values are expressed as mean ± S.E.M., n = 6.
TG – Triglyceride; TC – Total cholesterol; HDL-c – High density lipoprotein cholesterol; LDL-C – Low density lipoprotein cholesterol; VLDL-C – Very low density lipoprotein cholesterol, LDL-C, VLDL-c calculated using friedewald formula.
VLDL = TG/5; LDL = TC − (HDL + TG/5).
Significantly different from control, p < 0.05.
Table 6.
Effect of plant extract on serum total cholesterol levels in rats.
| Treatment | Serum total cholesterol (mg/dl) ± SEM |
||
|---|---|---|---|
| On day 0 | Day 15 | Day 30 | |
| Normal control | 78.89 ± 3.22 | 77.82 ± 2.78 | 78.09 ± 3.87 |
| Diabetic control | 99.49 ± 2.82 | 102.42 ± 3.38 | 104.37 ± 4.95 |
| Pioglitazone (2 mg/kg) | 95.11 ± 2.72 | 88.90 ± 2.64a | 84.21 ± 3.18a |
| Ethanol extract of cashew leaves (100 mg/kg) | 96.32 ± 2.59 | 91.93 ± 2.51 | 87.91 ± 3.21a |
Each of the values are expressed as mean ± S.E.M., n = 6.
Significantly different from control, p < 0.05.
Table 7.
Effect of plant extract on renal function biomarkers in rats.
| Treatment | Serum biomarkers of Liver and Kidney on day 30 |
|||
|---|---|---|---|---|
| SGOT | SGPT | Urea | Creatinine | |
| Normal control | 273.14 ± 5.47 | 71.88 ± 4.91 | 61.18 ± 1.64 | 0.67 ± 0.02 |
| Diabetic control | 286.71 ± 28.46 | 85.34 ± 10.85 | 77.83 ± 2.12 | 0.81 ± 0.06 |
| Pioglitazone (2 mg/kg) | 291.83 ± 20.61 | 91.16 ± 7.28 | 67.60 ± 3.88 | 0.75 ± 0.02 |
| Ethanol extract of cashew leaves (100 mg/kg) | 222.19 ± 23.23 | 66.29 ± 4.27 | 66.93 ± 3.96 | 0.66 ± 0.01a |
Each of the values are expressed as mean ± S.E.M., n = 6.
Significantly different from control, p < 0.05.
Studies indicate that there is interlink and interplay of various pathological factors between liver and kidneys in diabetic conditions.29 However, no studies report that the toxicities caused to liver and kidneys due to STZ are related to the levels of blood glucose.30, 31 It is reported in studies that STZ has a non-specific action on the destruction of vasculature of other body organs, besides pancreas. The exogenous insulin is reported to accumulate more in the blood of experimental animals, after the destruction of pancreatic cells. Researchers suggested that the accumulation of insulin in the blood may be result of impaired renal and hepatic functions.32
Publications focusing on hepatic destruction in STZ induced diabetic rat models report disorientation in the cellular structure of liver in diabetic rats. The structural degeneration includes deposition of glycogen, location of nucleus at the periphery of cell membrane and acidophilic cytoplasm. The livers of diabetic rats are found to have necrotic cells, microvascular vacuolization and hydropic inflammation.33
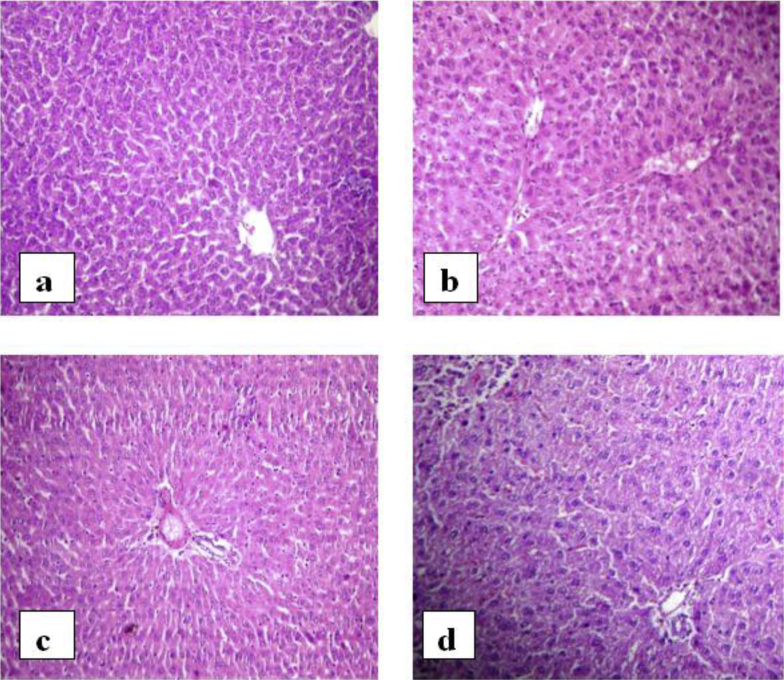
