Abstract
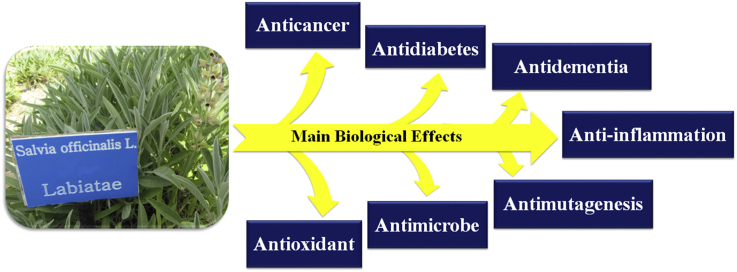
Salvia officinalis (Sage) is a plant in the family of Labiatae/Lamiaceae. It is native to Middle East and Mediterranean areas, but today has been naturalized throughout the world. In folk medicine, S. officinalis has been used for the treatment of different kinds of disorders including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremor, paralysis, diarrhea, and hyperglycemia. In recent years, this plant has been a subject of intensive studies to document its traditional use and to find new biological effects. These studies have revealed a wide range of pharmacological activities for S. officinalis. Present review highlights the up-to-date information on the pharmacological findings that have been frequently reported for S. officinalis. These findings include anticancer, anti-inflammatory, antinociceptive, antioxidant, antimicrobial, antimutagenic, antidementia, hypoglycemic, and hypolipidemic effects. Also, chemical constituents responsible for pharmacological effects of S. officinalis and the clinical studies on this plant are presented and discussed.
Keywords: Anticancer, Antimutagenic, Flavonoids, Sage, Salvia officinalis
Graphical abstract
1. Introduction
Salvia officinalis L. (Sage) is a perennial round shrub in the family of Labiatae/Lamiaceae (Fig. 1). Salvia is the largest genus of this family and includes near 900 species. Plants of this genus grow all over the world and the specie of S. officinalis is native to Middle East and Mediterranean areas. Today's, it has been naturalized throughout the world particularly in Europe and North America.1, 2, 3 The aerial parts of S. officinalis shrub has a long history of use in cookery and traditional medicine. Because of its flavoring and seasoning properties, this plant has been widely used in preparation of many foods. In folk medicine of Asia and Latin America, it has been used for the treatment of different kinds of disorders including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremor, paralysis, diarrhea, and hyperglycemia.4, 5 In traditional medicine of Europe, S. officinalis has been used to treat mild dyspepsia (such as heartburn and bloating), excessive sweating, age-related cognitive disorders, and inflammations in the throat and skin.6, 7, 8 German Commission E has accepted the use of S. officinalis for a number of medical applications included inflammation and dyspepsia.
Fig. 1.

Arial parts of Salvia officinalis L.
In recent years, many research studies have been conducted to document the traditional uses of S. officinalis and to find new biological effects for this plant. These studies have revealed a wide range of pharmacological activities including anticancer, anti-inflammatory, anti-nociceptive, antioxidant, antimicrobial, antimutagenic, antidementia, hypoglycemic, and hypolipidemic, effects. In this review, effort has been made to discuss all pharmacological findings that have been frequently reported for S. officinalis. Also, chemical constituents responsible for the biological effects of this plant are presented and discussed. Some of the unwanted effects and toxicity of S. officinalis are briefly outlined.
2. Bioactive compounds of S. officinalis
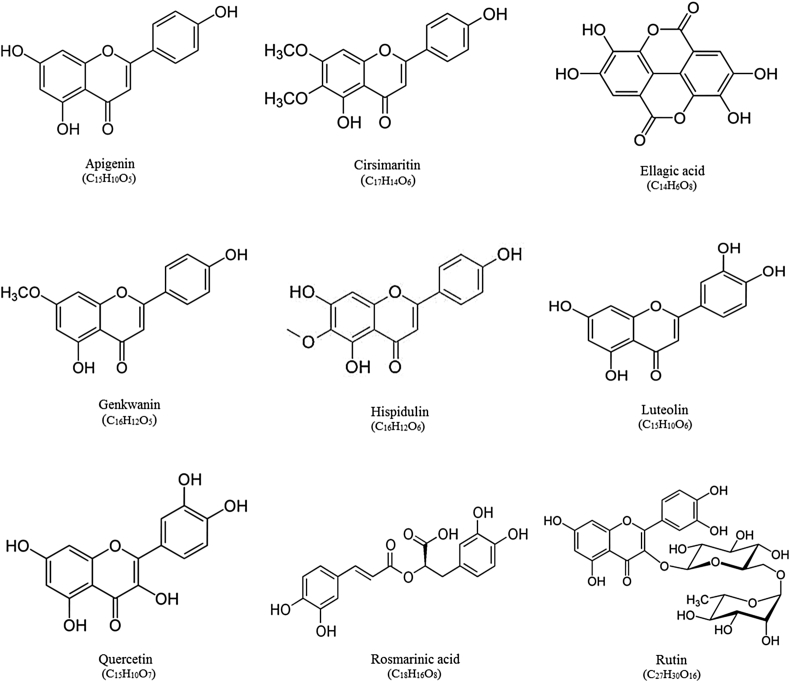
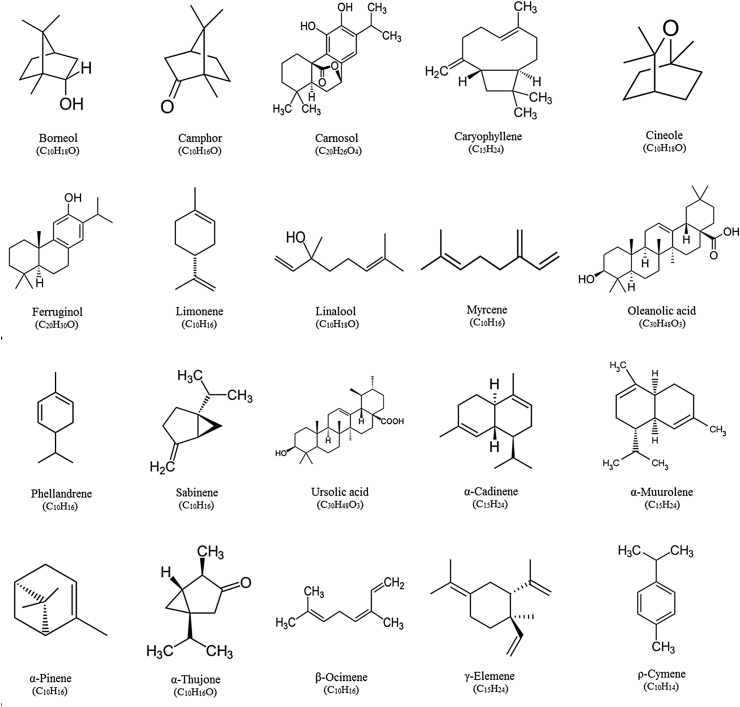
The major phytochemicals in flowers, leaves, and stem of S. officinalis are well identified. A wide range of constituents include alkaloids, carbohydrate, fatty acids, glycosidic derivatives (e.g., cardiac glycosides, flavonoid glycosides, saponins), phenolic compounds (e.g., coumarins, flavonoids, tannins), poly acetylenes, steroids, terpenes/terpenoids (e.g., monoterpenoids, diterpenoids, triterpenoids, sesquiterpenoids), and waxes are found in S. officinalis.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Structure of main flavonoids and terpenes/terpenoids isolated from S. officinalis is shown in Fig. 2 and Fig. 3, respectively. Most of the phytochemicals which are reported from S. officinalis have been isolated from its essential oil, alcoholic extract, aqueous extract, butanol fraction, and infusion preparation. More than 120 components have been characterized in the essential oil prepared from aerial parts of S. officinalis. The main components of the oil include borneol, camphor, caryophyllene, cineole, elemene, humulene, ledene, pinene, and thujone.9, 12, 13 Alcoholic and aqueous extracts of S. officinalis are rich in flavonoids particularly rosmarinic acid and luteolin-7-glucoside. Also the phenolic acids such as caffeic acid and 3-Caffeoylquinic acid have been found in methanolic extract of S. officinalis.17 Several flavonoids like chlorogenic acid, ellagic acid, epicatecin, epigallocatechin gallate, quercetin, rosmarinic acid, rutin, and luteolin-7-glucoside, as well as several volatile components such as borneol, cineole, camphor, and thujone have been identified in infusion prepared from S. officinalis.15, 26 Rosmarinic acid and ellagic acid are the most abundant flavonoids in S. officinalis infusion extract, followed by rutin, chlorogenic acid, and quercetin.26 The most abounding carbohydrates described in this plant are arabinose, galactose, glucose, mannose, xylose, uronic acids and rhamnose.10
Fig. 2.
