Abstract
Context
There are some reports about protective effects of Achillea on the heart.
Objective
We investigated the effect of Achillea wilhelmsii extract on cardiac function during ischemia–reperfusion (I/R) injury in the isolated rat heart.
Materials and methods
60 male Wistar rats were randomly divided into 6 groups; 1: Control group, 2: Control-ischemia (CI) 3: vitamin C (10 mg/kg), 4–6: Extract groups (E 100, E 200 and E 400 mg/kg). The animals received normal saline, vitamin C or A. wilhelmsii extract orally for 4 weeks. At the end of the treatment, the hearts were subjected to in vitro I/R Injury (20 min of global ischemia, followed by 40 min of reperfusion, Langendorff's mode). Heart rate (HR) and left ventricular pressure (LVP) were measured using a pressure transducer connected to a data acquisition system. Lactate dehydrogenase (LDH) and creatine kinase (CK) activities in the effluent were measured to determine the myocardial injury degree. The malondialdehyde (MDA), total thiol groups (-SH), superoxide anion dismutase (SOD) and catalase (CAT) in myocardial tissue were detected to determine the oxidative stress degree.
Results
Pretreatment with Achillea wilhlemsii significantly decreased the LDH, CK activities, and MDA level, while it increased the LVDP, ±dp/dtmax, rate-pressure product (RPP), SH groups, SOD and CAT activities, and also the coronary artery flow.
Discussion and conclusion
Our findings indicated that Achillea wilhelmsii could provide protection for heart against the I/R injury which may be related to the improvement of myocardial oxidative stress states.
Keywords: Achillea wilhelmsii, Ischemia–reperfusion, Cardiac function, Oxidative stress, Rat
Graphical abstract
1. Introduction
Ischemic heart disease (IHD) is a major cause of death in the world. The high mortality is due to poor recovery of hearts from IHD and cardiac remodeling induced by progressive necrosis and apoptosis in the myocardium.1, 2 As reduction of coronary blood flow leads to IHD, restitution of blood flow (reperfusion) of the affected area is the only logical treatment for this condition. However, reperfusion itself has been shown to exacerbate myocardial cellular injury, popularly known as ischemic–reperfusion (I/R) injury.3 I/R injury is known to increase the levels of reactive oxygen species (ROS) several-fold which can lead to apoptosis.4, 5 Bioactive natural substances having the additive and synergistic effects in plant food are responsible for their potent antioxidant activities.6, 7, 8, 9, 10 Medicinal plants have been used for over 2000 years and an increasing attention has been paid to herbal medicine products because of their effectiveness and lower cost in recent years. Achillea, is one of the most important genera of the compositae family and comprises more than 140 spices. Its several effects such as anti-inflammatory,6 antibacterial,7, 8, 9 antitumor,10, 11 antispasmotic,12, 13 choleretic,14 antiulcer,15 and hepatoprotective13 have been reported in previous studies. Literature search shows that the, flavonoids, polyphenols, terpenoids (sesquiterpenoids, monoterpenoids, diterpenes, triterpenes), lignans, amino acid derivatives, cineol, lignans, dicaffeoylquinic acids, rutin, β-pinen, sabinen, thujone, borneol, fatty acids and alkamides such as p-hydroxyphenethylamide IV have been identified in Achillea species.16, 17, 18, 19, 20, 21, 22 There are some reports on cardiovascular effects of Achillea on electrocardiogram and cardiac enzymes.23 Moreover the hypotensive,24 antihypertensive and antihyperlipidemia effects of this plant have been shown.25 Since Achillea genus is widespread all over the world, its species have been used by local people as folk or traditional herbal medicines. Bumadaran is a popular name for several species of Achillea in Persian language. They are reported as tonic, diaphoretic, diuretic and emmenagogic agents and have been used for treatment of chest pain (heart spasm), heart phlegm, hemorrhage, pneumonia, rheumatic pain and wounds healing in Persian traditional literature.26, 27 The composition of Iranian Achillea wilhelmsii (A. wilhelmsii) extract were also previously determined (Table 1).22, 28 Thus regarding to cardiovascular relieving properties of this plant, we evaluated the effects of A. wilhelmsii on cardiac functional parameters and oxidative stress injury in a model of I/R injury in the isolated rat heart.
Table 1.