In the histopathological investigations of the present study, the liver of control rats were found to be divided into the classical hepatic lobules; each formed of cords of hepatocytes radiating from the central vein to the periphery of the lobule. The cell cords were separated by narrow blood sinusoids. The histopathological examination of diabetic rats showed periportal necrosis of the hepatocytes near the portal areas. The liver also showed dilated and congested portal vessels as well as areas of inflammatory cell infiltration. In diabetic rats treated with standard Pioglitazone liver of control rats appears to be divided into the classical hepatic lobules; each is formed of cords of hepatocytes radiating from the central vein to the periphery of the lobule. The cell cords were separated by narrow blood sinusoids as in normal control rat. In diabetic rats treated with extract of leaves, the liver architecture appears more or less like control (Fig. 1).
Fig. 1.
Photomicrographs of liver. a) Normal rat, b) diabetic rat, c) diabetic rats treated with standard drug, d) diabetic rat treated with ethanol extract of leaves.
It is well known that diabetic nephropathy is one of the complications caused during progression of diabetes. The nephropathy characteristics are atrophy of glomerular cells, thickening of basement membrane of glomerular cells and hypertrophy. The tubular interstitium cells are often reported to have an accumulation of extracellular matrix constituents.34 The inflammatory reactions, degeneration of tubules in the kidney cortex coupled with hyperglycemia and hyperlipidemia lead to renal damage.35 Prolonged existence of these complications lead to acute inflammation in the kidneys of type 2 diabetic animals. The diabetic nephropathy is characterized by necrosis of tubules, atrophy of glomerulus, and capillary congestions. Extended periods of hyperlipidemia and hyperglycemia elevate oxidative stress conditions in-vivo. These conditions can be controlled by use of anti-oxidant rich compounds.36
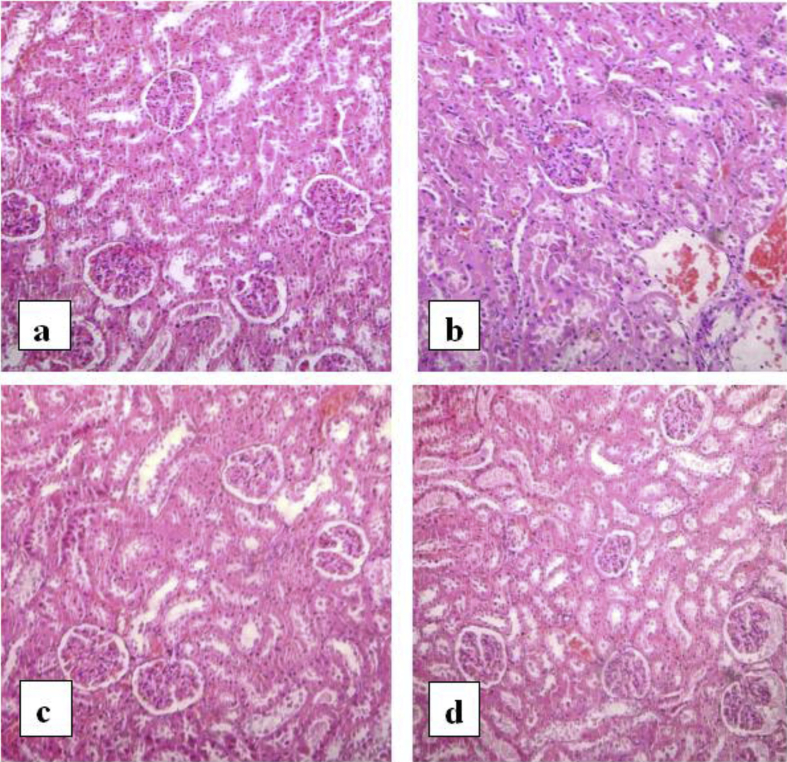
Examination of the kidney of the normal control rats revealed normal glomeruli with thin glomerular basement membranes, normal cellularity and patent capsular space surrounded by proximal and distal were normal. Light microscopy of the kidney sections from diabetic rats showed an increase in the mesangial cell and matrix of the glomeruli and hyalinization of the arterioles. In diabetic rats treated with pioglitazone, the kidney architecture appears more or less like normal control. In diabetic rats treated with leaves extract, the kidney architecture appears more or less like normal control with the exception of some inflammatory infiltration that appeared in the interstitium (Fig. 2).