Structure of main flavonoids isolated from Salvia officinalis.
Fig. 3.
Structure of main terpenes and terpenoids isolated from Salvia officinalis.
Comparing the phytochemicals in flowers, leaves, and stem of S. officinalis; linalool is the most present phytochemical in the stem; the flowers have the highest level of α-pinene and cineole; and bornyl acetate, camphene, camphor, humulene, limonene, and thujone are the most present phytochemicals in the leaves.22 However, it should be considered that, like other herbs, the chemical composition of S. officinalis would be varied depending on the environmental conditions such as climate, water availability, and altitude.20
3. Pharmacological activities
Experimental and clinical studies on pharmacological properties of S. officinalis are presented and discussed in the following sections. Table 1 summarizes clinical studies on S. officinalis.
Table 1.
Clinical studies of the pharmacological effects of S. officinalis.
| Category | Study design | Subjects | Dosage | Effects | References |
|---|---|---|---|---|---|
| Effects on memory and cognitive functions | Randomized placebo-controlled trial | Patients with Alzheimer's disease | 60 drops/day of alcoholic extract for week 16 | Improvement of cognitive functions | 88 |
| Randomized placebo-controlled trial | Healthy young participants | 300–600 mg encapsulated dried leaf | Improvement of mood and cognitive functions after single dose | 89 | |
| Randomized placebo-controlled trial | Healthy old participants | 167–1332 mg of ethanolic extract was administrated 1, 2.5, 4 and 6 h before assessment | Improvement of memory and attention | 87 | |
| Randomized controlled trial | Healthy adults participants | 5 drops of essential oil were placed into the testing cubicle | Improvement of prospective memory and cognitive performance | 85, 86 | |
| Effects on pain | Randomized controlled trial | Patient with pharyngitis | 15% spray containing 140 μl of the plant extract per dose | Reduction of the throat pain intensity | 72 |
| Randomized controlled trial | Patients undergoing tonsillectomy or adenoidectomy | Infusion of the plant was administrated as an oral rinse 4–8 h following surgery and then 6 times a day | The antinociceptive effect was not more powerful than the benzydamine hydrochloride | 73 | |
| Effects on glucose and lipids | Randomized placebo-controlled trial | Patients with newly diagnosed primary hyperlipidemia | 500 mg encapsulated hydroalcoholic extract every 8 h for 2 months | Reduction of the blood levels of total cholesterol, triglyceride, LDL and VLDL; Increase of HDL level | 102 |
| Randomized placebo-controlled trial | Hyperlipidemic type 2 diabetic patients | 500 mg encapsulated hydroalcoholic extract every 8 h for 3 months | Reduction of the blood levels of glucose, HbA1c, total cholesterol, triglyceride, and LDL; Increase of HDL level | 103 | |
| Randomized placebo-controlled trial | Type 2 diabetic patients | 150 mg sage extract 3 times a day for 3 months | Reduction of 2 h postprandial glucose and total cholesterol; No effect on fasting glucose, HbA1c, triglyceride, LDL and HDL | 95 | |
| A pilot study (non-randomized crossover trial) | Healthy female volunteers | 300 mL of sage tea twice daily for 4 weeks | Reduction of total cholesterol and LDL; No effect on fasting glucose; Increase of HDL level | 104 |
3.1. Anticancer and antimutagenic effects
Potential antitumor activity of S. officinalis has been studied on several cancerous cell lines and on animal models of cancer. It has been reported that sage tea drinking prevented initiation phases of colon carcinogenesis.27 Extracts of this plant showed pro-apoptotic and growth-inhibitory effects on cell lines of breast cancer (MCF-7), cervix adeno carcinoma (HeLa), colorectal cancer (HCT-116, HCT15, CO115, HT29), insulinoma (RINm5F), laryngeal carcinoma (Hep-2), lung carcinoma (A549), melanoma (A375, M14, A2058, B16), and oral cavity squamous cell carcinoma.5, 11, 20, 28, 29, 30, 31 In addition to antiproliferative action, S. officinalis has antimigratory and antiangiogenic effects.32, 33 The S. officinalis extract enhances TNF-α and nitric oxide release from macrophages therefore increasing its cytotoxic effect.31 These effects may be related to the presence of several cytotoxic and anticancer compounds in S. officinalis. Among terpenes and terpenoids isolated from S. officinalis, the caryophyllene and α-humulene have been shown to inhibit growth of MCF-7 and HCT-116 tumor cells.11 Manool, a diterpene, induces selective cytotoxicity on human cervical adenocarcinoma and human glioblastoma.34 Also, ursolic acid, a pentacyclic triterpenoid, inhibits angiogenesis, neoplastic proteases, and invasion of melanoma cells.35 Among flavonoids of S. officinalis, rosmarinic acid has been extensively studied for its anticancer effects. It inhibits the growth of various human cancer cells including breast adenocarcinoma, colon carcinoma, chronic myeloid leukemia, prostate carcinoma, hepatocellular carcinoma, and small cell lung carcinoma.30, 36 In animals studies, rosmarinic acid was able to prevent the formation of skin tumors in mice model of dimethylbenz(a)anthracene-induced skin carcinogenesis and to prevent bone metastasis from breast carcinoma.37, 38 The anticancer effects of this flavonoid seem to be due, at least in part, to the inhibition of Mitogen-Activated Protein Kinase/Extracellular Signal-regulated Kinase pathway, the suppression of reactive oxygen species (ROS) and nuclear transcription factor-kappa B, and the reduction of pro-inflammatory gene cyclooxygenase-2 expression.36, 39, 40 It also inhibits several phases of angiogenesis (proliferation, migration, adhesion and tube formation) in endothelial cells.41