Composition of the essential oil of Achillea wilhelmsii and list of its flavonoids.
| Components | % | Flavonoid Number | Names |
|---|---|---|---|
| 1,8-Cineole | 3.6 | I | Cynaroside |
| Linalool | 5.5 | II | Cosmosiin |
| alpha-Campholenal | 1.5 | V | Luteolin |
| Camphor | 9.0 | VI | Apigenin |
| Isoborneol | 0.1 | XX | Centaureidin |
| Borneol | 6.1 | XXI | Quercetin |
| para-Cymene | 0.5 | XXIII | 3'-methoxy luteolin |
| Chrysanthenol acetate | 2.8 | XXIV | Luteolin 7-O-glucoside |
| Bornyl acetate | 0.1 | XXV | Apigenin 7-O-glucoside |
| Isobornyl acetate | 0.5 | XXVII | 5-hydroxy 3′, 4′, 7-tetra methoxy flavone |
| Carvacrol | 2.0 | XXVIII | Salvigenin |
| Geranyl acetate | 1.2 | XXXIV | Galangin |
| Caryophyllene | 1.0 | XXXV | Eupatilin |
| cis-Nerolidol | 10.8 | ||
| Caryophyllene oxide | 12.5 | ||
| Oleic aldehyde | 6.7 | ||
| Farnesol | 1.4 | ||
| 2-Hydroxycyclopenta-decanone | 1.5 | ||
| cis-alpha-Santalol acetate | 3.3 | ||
| Essential oil yield (ml/100 g dry weight) | 0.4 |
2. Methods
2.1. Plant material and preparation of the extract
The aerial part of A. wilhelmsii was collected in summer (July–August 2014) from Khorasan Province, Neyshabour, Iran, and identified by Ferdowsi University Herbarium (voucher No. 164-2218-2) and then dried at room temperature. Four hundred grams of aerial parts of the plant were soaked in ethanol (50%) for 48 h and paper filter was used to filter the solute after mixing. The solution was then dried using a 40 °C oven for 72 h. The dried extract was dissolved in the distilled water to obtain the doses of 100, 200 and 400 mg/kg.
2.2. Animals and experimental design
In this study 60 male Wistar rats weighing 250–280 g were divided into 6 groups, n = 10 for each. Animals were maintained under 12 h light and 12 h of darkness, unrestricted access to food and water at 22 ± 4 °C. All experiments were conducted in accordance with the internationally accepted principles for laboratory animal use and care and with institutional guidelines. The animals received different doses of the extract (100, 200 and 400 mg/kg), vitamin C (Vit C) (10 mg/kg) and equal volume of saline (Control-Ischemia, CI) by gavage for 4 weeks.
Vitamin C was used as a positive control29 and also for comparison of its efficacy as an antioxidant with that of A. wilhelmsii. At the end of this period, the animals were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and after tracheotomy and opening the chest, the hearts were excised quickly and cooled in ice-cold saline until contractions stopped. Hearts were then mounted on a Langendorff apparatus and perfused isovolumically at a constant pressure of 70 mmHg with a non-recirculating Krebs – Ringer bicarbonate solution of the following composition (in mmol/L): NaCl, 120; HNaCO3, 25; KCl, 4.8; MgSO4, 1.33; KH2PO4, 1.2; CaCl2, 1.6; Na2 EDTA, 0.02; and glucose, 10. The perfusate was gassed with 95 % O2 and 5% CO2 (pH 7.4) and kept at a constant temperature of 37 °C. Isolated hearts underwent 30 min stabilization, then 20 min global ischemia (achieved by total perfusion arrest) and 40 min reperfusion.
2.3. Measurement of heart hemodynamic parameters
For measurement of left ventricular pressures, the left atrium was removed, and a latex balloon connected to pressure transducer was inserted into the left ventricle through the mitral valve. The volume of the balloon was adjusted to obtain left ventricular end-diastolic pressure of 10 mmHg. The indices of myocardial function were left ventricular developed pressure (LVDP), which was defined as peak systolic pressure minus end diastolic pressure, heart rate (HR; cardiac spontaneous rhythm was counted per min), peak rate of contraction (+dp/dtmax), peak rate of relaxation (−dp/dtmin) and the rate pressure product (RPP = LVDP × HR) which were obtained using a digital data acquisition system (AD instrument, Australia). Coronary flow (CF) was measured by timed collections of the coronary effluent.
2.4. Enzymes activities assays
To determine lactate dehydrogenase (LDH) and creatine kinase (CK) activity, as myocardial damage markers in the perfusate, samples were collected from the coronary effluent before 2 min of ischemia and during first 8 min of reperfusion. LDH and CK were assayed spectrophotometrically using commercial kits (Pars Azmoon, Iran).
