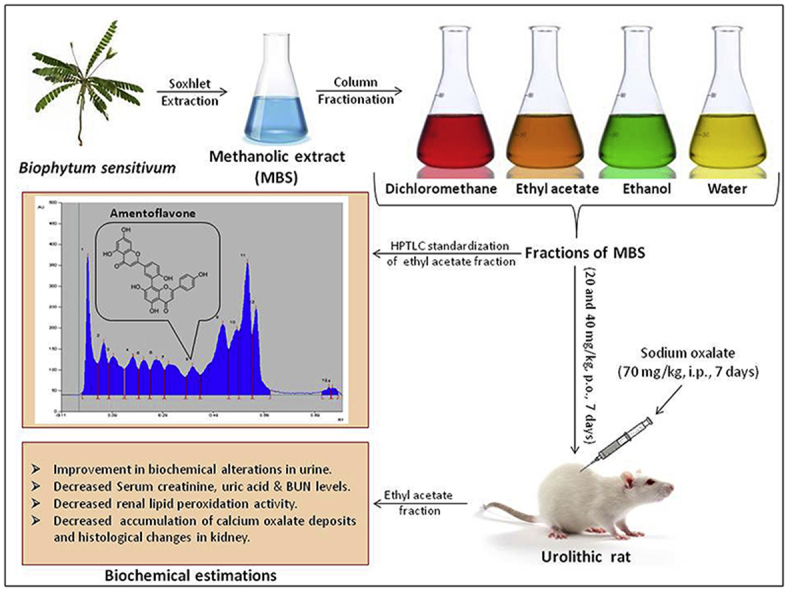
Abstract
The methanolic whole plant extract of Biophytum sensitivum (感应草gǎnyìng cǎo) has been found to possess antiurolithiatic effect. The present study was undertaken to evaluate the antiurolithiatic effect of some fractions of methanolic whole plant extract of B. sensitivum (MBS) in rats as a step toward activity-directed isolation of antiurolithiatic component. The MBS was successively extracted with dichloromethane, ethyl acetate, ethanol and water to obtain fractions. Sodium oxalate (70 mg/kg, i.p.) was administered to rats for seven days to develop calcium oxalate urolithiasis. These rats were treated with two doses (20 and 40 mg/kg, p.o.) of the fractions, 1 h before sodium oxalate injections. Antiurolithiatic activity was assessed by estimating biochemical changes in urine, serum and kidney homogenate along with histological changes in kidney tissue. Sodium oxalate administration caused biochemical alterations in urine which was found to be prevented significantly by the ethyl acetate fraction. Supplementation with ethyl acetate fraction prevented the elevation of serum creatinine, uric acid and blood urea nitrogen levels. The elevated calcium, oxalate and phosphate levels in the kidney tissue homogenate of lithiatic rats were significantly reduced by the treatment with ethyl acetate fraction. The ethyl acetate fraction also caused significant decrease in lipid peroxidation activity, accumulation of calcium oxalate deposits and histological changes in the kidney tissue. The results showed that the antiurolithiatic component of the methanolic whole plant extract of the plant is contained in the ethyl acetate fraction. The effect is attributed to its diuretic, antioxidant, nephroprotective properties and effect on lowering the concentration of urinary stone-forming constituents.
Keywords: Biophytum sensitivum, Ethyl acetate fraction, Oxalidaceae, Sodium oxalate, Urolithiasis
Graphical abstract
1. Introduction
Urolithiasis i.e. formation of urinary calculi (stone) anywhere in the urinary tract is one of the most painful and third prevalent ailments, has beset humans from centuries.1 It is reported as complaint with an increasing incidence and prevalence worldwide. It is estimated that it affect about 12% of world population and expected to rise further with the advancement in the industrialization.2, 3, 4 This increased incidence of urinary stones over the last few years, are associated with decrease in age of onset has an important effect on the healthcare system.5 Recurrence is another major factor that makes it more serious issue to address. On recurrence, the subsequent relapse risk is raised and the interval between recurrences is shortened.6 Common features associated with recurrence include a young age of onset, family history, frequent infections and underlying medical conditions.7
The current medical management of urolithiasis involves administration of symptomatic drugs like diuretics, alkanizers, anti-inflammatory etc., and other techniques like extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy.7, 8, 9 However, these treatment options have certain hurdles such as limited therapeutic outcome, comparatively high cost and chances of frequent recurrence.10 Furthermore, they also causes wide range of undesirable effects such as hemorrhage, hypertension, tubular necrosis followed by subsequent fibrosis of the kidney leading to cell injury.11 The continuous research is going on to overcome these drawbacks and to develop more suitable and safe treatment options, however till today no satisfactory therapy has been developed that indicates need for effective alternative remedy for the prevention and treatment of urolithiasis.
In the Indian traditional systems of medicine i.e. Ayurveda, a number of plants have been claimed to be efficient to cure and correct urinary stones.12 These plants and their products are also reported to be effective in the treatment as well as prevention of recurrence of renal calculi with minimal or no side effects.10 In Ayurveda, the whole plant of Biophytum sensitivum (感应草gǎnyìng cǎo) is claimed for its usefulness in the treatment of various health aliments including urinary stones.13, 14 B. sensitivum (感应草gǎnyìng cǎo) belonging to family oxalidaceae is distributed in tropical Asia, Africa, America and Philippines. It has been extensively studied for its analgesic, anti-pyretic, anti-inflammatory, immunomodulatory, antitumor, antidiabetic, antioxidant, antibacterial, antihypertensive, chemoprotective, radioprotective and antifertility effects.15 We have previously reported antiurolithiatic potential of B. sensitivum (感应草gǎnyìng cǎo) against zinc disc implantation-induced lithiasis and ethylene glycol and ammonium chloride-induced lithiasis in rats.16 The antiurolithiatic constituent of the plant is yet to be isolated. The present study was carried out to evaluate antiurolithiatic activity of fractions of B. sensitivum (感应草gǎnyìng cǎo) against sodium oxalate-induced calcium oxalate urolithiasis in rats in order to determine the fraction containing antiurolithiatic component of the plant.
2. Materials and methods
2.1. Collection and extraction of plant materials
The plant material was identified, collected and thereafter extracted as previously reported.16 Briefly, whole plant material of B. sensitivum (感应草gǎnyìng cǎo) was collected from the local region of Pune, India. It was authenticated by Dr. J. Jayanthi, Scientist, Botanical Survey of India, Pune, India. The plant material i.e. whole plant of B. sensitivum (感应草gǎnyìng cǎo) was washed and dried in shade for 10–15 days. The dried whole plant was coarsely powdered, packed into soxhlet column and extracted with 70% v/v methanol in water for 22 h. This methanolic extracts of plant (MBS) was then evaporated at 45 °C and then dried in oven. The dried extract was stored in airtight container.
2.2. Fractionation of extract
The MBS was subjected to fractionation by earlier reported method.17 In brief, 5 g of MBS was fractioned by filter column chromatography over 100 g silica gel 60–120 (S), and eluted with approximately 3 L of solvents dichloromethane, ethyl acetate, ethanol, and water, in the order of increasing polarity, until a clear elute was obtained at the end of the elution. The collected elutes were subjected to evaporation at 45 °C to obtain dichloromethane (DCM-MBS), ethyl acetate (EA-MBS), ethanol (E-MBS), and water (AQ-MBS) fractions of MBS. Fractions were stored at 4 °C until assayed.
2.3. Evaluation of antiurolithiatic activity of fractions
All fractions (i.e. DCM-MBS, EA-MBS, E-MBS and AQ-MBS) were evaluated for antiurolithiatic activity at the dose levels of 20 and 40 mg/kg (p.o.),16 for seven days treatment,18, 19 against sodium oxalate-induced calcium oxalate urolithiasis in rats.
2.4. Animals
Male Wistar albino rats weighing between 150–200 g were used for the study. They were procured from National Institute of Biosciences, Pune, India. The rats were allowed for acclimatization for ten days under standard conditions in the CPCSEA approved animal house of MAEER's Maharashtra Institute of Pharmacy, Pune, India. The animals were given standard diet supplied by Nutrivet Life Sciences, Pune, India. The study protocol was approved by the Institutional Animal Ethics Committee (Ref. No.: MIP/IAEC/2014-15/M1/Appr/004) of MAEER's Maharashtra Institute of Pharmacy, Pune, India.
2.5. Chemicals and apparatus
Sodium oxalate (Qualigens Fine Chemicals, India) was used for the study. All other chemicals and reagents used were of analytical grade and procured from approved vendors. Apparatus such as the metabolic cages (New Neeta Chemicals, India), cold centrifuge (BioEra, India), semi-automated clinical analyzer (Avantor Performance Materials, India) and UV-spectrophotometer (LabIndia, India) were used in the study.