There is increasing evidence that S. officinalis can act as inhibitor of mutagenesis. Its essential oil has been shown to reduce UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae.42 Its tea infusion reduces the frequency of mutations induced by methyl methanesulphonate in Drosophila melanogaster.43 Its methanolic extract shows protective effect against cyclophosphamide-induced genotoxicity in rats.44 This plant also reduces hydrogen peroxide- and dimethoxy-1,4-naphthoquinone-induced oxidative DNA damage in HepG2 cells.45 Antimutagenic effect of S. officinalis is mainly attributed to its monoterpene compounds such as thujone, camphor, limonene, and 1,8-cineole.42, 46, 47, 48 The protective effect of S. officinalis on DNA could be explained by its antioxidant activity.44, 45
3.2. Antioxidant activities
Oxidative stress plays an important role in the initiation and progression of several diseases, such as cancer, cardiovascular disorders, diabetes, and neurological diseases.49, 50, 51, 52 Enhanced oxidative stress occurs when the generation of ROS by mitochondrial electron-transport chain, NADPH oxidase, uncoupled nitric oxide syntheses, and xanthine oxidase, exceeds the potential of antioxidant defenses including catalase, glutathione per oxidase, and superoxide dismutase activities.51 Natural antioxidants protect cells against ROS over production and therefore can counteract oxidative stress-mediated tissue damage. Evidence from several studies suggests that S. officinalis has potent antioxidant activities. Enriching the drinking water of rats with S. officinalis extract increases resistance of rat hepatocytes against oxidative stress.53 It protects hepatocytes against dimethoxy naphthoquinone- and hydrogen peroxide-induced DNA damage through elevation of glutathione peroxidase activity.45 The most effective antioxidant constituents of S. officinalis are carnosol, rosmarinic acid, and carnosic acid, followed by caffeic acid, rosmanol, rosmadial, genkwanin, and cirsimaritin.54 The radical scavenging effect of carnosol is comparable to that of α-tocopherol.55, 56 The superoxide scavenging activity of the rosmarinic acid derivatives are 15–20 times more than trolox, a synthetic water-soluble vitamin E. In streptozotocin-induced diabetic rats, rosmarinic acid increases activities of pancreatic catalase, glutathione peroxidase, glutathione-S-transferase, and superoxide dismutase.57 In addition to rosmarinic acid, other flavonoids of S. officinalis particularly quercetin and rutin have strong antioxidant activities.58 For example, in our previous work we showed that rutin reversed hexachlorobutadiene-induced elevation of lipid peroxidation and depletion of thiol content in the kidney.59
3.3. Anti-inflammatory and antinociceptive properties
Inflammation and pain are the two main symptoms which are occur in response to tissue damage. Non-steroidal anti-inflammatory drugs are still a key component of the pharmacological treatment of these symptoms. However, the clinical uses of these drugs are accompanied with unpleasant side effects such as gastrointestinal and cardiovascular complications.60 Therefore, the search for new anti-inflammatory and antinociceptive agents with lesser unwanted actions remains an attractive subject. Pharmacological studies have shown that S. officinalis has anti-inflammatory and antinociceptive effects.61, 62, 63, 64, 65, 66, 67, 68, 69 For example, it has been shown that this plant helps to control neuropathic pain in chemotherapy-induced peripheral neuropathy.61 Among different extracts of S. officinalis, the chloroform one shows more anti-inflammatory action, while the methanolic extract and essential oil demonstrate low action.70 Flavonoids and terpenes are the compounds that most likely contribute to the anti-inflammatory and antinociceptive actions of the herb.58, 64, 67, 70 Mansourabadi et al reported that flavonoids extracted from S. officinalis reduce inflammation in the mouse carrageenan model and induce analgesic effect in a dose-dependent manner.64 Osakabe et al showed that topical application of rosmarinic acid inhibits epidermal inflammation.71 Manool, carnosol, and ursolic acid are of the terpenes/terpenoids with anti-inflammatory potential.65, 67, 70 The anti-inflammatory action of ursolic acid is twofold more potent than that of indomethacin.70 This action of S. officinalis constituents may be responsible for its antinociceptive effect in patient with pharyngitis.72 However, this effect of S. officinalis is not more powerful than the benzydamine hydrochloride in controlling postoperative pain after tonsillectomy or adenoidectomy.73
3.4. Antiseptic effects