2.5. Assay of oxidative stress
When the perfusions finished, we froze the hearts under the condition of −70 °C to prepare for further testing. The frozen hearts were crushed to powder by liquid nitrogen-chilled tissue pulverizer. For tissue analyses, weighed amounts of the frozen tissues were homogenized in appropriate buffer using microcentrifuge tube homogenizer.
2.6. Assessment of lipid peroxidation
MDA level is as an index of lipid peroxidation. MDA reacts with thiobarbituric acid (TBA) as a TBA reactive substance (TBARS) and produces a red complex. Briefly, 1 mL of homogenates was added to 2 mL of a complex solution containing TBA/trichloroacetic acid (TCA))/hydrochloric acid (HCL) and it was then boiled in a water bath for 40 min. After reaching to the room temperature, the solution was centrifuged at 1000 g for 10 min. The absorbance was read at 535 nm30 and was expressed as TBARS in nmol/g tissue weight, using an extinction coefficient of 1.56 × 105 cm−1 M−1.
2.7. Determination of thiol concentration
DTNB (2, 2′-dinitro-5, 5′-dithiodibenzoic acid) reagent, which reacts with the SH group, was used to determine the total thiol groups. The produced yellow complex has a peak absorbance at 412 nm. Briefly, 50 μL of tissue homogenates was added to 1 mL Tris-EDTA) ethylenediaminetetraacetic acid) buffer (pH = 8.6) and the absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then, 20 μL of 10 mM solution of DTNB was mixed with the solution and it was stored in room temperature for 15 min and the absorbance was read again (A2). The absorbance of DTNB reagent was also read as blank (B).31 The thiol levels were determined by a spectrophotometric method based on the use of Ellman's reagent.
| Total thiol concentration (mM) = (A2 − A1 − B) × 1.07 / (0.05 × 14,150) |
2.8. Determination of superoxide dismutase (SOD) activity
SOD activity was measured by the procedure of Madesh and Balasubramanian. A colorimetric assay involving generation of superoxide by pyrogallol auto-oxidation and the inhibition of superoxide-dependent reduction of the tetrazolium dye, MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide) to its Formosan by SOD was measured at 570 nm. One unit of SOD activity was defined as the amount of enzyme causing 50% inhibition in the MTT reduction rate.32
2.9. Determination of catalase (CAT) activity
Catalase activity was measured according to the Aebi method. The principle of the assay is based on the determination of the rate constant, k, (dimension: s−1, k) of hydrogen peroxide decomposition. By measuring the decrease in absorbance at 240 nm per minute, the rate constant of the enzyme was determined. Activities were expressed as k (rate constant) per liter.33
2.10. Analysis of results and statistical analysis
Values are mean ± SEM. Comparisons between datasets were made using ANOVA followed by the Tukey's test. Significance was set at p < 0.05.
3. Results
Since the changes in all cardiac parameters were almost similar in all of the time duration before ischemia and as well as those of after reperfusion we mentioned only the average changes of 2 min before ischemia and the first 10 min of reperfusion period. As it is obvious in following, cardiac function factors and CF in CI group significantly suppressed during ischemic period compared to control group (p < 0.001). Moreover in order to prevention of much more chaos in figures, comparisons were made between the best responded group and CI group.
3.1. The effects of A. wilhelmsii on cardiac function and coronary flow (CF)
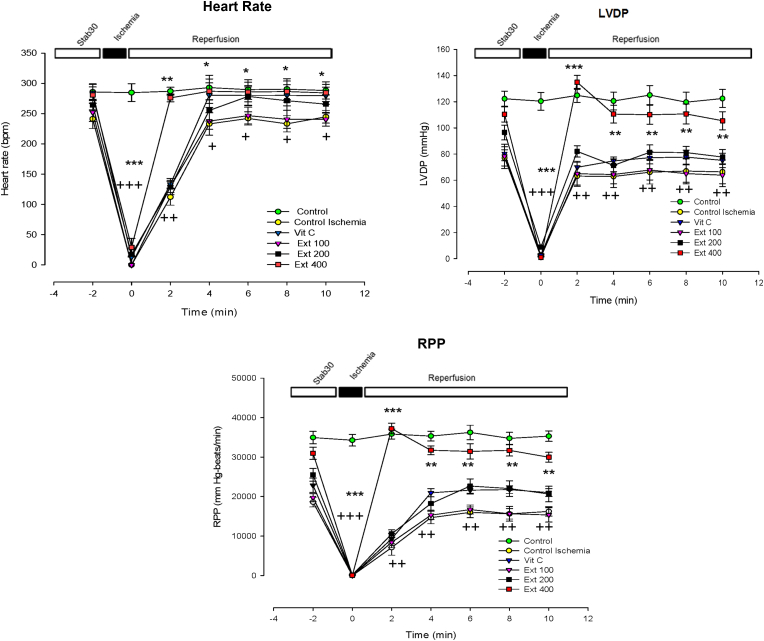
In the first 2 min after the reperfusion, heart rate in group of extract 400 showed a significant increase compared to the CI group (p < 0.01), however this increasing trend continued slowly by the end of reperfusion period (p < 0.05) (Fig. 1).