2.6. Experimental design
Sodium oxalate-induced calcium oxalate urolithiasis model was used to evaluate antiurolithiatic effect of fractions of MBS in rats.18, 19 Animals were randomly divided into ten groups each containing six animals. Group I served as vehicle control, animals of this group were maintained on standard rat food and drinking water ad libitum and were received vehicle, i.e. 0.5% w/v gum acacia solution (5 ml/kg, p.o.). All remaining groups received calculi inducing treatment, comprised of sodium oxalate (70 mg/kg, i.p.) administration for seven days. Group II served as lithiatic control and received 0.5% w/v gum acacia solution (5 ml/kg, p.o.). Groups III and IV served as DCM-MBS treatment groups and received DCM-MBS at doses of 20 and 40 mg/kg respectively for seven days. Groups V and VI served as EA-MBS treatment groups and received EA-MBS at doses of 20 and 40 mg/kg respectively for seven days. Groups VII and VIII served as E-MBS treatment groups and received E-MBS at doses of 20 and 40 mg/kg respectively for seven days. Groups IX and X served as AQ-MBS treatment groups and received AQ-MBS at doses of 20 and 40 mg/kg respectively for seven days. The fractions were suspended in distilled water using 0.5% w/v gum acacia solution and given once daily by oral route (5 ml/kg body weight) 1 h prior to sodium oxalate challenge.
2.7. Collection and analysis of urine
All animals were kept in individual metabolic cages and 24 h urine samples were collected on 0 and 7th day of experimental period. The pH of freshly collected urine sample was measured and 24 h urine samples were analyzed for its volume, calcium, oxalate,20 phosphate, magnesium,21, 22 uric acid, citrate,23 and total protein contents. Commercial kits for estimating urinary levels of calcium (Transasia Bio-medicals Ltd., India), phosphate (Coral Clinical Systems, India), uric acid (Transasia Bio-medicals Ltd., India) and total protein (Transasia Bio-medicals Ltd., India) were used according to the manufacturer's protocol.
2.8. Serum analysis
After urine collection, blood was collected from retro-orbital sinus under ether anesthesia. Serum was separated by centrifugation at 10,000 ×g for 10 min and analyzed for creatinine, uric acid and blood urea nitrogen (BUN) levels by using commercial kits (Transasia Bio-medicals Ltd., India).
2.9. Kidney tissue analysis
After blood collection, all rats were sacrificed by cervical dislocation. The abdomen was cut open to remove both kidneys from each animal. Isolated kidneys were cleaned-off extraneous tissue, rinsed in ice-cold physiological saline and used for tissue homogenate and histopathological analysis.
2.10. Homogenate analysis
The left kidney was finely minced and 20 % homogenate was prepared in Tris–Hcl buffer (0.02 mol/l, pH 7.4). The kidney homogenate was used to analyze tissue calcium, oxalate,20 phosphate and lipid peroxidation24 levels. Commercial kits were used for estimating renal levels of calcium (Transasia Bio-medicals Ltd., India) and phosphate (Coral Clinical Systems, India) according to the manufacturer's protocol.
2.11. Histopathology
The right kidney was fixed in 10% neutral buffered formalin, processed in a series of graded alcohol and xylene, embedded in paraffin wax, sectioned at 5 μm and stained with Hematoxylin and Eosin for examination under light microscope to estimate damage index (DI). The histological changes such as congestion, hemorrhages, focal tubular swelling, granular and vacuolar changes in cytoplasm, tubular degeneration, sloughing of tubular epithelium, cystic tubular changes, necrotic changes of tubular epithelium, infiltration of mononuclear cells, glomerular changes and interstitial fibrosis were observed for damage index with semiquantitative grading system as grade 0-no visible changes, grade 1-minimal changes (<25%), grade 2-mild changes (25%–50%), grade 3-moderate changes (51%–75%) and grade 4-severe changes (>75%). The kidney section was also stained by method reported by Pizzolato method,25 which selectively stains calcium oxalate crystals, and observed under light microscope to estimate total number of calcium oxalate deposits.
To estimate DI and number of calcium oxalate deposits, a saggital section of each renal specimen was divided into 8 equal sized regions by four virtual lines according to previously reported method.26 A 100x field of Hematoxylin and Eosin or Pizzolato stained slide was then randomly selected from each region, graded for damage index or number calcium oxalate deposits. The average of eight readings was reported as damage index and number of calcium oxalate deposits for each specimen.
2.12. HPTLC analysis of EA-MBS and E-MBS
The most effective fractions i.e. EA-MBS and E-MBS were standardized for the content of marker compound, amentoflavone by HPTLC method.27 In brief, sample solutions were applied on pre-washed and activated pre-coated silica gel aluminium HPTLC plate 60F254 (20 cm × 10 cm with 250 μm thickness) in the form of band of 6 mm width with a Camag syringe (100 μl) using a Camag Linomat V sample applicator. HPTLC plates were then developed with 20 ml mobile phase consisting of toluene:ethyl formate:formic acid (5:4:1, v/v/v). Linear ascending development was carried out in 20 cm × 10 cm twin trough glass chamber saturated with the solvent system. The chamber saturation time for solvent system was 15 min at room temperature 25 ± 2 °C and relative humidity of 60 ± 5%. After chromatography, plates were dried in an air current. Densitometric scanning was performed by using Camag TLC scanner III with winCATS software version 1.4.4 at 366 nm.
2.13. Statistical analysis
All the results were expressed as mean ± standard error of mean (SEM). The statistical significance between lithiatic control group and vehicle control group was calculated by student's t-test, whereas one-way analysis of variance (ANOVA) followed by Dunnett's comparison test was used to calculate statistical significance between fraction-treated groups and lithiatic control group. p < 0.05 was considered significant.
3. Results
3.1. Fractionation of MBS
The yield of DCM-MBS, EA-MBS, E-MBS and AQ-MBS were found to be 5.80, 4.80, 2.80 and 7.20% w/w respectively.
3.2. Effect on urine parameters
The basal urinary parameters i.e. urine volume and urinary excretion of calcium, oxalate, phosphate, total protein, uric acid, magnesium and citrate of rats were found to be equivalent (Table 1).
Table 1.
Effect of MBS-fractions on urinary parameters in urolithiasis-induced rats.
| Days | Vehicle control | Lithiatic control | DCM-MBS (20 mg/kg) | DCM-MBS (40 mg/kg) | EA-MBS (20 mg/kg) | EA-MBS (40 mg/kg) | E-MBS (20 mg/kg) | E-MBS (40 mg/kg) | AQ-MBS (20 mg/kg) | AQ-MBS (40 mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (ml/24 h) | ||||||||||
| 0 | 6.43 ± 0.46 | 6.28 ± 0.57 | 5.95 ± 0.58 | 6.08 ± 0.61 | 6.00 ± 0.65 | 6.15 ± 0.58 | 6.23 ± 0.58 | 6.10 ± 0.58 | 6.33 ± 0.48 | 6.45 ± 0.53 |