Several lines of evidence support antimicrobial effects of S. officinalis. The essential oil and ethanolic extract of S. officinalis show strong bactericidal and bacteriostatic effects against both Gram-positive and Gram-negative bacteria. Among Gram-positive pathogens, Bacillus cereus, Bacillus megaterium, Bacillus subtilis, Enterococcus faecalis, Listeria monocytogenes, and Staphylococcus epidermidis show high sensitivity to S. officinalis.12, 19, 22, 74, 75 Effects of S. officinalis on Gram-negative bacteria depend on the type of extract used. While essential oil of S. officinalis has significant inhibitory effect on the growth of Aeromonashydrophila, Aeromonassobria, E. coli, Klebsiella oxytoca, Klebsiella pneumonia, Pseudomonas morgani, Salmonella anatum, Salmonella enteritidis, Salmonella typhi, and Shigellasonei, effect of ethanolic extract on E. coli, Pseudomonas aeruginosa, and S. enteritidis is weak.12, 19, 22, 74, 75
In addition to antibacterial action, S. officinalis has been reported to induce antifungal, antiviral and anti-malarial effects.9, 76, 77, 78 The antifungal activity has been reported against Botrytis cinerea, Candida glabrata, Candida albicans, Candida krusei, and Candida parapsilosis.9, 78 Antimicrobial effects of S. officinalis are attributed to terpens and terpenoids compounds found in this plant. It has been shown that camphor, thujone, and 1,8-cineole have antibacterial effects against Aeromonas hydrophila, Aeromonas sobria, B. megatherium, B. subtilis, B. cereus, and Klebsiella oxytoca.75 Also, oleanolic acid and ursolic acid, two triterpenoids of S. officinalis, have inhibitory action on growth of multidrug-resistant bacteria such as vancomycin-resistant enterococci, penicillin-resistant Streptococcus pneumonia, and methicillin-resistant Staphylococcus aureus. The effect of ursolic acid on Enterococcus faecium and multidrug-resistant bacteria is stronger than that of ampicillin.79 Carnosol, a diterpenoid, and its related compound carnosic acid are two other antibacterial compounds obtained from S. officinalis. These compounds potentiate the effects of aminoglycosides on methicillin-resistant S. aureus.80 The antiviral activity of S. officinalis is most probably is mediated by safficinolide and sage one, two diterpenoids which are found in its aerial parts.77
3.5. Cognitive- and memory-enhancing effects
There is increasing evidence to suggest that S. officinalis has cognitive- and memory-enhancing effects. In animal studies, it has been shown that ethanoic extract of S. officinalis increases memory retention of passive avoidance learning in rats.81 Hydroalcoholic extract from S. officinalis and its main flavonoid rosmarinic acid improve cognition in healthy rats and prevent learning and memory deficits induced by diabetes.82 Also, S. officinalis hydroalcoholic extract attenuates morphine-induced memory impairment.83
Clinical trials confirm the results of animal studies and demonstrated that S. officinalis enhances cognitive performance both in healthy participants and patients with cognitive impairment or dementia.84 Moss et al reported that the aroma of S. officinalis essential oil could enhance prospective memory performance in healthy adults.85, 86 Also, Scholey et al showed that ethanolic extract of this plant improved memory and attention in healthy older subjects.87 A randomized controlled trial by Akhondzadeh et al showed that a 4-month treatment with hydroalcoholic extract of S. officinalis improved cognitive functions in patients with mild to moderate Alzheimer's disease.88
With regards the mechanisms responsible for cognitive- and memory-enhancing effects of S. officinalis, a potential interaction with cholinergic system has been suggested. Eidi and coworkers found that activation of muscarinic and nicotinic receptors by pilocarpine and nicotine, respectively, potentiated memory-enhancing effects of S. officinalis. On the other hand, blockade of muscarinic and nicotinic receptors by scopolamine and mecamylamine, respectively, attenuated this effect.81 In addition, S. officinalis has been reported to inhibit acetylcholinesterase activity.89, 90 To date, inhibitors of acetylcholinesterase are the leading therapeutics of Alzheimer's disease and S. officinalis might be a promising source for developing therapeutic agents for this disease.
3.6. Metabolic effects
Experimental and clinical studies have confirmed the beneficial effects of some medicinal plants on body metabolism particularly glycemic status, serum lipids, lipolysis, and adipogenesis.26, 91, 92, 93, 94 Recent pharmacological investigations demonstrated that different extracts of aerial parts of S. officinalis are able to decrease blood glucose in normal and diabetic conditions.95, 96, 97, 98, 99 The mechanisms suggested for hypoglycemic effect of S. officinalis include an inhibition of hepatocyte gluconeogenesis and decrease of insulin resistance through stimulation of peroxisome proliferator-activated receptor γ (PPARγ).100, 101 Recently, one study group reported that S. officinalis extract increased plasma insulin in streptozotocin-induced diabetic rats.97 However, in their previous work they observed that the extract did not affect insulin releasing from the pancreas of normal or diabetic rats.96 Therefore further studies required to elucidate whether stimulation of insulin release mediates hypoglycemic effect of S. officinalis.
