Abstract
Natural antioxidants derived from plants have shown a tremendous inhibitory effect on free radicals in actively metabolizing cells. Overproduction of free radicals increases the risk factor of chronic diseases associated with diabetes, cancer, arthritis and cardiovascular disease. Andrographis paniculata, Cinnamon zeylanicum, Curcuma xanthorrhiza, Eugenia polyantha and Orthosiphon stamineus are ethnomedicinal plants used in the Asian region to treat various illnesses from a common fever to metabolic disease. In this study, we have quantified the total phenolic (TPC) and flavonoid content (TFC) in these plants and its inhibitory effect on 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radicals as well as the cytotoxicity effect on cell lines proliferation and zebrafish embryogenesis. Results showed that Cinnamon zeylanicum and E. polyantha have the highest phenolic and flavonoid content. Furthermore, both herbs significantly inhibited the formation of DPPH and ABTS free radicals. Meanwhile, O. stamineus exhibited minimum cytotoxicity and embryotoxicity on tested models. Good correlation between IC50 of 3T3-L1 cells and LC50 embyrotoxicity was also found. This study revealed the potent activity of antioxidant against free radical and the toxicology levels of the tested herbal plants.
Keywords: Herbal, Cytotoxicity, Embryotoxicity, Total phenolic content, Antioxidant
Graphical abstract
1. Introduction
Reactive oxygen species (ROS); superoxide anion (O2−), hydroxyl radical (OH−) and hydrogen peroxide (H2O2) are byproduct molecules generated during aerobic cell metabolism. The presence of these molecules is crucial as they modulate diverse physiological signaling and homeostasis. In turn, overproduction of ROS results in oxidative stress and contributes to the development of cancer, diabetes, aging, inflammation, cardiovascular disease and neurodegenerative diseases.1, 2, 3 Synthetic and naturally derived antioxidants exhibit tremendous protective responses against ROS through free radicals scavenging, metals chelating, quenching of single and breaking the autoxidative chain reaction and restore the ‘redox homeostasis’ state to its original level.4, 5, 6, 7, 8, 9 Evidences showed that natural antioxidants deliver better effectiveness as compared to synthetic antioxidants. A study of vitamin E on human plasma and tissues revealed that naturally derived vitamin E gives twice higher absorption rate as compared to synthetic vitamin E.10 In addition, dietary supplement from fruits, vegetables, cereals, beverages, spices and herbs not only offer targeted beneficiary antioxidant compounds, but a whole range of antioxidants, vitamins and phytochemicals. Andrographis paniculata (AP), Cinnamon zeylanicum (CZ), Curcuma xanthorrhiza (CX), Eugenia polyantha (EP) and Orthosiphon stamineus (OS) are commonly used in daily culinary as well as traditional medicinal preparations in South Asian region. Rich with aromatic flavors, pharmacological studies discover that these herbs contained a variety of potent phytochemicals that help to treat various illnesses.
Even though most herbs and spices are generally recognized as safe, but adverse events sometimes occur after consuming herbal products. In 2013, statistical evaluation by Malaysian Adverse Drug Reaction Advisory Committee (MADRAC), National Pharmaceutical Control Bureau and Ministry of Health showed that 11,473 cases of adverse drug reactions were reported and 0.2% were caused by herbal products.11 In most countries, safety and toxicology evaluation are not a mandatory requirement for herbal based product registration. In addition, lack of policies focusing on herbal product production contributes to low quality, ineffective and potentially hazardous consumption. ‘Natural’ term has misled the consumers' perceptions especially on the possible adverse effects that might arise from inappropriate usage of herbal medications. Adverse effect events may cause from several factors including the side effects of active compounds, contamination or substitution with toxic herbs, heavy metal contamination and herbs-drugs interactions.12 These factors responsible for causing liver, kidney and lung failure, high blood pressure, heart attack and stroke have been reported.12, 13, 14, 15, 16
Current safety and toxicology testing for preclinical studies comprise of in vitro and in vivo models. Typically, these tests are time consuming, expensive and required a large number of animals. Potentially, zebrafish (Danio rerio), an emerging model in the early drug discovery and toxicological screening offers several advantages on physiological, biological and molecular alteration.17, 18, 19 Its low maintenance, high fertility, ex utero development and transparent eggs offer clear visualization in all stages of organogenesis monitoring. Fish embryo acute toxicity test (FET) is designed by the OECD as a guideline to evaluate the embryotoxicity effects of certain compounds on the early 96 h of developmental stages of embryos.20 The use of zebrafish is not only limited to toxicity screening. Over the past years, scientists have developed transgenic zebrafish through gene alteration and targeted mutation for specific diseases such as cardiovascular, neurogenesis, digestive system, muscles, cancer, immunology, diabetes and inflammation.21, 22, 23, 24, 25, 26
In the present study, the water extracts of AP, CX, CZ, EP and OS were examined for its total phenolic and flavonoid content as well as their antioxidant inhibitory effect on DPPH and ABTS radicals. Furthermore, the toxicity effects of these herbs were evaluated using 3T3-L1, 1.1B4 and WRL-68 cell lines and compared with zebrafish embryotoxicity assay.
2. Materials and methods
2.1. Materials
0.2 N Follin-Ciocaltue reagent, sodium carbonate (Na2CO3), gallic acid, sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium hydroxide (NaOH), 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium persulphate (K2O8S2), were purchased from Sigma Aldrich (St. Louis, MO, USA). Ascorbic acid and methanol was purchased from Qrëc™ (Selangor, Malaysia). Mouse 3T3-L1 pre-adipocyte and normal liver cells WRL-68 were purchased from American Type Culture Collection, ATCC, Manassas, USA and pancreatic beta cells 1.1B4 from Public Health England, Salisbury, UK. DMEM, RPMI, fetal bovine serum (FBS), fetal calf serum (FCS) and penicillin strep (PS) were purchased from Gibco, Life Technologies (Rockville, MD, USA). Adult, wild type, zebrafish were obtained from commercial supplier, Jurassic Fish, Johor, Malaysia.
2.2. Herbs and extraction
A. paniculata (AP) (leaves), Cinnamon zeylanicum (CZ) (bark), C. xanthorrhiza (CX) (rhizome), E. polyantha (EP) (leaves) and O. stamineus (OS) (whole plant) were purchased from NatureMedic Supply (Terengganu, Malaysia) and taxonomically confirmed by Prof Dr. Fadzilah Adibah Abdul Majid. All specimens were deposited at Tissue Culture Engineering Research Group, Universiti Teknologi Malaysia under vouchers no AP-TCERG-2013, CZ-TCERG-2013, CX-TCERG-2013, EP-TCERG-2013 and OS-TCERG-2013. Raw materials were ground into coarse form and extracted with filtered water by the ratio 1.5: 10 for 3 h at 60 °C. Spray drying were carried out in Institute of Bioproduct Development (IBD) (Johor, Malaysia). Extracts were stored at 4 °C until future purposes.
2.3. Biomarker fingerprinting and chromatography technique
2.3.1. FT–IR detection
Samples were subjected to IR spectroscopic study using Perkin Elmer FT–IR spectrophotometer by employing standard potassium bromide (KBr) pellet technique. Samples were ground with KBr in the ration of 1:100. Mixture was placed in the mold and pressed. The pellet was scanned over the wavelength ranged from 4000–370 cm−1. FTIR spectrums were expressed as percent transmission (%T).
2.3.2. GS–MS analysis
Extracts were analyzed by gas chromatography–mass spectrometry (GC–MS) (Shimadzu QP2010 Ultra) equipped with a BP5MS capillary column (30 m length, 0.25 mm in diameter, 0.25 mm film thickness). Analyses were carried out using a programmed temperature from 50 °C to 300 °C and helium as a carrier gas. Compound identification was established based on the comparison of the GC retention factors with standards and the comparison of the mass spectra with the Wiley 138 library data of the GC–MS system.
2.3.3. Selected biomarkers identification and quantification
One hundred mg of AP, CX, CZ, EP and OS were dissolved in 25 ml of methanol, vortexed for 5 min to ensure dissolution and filtered through a 0.45 μm nylon filter. Standard stock solutions of biomarkers were prepared by dissolving 1 mg of biomarkers in 1 ml of methanol and sonicated for 5 min. Quantification of andrographolide, curcumin, catechin, gallic acid and rosmarinic acid were determined by HPLC fingerprinting detection; Waters 2690 Alliance Separation Module with LiChrospher® 100 RP-18 endcapped, Merck column cartridge (250 × 4.6 mm, 5 μm). The chromatograms were monitored at wavelengths 340 nm (rosmarinic acid), 272 nm (gallic acid), 223 nm (andrographolide), 280 nm (cathechin) and 420 nm (curcumin).
2.4. Total phenolic content
Quantification of total phenolic content (TPC) was carried out by Folin–Ciocalteu method27 with slight modifications. Briefly, 100 μl of extract (1 mg/ml diluted in distilled water) was mixed with 100 μl of 0.2 N Folin–Ciocalteu reagents. After 5 min, 80 μl of 7.5% sodium carbonate (Na2CO3) solution was added and incubated for 2 h at RT. The absorbance was measured at 750 nm against the blank. The calibration curve was prepared using the standard gallic acid solution. The total phenolic content was expressed in mg of Gallic acid equivalent (GAE/100 g of sample).
2.5. Total flavonoid content
Total flavonoid content (TFC) was determined using aluminum chloride colorimetric method28 with slight modifications. Briefly, 200 μl of extract was mixed with 12 μl NaNO2 and 12 μl AlCl3. After 5 min incubation at RT, 80 μl of NaOH was added and re-incubated for 30 min. The absorbance was read at 510 nm. The calibration curve was prepared using standard catechin solution. The total flavonoid content was expressed in mg of g catechin equivalent (CE/100 g of sample).