| 7 | 6.20 ± 0.52 | 6.10 ± 0.50 | 10.07 ± 1.37 | 14.20 ± 2.36∗ | 10.53 ± 0.83 | 13.78 ± 2.16∗ | 10.20 ± 1.25 | 17.73 ± 2.85∗∗∗ | 12.47 ± 1.32 | 21.92 ± 2.02∗∗∗ |
| Oxalate (mg/24 h) | ||||||||||
| 0 | 4.55 ± 0.28 | 4.48 ± 0.26 | 4.47 ± 0.31 | 4.60 ± 0.26 | 4.59 ± 0.23 | 4.54 ± 0.31 | 4.52 ± 0.27 | 4.43 ± 0.28 | 4.57 ± 0.25 | 4.48 ± 0.27 |
| 7 | 4.46 ± 0.25 | 6.34 ± 0.32††† | 6.30 ± 0.36 | 6.20 ± 0.29 | 4.94 ± 0.34∗ | 4.60 ± 0.27∗∗ | 5.41 ± 0.30 | 4.90 ± 0.33∗ | 5.96 ± 0.30 | 5.55 ± 0.36 |
| Calcium (mg/24 h) | ||||||||||
| 0 | 0.49 ± 0.02 | 0.51 ± 0.03 | 0.53 ± 0.02 | 0.50 ± 0.02 | 0.53 ± 0.02 | 0.50 ± 0.02 | 0.54 ± 0.02 | 0.51 ± 0.03 | 0.50 ± 0.02 | 0.52 ± 0.02 |
| 7 | 0.54 ± 0.02 | 0.33 ± 0.02††† | 0.33 ± 0.02 | 0.31 ± 0.02 | 0.40 ± 0.02∗ | 0.50 ± 0.02∗∗∗ | 0.39 ± 0.02 | 0.43 ± 0.02∗∗ | 0.35 ± 0.02 | 0.41 ± 0.02∗ |
| Phosphate (mg/24 h) | ||||||||||
| 0 | 5.78 ± 0.11 | 5.43 ± 0.12 | 5.48 ± 0.11 | 5.51 ± 0.15 | 5.58 ± 0.14 | 5.45 ± 0.13 | 5.56 ± 0.13 | 5.56 ± 0.13 | 5.69 ± 0.16 | 5.74 ± 0.13 |
| 7 | 5.45 ± 0.11 | 6.88 ± 0.22††† | 6.79 ± 0.17 | 6.64 ± 0.22 | 5.91 ± 0.13∗∗ | 5.61 ± 0.15∗∗∗ | 6.11 ± 0.26∗ | 5.89 ± 0.19∗∗ | 6.37 ± 0.17 | 6.04 ± 0.12∗ |
| Total protein (mg/24 h) | ||||||||||
| 0 | 4.01 ± 0.20 | 3.90 ± 0.34 | 3.67 ± 0.52 | 3.71 ± 0.54 | 3.84 ± 0.50 | 4.00 ± 0.39 | 3.84 ± 0.41 | 4.04 ± 0.36 | 3.90 ± 0.25 | 3.98 ± 0.30 |
| 7 | 3.67 ± 0.43 | 6.06 ± 0.35†† | 6.08 ± 0.26 | 5.38 ± 0.59 | 3.93 ± 0.15∗∗∗ | 3.41 ± 0.22∗∗∗ | 4.72 ± 0.38∗ | 3.80 ± 0.24∗∗∗ | 4.78 ± 0.33 | 4.08 ± 0.28∗∗∗ |
| Uric acid (mg/24 h) | ||||||||||
| 0 | 1.41 ± 0.06 | 1.43 ± 0.05 | 1.40 ± 0.06 | 1.39 ± 0.06 | 1.36 ± 0.07 | 1.38 ± 0.06 | 1.36 ± 0.05 | 1.40 ± 0.06 | 1.38 ± 0.06 | 1.41 ± 0.05 |
| 7 | 1.38 ± 0.05 | 1.75 ± 0.07†† | 1.78 ± 0.08 | 1.74 ± 0.07 | 1.49 ± 0.06 | 1.40 ± 0.07∗∗ | 1.57 ± 0.05 | 1.48 ± 0.06∗ | 1.71 ± 0.06 | 1.55 ± 0.06 |
| Magnesium (mg/24 h) | ||||||||||
| 0 | 2.47 ± 0.08 | 2.51 ± 0.07 | 2.46 ± 0.06 | 2.44 ± 0.06 | 2.39 ± 0.07 | 2.42 ± 0.07 | 2.46 ± 0.08 | 2.44 ± 0.07 | 2.45 ± 0.07 | 2.51 ± 0.08 |
| 7 | 2.44 ± 0.11 | 1.82 ± 0.08††† | 1.80 ± 0.07 | 1.84 ± 0.07 | 2.23 ± 0.07∗∗ | 2.35 ± 0.06∗∗∗ | 2.11 ± 0.06∗ | 2.25 ± 0.10∗∗∗ | 1.89 ± 0.07 | 2.09 ± 0.07 |
| Citrate (mg/24 h) | ||||||||||
| 0 | 5.24 ± 0.23 | 5.32 ± 0.24 | 5.26 ± 0.21 | 5.35 ± 0.28 | 5.26 ± 0.13 | 5.53 ± 0.26 | 5.47 ± 0.24 | 5.30 ± 0.19 | 5.16 ± 0.25 | 5.28 ± 0.22 |
| 7 | 5.35 ± 0.29 | 4.30 ± 0.21† | 4.50 ± 0.21 | 4.48 ± 0.17 | 5.01 ± 0.31 | 5.31 ± 0.22∗ | 4.51 ± 0.19 | 5.45 ± 0.24∗∗ | 4.74 ± 0.23 | 5.21 ± 0.29 |
Values are expressed as mean ± SEM, n = 6, †p < 0.05; ††p < 0.01; †††p < 0.001 vs. vehicle control, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. lithiatic control. Values of lithiatic control group were compared with vehicle control and those of drug-treated groups with lithiatic control. SEM = Standard error of mean.
The induction of lithiasis did not show any significant alteration in the urine volume as compared to vehicle control rats (Table 1). Preventive treatment with the 40 mg/kg dose of DCM-MBS, EA-MBS, E-MBS and AQ-MBS produced significant increase in urine volume as compared to vehicle control animals. The AQ-MBS and E-MBS were found to be more potent than other fractions, in increasing urine volume (Table 1).
Calculi-inducing treatment caused significant (p < 0.001) increase in the urinary oxalate excretion from 4.46 ± 0.25 mg/24 h to 6.34 ± 0.32 mg/24 h at the end of experimental period (Table 1). This increased urinary oxalate excretion was significantly decreased by the treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) and higher dose of E-MBS (i.e. 40 mg/kg). The EA-MBS was found most effective fraction in this regard (Table 1).
The mean urinary calcium excretion of vehicle control rats was 0.54 ± 0.02 mg/24 h at the end of experimental period, which was significantly (p < 0.001) decreased to 0.33 ± 0.02 mg/24 h in the calculi-induced rats (Table 1). This decreased urinary calcium excretion was significantly increased by treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) as well as higher dose of E-MBS and AQ-MBS (i.e. 40 mg/kg). The EA-MBS was found to be most effective fraction in this regard (Table 1).
Calculi-inducing treatment caused significant (p < 0.001) increase in urinary phosphate excretion from 5.45 ± 0.11 mg/24 h to 6.88 ± 0.22 mg/24 h in the lithiatic control rats at the end of experimental period (Table 1). This increased urinary phosphate excretion was significantly decreased by preventive treatment with all doses of EA-MBS and E-MBS (i.e. 20 and 40 mg/kg) in dose dependent manner. On the other hand, only higher dose of AQ-MBS (i.e. 40 mg/kg) was found to be effective in producing significant decrease in urinary phosphate excretion as compared to lithiatic control animals. The EA-MBS was found most effective fraction in this regard (Table 1).
The mean urinary total protein excretion of vehicle control rats was 3.67 ± 0.43 mg/24 h at the end of experimental period, which was significantly (p < 0.001) increased to 6.06 ± 0.35 mg/24 h in lithiatic control rats (Table 1). This increased urinary total protein excretion was significantly decreased by preventive treatment with all doses of EA-MBS and E-MBS (i.e. 20 and 40 mg/kg) as well as higher dose of AQ-MBS (i.e. 40 mg/kg). The EA-MBS was found most effective fraction in this regard (Table 1).
Induction of lithiasis caused significant (p < 0.01) increase in urinary uric acid excretion from 1.38 ± 0.05 mg/24 h to 1.75 ± 0.07 mg/24 h at the end of experimental period (Table 1). This increased urinary uric acid excretion was significantly decreased by preventive treatment with higher dose of EA-MBS and E-MBS (i.e. 40 mg/kg). The EA-MBS was found most effective fraction in this regard (Table 1).
The mean urinary magnesium excretion was significantly (p < 0.01) decreased from 2.44 ± 0.11 mg/24 h to 1.82 ± 0.08 mg/24 h in the lithiatic control rats at the end of experimental period when compared against vehicle control rats (Table 1). This decreased urinary magnesium excretion was significantly increased by preventive treatment with all doses of EA-MBS and E-MBS (i.e. 20 and 40 mg/kg) in dose dependent manner. The EA-MBS was found most effective fraction in this regard (Table 1).
Calculi-inducing treatment caused significant (p < 0.05) decrease in urinary citrate excretion from 5.35 ± 0.29 mg/24 h to 4.30 ± 0.21 mg/24 h in the lithiatic rats at the end of experimental period as compared to vehicle control rats (Table 1). This decreased urinary citrate excretion was significantly increased by treatment with higher dose of EA-MBS and E-MBS (i.e. 40 mg/kg). The E-MBS was found most effective fraction in this regard (Table 1).
3.3. Effect on serum parameters
Basal serum creatinine, uric acid and BUN level of rats did not show any significant difference (Table 2).
Table 2.