Pharmacological studies also revealed that different extracts of S. officinalis reduces serum lipids. Hernandez-Saavedra et al Reported that infusion prepared from this plant reduced serum triglycerides, total cholesterol, and low density lipoproteins (LDL) levels in diet-induced obese rats.26 It also decreased body weight and abdominal fat mass in these animals. The beneficial effects of S. officinalis on lipid profile have been also shown in diabetic animals. It could decrease the level of triglyceride, cholesterol, urea, uric acid, creatinine, aspartate amino transferase (AST), and alanine amino transferase (ALT) in streptozotocin-induced diabetic rats.97, 98 In clinical trials, extract of S. officinalis leaf could lower the blood levels of triglyceride, total cholesterol, LDL, very low density lipoproteins (VLDL) and 2 h postprandial glucose in patients with hyperlipidemia and diabetes.95, 102, 103 The beneficial properties of S. officinalis tea consumption on serum lipid profile have been also reported on non-diabetic healthy volunteers.104 Because hyperlipidemia is a common metabolic disorder contributing to mortalities and morbidities due to cerebrovascular and cardiovascular diseases, S. officinalis may be valuable for the management of dyslipidemia in high risk patients like those with diabetes mellitus or hypercholesterinemia. The beneficial action of S. officinalis on dyslipidemia may be related to flavonoids present in the plant. For example, rosmarinic acid treatment reduces the levels of triglycerides and cholesterol in serum of high fat diet- and streptozotocin-induced type 2 diabetic rats.57 Also, administration of rutin reduces adipose tissue mass and body weight in high-fat diet-induced obese rats. In addition, this flavonoid increases mitochondrial size, mitochondrial DNA content, and gene expression related to mitochondrial biogenesis (e.g., PPARγ coactivator-1α, nuclear respiratory factor-1, transcription factor A, and nicotinamide adenine dinucleotide-dependent deacetylase) in skeletal muscle.105
4. Toxicological studies
A number of clinical trials have reported that consumption of S. officinalis does not induce severe side effects.88, 102, 104 However, in the case of prolonged use or following overdose of ethanolic extract and volatile oil of S. officinalis (corresponding to more than 15 g of the leaves) some unwanted effects such as vomiting, salivation, tachycardia, vertigo, hot flushes, allergic reactions, tongue swallowing, cyanosis, and even convulsion may occur.1, 3, 106 The proconvulsant action of S. officinalis oil is due to its direct effect (at doses more than 0.5 g/kg) on nervous system.106 Camphor, thujone, and terpene ketones are considered as the most toxic compounds in S. officinalis. These compounds may induce toxic effects on the fetus and newborn. Therefore consumption of S. officinalis is not recommended in pregnancy and lactation.1, 3, 106, 107 Results from animal studies have demonstrated that the LD50 of S. officinalis oil (when consumed orally) and the methanolic extract (when injected intraperitoneally) is 2.6 g/kg and 4 g/kg, respectively.96, 106 It has been reported that S. officinalis tea enhances CCl4-induced hepatotoxicity in mice.16 However, in clinical studies no hepatotoxic effects were reported.102, 104
5. Conclusion
Today, there is lot of interest towards traditional medicines and herbal-based treatment all over the world. Therefore numerous experimental and clinical studies are being undertaken on medicinal plants and there is a need for updating and integrating the findings. In this article effort has been made to discuss available pharmacological findings that have been frequently reported for S. officinalis. On the basis of the available literature evidence, this plant shows anticancer, anti-inflammatory, antinociceptive, antioxidant, antimicrobial, hypoglycemic, hypolipidemic, and memory-enhancing effects. The effectiveness of S. officinalis as an antinociceptive, hypolipidemic, and memory-enhancing medicinal plant has been confirmed with clinical trials. In addition to the above mentioned effects, a number of other biological actions such as activating benzodiazepine receptors and inhibiting pentylenetetrazole-induced seizure have been shown for S. officinalis in literature.63, 108 The possible therapeutic applications for these effects of S. officinalis need to be elucidated in future studies. Also, future works is necessary to understand the exact molecular mechanisms responsible for S. officinalis effects, its toxicity, and drug-drug interactions.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
Salary support was provided by Mashhad University of Medical Sciences and Esfarayen Faculty of Medical Sciences for the first and second authors, respectively.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bisset N.G., Wichtl M. 2nd ed. CRC Press; Boca Raton, FI: 2001. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis with Reference to German Commision E Monographs; pp. 440–443. [Google Scholar]
- 2.Miura K., Kikuzaki H., Nakatani N. Apianane terpenoids from Salvia officinalis. Phytochemistry. 2001;58:1171–1175. doi: 10.1016/s0031-9422(01)00341-7. [DOI] [PubMed] [Google Scholar]
- 3.Physicians' Desk Reference (PDR) for Herbal Medicines. 3rd ed. Thompson; Montvale, NJ: 2004. pp. 698–701. [Google Scholar]
- 4.Zargari A. 4th ed. Tehran University Press; Tehran: 1990. Medicinal Plants; pp. 59–64. [Google Scholar]
- 5.Garcia C.S.C., Menti C., Lambert A.P.F. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): antioxidant, and antitumor in mammalian cells. An Acad Bras Ciênc. 2016;88:281–292. doi: 10.1590/0001-3765201520150344. [DOI] [PubMed] [Google Scholar]
- 6.Perry E.K., Pickering A.T., Wang W.W., Houghton P.J., Perry N.S. Medicinal plants and Alzheimer's disease: from ethnobotany to phytotherapy. J Pharm Pharmacol. 1999;51:527–534. doi: 10.1211/0022357991772808. [DOI] [PubMed] [Google Scholar]
- 7.Adams M., Gmünder F., Hamburger M. Plants traditionally used in age related brain disorders–a survey of ethnobotanical literature. J Ethnopharmacol. 2007;113:363–381. doi: 10.1016/j.jep.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency . European Medicines Agency; London: 2009. Community Herbal Monograph on Salvia officinalis L., Folium. [Google Scholar]
- 9.Badiee P., Nasirzadeh A.R., Motaffaf M. Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J Pharm Technol Drug Res. 2012;1:7. [Google Scholar]
- 10.Capek P., Hríbalová V. Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity. Phytochemistry. 2004;65:1983–1992. doi: 10.1016/j.phytochem.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 11.El Hadri A., del Río M.Á.G., Sanz J. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An R Acad Nac Farm. 2010;76:343–356. [Google Scholar]