2.6. Antioxidant assay
2.6.1. DPPH radical scavenging activity assay
The DPPH radical scavenging activity was determined according to Guilong Yan protocol with modifications.29 Briefly, 0.2 mM of DPPH-methanol solution was added to equal volume of extract dissolved in methanol at various concentrations (5 μg–500 μg/ml). The mixture was vortexed vigorously and incubated for 30 min at RT. The absorbance was spectrophotometrically measured at 517 nm. Ascorbic acid was chosen as positive control. The scavenging activity was calculated using the following equation:
where Ac is the absorbance of reaction without samples and As is the absorbance of tested extract. The antioxidant activity was expressed as median effective concentration (EC50) where the concentration caused 50% reduction of DPPH.
2.6.2. ABTS radical scavenging activity
ABTS inhibition was measured based on Iqbal method with modifications.30 ABTS stock solution was dissolved in water to a 7 mM concentration. ABTS radical cation was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate and the mixture was allowed to stand in dark at room temperature for 12–16 h. Prior to use, ABTS radical cation was diluted in methanol until the absorbance at 0.7 ± 0.05. ABTS solution (300 μl) was added to 30 μl sample and incubated for 6 min at RT. The absorbance was read at 750 nm. Ascorbic acid was chosen as positive control. The radical-scavenging activity was expressed as inhibition percentage, and calculated using the following formula.
where Ac is the absorbance of reaction without samples and As is the absorbance of tested extract. The antioxidant activity was expressed as median effective concentration (EC50) where the concentration caused 50% reduction of ABTS.
2.7. Cytotoxicity assay and embryotoxicity assay
2.7.1. Cell culture
3T3-L1, 1.1B4 and WRL-68 cells were cultured according to the protocol provided by the depositor. 3T3-L1 and WRL-68 were grown in DMEM supplemented with 10% FCS and 10% FBS respectively with additional 1% PS at 37 °C under a humidified atmosphere of 5% CO2. 1.1B4 was cultured in RPMI supplemented with 10% FBS and 1% PS at 37 °C under a humidified atmosphere of 5% CO2.
2.7.2. MTT cytotoxicity assay
Cells were seeded in 96 well plate and incubated overnight. Samples treatment was carried out by replacing spent medium with various concentrations (0.005 mg/ml to 10 mg/ml) of extract diluted in medium. After 24 h of incubation, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to each well and incubated for 4 h. Developed MTT formazon was dissolved in DMSO. Color development was analyzed at 570 nm. Samples were run in 6 replicates. Untreated cells were used as a control.
2.7.3. Animal test
The study design was approved by the Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC) (Approval Number: UTM/FKK/2013/Fadzilah/15-May/506-June-2013-June-2016). Maintenance and breeding procedures were followed the standard OECD guidelines.20 Fish were maintained in 1 ft × 1 ft aquaria with 12 h light:12 h dark cycle at ±28 °C and fed twice daily with dry flake food.
2.7.4. Egg production, collection
Embryos were obtained from spawning zebrafish adults in breeding tanks with sex ratio 3 male:5 female. Spawning was induced in the morning and after 2 h, embryos were collected and washed using embryo medium. Fertilized and dead embryos were separated according to the descriptions of Kimmel.
2.7.5. Embryo short-term toxicity test (FET)
Toxicity test was referred to the OECD guideline; No. 236: Fish Embryo Acute Toxicity (FET) Test.20 Fertilized embryos were transferred into 24 well plates (30 embryos/well) containing a series of diluted extract ranging from 0.005 mg/ml to 10 mg/ml. Exposure involved semi-static condition, ±28 °C and 12 dark: 12 light cycle period. Plates were sealed with parafilm to minimize evaporation. Embryo development was monitored 24 h interval for 96 h. Four morphological characteristics were evaluated including coagulation of eggs, tail detachment, present of heart beat and hatching rates using Carl-Zeiss Inverted Microscope (F51) equipped with phase contrast function, camera and software for image optimization. Experiments were conducted in triplicates.
2.7.6. Calculation of IC50, LC50
Median inhibitory concentrations (IC50) and median lethal concentration (LC50) were statistically analyzed by GraphPad Prism Version 6 software. Non-linear regressions of log (inhibitor) vs. response with four parameters was selected for IC50 and LC50 estimation. The bottom and top constrain were 0% and 100% respectively.
2.8. Statistical analysis
All data were expressed in mean ± SEM. Statistical analysis was performed using SPSS program with one way ANOVA and Tukey test. Significant difference was considered as p < 0.05.
3. Results
3.1. Functional group and biomarker fingerprinting
3.1.1. FTIR spectra analysis
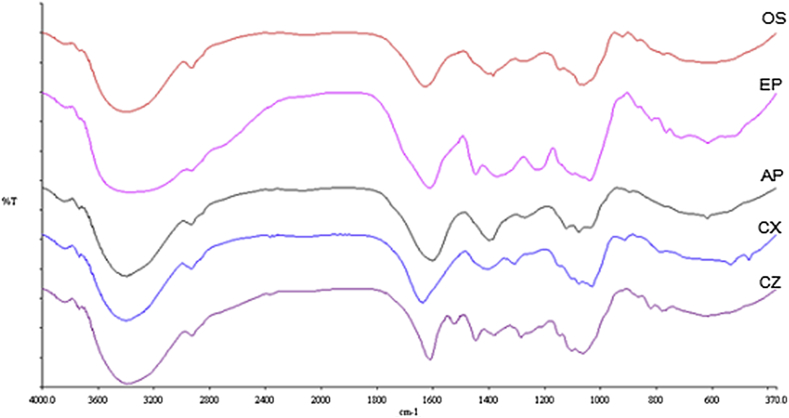
Characterization of active components based on their respective functional groups can be predicted by Fourier transform infrared spectroscopy (FTIR). Fig. 1 demonstrated the spectra peak stretched from 4000 to 370 cm−1 for AP, CX, CZ, EP and OS. Similar trends were observed in all extracts suggesting the existence of alkanes, alkenes, aromatic rings, hydroxyl compounds, carbonyl compounds, amino acid, aldehydes, amines, nitriles, amides and ethers groups as listed in Table 1.
Fig. 1.
FTIR spectra (4000 cm−1 to 370 cm−1) of Andrographis paniculata (AP), Cinnamon zeylanicum (CZ), Curcuma xanthorrhiza (CX), Eugenia polyantha (EP) and Orthosiphon stamineus (OS) water extracts. Similar transmission trends were detected in all extracts.
Table 1.
List of chemical functional groups present in AP, CX, CZ, EP and OS.
| Functional group | Vibration (Stretch/Bend) | Range | AP | CX | CZ | EP | OS |
|---|---|---|---|---|---|---|---|
| Alkanes | C–H stretch | 3000–2800 | ✓ | ✓ | ✓ | ✓ | ✓ |
| C–H bend | 1500–1440 | – | – | ✓ | ✓ | – | |
| Alkenes | C–H stretch | 3200–3000 | – | – | – | – | – |
| C C stretch | 1680–1600 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Aromatic rings | (sp2) = C–H stretch | 3200–3000 | – | – | – | – | – |
| C C stretch | 1600–1400 | ✓ | ✓ | ✓ | |||
| Hydroxyl compounds | O–H stretch | 3600–3200 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Carbonyl compounds | C O stretch | 1610–1550 | ✓ | – | – | – | – |
| Amino acid | C O stretch | 1600–1660 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Aldehydes | C O stretch | 1750–1625 | – | ✓ | – | – | ✓ |
| C–H stretch of C O | 2700–2850 | – | – | – | – | – | |
| Amines | N–H stretch | 3500–3100 | ✓ | ✓ | ✓ | ✓ | ✓ |
| N–H Bend | 1640–1550 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Nitriles | C N stretch | 2500–2000 | ✓ | ✓ | ✓ | – | ✓ |
| Amides | N–H stretch | 3500–3100 | ✓ | ✓ | ✓ | ✓ | ✓ |
| C O stretch | 1670–1600 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| N–H bend | 1640–1550 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Ethers | C–O stretch | 1040 & 1260 | ✓ | ✓ | – | – | ✓ |
In the alkane vibration range, all extracts displayed the C-H stretching (3000–2800 cm−1) vibration, but only CZ and EP contained both C-H stretching and bending (1500–1440 cm−1) vibration. For the alkene group, no C–H stretch (3200–3000 cm−1) was detected, instead the C C (1680–1600 cm−1) stretch were present in all extracts. In the aromatic ring region, the existence of (sp2) = C–H stretches were not detected. However, most extracts have C C stretch in the region 1600–1400 cm−1 with exception to OS and AP. The absorbance was quite strong in the region 3600–3200 cm−1 and all extracts exhibit the behavior of O–H group.
The existence of carbonyl compound (1610–1550 cm−1) was detected only in AP which signifying the existence of ketone group. In amino acid C O region (1600–1660 cm−1), all extracts exhibited similar vibrations trends, confirming the existence of this functional group. Absorbance in the aldehyde range, C O (1750–1625 cm−1) stretch was detectable in CX and OS. However, the C-H stretch (2700–2850 cm−1) for the aldehyde functional group was not detected at all. The absorbance of N–H bend (3500–3100 cm−1) and stretches (1640–1550 cm−1) were identified in all extracts suggesting the amino group exists in every extracts. Meanwhile, the C N stretch (2500–2000 cm−1) was detected in OS, AP, CX, and CZ. The presence of amide functional groups (N–H Stretch, C O Stretch, N–H Bend) was also detected in all extracts. The presence of ether functional group (1040–1260 cm−1) was also detected in OS, AP, and CX.