Effect of MBS-fractions on serum parameters in urolithiasis induced rats.
| Days | Vehicle control | Lithiatic control | DCM-MBS (20 mg/kg) | DCM-MBS (40 mg/kg) | EA-MBS (20 mg/kg) | EA-MBS (40 mg/kg) | E-MBS (20 mg/kg) | E-MBS (40 mg/kg) | AQ-MBS (20 mg/kg) | AQ-MBS (40 mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | ||||||||||
| 0 | 0.63 ± 0.05 | 0.56 ± 0.03 | 0.56 ± 0.03 | 0.57 ± 0.03 | 0.61 ± 0.04 | 0.57 ± 0.02 | 0.57 ± 0.04 | 0.59 ± 0.03 | 0.59± 0.04 | 0.57 ± 0.04 |
| 7 | 0.57 ± 0.03 | 0.84 ± 0.05††† | 0.80 ± 0.06 | 0.82 ± 0.04 | 0.66 ± 0.04∗ | 0.60 ± 0.03∗∗ | 0.69 ± 0.04 | 0.64 ± 0.03∗ | 0.76 ± 0.05 | 0.68 ± 0.04 |
| Uric acid (mg/dl) | ||||||||||
| 0 | 1.35 ± 0.04 | 1.39 ± 0.03 | 1.36 ± 0.04 | 1.38 ± 0.04 | 1.41 ± 0.03 | 1.42 ± 0.03 | 1.39 ± 0.03 | 1.36 ± 0.03 | 1.38 ± 0.03 | 1.33 ± 0.02 |
| 7 | 1.33 ± 0.04 | 1.75 ± 0.05††† | 1.80 ± 0.06 | 1.85 ± 0.04 | 1.48 ± 0.05∗∗ | 1.40 ± 0.06∗∗∗ | 1.63 ± 0.06 | 1.50 ± 0.04∗∗ | 1.69 ± 0.06 | 1.54 ± 0.03∗ |
| BUN (mg/dl) | ||||||||||
| 0 | 14.80 ± 0.42 | 15.81 ± 0.47 | 15.74 ± 0.51 | 15.81 ± 0.45 | 16.46 ± 0.70 | 16.15 ± 0.46 | 14.54 ± 0.44 | 16.16 ± 0.61 | 14.95 ± 0.46 | 14.85 ± 0.51 |
| 7 | 15.03 ± 0.51 | 38.84 ± 2.01††† | 35.40 ± 1.88 | 30.83 ± 1.97 | 27.06 ± 2.13∗∗ | 22.83 ± 1.45∗∗∗ | 32.84 ± 2.22 | 26.92 ± 2.39∗∗ | 36.89 ± 2.52 | 27.80 ± 2.41∗∗ |
Values are expressed as mean ± SEM, n = 6, †p < 0.05; ††p < 0.01; †††p < 0.001 vs. vehicle control, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. lithiatic control. Values of lithiatic control group were compared with vehicle control and those of drug-treated groups with lithiatic control. BUN = Blood urea nitrogen, SEM = Standard error of mean.
Serum creatinine level of vehicle control rats was 0.57 ± 0.03 mg/dl, which was significantly (p < 0.001) increased to 0.84 ± 0.05 mg/dl in the lithiatic rats at the end of experimental period (Table 2). This increased serum creatinine level was significantly decreased by preventive treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) and higher dose of E-MBS (i.e. 40 mg/kg). The EA-MBS was found to be most effective in this regard (Table 2).
At the end of experimental period, serum uric acid level was significantly (p < 0.001) increased from 1.33 ± 0.04 mg/dl to 1.75 ± 0.05 mg/dl in lithiatic control rats when compared against vehicle control rats (Table 2). This increased serum uric acid level was significantly decreased by treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) as well as higher dose of E-MBS and AQ-MBS (i.e. 40 mg/kg). The EA-MBS was found to be most effective fraction in this regard (Table 2).
Calculi-inducing treatment caused significant (p < 0.001) increase in serum BUN level from 15.03 ± 0.51 mg/dl to 38.84 ± 2.01 mg/dl in the lithiatic control rats at the end of experimental period as compared to vehicle control rats (Table 2). This increased serum BUN level was significantly decreased by preventive treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) as well as higher dose of E-MBS and AQ-MBS (i.e. 40 mg/kg). The EA-MBS was found to be most effective fraction in this regard (Table 2).
3.4. Effect on renal parameters
The mean calcium level in kidney tissue homogenate of vehicle control rats was 0.16 ± 0.01 mg/g, which was significantly increased to 0.25 ± 0.02 mg/g in the lithiatic control rats (Table 3). This increased calcium level was significantly decreased by the treatment with all doses of EA-MBS and E-MBS (i.e. 20 and 40 mg/kg) in dose dependent manner. The EA-MBS was found to be most effective in this regard (Table 3).
Table 3.
Effect of MBS-fractions on renal parameters in urolithiasis induced rats.
| Parameters | Vehicle control | Lithiatic control | DCM-MBS (20 mg/kg) | DCM-MBS (40 mg/kg) | EA-MBS (20 mg/kg) | EA-MBS (40 mg/kg) | E-MBS (20 mg/kg) | E-MBS (40 mg/kg) | AQ-MBS (20 mg/kg) | AQ-MBS (40 mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Calcium (mg/g) | 0.16 ± 0.01 | 0.25 ± 0.02†† | 0.27 ± 0.03 | 0.28 ± 0.02 | 0.17 ± 0.02∗ | 0.14 ± 0.01∗∗ | 0.17 ± 0.01∗ | 0.14 ± 0.03∗∗ | 0.21 ± 0.02 | 0.18 ± 0.02 |
| Oxalate (mg/g) | 1.24 ± 0.09 | 3.53 ± 0.19††† | 3.48 ± 0.16 | 3.56 ± 0.13 | 1.82 ± 0.13∗∗∗ | 1.60 ± 0.12∗∗∗ | 2.49 ± 0.12∗∗∗ | 2.25 ± 0.13∗∗∗ | 3.46 ± 0.12 | 3.15 ± 0.12 |
| Phosphate (mg/g) | 1.97 ± 0.09 | 3.05 ± 0.12††† | 2.90 ± 0.10 | 2.81 ± 0.12 | 2.34 ± 0.11∗∗∗ | 2.20 ± 0.11∗∗∗ | 2.66 ± 0.12 | 2.44 ± 0.10∗∗ | 2.72 ± 0.10 | 2.51 ± 0.12∗∗ |
| Lipid peroxidation (%) | 33.39 ± 2.13 | 100.00 ± 5.18††† | 92.83 ± 4.93 | 95.27 ± 4.98 | 71.57 ± 5.47∗∗ | 58.36 ± 4.92∗∗∗ | 69.41 ± 4.66∗∗ | 63.74 ± 5.38∗∗∗ | 90.75 ± 6.27 | 79.73 ± 6.27 |
| Damage index (Arbitrary units) | 0.00 ± 0.00 | 2.88 ± 0.10 | 2.46 ± 0.13 | 2.42 ± 0.15 | 1.79 ± 0.17∗∗∗ | 1.56 ± 0.12∗∗∗ | 2.17 ± 0.14∗∗ | 2.08 ± 0.14∗∗ | 2.25 ± 0.17∗ | 2.13 ± 0.16∗∗ |
| CaOx deposits (No.) | 0.00 ± 0.00 | 45.98 ± 2.28 | 42.42 ± 2.04 | 40.81 ± 1.84 | 15.00 ± 2.53∗∗∗ | 8.69 ± 1.11∗∗∗ | 26.71 ± 1.64∗∗∗ | 18.58 ± 1.97∗∗∗ | 36.65 ± 2.26∗ | 33.63 ± 2.53∗∗∗ |
Values are expressed as mean ± SEM, n = 6, †p < 0.05; ††p < 0.01; †††p < 0.001 vs. vehicle control, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. lithiatic control. Values of lithiatic control group were compared with vehicle control and those of drug-treated groups with lithiatic control. CaOx = Calcium oxalate, SEM = Standard error of mean.
Calculi-inducing treatment caused significant (p < 0.001) increase in oxalate level in kidney tissue homogenate from 1.24 ± 0.09 mg/g to 3.53 ± 0.19 mg/g in the lithiatic control rats when compared against vehicle control rats (Table 3). This increased oxalate level was significantly decreased by the treatment with all doses of EA-MBS and E-MBS (i.e. 20 and 40 mg/kg). Both EA-MBS and E-MBS were found to be equipotent, in decreasing oxalate level in kidney tissue homogenate (Table 3).
The mean phosphate level in kidney tissue homogenate of vehicle control rats was 1.97 ± 0.09 mg/g, which was significantly (p < 0.001) increased to 3.05 ± 0.12 mg/g in the lithiatic control rats (Table 3). This increased phosphate level was significantly decreased by treatment with all doses of EA-MBS (i.e. 20 and 40 mg/kg) and higher dose of E-MBS as well as AQ-MBS (i.e. 40 mg/kg). The EA-MBS was found to be most effective in this regard (Table 3).
Calculi-inducing treatment caused significant (p < 0.001) increase in lipid peroxidation activity in renal tissue from 33.39 ± 2.13% to 100.00 ± 5.18% in the lithiatic control rats (Table 3). This increased lipid peroxidation activity was significantly decreased by treatment with all doses (i.e. 20 and 40 mg/kg) of EA-MBS and E-MBS. The EA-MBS was found to be most effective in this regard (Table 3).