Fig. 2.
Photomicrographs of kidney. a) Normal rat, b) diabetic rat, c) diabetic rats treated with standard drug, d) diabetic rat treated with ethanol extract of leaves.
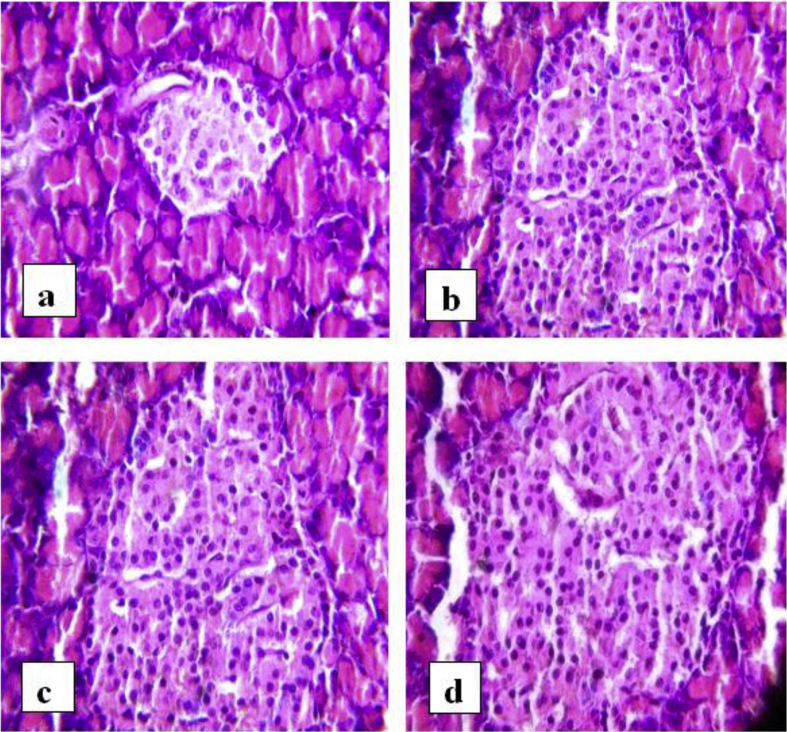
The hepatic and renal processes contribute significantly to the vital functions of excretion and elimination and pancreas contributes in metabolic regulation. The structural changes in pancreas reflect changes in metabolic processes of secretion; sensitivity and regulation of insulin.33 Atrophy of islets, decrease in the beta cells, and cellular degeneration are indicating features of pancreatic destruction. Fat and amyloid tissue deposition occurs in the islets, and the number of beta cells is drastically reduced in terminal stages of diabetes.33 In the pancreas of normal control rats of this present study, many round and elongated islets were evenly distributed throughout the cytoplasm, with their nucleus lightly stained than the surrounding acinar cells. In diabetic rats, the islets were damaged, shrunken in size and infiltration of lymphocytes was observed. In rats treated with plant extracts and standard Pioglitazone, islets were comparable to normal rats and there was not much shrinkage in size of the islet although slight damage was observed (Fig. 3).
Fig. 3.
Photomicrographs of pancreas. a) Normal rat, b) diabetic rat, c) diabetic rats treated with standard drug, d) diabetic rat treated with ethanol extract of leaves.