Fig. 1.
Time course of change in Heart rate, left ventricular developed pressure (LVDP) and rate pressure product (RPP) in control, control ischemia, Vit C and different doses of Achillea wilhelmsii extract (100, 200 and 400 mg/kg; Ext 100, Ext 200, Ext 400) treated rat groups during ischemia–reperfusion. The data provided are mean ± SEM. +p < 0.05, ++p < 0.01 and +++p < 0.001 compared to control group. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to control ischemia group.
In the first 10 min after the establishments of reperfusion, LVDP and RPP in group of extract 400 increased compared to the CI group (p < 0.01 to p < 0.001). Comparing the trend of increase in LVDP between all groups using repeated measures ANOVA test, also showed a significant increase in the extract 400 group compared to the CI group (p < 0.05) (Fig. 1).
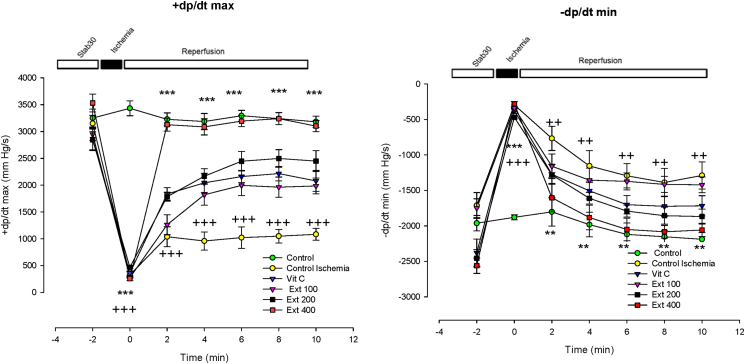
+dp/dtmax also showed increased trend during the first 10 min of reperfusion in group of extract 400 compared to CI group (p < 0.001). In the other hand, −dp/dtmin in the groups of extract 400 decreased compared to the CI group (p < 0.01) (Fig. 2).
Fig. 2.
Time course of change in the rate of ventricular pressure development (+dp/dt) and the rate of ventricular pressure decline (−dp/dt) in control, control ischemia, Vit C and different doses of Achillea wilhelmsii extract (100, 200 and 400 mg/kg; Ext 100, Ext 200, Ext 400) treated rat groups during ischemia–reperfusion. The data provided are mean ± SEM. ++p < 0.01 and +++p < 0.001 compared to control group. **p < 0.01 and ***p < 0.001 compared to control ischemia group.
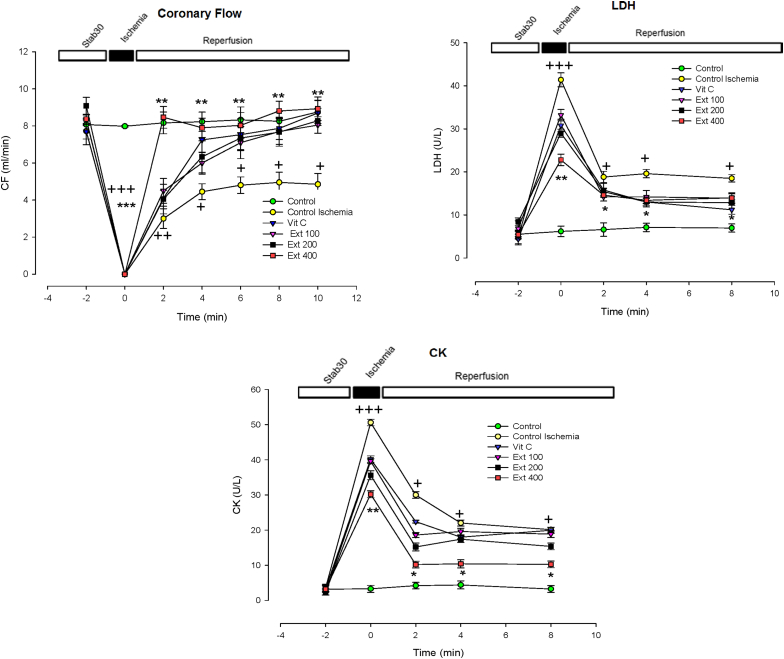
CF in all three groups of the extract increased compared to the CI group during the first 10 min of reperfusion however this increase was significant only in group of extract 400 (p < 0. 01) ( (Fig. 3).