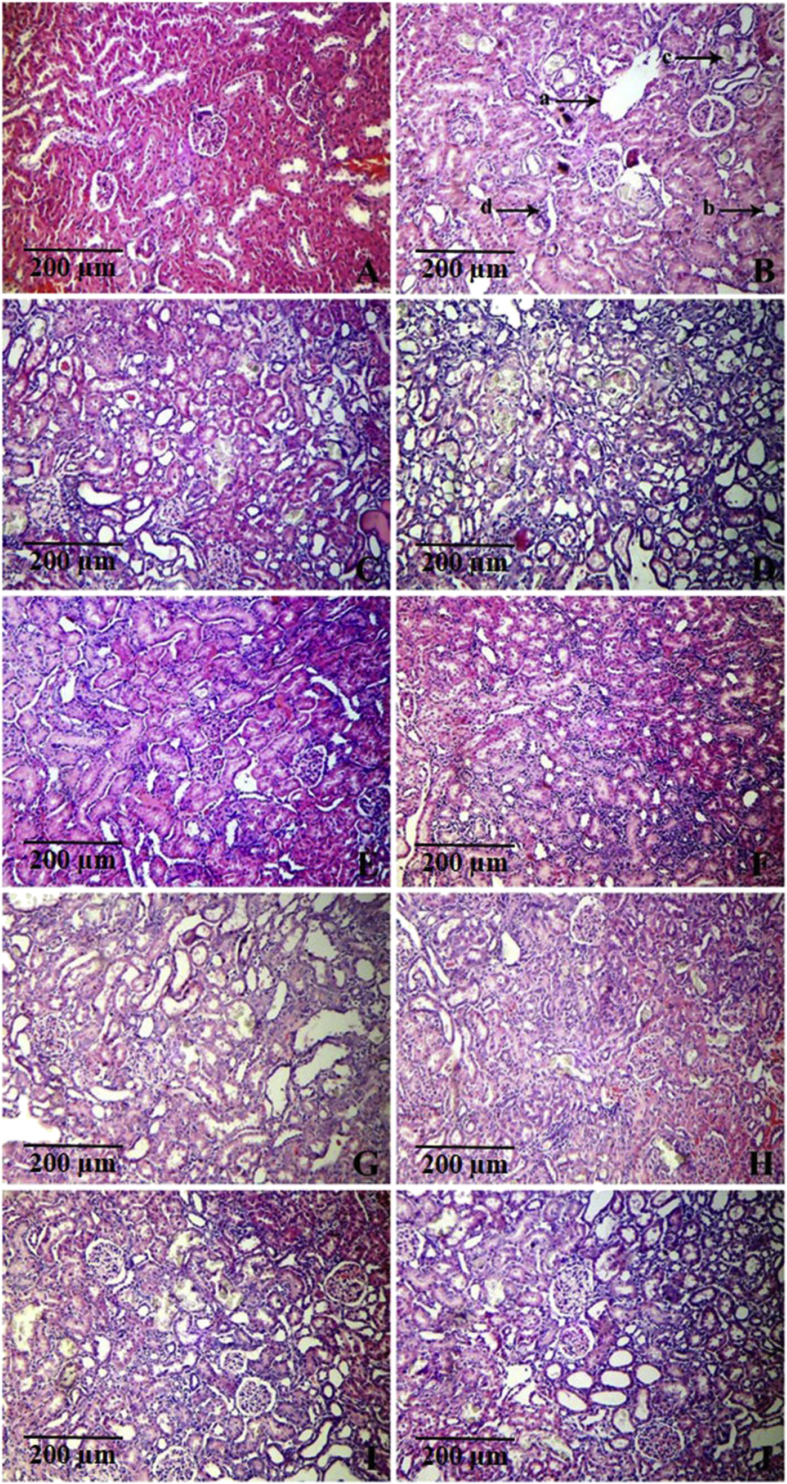
Calculi-inducing treatment caused marked histological changes such as tubular dilatation and initial cystic changes, tubular atrophy, calcium oxalate crystal deposits and interstitial mononuclear cell infiltration in the kidneys (Fig. 1). This resulted in marked increase in the kidney damage index. The kidney damage index of vehicle control rats was 0.00 ± 0.00, which was increased to 2.88 ± 0.10 in the lithiatic control rats (Table 3). Treatment with all doses of EA-MBS, E-MBS and AQ-MBS (i.e. 20 and 40 mg/kg) prevented these histological changes (Fig. 1) and thereby caused significant decrease in the damage index (Table 3).
Fig. 1.
Light microscopic images of kidney sections from animals of (A) vehicle control; (B) lithiatic preventive control showing severe histological changes including tubular dilatation and initial cystic changes (marked as ‘a’), tubular atrophy (marked as ‘b’), calcium oxalate crystals deposits (marked as ‘c’) and interstitial mononuclear cell infiltration (marked as ‘d’); (C and D) treatment with DCM-MBS at 20 and 40 mg/kg respectively, showing severe histological changes; (E and F) treatment with EA-MBS at 20 and 40 mg/kg respectively, showing mild histological changes; (G and H) treatment with E-MBS at 20 and 40 mg/kg respectively, showing moderate histological changes; (I and J) treatment with AQ-MBS at 20 and 40 mg/kg respectively, showing moderate histological changes. (H&E × 100).
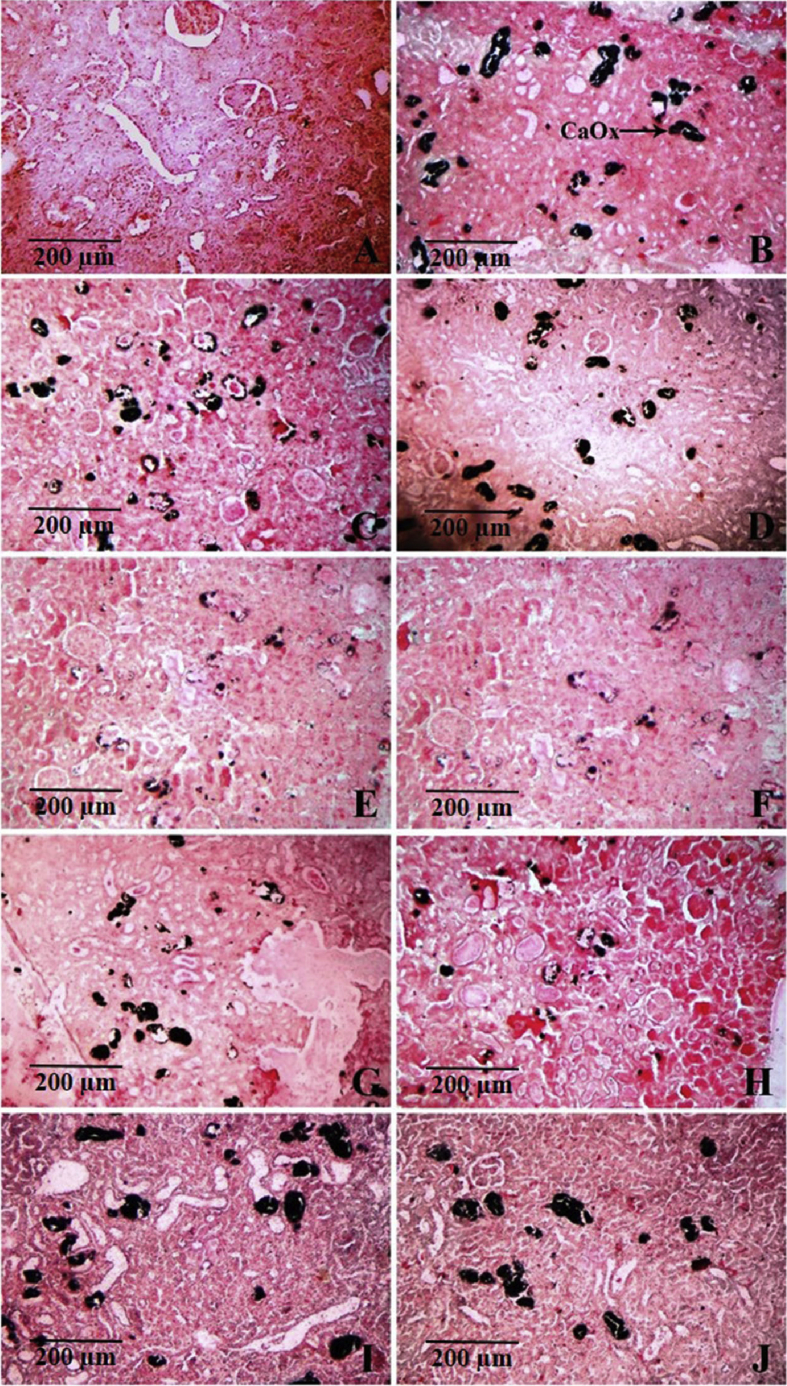
The Pizzolato's staining method showed calcium oxalate deposits (stained black) in tubules of all regions of kidney (cortex, medulla and papilla) of calculi-induced animals (Fig. 2). The mean calcium oxalate deposits (No.) in kidney tissue of calculi induced rats was 45.98 ± 2.28 (Table 3). This accumulation of calcium oxalate deposits in kidney tissue was significantly decreased by treatment with EA-MBS, E-MBS and AQ-MBS. The EA-MBS and E-MBS were found to be equipotent and more effective than other fractions, in decreasing calcium oxalate deposits in kidney tissue. The calcium oxalate deposits in kidneys of rats treated with EA-MBS, E-MBS and AQ-MBS were small and less abundant as compared to the lithiatic control rats (Fig. 2).