3.1.2. GC–MS detection
Extracts were further analyzed by GC-MS to identify the presence of volatile compounds. Table 2 summarizes the five major compounds detected in each extract. These compounds have shown beneficial pharmacological activities as anti-viral, anti-inflammatory, antioxidant, anticancer, antimicrobial, anti-fungal, antibacterial, anti-acne, anti-hyperglycemic, anti-hypertensive, antiulcer, anti-arthritis, anti-coagulant, anti-tubercular, anti-convulsant, anti-obesity, anti-tumor, anti-HIV agents, CNS-active compounds, nephroprotective and hepatoprotective activities. Octadecanoic acid methyl ester detected in all extracts is acting as an anti-viral and anti-microbial properties against measles virus.31 Meanwhile coumarin identified in CX and OS is an anti-inflammatory, anti-coagulant, antibacterial, anti-fungal, anti-viral, anti-hypertensive, anti-tubercular, anti-convulsant, anti-obesity, anti-hyperglycemic, antioxidant, and neuroprotective, anti-tumor, anti-HIV agents, CNS-active compounds.32, 33, 34 Other important compounds were xanthorhixol and cinnamadehyde detected in CX and CZ respectively. Both compounds showed multiple biological effects including as antioxidant, anti-microbial, anti-inflammatory, anti-arthritis, anti-cancer, anti-hyperglycemic, nephroprotective and hepatoprotective agents.35, 36
Table 2.
List of five major compounds, chromatogram percentage area, height, retention time and biological activity of AP, CX, CZ, EP and OS detected by GC–MS.
| Herb | Compound | Area% | Height% | Retention time | Biological Activity |
|---|---|---|---|---|---|
| AP | Octadecanoic acid methyl ester Other names: Stearic acid methyl ester, Methyl stearate Mol. Formula: C18H38O2 |
22.27 | 27.95 | 36.809 | Anti-viral, anti-microbial31 |
| 1,2-Cyclopentanedione Other names: - Mol. Formula: C5H6O2 |
8.50 | 12.79 | 6.863 | NA | |
| Octadecanoic acid, 2,3-dihydroxypropyl ester Other names: - Mol. Formula: C21H42O4 |
6.82 | 4.90 | 46.755 | Co-solvents, oil carrier, antioxidant, anti-acne66 | |
| n-Undecane Other names: Hendecane Mol. Formula: C11H24 |
6.47 | 4.70 | 11.827 | NA | |
| Tetraacetyl-d-xylonic nitrile Other names: (2,3,4-triacetyloxy-5-cyano-5-oxopentyl) acetate Mol. Formula: C14H17NO9 |
3.97 | 1.98 | 25.861 | Antioxidant, anti-cancer67 | |
| CX | Octadecanoic acid, methyl ester Other names: Stearic acid methyl ester, Methyl stearate Mol. Formula: C18H38O2 |
19.56 | 20.78 | 36.798 | Anti-viral, anti-microbial31 |
| Xanthorrhizol Other names: 2-methyl-5-[(2r)-6-methylhept-5-en-2-yl]phenol Mol. Formula: C15H22O |
18.12 | 19.29 | 29.201 | Antioxidant, anti-microbial, anti-inflammatory, anti-hyperglycemic, nephroprotective and hepatoprotective35 | |
| Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- Other names: Curcumene, Ar-Curcumene, 2-Methyl-6-p-tolyl-2-heptene, Alpha-Curcumene Mol. Formula: C15H22 |
6.43 | 7.37 | 22.690 | Antioxidant, anti-viral, anti-ulcer68 | |
| 4-vinylquaiacol Other names: 2-Methoxy-4-vinylphenol Mol. Formula: C9H10O2 |
4.62 | 5.08 | 18.069 | Antioxidant, anti-inflammatory69 | |
| beta.-Elemenone Other names: – Mol. Formula: C15H22O |
4.39 | 4.51 | 25.716 | NA | |
| CZ | Coumarin Other names: 2H-Chromen-2-one Mol. Formula: C9H6O2 |
21.14 | 21.93 | 21.505 | Anti-inflammatory, anti-coagulant, antibacterial, anti-fungal, anti-viral, – anti-hypertensive, anti-tubercular, anti-convulsant, anti-obesity, anti-hyperglycemic, anti-oxidant, and neuroprotective, anti-tumor, anti-HIV agents, CNS-active compounds32, 33, 50, 51 |
| Octadecanoic acid, methyl ester Other names: Stearic acid methyl ester, Methyl stearate Mol. Formula: C18H38O2 |
14.92 | 17.64 | 36.797 | Anti-viral, anti-microbial31 | |
| n-Undecane Other names: Hendecane Mol. Formula: C11H24 |
5.16 | 3.35 | 11.824 | NA | |
| Cinnamaldehyde Other names: Trans-Cinnamaldehyde Mol. Formula: C9H8O |
4.20 | 4.64 | 16.889 | Anti-microbial, anti-inflammatory, anti-arthritis, anti-cancer, anti-oxidant, anti-hyperglycemic, anti-obesity, neuroprotective,43, 52 | |
| Formic acid, 2-propenyl ester Other names: Formic acid, allyl ester, Allyl formate Mol. Formula: C4H6O2 |
3.26 | 2.46 | 8.471 | NA | |
| EP | Octadecanoic acid, methyl ester Other names: Stearic acid methyl ester, Methyl stearate Mol. Formula: C18H38O2 |
22.09 | 15.67 | 36.734 | An-tiviral, anti-microbial31 |
| Coumarin Other names: 2H-Chromen-2-one Mol. Formula: C9H6O2 |
20.56 | 14.56 | 21.515 | Anti-inflammatory, anti-coagulant, antibacterial, anti-fungal, anti-viral, – anti-hypertensive, anti-tubercular, anti-convulsant, anti-obesity, anti-hyperglycemic, anti-oxidant, and neuroprotective, anti-tumor, anti-HIV agents, CNS-active compounds32, 33, 50, 51 | |
| Octadecanoic acid, 2,3-dihydroxypropyl ester Other names: - Mol. Formula: C21H42O4 |
7.23 | 6.43 | 46.697 | Co-solvents, oil carrier, antioxidant, anti-acne66 | |
| Eugenol Other names: 2-Methoxy-4-(2-propenyl)phenol, Eugenic acid, Mol. Formula: C10H12O2 |
3.87 | 3.21 | 4.562 | Anti-cancer, antioxidant, anti-mutagenic, anti-genotoxic, anti-inflammatory6, 53 | |
| n-Undecane Other names: Hendecane Mol. Formula: C11H24 |
3.55 | 2.45 | 11.854 | NA | |
| OS | Octadecanoic acid, methyl ester Other names: Stearic acid methyl ester, Methyl stearate Mol. Formula: C18H38O2 |
11.08 | 15.95 | 36.811 | Anti-viral, anti-microbial31 |
| 2-hydroxy-4-methylbenzaldehyde Other names: 4-Methylsalicylaldehyde Mol. Formula: C8H8O2 |
8.27 | 8.36 | 21.922 | NA | |
| Butyrate, 3-methyl-, 3-butenyl-3-methyl- Other names: 3-methylbut-3-enyl butanoate Mol. Formula: C9H16O2 |
7.37 | 7.76 | 11.833 | NA | |
| Octadecanoic acid, 2,3-dihydroxypropyl ester Other names: - Mol. Formula: C31H62O4 |
7.33 | 4.60 | 46.760 | Antioxidant, anti-acne66 | |
| Coumarin Other names: 2H-Chromen-2-one Mol. Formula: C9H6O2 |
7.22 | 2.75 | 21.512 | Anti-inflammatory, anti-coagulant, anti-bacterial, anti-fungal, anti-viral, – anti-hypertensive, anti-tubercular, anti-convulsant, anti-obesity, anti-hyperglycemic, anti-oxidant, and neuroprotective, anti-tumor, anti-HIV agents, CNS-active compounds32, 33, 50, 51 |
NA− Not available.
3.1.3. Identification and quantification of active biomarkers by HLPC
Five biomarkers were selected for HPLC fingerprinting including andrographolide, curcumin, catechin, gallic acid and rosmarinic acid as previously detected in AP, CX, CZ, EP and OS respectively.37, 38, 39, 40, 41 Quantification of biomarkers (Table 3) showed that 17.78 μg/mg of andrographolide was detected in AP, 4.65 μg/mg of curcumin in CX, 4.29 μg/mg of catechin in CZ, 12.08 μg/mg of gallic acid in EP and 6.39 μg/mg of rosmarinic acid in OS.
Table 3.
Amount of selected biomarkers in AP (andrographolide), CX (curcumin), CZ (catechin), EP (gallic acid) and OS (rosmarinic acid) quantified by HPLC fingerprinting.
| Biomarkers | Andrographolide (μg/mg) (RT: 2.940) |
Curcumin (μg/mg) (RT: 2.631) |
Catechin (μg/mg) (RT: 4.674) |
Gallic acid (μg/mg) (RT: 2.814) |
Rosmarinic acid (μg/mg) (RT: 3.326) |
|---|---|---|---|---|---|
| AP | 17.78 | – | – | – | – |
| CX | – | 4.65 | – | – | – |
| CZ | – | – | 4.29 | – | – |
| EP | – | – | – | 12.08 | – |
| OS | – | – | – | – | 6.39 |
3.2. Total phenolic and flavonoid content
Table 4 summarizes the TPC and TFC in AP, CX, CZ, EP and OS. The TPC values varied widely, ranging from 20.1 mg GAE/100 g samples to 269.9 mg GAE/100 g samples. CZ exhibited the highest phenolic content at 269.9 mg GAE/100 g sample followed by EP (219.4 mg GAE/100 g sample), OS (94.9 mg GAE/100 g sample), AP (21.2 mg GAE/100 g sample) and CX (20.1 mg GAE/100 g sample). Meanwhile, the TFC detected varied from 21.1 CE/100 g sample to 62.1 mg CE/100 g sample. The highest flavonoid content was in CZ at 62.1 mg CE/100 g sample followed by OS (29.3 mg CE/100 g sample), AP (24.5 mg CE/100 g sample), CX (21.1 mg CE/100 g sample) and EP (20.33 mg CE/100 g sample).