- 12.Hayouni E.A., Chraief I., Abedrabba M. Tunisian Salvia officinalis L. and Schinusmolle L. essential oils: their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int J Food Microbiol. 2008;125:242–251. doi: 10.1016/j.ijfoodmicro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Langer R., Mechtler C., Jurenitsch J. Composition of the essential oils of commercial samples of Salvia officinalis L. and S. fruticosa Miller: a comparison of oils obtained by extraction and steam distillation. Phytochem Anal. 1996;7:289–293. [Google Scholar]
- 14.Lima C.F., Carvalho F., Fernandes E. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol In Vitro. 2004;18:457–465. doi: 10.1016/j.tiv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Lima C.F., Andrade P.B., Seabra R.M., Fernandes-Ferreira M., Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J Ethnopharmacol. 2005;97:383–389. doi: 10.1016/j.jep.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Lima C.F., Fernandes-Ferreira M., Pereira-Wilson C. Drinking of Salivia officinalis tea increases CCl4-induced hepatotoxicity in mice. Food Chem Toxicol. 2007;45:456–464. doi: 10.1016/j.fct.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Lima C.F., Valentao P.C.R., Andrade P.B., Seabra R.M., Fernandes-Ferreira M., Pereira-Wilson C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chem Biol Interact. 2007;167:107–115. doi: 10.1016/j.cbi.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y., Foo L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 2000;55:263–267. doi: 10.1016/s0031-9422(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 19.Mitic-Culafic D., Vukovic-Gacic B., Knezevic-Vukcevic J., Stankovic S., Simic D. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.) Arch Biol Sci. 2005;57:173–178. [Google Scholar]
- 20.Russo A., Formisano C., Rigano D. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem Toxicol. 2013;55:42–47. doi: 10.1016/j.fct.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Seidel V. Initial and bulk extraction. In: Sarker S.D., Latif Z., Gray A.I., editors. Natural Product Isolation. Humana Press; New Jersey, NY: 2006. pp. 27–46. [Google Scholar]
- 22.Veličković D.T., Ranđelović N.V., Ristić M.S., Veličković A.S., Šmelcerović A.A. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J Serb Chem Soc. 2003;68:17–24. [Google Scholar]
- 23.Venskutonis P.R. Effect of drying on the volatile constituents of thyme (Thymus vulgaris L.) and sage (Salvia oficinalis L.) Food Chem. 1997;59:219–227. [Google Scholar]
- 24.Wang M., Li J., Rangarajan M. Antioxidative phenolic compounds from Sage (Salvia officinalis) J Agric Food Chem. 1998;46:4869–4873. [Google Scholar]
- 25.Wang M., Kikuzaki H., Zhu N., Sang S., Nakatani N., Ho C.T. Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.) J Agric Food Chem. 2000;48:235–238. doi: 10.1021/jf990761p. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Saavedra D., Perez-Ramirez I.F., Ramos-Gomez M., Mendoza-Diaz S., Loarca-Pina G., Reynoso-Camacho R. Phytochemical characterization and effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis infusions on obesity associated cardiovascular risk. Med Chem Res. 2016;25:163–172. [Google Scholar]
- 27.Pedro D.F., Ramos A.A., Lima C.F., Baltazar F., Pereira-Wilson C. Colon cancer chemoprevention by sage tea drinking: decreased DNA damage and cell proliferation. Phytother Res. 2016;30:298–305. doi: 10.1002/ptr.5531. [DOI] [PubMed] [Google Scholar]
- 28.Jedinák A., Mucková M., Kost'álová D., Maliar T., Masterova I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z Natur forsch. 2006;61:777–782. doi: 10.1515/znc-2006-11-1203. [DOI] [PubMed] [Google Scholar]
- 29.Sertel S., Eichhorn T., Plinkert P.K., Efferth T. Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1) HNO. 2011;59:1203–1208. doi: 10.1007/s00106-011-2274-3. [DOI] [PubMed] [Google Scholar]
- 30.Xavier C.P., Lima C.F., Fernandes-Ferreira M., Pereira-Wilson C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: the role in MAPK/ERK pathway. Nutr Cancer. 2009;61:564–571. doi: 10.1080/01635580802710733. [DOI] [PubMed] [Google Scholar]
- 31.Kontogianni V.G., Tomic G., Nikolic I. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120–129. doi: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 32.Keshavarz M., Mostafaie A., Mansouri K., Bidmeshkipour A., Motlagh H.R., Parvaneh S. In vitro and ex vivo antiangiogenic activity of Salvia officinalis. Phytother Res. 2010;24:1526–1531. doi: 10.1002/ptr.3168. [DOI] [PubMed] [Google Scholar]
- 33.Keshavarz M., Bidmeshkipour A., Mostafaie A., Mansouri K., Mohammadi-Motlagh H.R. Antitumor activity of Salvia officinalis is due to its anti-angiogenic, anti-migratory and anti-proliferative effects. Cell J. 2011;12:477–482. [Google Scholar]
- 34.de Oliveira P.F., Munari C.C., Nicolella H.D., Veneziani R.C., Tavares D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology. 2016;68:2139–2143. doi: 10.1007/s10616-015-9927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedinák A., Mucková M., Kost'álová D., Maliar T., Masterova I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z Naturforsch C. 2006;61:777–782. doi: 10.1515/znc-2006-11-1203. [DOI] [PubMed] [Google Scholar]
- 36.Yesil-Celiktas O., Sevimli C., Bedir E., Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010;65:158–163. doi: 10.1007/s11130-010-0166-4. [DOI] [PubMed] [Google Scholar]
- 37.Sharmila R., Manoharan S. Anti-tumor activity of rosmarinic acid in 7,12-dimethylbenz(a)anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Indian J Exp Biol. 2012;50:187–194. [PubMed] [Google Scholar]
- 38.Xu Y., Jiang Z., Ji G., Liu J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010;76:956–962. doi: 10.1055/s-0029-1240893. [DOI] [PubMed] [Google Scholar]
- 39.Moon D.O., Kim M.O., Lee J.D., Choi Y.H., Kim G.Y. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010;288:183–191. doi: 10.1016/j.canlet.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 40.Scheckel K.A., Degner S.C., Romagnolo D.F. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J Nutr. 2008;138:2098–2105. doi: 10.3945/jn.108.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S.S., Zheng R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006;239:271–280. doi: 10.1016/j.canlet.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Vuković-Gačić B., Nikčević S., Berić-Bjedov T., Knežević-Vukčević J., Simić D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem Toxicol. 2006;44:1730–1738. doi: 10.1016/j.fct.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Patenković A., Stamenković-Radak M., Banjanac T., Andjelković M. Antimutagenic effect of sage tea in the wing spot test of Drosophila melanogaster. Food Chem Toxicol. 2009;47:180–183. doi: 10.1016/j.fct.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Alkan F.U., Gürsel F.E., Ateş A., Özyürek M., Güçlü K., Altun M. Protective effects of Salvia officinalis extract against cyclophosphamide-induced genotoxicity and oxidative stress in rats. Turk J Vet Anim Sci. 2012;36:646–654. [Google Scholar]