Fig. 2.
Microscopic images of kidney sections under light microscope (100x) after Pizzolato's staining from animals of (A) vehicle control; (B) lithiatic preventive control respectively, showing excessive accumulation of calcium oxalate deposits (marked as ‘CaOx’); (C and D) preventive treatment with DCM-MBS at 20 and 40 mg/kg respectively; (E and F) preventive treatment with EA-MBS at 20 and 40 mg/kg respectively; (G and H) preventive treatment with E-MBS at 20 and 40 mg/kg respectively; (I and J) preventive treatment with AQ-MBS at 20 and 40 mg/kg respectively.
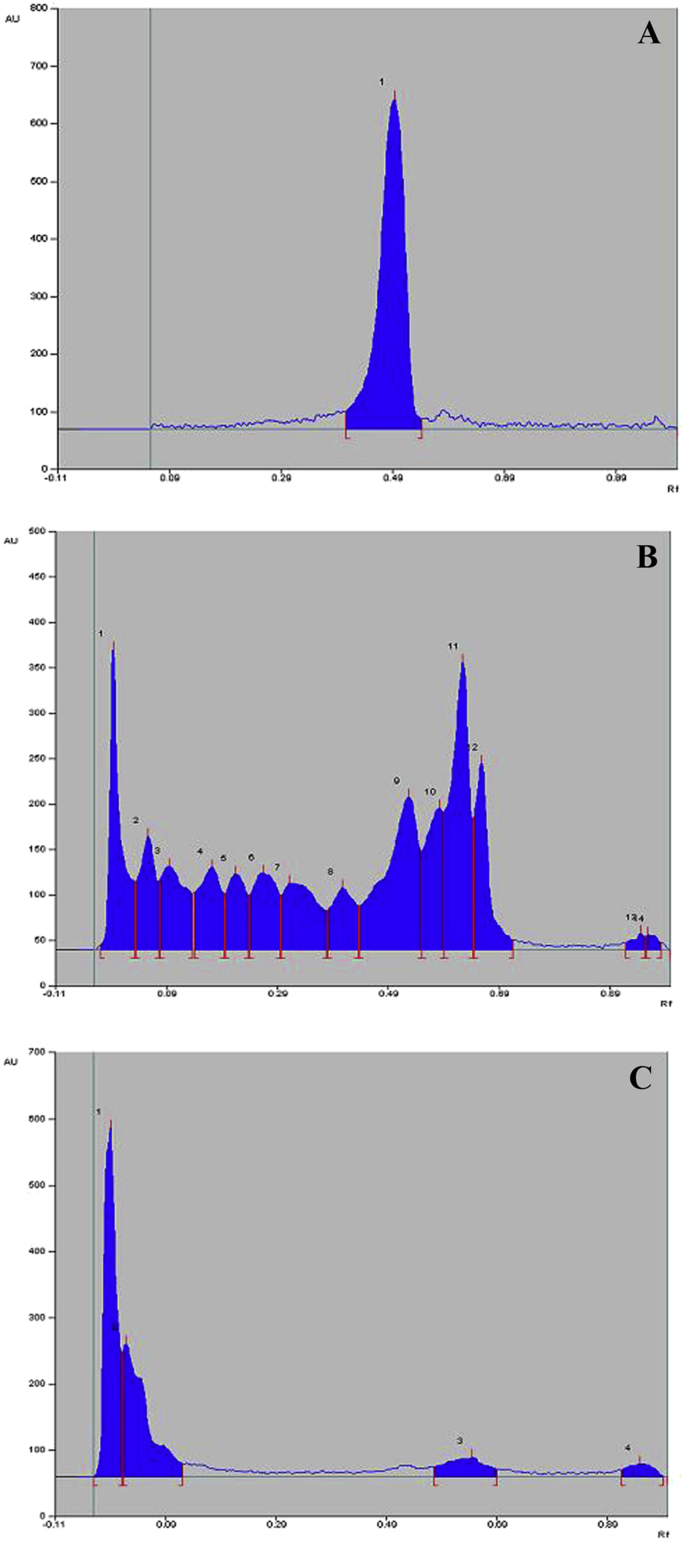
3.5. HPTLC analysis
The Rf-value of reference standard amentoflavone was found to be 0.48 (Fig. 3). Analysis of EA-MBS and E-MBS showed presence of amentoflavone in EA-MBS and absence in E-MBS. Based on peak area, percent content of amentoflavone in EA-MBS was found to be 0.15 % w/w (Fig. 3).
Fig. 3.
High performance thin layer chromatograph (HPTLC) densitogram of (A) Amentoflavone (peak no. 1); (B) EA-MBS standardized to amentoflavone (peak no. 8) and (C) E-MBS (absence of amentoflavone peak) scanned at 366 nm.
4. Discussion
A number of animal models using rats have been used to induce calcium oxalate urolithiasis.28 Out of these models, sodium oxalate induced hyperoxaluria rat model causes rapid formation calcium oxalate crystals in renal tubules of experimental animals and hence commonly employed for rapid screening of antiurolithiatic drugs.29 As yield of fractions of MBS was less, we investigated the antiurolithiatic potential of fractions of MBS on calcium oxalate urolithiasis using this model. The biochemical mechanisms of sodium oxalate-induced lithiasis are related to an increase in the urinary concentration of oxalate. Intraperitoneal administration of sodium oxalate to rats results in hyperoxaluria.18, 19 This causes precipitation of oxalate in urine as calcium oxalate due to its poor solubility. High oxalate levels and calcium oxalate crystals especially in nephron damages epithelial cells, leading to heterogeneous nucleation followed by causing aggregation of calcium oxalate crystals in the renal tubules of experimental animals.30, 31
Male rats were selected to induce urolithiasis because the urinary system of male rats has more resemblance to that of humans. Furthermore, earlier studies have reported that the amount of stone deposition in female rats was significantly less compared to male rats.32
The enhanced urine volume is expected to remove urine stone especially in early stage. In the present study, Treatment with DCM-MBS, EA-MBS, E-MBS and AQ-MBS produced significant increase in urine volume as compared to vehicle control animals. It is reported that the diuresis hastens the process of dissolving the preformed stones.32 It also facilitates the removal of small crystals and reduces the chance of these crystals to grow and aggregate and thereby prevents the formation of new stones in the urinary system. Diuresis also leads to urinary dilution of the constituents and thereby reduced the risk of stone formation by reducing the saturation product of calcium oxalate.1 The exact reason of diuresis caused by specified fractions may be revealed upon overall results, however irrespective of mechanism enhanced urine output is beneficial to restore normal condition.
Calculi induction with sodium oxalate administration results into hyperoxaluria.29 Consistent hyperoxaluria is a more significant risk factor in the pathogenesis of renal stone.33, 34 In addition, hyperoxaluria may further leads to calcium oxalate deposition in multiple organs. In the present study, increased urinary oxalate excretion was observed in lithiatic control rats which is in accordance with previously published reports.34, 35 However, treatment with EA-MBS and E-MBS caused significant decrease in urinary oxalate excretion as compared to lithiatic control rats. The EA-MBS was found to be most potent. This effect is might be due to interference of E-MBS with oxalate metabolism.
In the present study, decreased urinary calcium excretion was observed in lithiatic control rats. It is reported that hyperoxaluria leads to precipitation of the oxalate in the urine as calcium oxalate30, 31 and this might be the reason of observed decrease in urinary calcium excretion. This calcium oxalate precipitation further result in damage to the renal architecture at variable range and thereby induces functional damage too. Precipitation and long term deposition of calcium oxalate make the system more vulnerable and elicit onset of complications. However, treatment with EA-MBS, E-MBS and AQ-MBS caused significant improvement in urinary calcium excretion. Out of all these fractions, EA-MBS was found to most effective in this regard. The improvement in urinary calcium excretion indicating potential of EA-MBS to prevent damage and thereby halts possible functional abnormality which is most widely expected result from any therapy. The effect might be due to either prevention of hyperoxaluria or inhibition of binding of calcium with oxalate by the EA-MBS. In urolithiasis management, prevention of stone formation is considered to be first step especially during recurrence which can avoid further symptoms and complications.