Table 4.
Mean of TPC, TFC and EC50 of DPPH and ABTS radical scavenging activity.
| Herbs | Total phenolic content (mg GAE/100 g sample) | Total flavonoid content (mg CE/100 g sample) | EC50 – DPPH RSA (μg/ml) | EC50 – ABTS RSA (μg/ml) |
|---|---|---|---|---|
| AP | 21.2 ± 1.345 | 24.5 ± 0.741 | 143.7 | 915.0 |
| CX | 20.1 ± 0.712 | 21.1 ± 0.217 | 326.3 | 1076.0 |
| CZ | 269.9 ± 28.6 | 62.1 ± 3.064 | 11.03 | 78.26 |
| EP | 219.4 ± 9.586 | 20.3 ± 0.512 | 15.48 | 94.27 |
| OS | 94.9 ± 0.310 | 29.3 ± 1.271 | 53.51 | 284.9 |
| Ascorbic acid | – | – | 2.563 | 34.58 |
Each value in the table is represented as mean (n = 3).
3.3. DPPH and ABTS radical scavenging activities
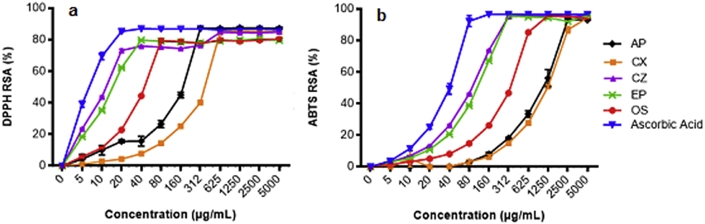
Investigation on the DPPH and ABTS radical scavenging of AP, CX, CZ, EP and OS have demonstrated dose-dependent radical scavenging activities as presented in Fig. 2. The EC50 calculated for DPPH (Table 4) were 11.03 μg/ml, 15.48 μg/ml, 53.51 μg/ml, 143.7 μg/ml and 326.3 μg/ml for CZ, EP, OS, AP and CX respectively. Meanwhile, a similar trend was observed in ABTS in which the EC50 for CZ was 78.26 μg/ml followed by EP (94.27 μg/ml), OS (284.9 μg/ml), AP (915.0 μg/ml) and CX 1075 μg/ml. Therefore, the antioxidant capacities were arranged in the following manner; CZ > EP > OS > AP > CX. The correlation between phenolic content and antioxidant activities were determined by plotting the TPC and TFC values against the values of DPPH and ABTS. With reference to Table 5, significant positive correlations in all herbs extracts were quantified.
Fig. 2.
Antioxidant activities of AP, CX, CZ, EP and OS at various concentrations ranging from 5 to 5000 μg/ml. a) DPPH radical scavenging activities. b) ABTS radical scavenging. Ascorbic acid was used as a standard. Results were expressed in mean ± SEM (n = 3).
Table 5.
Correlation coefficient (R2) between TPC and TFC against antioxidant activities. Positive correlations were determined for all extracts.
| Herbs | TPC |
TFC |
||
|---|---|---|---|---|
| DPPH | ABTS | DPPH | ABTS | |
| AP | 0.8441** | 0.6859*** | 0.8391**** | 0.6786*** |
| CX | 0.8012**** | 0.6546** | 0.7990**** | 0.6524** |
| CZ | 0.8227**** | 0.7545*** | 0.8030**** | 0.8068**** |
| EP | 0.7756*** | 0.7448*** | 0.7308*** | 0.8300**** |
| OS | 0.7850**** | 0.7372*** | 0.8043**** | 0.7930*** |
** Significant at p < 0.01.
*** Significant at p < 0.001.
**** Significant at p < 0.0001.
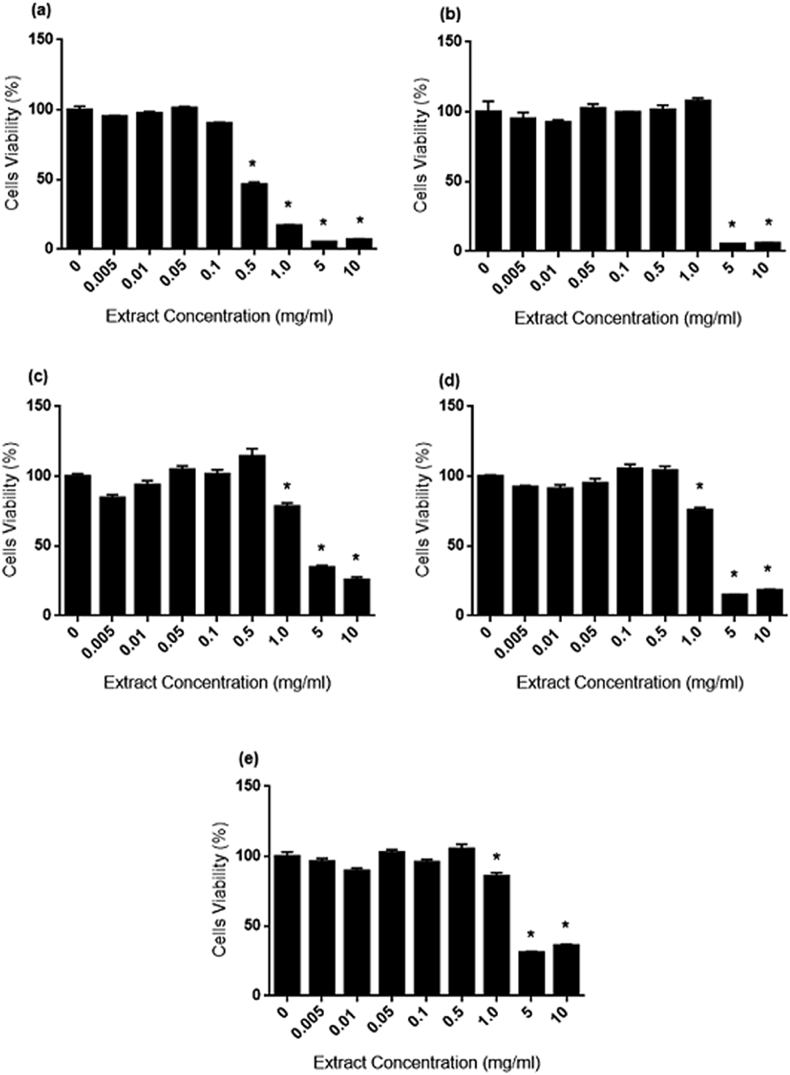
3.4. Cytotoxicity effects on cell proliferations
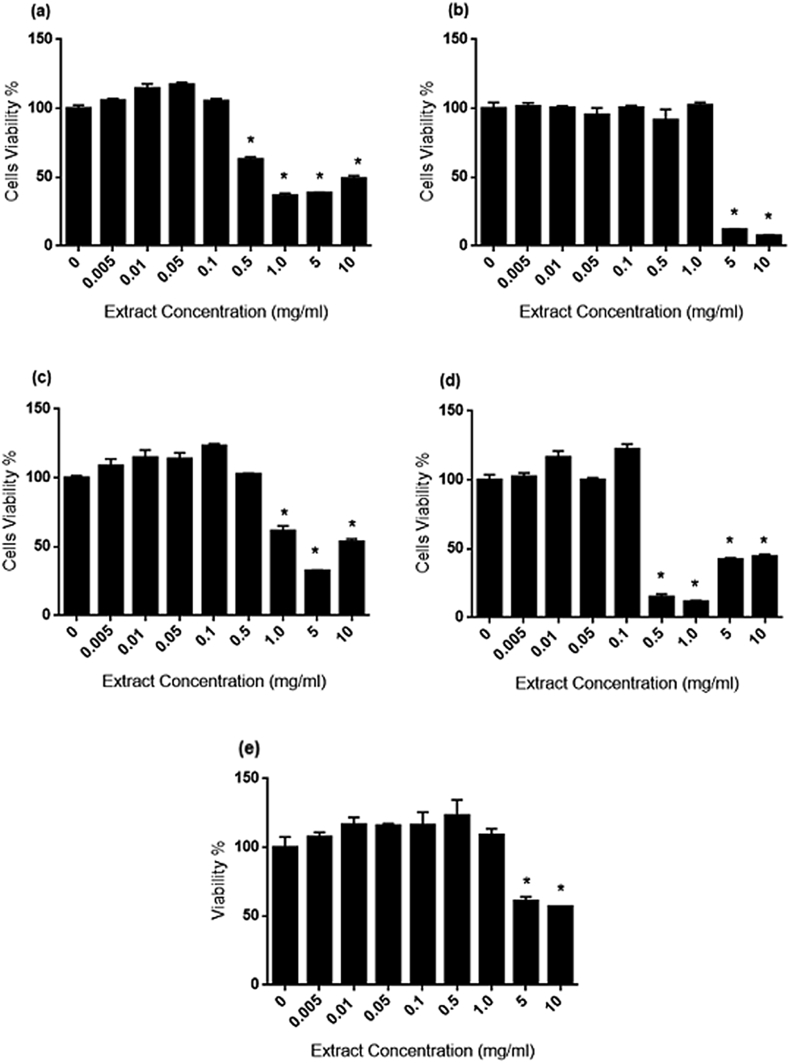
The cytotoxicity effects of AP, CX, CZ, EP and OS on cells proliferations were assessed via MTT assay. Fig. 3, Fig. 4, Fig. 5 illustrate the dose-dependent effect of extracts on 1.1B4, 3T3-L1 and WRL-68 cells viability during 24 h of treatment. Fig. 3 (A–E) showed significant decrease (p < 0.05) on 1.1B4 cells viability starting from 0.5 mg/ml for AP and EP, 1.0 mg/ml for CZ and 5.0 mg/ml for CX and OS. In addition, the IC50 values (Table 6) were arranged based on decreasing toxicity levels as follow; EP (0.365 mg/ml) > AP (2.345 mg/ml) > CX (3.099 mg/ml) > CZ (4.692 mg/ml) > OS (9.934 mg/ml).