Fig. 3.
Time course of change in rate of coronary flow (CF), outflow of LDH and CK enzymes in control, control ischemia, Vit C and different doses of Achillea wilhelmsii extract (100, 200 and 400 mg/kg; Ext 100, Ext 200, Ext 400) treated rat groups during ischemia–reperfusion. The data provided are mean ± SEM. +p < 0.05, ++p < 0.01 and +++p < 0.001 compared to control group. *p < 0.05, **p < 0.01 and **p < 0.01 compared to control ischemia group.
3.2. The effects of A. wilhelmsii on cardiac lactate dehydrogenase (LDH) and creatine kinase (CK) release
The extent of reperfusion injury in the all groups of hearts was determined from the release of a marker intracellular enzyme into the effluent. As shown in Fig. 3, after 20 min of ischemia, in the first moments, the leakage of LDH and CK markedly increased in CI group compared to control group (p < 0.001). The A. wilhelmsii pretreatment by dose of 400 mg/kg significantly reduced the I/R injury-induced increase in LDH and CK release in rat heart (p < 0.05 to p < 0.01) (Fig. 3)
3.3. The effects of A. wilhelmsii on oxidative stress state induced by I/R injury
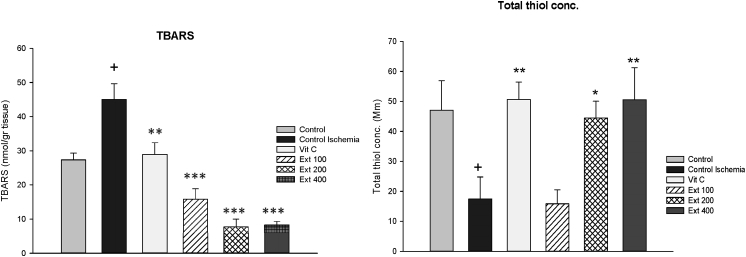
To identify the possible mechanisms of A. wilhelmsii on cardioprotection, the TBARS and thiol levels as well as SOD and CAT activities were determined in myocardial tissue. As shown in Fig. 4, the TBARS level, that had been elevated in CI group (p < 0.05 vs control) was significantly decreased in all groups of vit C and extracts compared to CI group (p < 0.01 to p < 0.001), the total thiol concentration, that had been decreased in CI group (p < 0.05 vs control) showed significant increase in groups of vit C, extract 200 and 400 compared to CI group (p < 0.05 to p < 0.01).
Fig. 4.
The level of formation of thiobarbituric acid reactive substances (TBARS), expressed as nmol MDA/g heart weight and total thiol concentration in the heart tissues of control, control ischemia, Vit C and different doses of Achillea wilhelmsii extract (100, 200 and 400 mg/kg; Ext 100, Ext 200, Ext 400) treated rat groups. +p < 0.05compared to control group, *p < 0.05, **p < 0.01 and ***p < 0.001 Compared to control ischemia group.
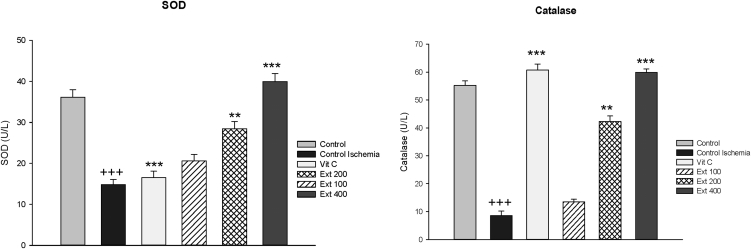
The SOD and CAT activities significantly increased in groups of extract 200 and 400 after reperfusion compared to CI group (p < 0.01 to p < 0.001), while these values showed significant reductions in CI group compared to control group (p < 0.001) (Fig. 5).
Fig. 5.
SOD and CAT enzyme activity values in the heart tissues of control, control ischemia, Vit C and different doses of Achillea wilhelmsii extract (100, 200 and 400 mg/kg; Ext 100, Ext 200, Ext 400) treated rat groups. +++ p < 0.001 compared to control group, **p < 0.01 and ***p < 0.001 Compared to control ischemia group.