Increased urinary excretion of phosphorus has been reported to be critical step in stone formers.36 It is documented that increased urinary phosphate excretion along with oxalate stress provides an environment suitable for stone formation by forming calcium phosphate crystals, which induces calcium oxalate deposition.1 In the current investigation, calculi-inducing treatment caused significant increase in urinary phosphate excretion. However, treatment with EA-MBS, E-MBS and AQ-MBS caused significant improvement in the urinary phosphate excretion. Out of all fractions, the MBS was found to be most effective in decreasing urinary phosphate excretion. Hence control on phosphate excretion by EA-MBS treatment itself is a limiting step towards stone formation which is appreciable outcome especially to avoid relapse.
Consistent with some previous reports, urinary total protein excretion of lithiatic rats was significantly increased as compared to vehicle control rats.34, 35 Supersaturation of urinary colloids results in precipitation of crystal initiation particle which when trapped acts as a nidus leading to subsequent crystal growth. This is associated with proteinuria that chiefly reflects proximal tubular dysfunction.1 This elevated urinary total protein excretion was significantly decreased by treatment with EA-MBS, E-MBS and AQ-MBS. The EA-MBS was found to be most effective among all fractions. This indicates that EA-MBS minimize the excretion of protein and thus might have prevented the nidus formation of crystal nucleation. The reduction in protein excretion by EA-MBS is indirect indication of either recovery of urinary dysfunction or prevention of crystal-induced damage or both. This action of EA-MBS is considered as most fruitful outcome because EA-MBS not only provides its potential towards symptomatic relief but also for its direct action at membrane level. These both potentially suggest its possible use in acute as well as chronic cases.
Increased uric acid excretion has been reported in kidney stone patients and hyperoxaluric rats.1, 34 Uric acid reported to interferes with calcium oxalate solubility. It also binds and inhibits urinary glycosaminoglycans.1 It is reported that glycosaminoglycans are inhibitors of calcium oxalate nucleation and calcium oxalate crystal growth.37 Therefore, uric acid binding to free urinary glycosaminoglycans blocks their inhibitory activity on calcium oxalate crystallization. The predominance of uric acid crystals in calcium oxalate stones and the observation that uric acid binding proteins are capable of binding to calcium oxalate and modulate its crystallization also suggests its primary role in stone formation.1, 36 In the present study, urinary uric acid excretion was significantly increased in the lithiatic control rats when compared against vehicle control rats. However, treatment with EA-MBS and E-MBS reduced this elevation in urinary uric acid excretion. Out of all fractions again EA-MBS was found to most effective in improving urinary uric acid excretion. This indicates that the EA-MBS reduced the excretion of uric acid and thereby reduce the risk of stone formation.
Normal urine contains many inorganic and organic inhibitors of crystallization, magnesium is one such well known urinary inhibitor. Low levels of magnesium are also encountered in stone-forming rats as well as in patients with renal stones. In addition, it is observed that magnesium levels return to normal on drug treatment in renal stone patients.1 Diets high in magnesium have been found to protect against deposition of calcium oxalate in the kidneys of vitamin B6 deficient rats. The beneficial effects in preventing recurrence of stone have been shown in patients treated with potassium ammonium citrate. Magnesium reported to form complex with oxalate and reduce the supersaturation of calcium oxalate and as a consequence reduced the growth and nucleation rate of calcium oxalate crystals.1, 36 In the present study, calculi-inducing treatment caused significant decrease in urinary magnesium excretion as compared to vehicle control rats which is in consistent with some previous published reports.34, 35 This reduced rate of urinary magnesium excretion was significantly increased by treatment with EA-MBS and E-MBS making it available to form complexes with oxalate and thereby prevent formation and growth of stones. Overall the EA-MBS was found to be most potent. The facilitation of natural process of beneficial complex formation is an ideal way that EA-MBS have shown and is major outcome towards patient friendly treatment.
Hypocitraturia is the major metabolic abnormality in patients with renal stones.36 In the present investigation, urinary citrate excretion was significantly decreased by calculi-inducting treatment during experimental period. This decreased urinary citrate excretion was significantly improved by treatment with EA-MBS and E-MBS. Out of all fractions again E-MBS was found to most effective in increasing urinary citrate excretion. This indicates that the EA-MBS and E-MBS interfere with tubular citrate reabsorption which is reported as the main mechanism regulating citrate excretion in lithiatic patients.36
Nucleation and subsequent growth of renal stone reported to cause lipid peroxidation and thereby renal damage which is indicated by the elevated serum creatinine, uric acid and BUN levels.34 In the present study, preventive treatment with EA-MBS, E-MBS and AQ-MBS prevented the elevation of serum levels of these markers indicating prevention of renal damage. Out of all extracts again EA-MBS was found to most effective in this regard. This effect of EA-MBS is considered to be one of the most valuable outcomes because renal damage may exhibit wide spectrum of functional abnormality and also may lead to initiation of variety of complications making complaint more difficult to manage.
Consistent with previous reports, calcium level in kidney tissue homogenate was significantly increased in the lithiatic control rats as compared to vehicle control rats.34 However, this elevated renal calcium level was significantly decreased by treatment with EA-MBS and E-MBS. The EA-MBS was found to be most potent among all fractions in decreasing renal calcium level. Initial subepithelial calcification of renal papilla is pre-requisite step in the development of calcium oxalate renal stone.38 Therefore, elevated renal calcium level in lithiatic control rats indicates calcification of renal papilla and development of calcium oxalate stone in the renal tissue. Furthermore, inhibition of renal calcium level by EA-MBS at specified doses indicates their role in the prevention of calcium oxalate papillary calculus formation.
Calculi-inducing treatment caused significant increase in oxalate level in kidney tissue homogenate which is in accordance with previously published report.34 This increased oxalate level was significantly decreased by treatment with EA-MBS and E-MBS. Increased renal oxalate level is indication of excessive formation followed by retention of calcium oxalate stones in the renal tissue. Therefore, decreased renal oxalate level is indication of potential of EA-MBS and E-MBS to prevent retention of stones which supports other findings of the study.
It is reported that majority of calcium oxalate stone formation starts with an initial calcium phosphate concretion (also called as Randall's plaque) that originates in the terminal collecting ducts.39 This calcium phosphate concretion further forms a nidus for calcium oxalate deposition. In the present study, renal phosphate level was significantly increased in lithiatic control rats indicating the formation of calcium phosphate concretion as well as development of calcium oxalate mixed with calcium phosphate stones. However, treatment with EA-MBS, E-MBS and AQ-MBS significantly reduced renal phosphate level as compared to lithiatic control rats. Overall the EA-MBS was found to be most effective. This also indicates role of EA-MBS in the prevention of formation as well as inhibition of the retention of stone in the renal tubules.
Calculi-inducing treatment caused significant increase in the lipid peroxidation activity in the kidney tissue homogenate which is in accordance with previously published reports.34, 40 This increased lipid peroxidation of kidney tissue was significantly decreased by the treatment EA-MBS and E-MBS. Out of all fractions again EA-MBS was found to most effective in decreasing lipid peroxidation activity in renal tissue. This effect might be mediated chiefly by antioxidant property of the plant.41 The present results indicate that the EA-MBS act by inhibiting the lipid peroxidation and thereby provide better control on progress of pathology.
Calculi-inducing treatment caused marked histological changes such as congestion and hemorrhages, cytoplasmic changes, tubular changes, infiltration of mononuclear cells and glomerular changes in the kidneys causing increase in the kidney damage index. Treatment with EA-MBS, E-MBS and AQ-MBS prevented these histological changes and thereby caused significant decrease in the damage index. This indicates that the EA-MBS, E-MBS and AQ-MBS reduce damage to the renal tubules. This effect might be chiefly due antioxidant property of the plant.41 The histological changes are considered to be direct method to evaluate effect of the treatment. These results clearly indicated potential of EA-MBS to prevent, halt and restore pathological changes which supports earlier observations which are of indirect method.
The Pizzolato's staining method revealed the presence of calcium oxalate deposits (stained black) in tubules of all regions of kidney (cortex, medulla and papilla) of calculi-induced animals. However, such deposits were small and less abundant in kidneys of animals treated with EA-MBS, E-MBS and AQ-MBS as compared to those in the calculi-induced kidneys. The size and number of calcium oxalate crystals in renal tissue is direct method to evaluate antiurolithiatic potential wherein the EA-MBS showed significant reduction which is in accordance with the other biochemical parameters of the study. These parameters i.e. size and number of calcium oxalate deposition is also directly related to the progress of pathophysiology and related severity of complications. Therefore the EA-MBS showing reduction in calcium oxalate deposition is suggestive of potential to prevent stone formation which is the most fruitful outcome of the study.
Overall this significant improvement in urine parameters, serum parameters and renal parameters by EA-MBS and E-MBS in sodium oxalate-induced urolithiasis models itself suggest wide spectrum of activity of these fractions covering all phases of pathogenesis. The magnitude of action exerted by plant material is largely governed by prominent presence of phytochemicals and their interaction with each other. The summary of the result is suggestive of antiurolithiatic potential of EA-MBS followed by E-MBS. The presence of amentoflavone in EA-MBS and its absence in E-MBS suggest that the amentoflavone might be the active principle of the B. sensitivum (感应草gǎnyìng cǎo) for urolithiasis. However, the coexistence of other biologically active constituent(s) cannot be excluded.