Compared to the other existing models of diabetes in rats, the neonatal STZ rat model is considered to be better for elucidation of the mechanisms related to renal, hepatic and pancreatic functions in type 2 diabetes.37 The ethnobotanical data for the leaves of A. occidentale, which are traditionally used by the South Cameroon population against type 2 diabetes, is confirmed in our ethno pharmacological studies. Our results signify that the n-STZ diabetic animal group developed a moderate type 2 diabetes; nevertheless the animals were in better conditions (with lower blood-sugar concentrations); which confirms that the (n-STZ) model is appropriate for study on type 2 diabetes.
4. Conclusion
It can be concluded that the traditional use of A. occidentale as a hypoglycaemic agent is justified. The extracts from the leaves of this plant show a significant activity which is comparable to the standard hypoglycemic drug pioglitazone.
Conflict of interest
None declared.
Acknowledgements
The authors are thankful to the Indian Council of Medical Research, India for funding this project.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Word Health Organization 2006. http://www.who.org web page: (accessed February, 2012)
- 2.Inzucchi S. Classification and diagnosis of diabetes mellitus. In: Porte D., Sherwin R., Baron A., editors. Elenberg and Rifkin's Diabetes Mellitus. McGraw Hill; New York: 2003. [Google Scholar]
- 3.Paris R., Plat M., Giono-Barder P., Linhard J., et Laurens A. Recherche chimique et pharmacologique sur les feuilles d’Anacardium occidentale L. (Anacardiace'es) Bull la Société deMédecine d'Afrique Noire Lang Française. 1977;22:275–281. [PubMed] [Google Scholar]
- 4.Felcman J., Braganca M.L. Chromium in plants: comparison between the concentration of chromium in Brasilian nonhypo and hypoglycemic plants. Biol Trace Elem Res. 1987;17:11–16. doi: 10.1007/BF02795443. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M., Arias T., Correa M., Lamba S. Ethnopharmacognostic observations on Panamanian medicinal plants. Part I Q J Crude Drug Res. 1979;17:115–130. [Google Scholar]
- 6.Goncalves J., Lopes R., Oliveira D. In vitro anti-rotavirus activity of some medicinal plants used in Brazil against diarrhea. J Ethnopharmacol. 2005;99:403–407. doi: 10.1016/j.jep.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Schmourloa G., Ricardo R., Mendonca F., Celuta S., Sonia S. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol. 2005;96:563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Wedson S., Mourãob J., Raynner B., Alvesb R. Parallels between zootherapeutic practices in ethnoveterinary and human complementary medicine in northeastern Brazil. J Ethnopharmacol. 2011;134:753–767. doi: 10.1016/j.jep.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Iwu M.M. CRC Press; Boca Raton, FL: 1993. Handbook of African Medicinal Plants. [Google Scholar]
- 10.Oliver-Bever B. Cambridge University Press; London: 1986. Medicinal Plants in Tropical West Africa. [Google Scholar]
- 11.Coe F.G., Anderson G.J. Ethnobotany of the Garifuna of Eastern Nicaragua. Econ Bot. 1996;50:71–108. [Google Scholar]
- 12.Gill L., Akinwumi C. Nigerian folk medicine: practices and beliefs of the Ondo people. J Ethnopharmacol. 1986;18:259–266. doi: 10.1016/0378-8741(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 13.Barrett B. Medicinal plants of Nicaragua's Atlantic coast. Econ Bot. 1994;48:8–20. [Google Scholar]
- 14.Konan N., Bacchi E., Lincopan N., Varela S., Varanda E. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.) J Ethnopharmacol. 2007;110:30–38. doi: 10.1016/j.jep.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Carvalhoa A., Annonia R., Silva P. Acute, subacute toxicity and mutagenic effects of anacardic acids from cashew (Anacardium occidentale Linn.) in mice. J Ethnopharmacol. 2011;135:730–736. doi: 10.1016/j.jep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Kamtchouing P., Selestin D., Sokeng P., Moundipa P., Hermine B., Lontsi D. Protective role of Anacardium occidentale extract against streptozotocin-induced diabetes in rats. J Ethnopharmacol. 1998;62:95–99. doi: 10.1016/s0378-8741(97)00159-1. [DOI] [PubMed] [Google Scholar]
- 17.Arya R., Babu V., Ilyas M., Nasim K.T. Phytochemical examination of the leaves of Anacardium occidentale. J Indian Chem Soc. 1989;66:67–68. [Google Scholar]
- 18.Laurens A., Paris R.R. Sur les polyphe'nols d’Anacardiacées Africaines et Malgaches: Poupartia birrea Aub., Poupartia caffra H. Perr. et Anacardium occidentale L. Plantes Médicinales Phytothérapie. 1977;11:16–24. [Google Scholar]
- 19.Swarnalakshni T., Gomathi K., Sulschana N., Baskar E.A., Parmar N.S. Anti-inflammatory activity of (-) epicatechin, a biflavonoid isolated from Anacardium occidentale Linn. Indian J Pharm Sci. 1981;43:205–208. [Google Scholar]
- 20.Verspohl E.J. Recommended testing in diabetes research. Planta Medica. 2000;68:581–590. doi: 10.1055/s-2002-32894. [DOI] [PubMed] [Google Scholar]
- 21.OECD/OCDE 423, OECD Guideline for Testing of Chemicals, Acute Oral Toxicity – Acute Toxic Class Method, Environment Directorate Organisation For Economic Co-Operation And Development, Paris, (Adopted: 17th December 2001).