4. Discussion
Various effects of Achillea may be due to the presence of a broad range of secondary active metabolites which have been frequently reported from various species of this plant. Previous studies determined over 70 compounds in the different Achillea species samples from several provinces of Iran.16, 22, 28, 34 The percentage of chemical composition of the plant can vary depending on the species, however, all it contains flavonoids. Achillea is a rich source of unique antioxidants due to its flavonoid content.18, 19, 20, 21, 23, 24
Flavonoids are known to protect against myocardial I/R injury by their multifaceted properties, such as anti-inflammatory, antioxidant, vasodilatory, and antiplatelet aggregation.35
I/R injury leads to heart dysfunction, which is one of the most significant etiological factors.36 The data obtained in this study showed, significant myocardial dysfunction, including changed hemodynamic parameters (HR, LVDP, RPP, ±dp/dtmax and CF) after reperfusion of the ischemic myocardium. This is in agreement with numerous reports, indicating reperfusion as a key trigger of a number of events leading to myocardial dysfunction associated with I/R injury.
Severe tissue damage is known to occur during reperfusion due to massive production of ROS.37 In the present study, administration of A. wilhelmsii extract improved the cardiac contractility and left ventricular function, as evidenced by the increase in LVDP, RPP and improvement of ±dp/dt. Since the A. wilhelmsii extract is available at the time of reperfusion in pretreated groups, it can scavenge the free radicals and reduce the reperfusion-induced injury to the myocardium. In this regard also we found the former study that showed inotropic and chronotropic actions of another species of this plant, Achillea millefolium, in sheep.23 This is further supported by the decreased levels of cardiac marker enzymes LDH and CK indicating less tissue damage in the A. wilhelmsii extract-treated myocardium as compared to I/R heart.
Vasodilatory action of A. wilhelmsii has been reported previously, thus the augmentation of CF in extract groups could be attributed to this vasorelaxation which may be mediated by inhibition of extracellular Ca2+ influx through voltage- and receptor-operated Ca2+ channels (VDDCs and ROCCs) in vascular smooth muscle cells.38 Although considering to the good effects of A. wilhelmsii on LVDP in this study, it seems the reduction of diastolic LVP ameliorates CF.
Augmentation of endogenous antioxidants (SOD, CAT, thiol) has been recognized as an important pharmacological property, present in natural as well as many synthetic compounds like vitamin C.39, 40 This constitutes a major mechanism of protection against oxidative stress, offered by them.41, 42 The most abundant ROS generated in living system is superoxide radical which is acted upon by SOD to produce hydrogen peroxide which in turn is inactivated by catalase into water and oxygen. Thus an increase in both SOD and catalase along with elevation of thiol groups is considered to be more beneficial in the event of oxidative stress.43 Increase in myocardial MDA which is a marker of lipid peroxidation and depletion of myocardial endogenous antioxidants support the occurrence of oxidative stress in the control hearts following ischemia–reperfusion in the present study. Hearts from A. wilhelmsii fed rats in higher doses were protected against oxidative stress, as evidenced by inhibition of increase in MDA, depletion of SOD, catalase, and thiol group following ischemia–reperfusion. Administration of A. wilhelmsii and vitamin C prevented the I/R induced oxidative stress and maintained the levels of the anti-oxidant enzymes almost comparable to that of the normal control. The mechanism of such protection can be related to the augmented endogenous antioxidant reserve of heart in the higher dose that is in agreement with previous report of protective antioxidant properties of A. wilhelmsii,34 specially the recovery of cardiodynamics and oxidative stress data in the group of vitamin C could be a reason for this claim. As it is obvious in our data, the higher dose of extract had more preventive effects against oxidative stress as well as in ameliorating the cardiodynamics disorders caused by I/R injury, even better than vitamin C. This improvement may be attributed to the higher amount of flavonoids, which are known for their interesting activities in cardiovascular diseases, in higher doses of plant extract.
In the other hand, former reports about the beneficial effects of other different plant extracts on the I/R induced injury revealed that the improved conditions had been due to the antioxidant properties of their constituents.44, 45, 46
Regarding to the observations made in the present study, administration of A. wilhelmsii significantly enhanced the resumer of I/R-altered cardiac function by blunting the reduction of LVDP, maximum up/down rate of left ventricular pressure (±dp/dtmax), and CF decreased by I/R injury. Also, A. wilhelmsii treatment resulted in significant modulation of cardioprotection content, the MDA and thiol level and also SOD and CAT activity.