Fig. 3.
1.1B4 cell proliferation during AP, CX, CZ, EP and OS treatment for 24 h. Different levels of cytotoxicity effect were identified in each herb. Significant decrease (p < 0.05) in viability was observed starting from 0.5 mg/ml for AP (Fig. 3A) and EP (Fig. 3D), 1.0 mg/ml for CZ (Fig. 3C) and 5.0 mg/ml for CX (Fig. 3B) and OS (Fig. 3E). AP and EP, 500 μg/ml for CZ and 1000 μg/ml for CX and OS.
Fig. 4.
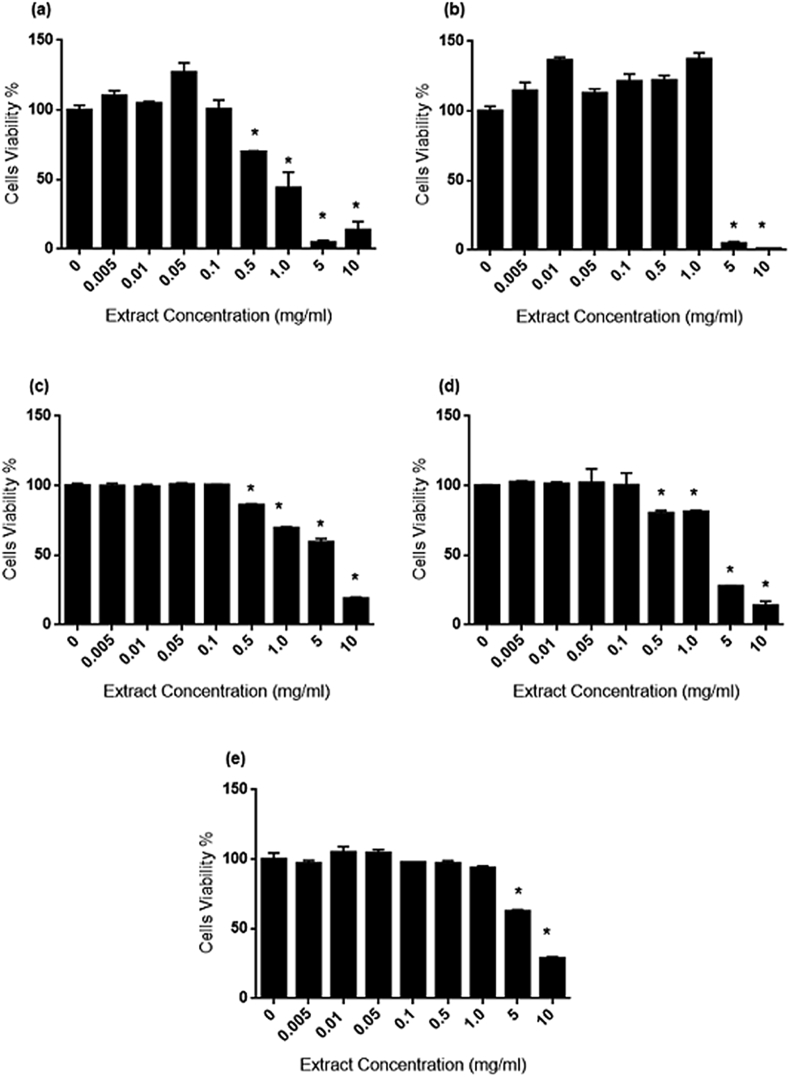
3T3-L1 cell proliferation during AP, CX. CZ, EP and OS treatment for 24 h. Significant decrease (p < 0.05) in viability was observed starting from 0.5 mg/ml for AP (Fig. 4A), CZ (Fig. 4C) and EP (Fig. 4D) and 5.0 mg/ml for CX (Fig. 4B) and OS (Fig. 4E).
Fig. 5.
WRL-68 cell proliferation during AP, CX. CZ, EP and OS treatment for 24 h. Significant decrease (p < 0.05) in viability was observed starting from 0.5 mg/ml for AP (Fig. 5A), 1.0 mg/ml for CZ (Fig. 5C), EP (Fig. 5D) and OS (Fig. 5E). CX (Fig. 5B) was shown to inhibit cell proliferation at 5.0 mg/ml.
Table 6.
IC50 of extracts that cause 50% inhibition on 1.1B4, 3T3-L1 and WRL-68 cell viability.
| Herbs | 1.1B4 (mg/ml) | 3T3-L1 (mg/ml) | WRL-68 (mg/ml) |
|---|---|---|---|
| AP | 2.345 | 0.6498 | 0.440 |
| CX | 3.099 | 3.786 | 3.406 |
| CZ | 4.692 | 3.928 | 3.681 |
| EP | 0.365 | 2.430 | 2.213 |
| OS | 9.934 | 6.335 | 4.014 |
Referring to Fig. 4 (A–E), 0.5 mg/ml of AP, EP and CZ significantly (p < 0.05) inhibited 3T3-L1 viability. Meanwhile CX and OS caused significant (p < 0.05) cytotoxicity at 5.0 mg/ml. The IC50 of extracts for 3T3-L1 cells were arranged as follow; AP (0.6498 mg/ml) > CX (3.786 mg/ml) > CZ (3.928 mg/ml) > OS (6.335 mg/ml) > EP (6.690 mg/ml). The cytotoxicity effect on WRL-68 (Fig. 5A–E) displayed significant reduction (p < 0.05) in viability starting from 0.5 mg/ml for AP, 0.1 mg/ml of CZ, EP and OS. Meanwhile CX has only showed to be toxic to WRL-68 at 5.0 mg/ml. Based on IC50 values, the cytotoxicity effect of extracts on WRL-68 were arranged as follows: AP (0.440 mg/ml) > EP (2.213 mg/ml) > CX (3.406 mg/ml) > CZ (3.691 mg/ml) > OS (4.014 mg/ml).
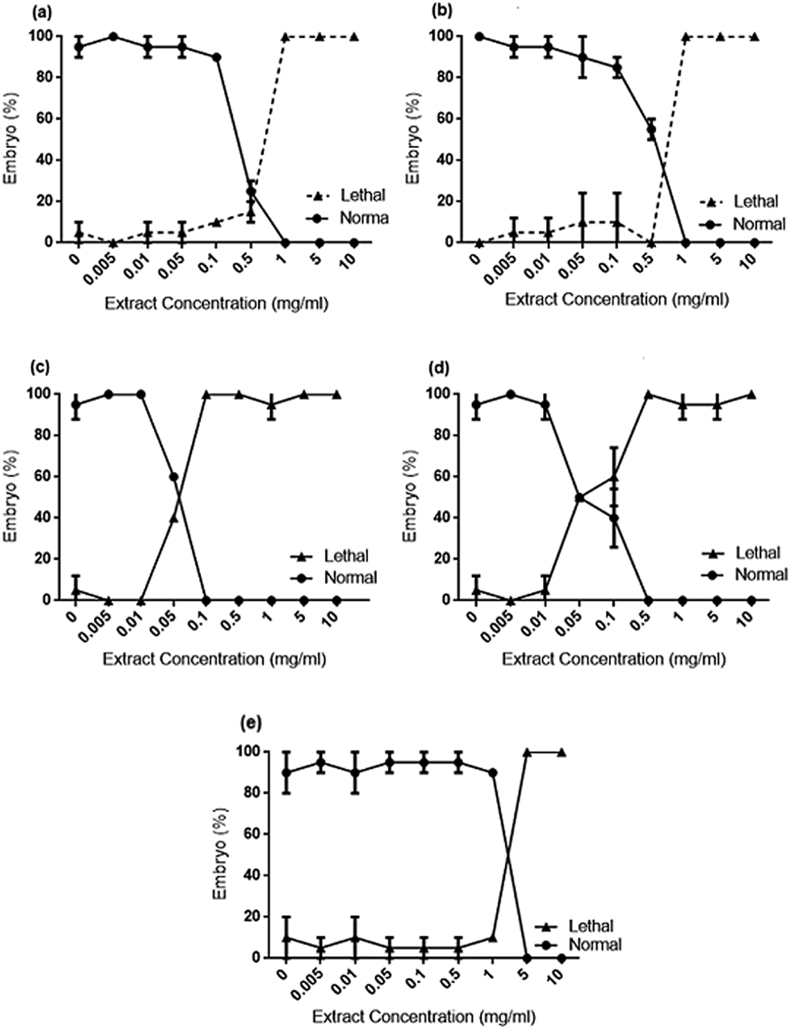
3.5. Fish embryo acute toxicity (FET) test on zebrafish
FET test performed on zebrafish revealed to be more toxico-sensitive towards the embryos development. Measurement on survival rate and embryogenesis end points were carried out every 24 h by optical monitoring. Fig. 6 (A–E) demonstrated the dose-dependent effects on embryos mortality and survival curves during the 96 h of extracts treatment. With reference to Table 7, CZ exhibited the highest toxicity with calculated LC50 of 0.0508 mg/ml, followed by EP (0.06039 mg/ml), AP (0.5256 mg/ml), CX (0.7037 mg/ml) and OS (1.685 mg/ml).
Fig. 6.