Herbal medicines are found effective for the urolithiasis and have lesser side effects compared to modern medicines.10 Large number of plant species is described in the indigenous systems of medicine worldwide as antiurolithiatic agents. However, it is found that either preliminary or no scientific study has not been carried out on these medicines.42 The consequence of this is an inadequate knowledge of their mode of action, potential adverse reactions and contraindications of these herbal medicines. Furthermore, herbs are also commonly used as an adjunct of modern medicines to reduce recurrence of the disease and therefore herbal medicines may interact with these modern medicines given simultaneously for the utolithiasis. The current study indicates only the potential of EA-MBS as an antiurolithiatic agent. Further studies on isolation of active phytoconstitutent(s) from EA-MBS along with in vivo studies are needed for better understanding of safety, efficacy and mechanism of action of these active phytoconstitutent(s) of EA-MBS as antiurolithiatic agent.
5. Conclusion
The present investigation showed that the ethyl acetate fraction of methanolic extract of B. sensitivum (感应草gǎnyìng cǎo) prevented the growth of urinary stones. It is concluded that the antiurolithiatic component of the methanolic extract of the plant is contained in the ethyl acetate fraction. The mechanism underlying this effect is mediated collectively through diuretic, antioxidant, nephroprotective properties and lowering the concentration of urinary stone-forming constituents.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
The authors wish to express their gratitude to the Management and Principal of MAEER's, Maharashtra Institute of Pharmacy, Pune for providing facility and necessary support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Selvam R., Kalaiselvi P., Govindaraj A. Effect of A. lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 2.Hesse A., Brandle E., Wilbert D. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 3.Coward R.J., Peters C.J., Duffy P.G. Epidemiology of paediatric renal stone disease in the UK. Arch Dis Child. 2003;88:962–965. doi: 10.1136/adc.88.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor E.N., Stampfer M.J., Curhan G.C. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 5.Knoll T. Stone disease. Eur Urol Suppl. 2007;6:717–722. [Google Scholar]
- 6.Tiselius H.G. Epidemiology and medical management of stone disease. BJU Int. 2003;91:758–767. doi: 10.1046/j.1464-410x.2003.04208.x. [DOI] [PubMed] [Google Scholar]
- 7.Moe O.W. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 8.Pietrow P.K., Karellas M.E. Medical management of common urinary calculi. Am Fam Physician. 2006;74:86–94. [PubMed] [Google Scholar]
- 9.Semins M.J. Medical evaluation and management of urolithiasis. Ther Adv Urol. 2010;2:3–9. doi: 10.1177/1756287210369121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad K., Sujatha D., Bharathi K. Herbal drugs in urolithiasis – a review. Pharmacogn Rev. 2007;1:175–179. [Google Scholar]
- 11.Terlecki R.P., Triest J.A. A contemporary evaluation of the auditory hazard of extracorporeal shock wave lithotripsy. Urology. 2007;70:898–899. doi: 10.1016/j.urology.2007.06.1151. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal A., Singla S.K., Tandon C. Urolithiasis: phytotherapy as an adjunct therapy. Indian J Exp Biol. 2014;52:103–111. [PubMed] [Google Scholar]
- 13.Warrier P.K., Nambiar V.P.K., Ramankutty C. Orient Longman Publishers; Chennai, India: 1994. Indian Medicinal Plants – A Compendium of 500 Species. [Google Scholar]
- 14.Kirtikar K.R., Basu B.D. International Book Distributor; Dehradun, India: 2005. Indian Medicinal Plants. [Google Scholar]
- 15.Pawar A.T., Vyawahare N.S. Phytochemical and pharmacological profile of Biophytum sensitivum (L.) DC. Int J Pharm Pharm Sci. 2014;6:18–22. [Google Scholar]
- 16.Pawar A.T., Vyawahare N.S. Protective effect of standardized extract of Biophytum sensitivum against calcium oxalate urolithiasis in rats. Bull Fac Pharm Cairo Univ. 2015;53:161–172. [Google Scholar]
- 17.Martins S., Amorim E.L.C., Peixoto Sobrinho T.J.S. Antibacterial activity of crude methanolic extract and fractions obtained from Larrea tridentata leaves. Ind Crops Prod. 2013;41:306–311. [Google Scholar]
- 18.Takawale R.V., Mali V.R., Kapase C.U. Effect of Lagenaria siceraria fruit powder on sodium oxalate induced urolithiasis in Wistar rats. J Ayurveda Integr Med. 2012;3:75–79. doi: 10.4103/0975-9476.96522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N., Badole S., Gupta P. Effect of ethanolic extract of leaves of Cocculus hirsutus (L.) diels on experimentally induced urothiliasis in rats. J Nat Rem. 2008;8:24–31. [Google Scholar]
- 20.Hodgkinson A. Determination of oxalic acid in biological material. Clin Chem. 1970;16:547–557. [Google Scholar]
- 21.Neill D.W., Neely R.A. The estimation of magnesium in serum using titan yellow. J Clin Pathol. 1956;9:162–163. doi: 10.1136/jcp.9.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaton F.W. Determination of magnesium by the titan yellow and ammonium phosphate method. J Clin Pathol. 1960;13:358–360. doi: 10.1136/jcp.13.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajagopal G. A simple colorimetric procedure for estimation of citric acid in urine. Ind J Exp Biol. 1984;22:391–392. [PubMed] [Google Scholar]
- 24.Shah Z.A., Gilani R.A., Sharma P. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol. 2005;101:299–307. doi: 10.1016/j.jep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Ghalayini I.F., Al-Ghazo M.A., Harfeil M.N. Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice. Int Braz J Urol. 2011;37:259–266. doi: 10.1590/s1677-55382011000200013. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C.H., Chen Y.C., Chen L.D. A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res. 2008;36:17–24. doi: 10.1007/s00240-007-0122-4. [DOI] [PubMed] [Google Scholar]
- 27.Ravishankara M.N., Pillai A.D., Padh H. A sensitive HPTLC method for estimation of amentoflavone, a bioactive principle from Biophytum sensitivum (Linn.) DC. and Putranjiva roxburghii Wall. J Planar Chromatogr. 2003;16:201–205. [Google Scholar]
- 28.Liu J., Cao Z., Zhang Z. A comparative study on several models of experimental renal calcium oxalate stones formation in rats. J Huazhong Univ Sci Technol Med Sci. 2007;27:83–87. doi: 10.1007/s11596-007-0124-z. [DOI] [PubMed] [Google Scholar]
- 29.Gupta P., Patel N., Bhatt L. Anti-urolithiatic effect of petroleum ether extract stem bark of Crataeva adansonii in rats. Pharm Biol. 2006;44:160–165. [Google Scholar]
- 30.Thamilselvan S., Khan S.R., Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 31.Scheid C.R., Cao L.C., Honeyman T. How elevated oxalate can promote kidney stone disease: changes at the surface and in the cytosol of renal cells that promote crystal adherence and growth? Front Biosci. 2004;9:797–808. doi: 10.2741/1265. [DOI] [PubMed] [Google Scholar]
- 32.Karadi R.V., Gadge N.B., Alagawadi K.R. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Ingale K.G., Thakurdesai P.A., Vyawahare N.S. Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J Pharmacol. 2012;44:639–642. doi: 10.4103/0253-7613.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divakar K., Pawar A.T., Chandrasekhar S.B. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Bashir S., Gilani A.H. Antiurolithiatic effect Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122:106–116. doi: 10.1016/j.jep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Soundararajan P., Mahesh R., Ramesh T. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 37.Grases F., Gil J.J., Conte A. Glycosaminoglycans: inhibition of calcium oxalate crystalline growth and promotion of crystal aggregation. Colloids Surfaces. 1989;36:29–38. [Google Scholar]
- 38.Grases F., Prieto R.M., Fernandez-Cabot R.A. Effects of polyphenols from grape seeds on renal lithiasis. Oxid Med Cell Longev. 2015;2015:813737. doi: 10.1155/2015/813737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matlaga B.R., Coe F.L., Evan A.P. The role of Randall's plaques in the pathogenesis of calcium stones. J Urol. 2007;177:31–38. doi: 10.1016/j.juro.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 40.Ghodasara J., Pawar A., Deshmukh C. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacogn Res. 2010;2:388–392. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guruvayoorappan C., Afira A.H., Kuttan G. Antioxidant potential of Biophytum sensitivum extract in vitro and in vivo. J Basic Clin Physiol Pharmacol. 2006;17:255–267. doi: 10.1515/jbcpp.2006.17.4.255. [DOI] [PubMed] [Google Scholar]
- 42.Firenzuoli F., Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Altern Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]