- 45.Kozics K., Klusová V., Srančíková A. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem Toxicol. 2013;141:2198–2206. doi: 10.1016/j.foodchem.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 46.Vujošević M., Blagojević J. Antimutagenic effects of extracts from sage (Salvia officinalis) in mammalian system in vivo. Acta Vet Hung. 2004;52:439–443. doi: 10.1556/AVet.52.2004.4.6. [DOI] [PubMed] [Google Scholar]
- 47.Nikolić B., Mitić-ćulafić D., Vuković-Gačić B., Knežević-Vukčevi J. The antimutagenic effect of monoterpenes against UV-irradiation-, 4NQO- and T-BOOH-induced mutagenesis in coli. Arch Biol Sci Belgr. 2011;63:117–128. [Google Scholar]
- 48.Simić D., Vuković-Gačić B., Knežević-Vukčević J. Detection of natural bioantimutagens and their mechanisms of action with bacterial assay-system. Mutat Res. 1998;402:51–57. doi: 10.1016/s0027-5107(97)00281-9. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho A.N., Firuzi O., Gama M.J., van Horssen J., Saso L. Oxidative stress and antioxidants in neurological diseases: is there still hope? Curr Drug Targets. 2016 doi: 10.2174/1389450117666160401120514. (in press), [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Jha J.C., Banal C., Chow B.S., Cooper M.E., Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H., Horke S., Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Toyokuni S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch Biochem Biophys. 2016;595:46–49. doi: 10.1016/j.abb.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Horváthová E., Srančíková A., Regendová-Sedláčková E. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis. 2016;31:51–59. doi: 10.1093/mutage/gev056. [DOI] [PubMed] [Google Scholar]
- 54.Cuvelier M.E., Richard H., Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chemists' Soc. 1996;73:645–652. [Google Scholar]
- 55.Dianat M., Esmaeiliziadeh M., Badavi M., Samarbafzadeh A., Naghizadeh B. Cardiac protective effects of crocin on hemodynamic parameters and infarct size in compare vitamin E after ischemia reperfusion in isolated rat heart. Planta Med. 2014;80:393–398. doi: 10.1055/s-0033-1360383. [DOI] [PubMed] [Google Scholar]
- 56.Miura K., Kikuzaki H., Nakatani N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J Agric Food Chem. 2002;50:1845–1851. doi: 10.1021/jf011314o. [DOI] [PubMed] [Google Scholar]
- 57.Govindaraj J., Sorimuthu Pillai S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats. Mol Cell Biochem. 2015;404:143–159. doi: 10.1007/s11010-015-2374-6. [DOI] [PubMed] [Google Scholar]
- 58.Azevedo M.I., Pereira A.F., Nogueira R.B. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadeghnia H.R., Yousefsani B.S., Rashidfar M., Boroushaki M.T., Assadpour E., Ghorbani A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Ren Fail. 2013;35:1151–1155. doi: 10.3109/0886022X.2013.815546. [DOI] [PubMed] [Google Scholar]
- 60.Brune K., Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res. 2015;8:105–111. doi: 10.2147/JPR.S75160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abad N.A.A., Nouri M.H.K., Tavakkoli F. Effect of Salvia officinalis hydroalcoholic extract on vincristine-induced neuropathy in mice. Chin J Nat Med. 2011;9:354–358. [Google Scholar]
- 62.Abad M., Moreno A., Palacios A., Narita M., Blanco F. The tumor suppressor ING1 contributes to epigenetic control of cellular senescence. Aging Cell. 2011;10:158–171. doi: 10.1111/j.1474-9726.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 63.Khayate-Nouri M.H., Namvaran-Abbasabad A., Tavakkoli F. Salvia Officinalis and cisplatin effects on pentylenetetrazole induced seizure threshold print. Zahedan J Res Med Sci. 2013;15:1–3. [Google Scholar]
- 64.Mansourabadi A.M., Sadeghi H.M., Razavi N., Rezvani E. Anti-inflammatory and analgesic properties of salvigenin, Salvia officinalis flavonoid extracted. Adv Herb Med. 2015;1:31–41. [Google Scholar]
- 65.Nicolella H., Senedese J., Furtado R., Veneziani R., Tavares D. Evaluation of anti-inflammatory potential of diterpene manool in macrophages by quantification of nitric oxide. J Int Soc Antioxid Nutr Health. 2015;1:124–128. [Google Scholar]
- 66.Qnais E.Y., Abu-Dieyeh M., Abdulla F.A., Abdalla S.S. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm Biol. 2010;48:1149–1156. doi: 10.3109/13880200903530763. [DOI] [PubMed] [Google Scholar]
- 67.Rodrigues M.R., Kanazawa L.K., das Neves T.L. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J Ethnopharmacol. 2012;139:519–526. doi: 10.1016/j.jep.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 68.Pra V.D., Bisol L.B., Detoni S., Denti M., Grando J. Anti-inflammatory activity of fractionated extracts of Salvia officinalis. J Appl Pharm Sci. 2011;1:67–71. [Google Scholar]
- 69.Schröder S., Beckmann K., Franconi G. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713. doi: 10.1155/2013/423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baricevic D., Sosa S., Della Loggia R. Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol. 2001;75:125–132. doi: 10.1016/s0378-8741(00)00396-2. [DOI] [PubMed] [Google Scholar]
- 71.Osakabe N., Yasuda A., Natsume M., Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 72.Hubbert M., Sievers H., Lehnfeld R., Kehrl W. Efficacy and tolerability of a spray with Salvia officinalis in the treatment of acute pharyngitis – a randomised, double-blind, placebo-controlled study with adaptive design and interim analysis. Eur J Med Res. 2006;11:20–26. [PubMed] [Google Scholar]
- 73.Lalićević S., Djordjević I. Comparison of benzydamine hydrochloride and Salvia officinalis as an adjuvant local treatment to systemic nonsteroidal anti-inflammatory drug in controlling pain after tonsillectomy, adenoidectomy, or both: an open-label, single-blind, randomized clinical trial. Curr Ther Res Clin Exp. 2004;65:360–372. doi: 10.1016/j.curtheres.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bozin B., Mimica-Dukic N., Samojlik I., Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 75.Delamare A.P.L., Moschen-Pistorello I.T., Artico L., Atti-Serafini L., Echeverrigaray S. Antibacterial activity of the essential oils of Salvia offcinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007;100:603–608. [Google Scholar]
- 76.Akkawi R., Valente A.L., Badawy S.Z. Large mesonephric cyst with acute adnexal torsion in a teenage girl. J Pediatr Adolesc Gynecol. 2012;25:e143–e145. doi: 10.1016/j.jpag.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Tada M., Okuno K., Chiba K., Ohnishi E., Yoshii T. Antiviral diterpens from Saliva officinialis. Phytochemistry. 1994;35:539–541. [Google Scholar]