Mortality and survival curves of 96 hpf zebrafish embryos during AP, CX. CZ, EP and OS treatment. At a concentration of 0.05 mg/ml, CZ (Fig. 6C) and EP (Fig. 6D) caused significant mortality. Meanwhile, AP (Fig. 6A) and CX (Fig. 6B) caused mortality at 0.5 mg/ml and OS (Fig. 6E) at 5 mg/ml.
Table 7.
LC50 of extracts at 48 hpf and 96 hpf of FET test.
| Herbs | LC50-48 hpf (mg/ml) | LC50-96 hpf (mg/ml) |
|---|---|---|
| AP | 0.5255 | 0.5256 |
| CX | 0.7486 | 0.7037 |
| CZ | 0.9858 | 0.05058 |
| EP | 0.9212 | 0.06039 |
| OS | 1.685 | 1.685 |
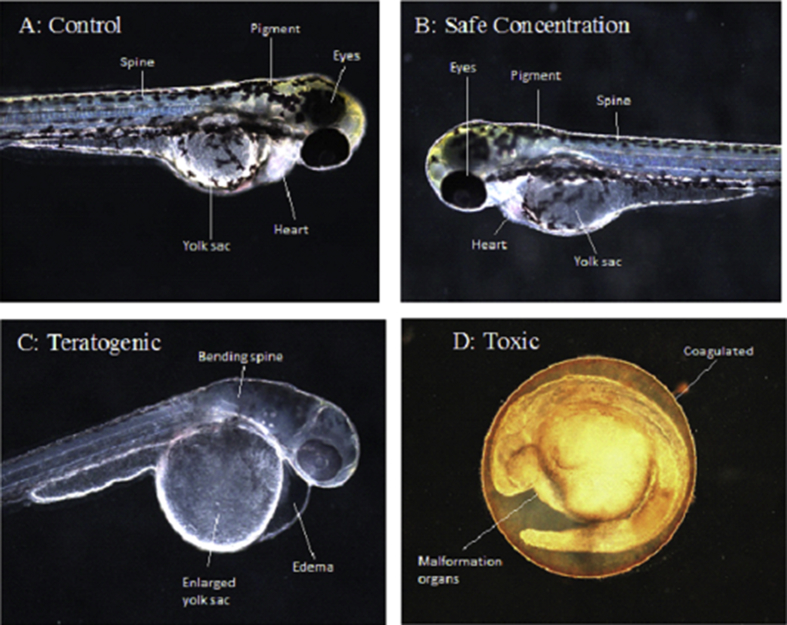
Assessment of the morphological aspects revealed a straight spine, normal body shape and heart rate, round yolk sac and pigmentation on the body and eyes for the vehicle control (Fig. 7A) and non-toxic concentrations (Fig. 7B). In contrast, teratogenic concentration (Fig. 7C), the concentration that cause abnormal organ development demonstrated bent spine, enlarged yolk sac, pericardial edema, slow heartbeat and delayed hatching (>72 hpf). Exposure on the toxic concentration (Fig. 7D) exhibited egg coagulation and undeveloped organs, especially on the formation of the spine, tail, cartilage, heart and jaw.
Fig. 7.
Morphological effect of extracts on zebrafish embryogenesis after 96 h of exposure (hpf). (A–B) 96 hpf embryo during control and non-toxic concentration exposure demonstrated straight spine, normal body shape, normal heartbeat, round yolk sac and high intensity of pigmentation on the body and eye. (C) 96 hpf embryos after exposed to teratogen concentration exhibited morphological defects such as bending spine, pericardial edema, enlarged yolk sac and hatching delayed. (D) Coagulated eggs with organ malformation after exposure to toxic concentration.
3.6. Cytotoxicity and embryotoxicity correlation
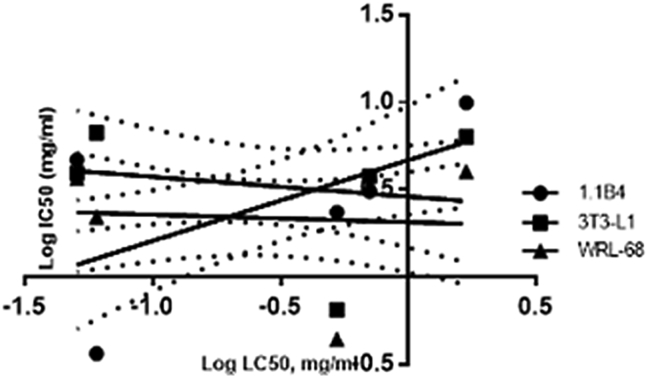
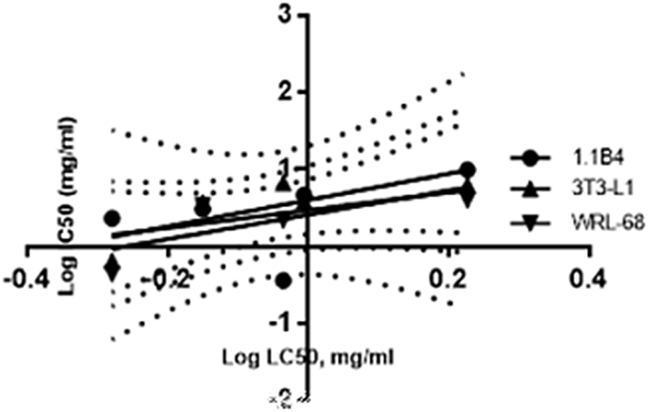
The correlation between cytotoxicity and embryotoxicity was expressed in logarithmic IC50 versus the corresponding LC50. Fig. 8 demonstrated that LC50-96 hpf was weakly correlated with IC50-1.1B4, IC50-3T3-L1 and IC50-WRL-68 (Eq. 1.1, 1.2, 1.3). Interestingly, better correlations were calculated for LC50-48 h (Fig. 9) (Eq. 2.1, 2.2, 2.3). Prediction between LC50-48 hpf and IC50-3T3-L1 was the closest correlation as represented in Eq. 2.2 (R2 = 0.6077).
| 1.1B4; Log (LC50 mg/ml) = 0.4620 log (IC50 mg/ml) + 0.6697 [R2 = 0.3444] | 1.1 |
| 3T3-L1; Log (LC50 mg/ml) = −0.1138 log (IC50 mg/ml) + 0.4606 [R2 = 0.03492] | 1.2 |
| WRL-68; Log (LC50 mg/ml) = −0.04306 log (IC50 mg/ml) + 0.3146 [R2 = 0.0053] | 1.3 |
| 1.1B4; Log (LC50 mg/ml) = 1.151 log (IC50 mg/ml) + 0.4748 [R2 = 0.1642] | 2.1 |
| 3T3-L1; Log (LC50 mg/ml) = 1.712 log (IC50 mg/ml) + 0.6064 [R2 = 0.6077] | 2.2 |
| WRL-68; Log (LC50 mg/ml) = 1.581 log (IC50 mg/ml) + 0.0.4155 [R2 = 0.5495] | 2.3 |
Fig. 8.
Correlation between LC50-96 hpf and IC50 of three tested cells. 1.1B4 displayed the closest relation between cytotoxicity and embryotoxicity with R2 = 0.3444. Linear regression indicated a correlation between LC50-96 hpf and IC50 with positive slope of 0.4620 and y-intercept at 0.6697.
Fig. 9.
Correlation between LC50-48 hpf and IC50 of three tested cells. Better relationships were observed between LC50-48 hpf and IC50 as compared to LC50-96 hpf and IC50 for 3T3-L1 cells and WRL-68. 3T3-L1 exhibited the highest correlation with LC50-48 hpf with R2 = 0.6077. The slope was 1.712 and y-intercept at 0.6064.
4. Discussions
For centuries, herbaceous plants have been frequently used for their scent and flavor in daily culinary as well as dietary supplement for health promotion. The present study examined the inhibitory capacity of water extracts of A. paniculata (AP), Cinnamon zeylanicum (CZ), C. xanthorrhiza (CX), E. polyantha (EP) and O. stamineus (OS) on the formation of DPPH and ABTS free radicals. Furthermore, the toxicity effect of extracts was measured under specific condition via in vitro cytotoxicity assay and zebrafish embryo acute test.