- 22.Ogbonnia S.O., Mbaka G.O., Igbokwe N.H., Anyika E.N., Nwakakwu N. Antimicrobial evaluation, acute and subchronic toxicity studies of Leone Bitters, a Nigerian polyherbal formulation in rodents. Agric Biol J N Am. 2010;1:366–376. [Google Scholar]
- 23.Saha P., Mazumber U.K., Halder P.K., Islam A., Kumar S.R.B. Evaluation of acute and subchronic toxicity of Lagenaria siceraria aerial part. Int J Pharm Sci Res. 2011;2:1507–1512. [Google Scholar]
- 24.Nandy S., Datta R. Acute and subacute toxicity studies of methanolic leaves extract of Pterospermum acerifolium L. wild in rodents. Int J Pharm Life Sci. 2012;3:1519–1529. [Google Scholar]
- 25.Kulkarni C.P., Bodhankar S.L., Ghule A.K., Mohan V., Thakurdesai P.A. Antidiabetic activity of trigonella foenumgraecum l. Seeds extract (ind01) in neonatal streptozotocin-induced (n-stz) rats. Diabetol Croat. 2012;41:29–40. [Google Scholar]
- 26.Patil M.A., Suryanarayana P., Putcha U.K., Srinivas M., Reddy G.B. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/463264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoguchi T., Li P., Umeda F. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi R., Nikfar S., Larijani B., Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Poretsky L. Springer; Boston, MA, USA: 2010. Principles of Diabetes Mellitus. [Google Scholar]
- 30.Palm F., Ortsater H., Hansell P., Liss P., Carlsson P.O. Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin-diabetic rat model. Diabetes Metab Res Rev. 2004;20:452–459. doi: 10.1002/dmrr.472. [DOI] [PubMed] [Google Scholar]
- 31.Rauter A.P., Martins A., Borges C. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother Res. 2010;24(suppl 2):S133–S138. doi: 10.1002/ptr.3017. [DOI] [PubMed] [Google Scholar]
- 32.Qinna N.A., Badwan A.A. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Dev Ther. 2015;9:2515–2525. doi: 10.2147/DDDT.S79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J.Y., Zhou S.W., Zhang K.B. Chronic effects of berberine on blood, liver glycolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull. 2008;31:1169–1176. doi: 10.1248/bpb.31.1169. [DOI] [PubMed] [Google Scholar]
- 34.Lane T.A., Lamkin G.E., Wancewicz E.V. Protein kinase C inhibitors block the enhanced expression intercellular adhesion molecule-1 on endothelial cells activated by interleukin-1, lipopolysaccharide and tumor necrosis factor. Biochem Biophys Res Commun. 1990;172:1273–1281. doi: 10.1016/0006-291x(90)91587-i. [DOI] [PubMed] [Google Scholar]
- 35.Leegwater D.C., Kuper C.F. Evaluation of histological changes in the kidneys of the alloxan diabetic rat by means of factor analysis. Food Chem Toxicol. 1984;22:551–557. doi: 10.1016/0278-6915(84)90226-6. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigo R., Bosso C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C. 2006;142:317–327. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–472. [PubMed] [Google Scholar]