- 78.Carta C., Moretti M.D.L., Peana A.T. Activity of the oil of Salvia officinalis L. against Botrytis cinerea. J Essent Oil Res. 1996;8:399–404. [Google Scholar]
- 79.Horiuchi K., Shiota S., Hatano T., Yoshida T., Kuroda T., Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci. Biol Pharm Bull. 2007;30:1147–1149. doi: 10.1248/bpb.30.1147. [DOI] [PubMed] [Google Scholar]
- 80.Horiuchi K., Shiota S., Kuroda T., Hatano T., Yoshida T., Tsuchiya T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis. Biol Pharm Bull. 2007;30:287–290. doi: 10.1248/bpb.30.287. [DOI] [PubMed] [Google Scholar]
- 81.Eidi M., Eidi A., Bahar M. Effects of Salvia officinalis L. (sage) leaves on memory retention and its interaction with the cholinergic system in rats. Nutrition. 2006;22:321–326. doi: 10.1016/j.nut.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Hasanein P., Felehgari Z., Emamjomeh A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: possible hypoglycaemic and antioxidant mechanisms. Neurosci Lett. 2016;622:72–77. doi: 10.1016/j.neulet.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 83.Gomar A., Hosseini A., Mirazi N. Evaluation of Salvia officinalis L. (sage) leaves on morphine-induced memory impairment in adult male rats. Focus Altern Complement Ther. 2014;19:156–162. [Google Scholar]
- 84.Miroddi M., Navarra M., Quattropani M.C., Calapai F., Gangemi S., Calapai G. Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer's disease. CNS Neurosci Ther. 2014;20:485–495. doi: 10.1111/cns.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moss L., Rouse M., Wesnes K.A., Moss M. Differential effects of the aromas of Salvia species on memory and mood. Hum Psychopharmacol. 2010;25:388–396. doi: 10.1002/hup.1129. [DOI] [PubMed] [Google Scholar]
- 86.Moss M., Rouse M., Moss L. Aromas of salvia species enhance everyday prospective memory performance in healthy young adults. Adv Chem Eng Sci. 2014;4:339–346. [Google Scholar]
- 87.Scholey A.B., Tildesley N.T., Ballard C.G. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology. 2008;198:127–139. doi: 10.1007/s00213-008-1101-3. [DOI] [PubMed] [Google Scholar]
- 88.Akhondzadeh S., Noroozian M., Mohammadi M., Ohadinia S., Jamshidi A.H., Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. 2003;28:53–59. doi: 10.1046/j.1365-2710.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy D.O., Pace S., Haskell C., Okello E.J., Milne A., Scholey A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology. 2006;31:845–852. doi: 10.1038/sj.npp.1300907. [DOI] [PubMed] [Google Scholar]
- 90.Russo P., Frustaci A., Del Bufalo A., Fini M., Cesario A. From traditional European medicine to discovery of new drug candidates for the treatment of dementia and Alzheimer's disease: acetylcholinesterase inhibitors. Curr Med Chem. 2013;20:976–983. [PubMed] [Google Scholar]
- 91.Milne E., White S., Campbell R., Swettenham J., Hansen P., Ramus F. Motion and form coherence detection in autistic spectrum disorder: relationship to motor control and 2:4 digit ratio. J Autism Dev Disord. 2006;36:225–237. doi: 10.1007/s10803-005-0052-3. [DOI] [PubMed] [Google Scholar]
- 92.Ghorbani A. Phytotherapy for diabetic dyslipidemia: evidence from clinical trials. Clin Lipidol. 2013;8:311–319. [Google Scholar]
- 93.Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Braz J Pharm Sci. 2013;49:413–422. [Google Scholar]
- 94.Ghorbani A., MoradiMarjaneh R., Rajaei Z., Hadjzadeh M.A.R. Effects of Securigera securidaca extract on lipolysis and adipogenesis in diabetic rats. Cholestrol. 2014;2014:582106. doi: 10.1155/2014/582106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Behradmanesh S., Derees F., Rafieian-kopaei M. Effect of Salvia officinalis on diabetic patients. J Ren Inj Prev. 2013;2:51–54. doi: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eidi M., Eidi A., Zamanizadeh H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:310–313. doi: 10.1016/j.jep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 97.Eidi M., Eidi A. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab Syndr Clin Res Rev. 2009;3:40–44. [Google Scholar]
- 98.Khattab H.A.H., Mohamed R.A., Hashemi J.M. Evaluation of hypoglycemic activity of Salvia officinalis L. (Sage) infusion on streptozotocin-induced diabetic rats. J Am Sci. 2012;8:411–416. [Google Scholar]
- 99.Lima C.F., Azevedo M.F., Araujo R., Fernandes-Ferreira M., Pereira-Wilson C. Metformin-like effect of Salvia officinalis (common sage): is it useful in diabetes prevention? Br J Nutr. 2006;96:326–333. doi: 10.1079/bjn20061832. [DOI] [PubMed] [Google Scholar]
- 100.Christensen K.B., Jørgensen M., Kotowska D., Petersen R.K., Kristiansen K., Christensen L.P. Activation of the nuclear receptor PPARγ by metabolites isolated from sage (Salvia officinalis L) J Ethnopharmacol. 2010;132:127–133. doi: 10.1016/j.jep.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 101.Shafiee-Nick R., Ghorbani A., Vafaee Bagheri F., Rakhshandeh H. Chronic administration of a combination of six herbs inhibits the progression of hyperglycemia and decreases serum lipids and aspartate amino transferase activity in diabetic rats. Adv Pharmacol Sci. 2012;2012:789796. doi: 10.1155/2012/789796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kianbakht S., Abasi B., Perham M., Hashem Dabaghian F. Antihyperlipidemic effects of Salvia officinalis L. leaf extract in patients with hyperlipidemia: a randomized double-blind placebo-controlled clinical trial. Phytother Res. 2011;25:1849–1853. doi: 10.1002/ptr.3506. [DOI] [PubMed] [Google Scholar]
- 103.Kianbakht S., Dabaghian F.H. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L. leaf extract: a randomized placebo controlled clinical trial. Complement Ther Med. 2013;21:441–446. doi: 10.1016/j.ctim.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Sá C.M., Ramos A.A., Azevedo M.F., Lima C.F., Fernandes-Ferreira M., Pereira-Wilson C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int J Mol Sci. 2009;10:3937–3950. doi: 10.3390/ijms10093937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seo S., Lee M.S., Chang E. Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients. 2015;7:8152–8169. doi: 10.3390/nu7095385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mills S., Bone K. Elsevier; St Louis, Missouri: 2005. The Essential Guide to Herbal Safety; pp. 558–559. [Google Scholar]
- 107.Halicioglu O., Astarcioglu G., Yaprak I., Aydinlioglu H. Toxicity of Salvia officinalis in a newborn and a child: an alarming report. Pediatr Neurol. 2011;45:259–260. doi: 10.1016/j.pediatrneurol.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Kavvadias D., Monschein V., Sand P., Riederer P., Schreier P. Constituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptor. Planta Med. 2003;69:113–117. doi: 10.1055/s-2003-37712. [DOI] [PubMed] [Google Scholar]