The phenolic compounds are predominant constituents in plants and categorized as phenolic acid, flavonoids, tannins, curcuminoids, coumarins, lignins and quinones. Our study revealed that CZ and EP contained the highest phenolic and flavonoid substances. However, these values were relatively low in comparison to previous reports carried out using different types of extraction processes and solvents. Microwave assist extraction (MAE) of CZ produced 1679 mg CAE/100 g DW of phenolic content which was 6 fold higher than our results.42 Meanwhile, an advance subcritical water extraction process produced ±17,500 mg GAE/100 g DW of phenolic content from cinnamon bark.43 Extraction solvent also affected the yield of phenolic content. 80% ethanol (50 °C/2 h) produced 1460 mg GAE/100 g DW of EP.44
Two approaches have been developed particularly to quantify the antioxidant ability to suppress or inhibit the formation of ROS; 1) radical scavenging activity (RSA) of compounds (DPPH, ABTS, ORAC) and 2) reduction process of samples (FRAP, CUPRAC).45 Scavenging of DPPH radicals is mechanized by the donation of hydrogen atom to the unpaired electron of nitrogen bridge causing the purple color turn to yellowish. Meanwhile, the ABTS+ radical cation undergoes the reduction process by hydrogen donating antioxidant and can be spectrophotometrically measured. It is well accepted that the phenolic and flavonoid content significantly influence the antioxidant activities.46, 47, 48 In agreement, our findings showed that radical scavenging of DPPH and ABTS were positively correlated to the phenolic and flavonoid content of herbs. The presence of phytochemical compounds such as andrographolide, curcumin, catechin, gallic acid, rosmarinic acid, coumarin, cinnamaldehyde and eugenol might play important roles on inhibitory effect of ROS. Coumarin detected in CZ and EP is a naturally occurring polyphenol and a remarkable antioxidant agent.32, 49, 50, 51 Meanwhile, cinnamaldehyde, an active compound in CZ significantly reduced the formation of lipid peroxide in STZ-induced rat plasma, thus prevent the glycation of free radical scavenging enzymes (SOD, CAT and CPx) in pancreatic beta cells.52 Eugenol, a prime component in EP, played tremendous beneficial effects as anti-cancer, antioxidant and anti-inflammatory agent.6, 53
In vitro cytotoxicity assay is performed to quantify toxicity effect of tested compounds towards the basic cellular functions via ATP, MTT, MTS, LDH or NRU assay.54, 55, 56 In this study, water extracts of AP, CX, CZ, EP and OS resulted in a dose-dependent cytotoxicity effect on 1.1B4, 3T3-L1 and WRL-68 cell line, but with different IC50 values. OS exhibited the lowest cytotoxicity effect to all cells, meanwhile AP demonstrated highest cytotoxicity effects. Previous study on IC50 of AP aqueous partition on WRL-68, a normal liver cells gave value greater than 100 μg/ml whereas for butanol, chloroform and hexane partition, the values ranged from 52 to 62 μg/ml.57 In another study, 500 μg/ml aqueous and ethanol extract of AP was non-toxic to the human normal lung fibroblast cell line Hs888Lu.47 Moreover, AP exhibited greater sensitivity towards cancer cells. IC50 of ethanol partition of AP on human hepatocarcinoma HePG2 were between 17–23 μg/ml. AP showed an increase of apoptotic event in HePG2 through the elevating of ROS and LDH, reduction in MMP, increasing of cell membrane permeability and CYP C. The activation of downstream caspase (Caspase 8 and 9), the important markers in apoptotic pathways were elevated during AP treatment.57 On the contrary, WOS displayed the lowest cytotoxicity effect with IC50 of 9.934 mg/ml, 6.335 mg/ml and 4.014 mg/ml for 1.1B4, 3T3-L1 and WRL-68 respectively. According to Al-Saude, the partition B2 of OS supercritical carbon dioxide extraction demonstrated selective cytotoxicity effect on PC3 and MDA-MB cancer cells. Interestingly, MCF7, MDA-MB, HCT116 and CCD-12 CO were shown to have mild and negligible toxic towards all partitions of OS supercritical carbon dioxide extract.58
Early development of embryos is the most sensitive phase toward external stimuli; i.e. toxicant, chemicals and mechanical stress.59 Our results evidenced a significant increment of toxicity effect after 48 hpf particularly for CZ and EP, causing the decrease of the survival rate, malformation of organs, abnormal heart beat and delayed in hatching rates. Results suggest that the chorion substantially protected the embryos by slowing down the diffusion of extracts into the embryos hence delayed the effect of toxicant until hatching. Dechorionation of embryos is one step to overcome this problem by; 1) enzymatic softening of chorion using proteolytic proteinase enzymes 2) mechanical dechorionation using fine forceps or automated machines59 and 3) microinjection through penetrating the chorion to the perivetilline space. The delayed in hatching is also suspected due to the absence of hatching enzymes secreted by hatching gland of zebrafish such as zebrafish hatching enzyme 1 (ZHE1). Evidences on the effect of compounds causing hatching gland dysfunction are well documented during exposure of nanoparticles and methylmercury.60, 61 Furthermore, the development of heart and blood circulation is completed by approximately 24–48 hpf and it is the first internal organ to form and function properly. Consequently, toxic compounds flow to the specific affected organ thus affecting the later stages of embryo development. Several reports have displayed the sensitivity of zebrafish embryo on plant extracts. A study on the cardiovascular system (HBR and blood flow) using an ethanolic extract of Cynodon dactylon and Sida acuta showed an altered heart beat rate and the blood velocity during cardiac cycle in 3 hpf zebrafish embryos.62 The LC50 for C. dactylon and S. acuta were 32.6 μg/ml and 20.9 μg/ml respectively. Another study on seed cake of Jatropha curcus, a biodiesel production waste, the LC50 for zebrafish embryo was 1.61 g/l.63 High concentration of seed cake showed to induce yolk sac and pericardial edema in 72 hpf zebrafish embryo.
Comparison study between cytotoxicity and embryotoxicity was previously conducted using polymeric nanoparticles,64 polyamidoamine (PAMAM) and polypropylenimine (PPI) and the result showed higher sensitivity of embryos compared to cell lines. Similar results were found in our study in agreement with the previous study. The LC50 of zebrafish embryotoxicity is significantly lower as compared to the IC50 of cytotoxicity assay performed in HepG2 and DU145 cell lines which both derived from human. In contrast, in a study on the toxicity of insecticide propoxur, insignificant difference between the embryotoxicty LC50-96 hpf and IC50 of zebrafish gill cell value was calculated perhaps due to the similarity of models sources, providing a similarity on the toxicity mechanism itself.65
To summarize, the present study demonstrated that phenolic and flavonoid content varied between the tested extracts. In addition, the DPPH and ABTS scavenging activities were found to be significantly correlated with the amount of TPC and TFC. Cinnamon zeylanicum and Eugenia paniculata significantly inhibited ROS formation in both DPPH and ABTS assay at low concentration. Our findings also suggested that OS produced the lowest toxicity effect on cell lines and zebrafish embryos. Moreover, a good correlation was determined between this two toxicity models.
Funding
This research was supported by Research University Grant, Universiti Teknologi Malaysia (RUG: 10H27); NRGS, Ministry of Agriculture, Malaysia (4H016); and Fundamental Research Grant Scheme (FRGS), Ministry of Higher Education, Malaysia (59424).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Hassan Fahmi Ismail, Email: hassanfahmiismail@yahoo.com.
Zanariah Hashim, Email: zanariahhashim@utm.my.
Wong Tet Soon, Email: daniel_wongts@yahoo.com.
Nur Syukriah Ab Rahman, Email: syukriah_org@yahoo.com.
Ain Nabihah Zainudin, Email: ainnabihah.zainudin@gmail.com.
Fadzilah Adibah Abdul Majid, Email: f.adibah@umt.edu.my.
References
- 1.Alfadda A.A., Sallam R.M. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:14. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke M.S., Evans M.D., Dizdaroglu M., Joseph L. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 4.Jeyabalan J., Aqil F., Soper L., Schultz D.J., Gupta R.C. Potent chemopreventive/antioxidant activity detected in common spices of the Apiaceae family. Nutr Cancer. 2015;67(7):1201–1207. doi: 10.1080/01635581.2015.1075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moukette B.M., Pieme C.A., Njimou J.R., Biapa C.P., Marco B., Ngogang J.Y. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol Res. 2015;48:15. doi: 10.1186/s40659-015-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan K. Antioxidant potential of spices and their active constituents. Crit Rev Food Sci Nutr. 2014;54(3):352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- 7.Suantawee T., Wesarachanon K., Anantsuphasak K., et al. Protein glycation inhibitory activity and antioxidant capacity of clove extract. J Food Sci Technol. 2015;52(6):3843–3850. doi: 10.1007/s13197-014-1452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paur I., Carlsen M.H., Halvorsen B.L., Blomhoff R. In: Herbal Medicine: Biomolecular and Clinical Aspects. Benzie I.F.F., Wachtel-Galor S., editors. Llc; Boca Raton FL: 2011. Antioxidants in herbs and spices: roles in oxidative stress and redox signaling. [Google Scholar]
- 9.Brewer M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10(4):221–247. [Google Scholar]
- 10.Burton G.W., Traber M.G., Acuff R.V., et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67(4):669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 11.BPFK . 2013. Annual Report of the National Centre for Adverse Drug Reaction Monitoring. [Google Scholar]
- 12.BPFK Traditional medicine: a reminder to report all suspected adverse reactions. Berita Ubat-Ubatan. 2000 [Google Scholar]
- 13.Baghkhani L., Jafari M. Cardiovascular adverse reactions associated with Guarana: is there a causal effect? J Herb Pharmacother. 2002;2(1):57–61. [PubMed] [Google Scholar]
- 14.Phua D.H., Zosel A., Heard K. Dietary supplements and herbal medicine toxicities—when to anticipate them and how to manage them. Int J Emerg Med. 2009;2(2):69–76. doi: 10.1007/s12245-009-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller C.A., Dyer J.E., Ko R., Olson K.R. Making a diagnosis of herbal-related toxic hepatitis. West J Med. 2002;176(1):39–44. doi: 10.1136/ewjm.176.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012;1:44. doi: 10.4103/2277-9175.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugel S.M., Tanguay R.L., Planchart A. Zebrafish: a marvel of high-throughput biology for 21 century toxicology. Curr Environ Health Rep. 2014;1(4):341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allard P. Emerging toxicity models from emerging scientists. Toxicol Res. 2015;4(3):545–547. doi: 10.1039/C4TX00085D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25(9):904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 20.OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD Publishing.