5. Conclusions
Therefore, it can be concluded that the aqueous extract of A. wilhelmsii possesses obvious protective effects on myocardial I/R injury, which may be concerned with the improvement of myocardial oxidative stress states and this may be attributed to the cardiac relief property of A. wilhelmsii that has been mentioned in traditional usage.
However, further studies are required to establish the mechanism, underlying the augmentation of tissue antioxidants.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgment
This paper is extracted from a M.Sc. thesis. The authors would like to thank Research Affairs of Mashhad University of Medical Sciences for their financial support (grant No. 920429).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Fliss H., Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb R.A., Burleson K., Kloner R.A., Babior B., Engler R. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper H.M., Meuter K., Schafer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644–S648. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson M.D. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 5.Ryter S.W., Kim H.P., Hoetzel A. Mechanisms of cell death in oxidative stress. Antioxidants Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 6.Benedek B., Kopp B., Melzig M.F. Achillea millefolium L. sl–Is the anti-inflammatory activity mediated by protease inhibition? J Ethnopharmacol. 2007;113:312–317. doi: 10.1016/j.jep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Candan F., Unlu M., Tepe B. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae) J Ethnopharmacol. 2003;87:215–220. doi: 10.1016/s0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 8.Stojanović G., Radulović N., Hashimoto T., Palić R. In vitro antimicrobial activity of extracts of four Achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Mahady G.B., Pendland S.L., Stoia A. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 10.Tozyo T., Yoshimura Y., Sakurai K. Novel antitumor sesquiterpenoids in Achillea millefolium. Chem Pharm Bull. 1994;42:1096–1100. doi: 10.1248/cpb.42.1096. [DOI] [PubMed] [Google Scholar]
- 11.Csupor-Löffler B., Hajdú Z., Zupkó I. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium sl on cultured human tumour cell lines. Phytother Res. 2009;23:672–676. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 12.Lemmens-Gruber R., Marchart E., Rawnduzi P., Engel N., Benedek B., Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium sl on isolated Guinea-pig Ilea. Arzneimittelforschung. 2006;56:582–588. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 13.Yaeesh S., Jamal Q., Au Khan, Gilani A.H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother Res. 2006;20:546–551. doi: 10.1002/ptr.1897. [DOI] [PubMed] [Google Scholar]
- 14.Benedek B., Geisz N., Jäger W., Thalhammer T., Kopp B. Choleretic effects of yarrow (Achillea millefolium sl) in the isolated perfused rat liver. Phytomedicine. 2006;13:702–706. doi: 10.1016/j.phymed.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcanti A.M., Baggio C.H., Freitas C.S. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006;107:277–284. doi: 10.1016/j.jep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Dokhani S., Cottrell T., Khajeddin J., Mazza G. Analysis of aroma and phenolic components of selected Achillea species. Plant Foods Hum Nutr. 2005;60:55–62. doi: 10.1007/s11130-005-5100-9. [DOI] [PubMed] [Google Scholar]
- 17.Benedek B., Gjoncaj N., Saukel J., Kopp B. Distribution of phenolic compounds in Middleeuropean taxa of the Achillea millefolium L. aggregate. Chem Biodivers. 2007;4:849–857. doi: 10.1002/cbdv.200790072. [DOI] [PubMed] [Google Scholar]
- 18.Gherase F., Spac A., Dorneanu V., Stănescu U., Grigorescu E. [Pharmacognostic research of some species of Achillea. Note 1. Volatile oils analysis] Rev Med Chir Soc Med Nat Iasi. 2002;107:188–191. [PubMed] [Google Scholar]
- 19.Si X.T., Zhang M.L., Shi Q.W., Kiyota H. Chemical constituents of the plants in the genus Achillea. Chem Biodivers. 2006;3:1163–1180. doi: 10.1002/cbdv.200690119. [DOI] [PubMed] [Google Scholar]
- 20.Benetis R., Radusiene J., Janulis V. Variability of phenolic compounds in flowers of Achillea millefolium wild populations in Lithuania. Med (Kaunas, Lithuania) 2007;44:775–781. [PubMed] [Google Scholar]
- 21.Orav A., Arak E., Raal A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European countries. Nat Prod Res. 2006;20:1082–1088. doi: 10.1080/14786410500510849. [DOI] [PubMed] [Google Scholar]
- 22.Saeidnia S., Gohari A., Mokhber-Dezfuli N., Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011;19:173–186. [PMC free article] [PubMed] [Google Scholar]
- 23.Rahchamani R., Mokhber dezfouli M., Haji Akhoundi A. Para clinical studies of ethanol extract of Achillea millefolium L. on electrocardiogram, cardiac enzymes and serum electrolytes in sheep. J Med Plant. 2008;7:63–69. [Google Scholar]
- 24.Farokhfal K., Fatehi M., Fatehi Hasanabad Z. Cardiovascular effects of five native plants from southern of Khorasan state. Tabib-e-Shargh. 2005;7:31–38. [Google Scholar]
- 25.Asgary S., Naderi G., Sarrafzadegan N., Mohammadifard N., Mostafavi S., Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000;26:89–94. [PubMed] [Google Scholar]
- 26.Mirheydar H. 5th ed. Islamic Culture Press; Tehran, Iran: 2004. Encyclopedia of Medicinal Plant of Iran. [Google Scholar]
- 27.Zargari A. 4th ed. Tehran University Publication; Tehran, Iran: 1996. Medicinal Plants. [Google Scholar]
- 28.Afsharypuor S., Asgary S., Lockwood G.B. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med. 1996;62:77–78. doi: 10.1055/s-2006-957810. [DOI] [PubMed] [Google Scholar]
- 29.Tan D.X., Manchester L.C., Reiter R.J., Qi W., Kim S.J., El-Sokkary G.H. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J Pineal Res. 1998;25:184–191. doi: 10.1111/j.1600-079x.1998.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 30.Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 31.Sharma J.B., Sharma A., Bahadur A., Vimala N., Satyam A., Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet. 2006;94:23–27. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Madesh M., Balasubramanian K.A. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- 33.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 34.Bashi D.S., Fazly Bazzaz B.S., Sahebkar A., Karimkhani M.M., Ahmadi A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm Biol. 2012;50:1168–1176. doi: 10.3109/13880209.2012.662235. [DOI] [PubMed] [Google Scholar]
- 35.Akhlaghi M., Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2009;46:309–317. doi: 10.1016/j.yjmcc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Xia A., Xue Z., Wang W. Naloxone Postconditioning alleviates rat myocardial ischemia reperfusion injury by inhibiting JNK activity. Korean J Physiol Pharmacol. 2014;18:67–72. doi: 10.4196/kjpp.2014.18.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceconi C., Boraso A., Cargnoni A., Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003;420:217–221. doi: 10.1016/j.abb.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Niazmand S., Harandizadeh F., Mahmoudabady M., Hosseini M., Hasanzadeh M., Fereidouni E. Mechanism of vasorelaxation induced by Achillea wilhelmsii in rat isolated thoracic aorta. Adv Biomed Res. 2014;3 doi: 10.4103/2277-9175.128470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang G., Zhang P., Ye L. Protective effects of sitagliptin on myocardial injury and cardiac function in an ischemia/reperfusion rat model. Eur J Pharmacol. 2013;718:105–113. doi: 10.1016/j.ejphar.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Xin G., Zhou M., Han J., Han B., Sun X. Cardioprotective effect of the aqueous extract of lavender flower against myocardial ischemia/reperfusion injury. J Chem. 2014;2014 [Google Scholar]
- 41.Banerjee S.K., Dinda A.K., Manchanda S.C., Maulik S.K. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002;2:16. doi: 10.1186/1471-2210-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya S.K., Bhattacharya A., Sairam K., Ghosal S. Effect of bioactive tannoid principles of Emblica officinalis on ischemia-reperfusion-induced oxidative stress in rat heart. Phytomedicine. 2002;9:171–174. doi: 10.1078/0944-7113-00090. [DOI] [PubMed] [Google Scholar]
- 43.Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991;88:5360–5363. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allahyari S., Delazar A., Najafi M. Evaluation of general toxicity, anti-oxidant activity and effects of ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Adv Pharm Bull. 2014;4:577–582. doi: 10.5681/apb.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malakul W., Ingkaninan K., Sawasdee P., Woodman O.L. The ethanolic extract of Kaempferia parviflora reduces ischaemic injury in rat isolated hearts. J Ethnopharmacol. 2011;137:184–191. doi: 10.1016/j.jep.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Sadeghi N., Dianat M., Badavi M., Malekzadeh A. Cardioprotective effect of aqueous extract of Chichorium intybus on ischemia-reperfusion injury in isolated rat heart. Avicenna J Phytomed. 2015;5:568–575. [PMC free article] [PubMed] [Google Scholar]