- 21.Li L., Bonneton F., Tohme M., Bernard L., Chen X.Y., Laudet V. In vivo screening using transgenic zebrafish embryos reveals new effects of HDAC inhibitors trichostatin a and valproic acid on organogenesis. PLoS One. 2016;11(2):e0149497. doi: 10.1371/journal.pone.0149497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X., Chen X., Jin X., He J., Yin Z. Generation and characterization of gsualpha: EGFP transgenic zebrafish for evaluating endocrine-disrupting effects. Toxicol Appl Pharmacol. 2014;278(1):78–84. doi: 10.1016/j.taap.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Marín-Juez R., Jong-Raadsen S., Yang S., Spaink H.P. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J Endocrinol. 2014;222(2):229–241. doi: 10.1530/JOE-14-0178. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Kretz C.A., Maeder M.L., et al. Targeted mutagenesis of zebrafish antithrombin III triggers disseminated intravascular coagulation and thrombosis, revealing insight into function. Blood. 2014;124(1):142–150. doi: 10.1182/blood-2014-03-561027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ota S., Kawahara A. Zebrafish: a model vertebrate suitable for the analysis of human genetic disorders. Congenit Anom. 2014;54(1):8–11. doi: 10.1111/cga.12040. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S., Huang J., Ye J. A fresh look at zebrafish from the perspective of cancer research. J Exp Clin Cancer Res. 2015;34(1):1–9. doi: 10.1186/s13046-015-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatami T., Emami S.A., Miraghaee S.S., Mojarrab M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iran J Pharm Res. 2014;13(2):551–559. [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J.-Y., Tang C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. [Google Scholar]
- 29.Yan G., Ji L., Luo Y., Hu Y. Antioxidant activities of extracts and fractions from Eupatorium lindleyanum DC. Molecules. 2011;16(7):5998–6009. doi: 10.3390/molecules16075998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal P., Ahmed D., Asghar M.N. A comparative in vitro antioxidant potential profile of extracts from different parts of Fagonia cretica. Asian Pac J Trop Med. 2014;7S1:S473–S480. doi: 10.1016/S1995-7645(14)60277-7. [DOI] [PubMed] [Google Scholar]
- 31.Jerah R.E.A.L., Lihan S., Ahmad Ib. The effect of combination of octadecanoic acid, methyl ester and ribavirin against measles virus. Int J Sci Tech Res. 2013;2(10):4. [Google Scholar]
- 32.Borges F., Roleira F., Milhazes N., Santana L., Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem. 2005;12(8):887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 33.Musa M.A., Cooperwood J.S., Khan M.O. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15(26):2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni M.V., Kulkarni G.M., Lin C.H., Sun C.M. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr Med Chem. 2006;13(23):2795–2818. doi: 10.2174/092986706778521968. [DOI] [PubMed] [Google Scholar]
- 35.Oon S.F., Nallappan M., Tee T.T., et al. Xanthorrhizol: a review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015;15(1):1–15. doi: 10.1186/s12935-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao P.V., Gan S.H. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Altern Med. 2014;2014:12. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao P.R., Rathod V.K. Rapid extraction of andrographolide from Andrographis paniculata Nees by three phase partitioning and determination of its antioxidant activity. Biocatal Agri Biotech. 2015;4(4):586–593. [Google Scholar]
- 38.Lechtenberg M., Quandt B., Nahrstedt A. Quantitative determination of curcuminoids in Curcuma rhizomes and rapid differentiation of Curcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochem Anal. 2004;15(3):152–158. doi: 10.1002/pca.759. [DOI] [PubMed] [Google Scholar]
- 39.Mateos-Martin M.L., Fuguet E., Quero C., Perez-Jimenez J., Torres J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal Bioanal Chem. 2012;402(3):1327–1336. doi: 10.1007/s00216-011-5557-3. [DOI] [PubMed] [Google Scholar]
- 40.Lelono R.A., Tachibana S. Bioassay-guided isolation and identification of antioxidative compounds from the bark of Eugenia polyantha. Pak J Biol Sci. 2013;16(16):812–818. doi: 10.3923/pjbs.2013.812.818. [DOI] [PubMed] [Google Scholar]
- 41.Saidan N.H., Hamil M.S.R., Memon A.H., et al. Selected metabolites profiling of Orthosiphon stamineus Benth leaves extracts combined with chemometrics analysis and correlation with biological activities. BMC Complement Altern Med. 2015;15:350. doi: 10.1186/s12906-015-0884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo M., Ferracane R., Graziani G., Ritieni A., Fogliano V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules. 2010;15(9):6365–6374. doi: 10.3390/molecules15096365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khuwijitjaru P., Sayputikasikorn N., Samuhasaneetoo S., Penroj P., Siriwongwilaichat P., Adachi S. Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum) J Oleo Sci. 2012;61(6):349–355. doi: 10.5650/jos.61.349. [DOI] [PubMed] [Google Scholar]
- 44.Lelono R.A., Tachibana S., Itoh K. In vitro antioxidative activities and polyphenol content of Eugenia polyantha Wight grown in Indonesia. Pak J Biol Sci. 2009;12(24):1564–1570. doi: 10.3923/pjbs.2009.1564.1570. [DOI] [PubMed] [Google Scholar]
- 45.Christodouleas D.C., Fotakis C., Nikokavoura A., Papadopoulos K., Calokerinos A.C. Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal Methods. 2015;8(5):1294–1302. [Google Scholar]
- 46.Othman A., Mukhtar N.J., Ismail N.S., Chang S.K. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int Food Res. 2014;21(2):18. [Google Scholar]
- 47.Qader S.W., Abdulla M.A., Chua L.S., Najim N., Zain M.M., Hamdan S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules. 2011;16(4):3433–3443. doi: 10.3390/molecules16043433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong S.P., Leong L.P., William Koh J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99(4):775–783. [Google Scholar]
- 49.Kostova I., Bhatia S., Grigorov P., et al. Coumarins as antioxidants. Curr Med Chem. 2011;18(25):3929–3951. doi: 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- 50.Bubols G.B., Vianna Dda R., Medina-Remon A., et al. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13(3):318–334. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 51.Witaicenis A., Seito L.N., da Silveira Chagas A., et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21(3):240–246. doi: 10.1016/j.phymed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Subash-Babu P., Alshatwi A.A., Ignacimuthu S. Beneficial antioxidative and antiperoxidative effect of cinnamaldehyde protect streptozotocin-induced pancreatic β-cells damage in Wistar rats. Biomol Ther. 2014;22(1):47–54. doi: 10.4062/biomolther.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaganathan S.K., Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012;17(6):6290. doi: 10.3390/molecules17066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ukelis U., Kramer P.-J., Olejniczak K., Mueller S.O. Replacement of in vivo acute oral toxicity studies by in vitro cytotoxicity methods: opportunities, limits and regulatory status. Regul Toxicol Pharmacol. 2008;51(1):108–118. doi: 10.1016/j.yrtph.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Weyermann J., Lochmann D., Zimmer A. A practical note on the use of cytotoxicity assays. Int J Pharm. 2005;288(2):369–376. doi: 10.1016/j.ijpharm.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Fotakis G., Timbrell J.A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160(2):171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Bardi D.A., Halabi M.F., Hassandarvish P., et al. Andrographis paniculata leaf extract prevents thioacetamide-induced liver cirrhosis in rats. PLoS One. 2014;9(10):e109424. doi: 10.1371/journal.pone.0109424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Suede F.S.R., Khadeer Ahamed M.B., Abdul Majid A.S., et al. Optimization of Cat's Whiskers Tea (Orthosiphon stamineus) using supercritical carbon dioxide and selective chemotherapeutic potential against prostate cancer cells. Evid Based Complement Altern Med. 2014;2014:15. doi: 10.1155/2014/396016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandrell D., Truong L., Jephson C., et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom. 2012;17(1):66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samson J.C., Goodridge R., Olobatuyi F., Weis J.S. Delayed effects of embryonic exposure of zebrafish (Danio rerio) to methylmercury (MeHg) Aquat Toxicol. 2001;51(4):369–376. doi: 10.1016/s0166-445x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 61.Muller E.B., Lin S., Nisbet R.M. Quantitative adverse outcome pathway analysis of hatching in zebrafish with CuO nanoparticles. Environ Sci Technol. 2015;49(19):11817–11824. doi: 10.1021/acs.est.5b01837. [DOI] [PubMed] [Google Scholar]
- 62.Kannan R.R., Vincent S.G.P. Cynodon dactylon and Sida acuta extracts impact on the function of the cardiovascular system in zebrafish embryos. J Biomed Res. 2012;26(2):90–97. doi: 10.1016/S1674-8301(12)60017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hallare A., Ruiz P., Cariño J.C.E. Assessment of Jatropha curcas L. biodiesel seed cake toxicity using the zebrafish (Danio rerio) embryo toxicity (ZFET) test. Environ Sci Pollut Res. 2014;21(9):6044–6056. doi: 10.1007/s11356-014-2539-y. [DOI] [PubMed] [Google Scholar]
- 64.Rizzo L.Y., Golombek S.K., Mertens M.E., et al. In vivo nanotoxicity testing using the zebrafish embryo assay. J Mater Chem B Mater Biol. 2013;1 doi: 10.1039/C1033TB20528B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey M.R., Guo H. Evaluation of cytotoxicity, genotoxicity and embryotoxicity of insecticide propoxur using flounder gill (FG) cells and zebrafish embryos. Toxicol Vitro. 2014;28(3):340–353. doi: 10.1016/j.tiv.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Gnanavel V., Saral A.M. GC-MS analysis of petroleum ether and ethanol leaf extarcts from Abrus precatorius linn. Int J Pharm Bio Sci. 2013;4(3):8. [Google Scholar]
- 67.Hussein H.M., Hameed I.H., Ibraheem O.A. Antimicrobial activity and spectral chemical analysis of methanolic leaves extract of Adiantum capillus-veneris using GC-MS and FTIR spectroscopy. Int J Pharmacog Phyto Res. 2016;8(3):17. [Google Scholar]
- 68.Jollow D.J., Mitchell J.R., Zampaglione N., Gillete J.R. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4- bromobenzene oxide as a hepatotoxic metabolite. Pharmacology. 1974:1. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 69.Esatbeyoglu T., Ulbrich K., Rehberg C., Rohn S., Rimbach G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015;6(3):887–893. doi: 10.1039/c4fo00790e. [DOI] [PubMed] [Google Scholar]