Abstract
Several species of Bridelia have been used in the condition of pain & arthritis in Indian folk medicine. Present study revealed the preliminary phytochemical investigation and evaluation of analgesic, anti-inflammatory and anti-arthritic activity as well as underlying mechanism of bark of Bridelia retusa Spreng. (Euphorbiaceae). The bark was subjected to extraction using pet.ether, ethyl acetate and acetone. All the extracts were significantly inhibit abdominal writhings response and licking time in late phase of formalin test. Extracts could also significantly inhibit mean paw edema of rats induced by carrageenan & histamine at dose of 200 & 400 mg/kg, i.p. Test materials also showed significant dose dependent reduction in cotton pellet granuloma & acetic acid induced vascular permeability at 400 mg/kg. Oral administration of B. retusa fractions in CFA induced arthritic rats, physical, biochemical and hematological parameters observed in arthritic animals were altered significantly to near normal condition. The maximum paw edema inhibition at day 21 was observed at 400 mg/kg. It also proved significant protection against protein denaturation & RBC membrane damage.
The GC-MS analysis of EA extract revealed the presence of β-sitosterol, stigmasterol, lupeol and friedelin (Pentacyclic triterpenoid). Therefore present study has demonstrated the analgesic; anti-inflammatory and anti-arthritic activities of B. retusa bark and suggested that the molecular membrane might be associated with inhibition of biochemical and hematological parameters. Overall bioactive profile of B. retusa used phytomedicine in future for inflammatory conditions.
Keywords: Bridelia retusa Spreng, Anti-inflammatory, CFA, Euphorbiaceae, Protein denaturation, Leukocyte migration
Graphical abstract
1. Introduction
Inflammation and pain are common nonspecific manifestations of many diseases. It is a defence mechanism aimed to remove the injurious stimuli and initiate the tissue healing process.1 Various endogenous mediators such as histamine, serotonin, bradykinin, prostaglandins, etc. are most abundant in inflammatory cells and among them prostaglandins are ubiquitous substances that indicate and modulate cell and tissue responses involved in inflammation.2 However, prolonged inflammation can lead to numerous diseases including rheumatoid arthritis (RA), psoriasis, and inflammatory bowel disease. RA, a chronic inflammatory disease which is characterized by immune-mediated inflammatory sinusitis involving cartilage and bone destruction, joint malformation, functional impairment results into continued swelling around the joint, pain, synovial hyperplasia, pannus formation, and morphological changes.3, 4 The only available medicine in modern practice are cyclooxygenase (COX) inhibitors i.e. NSAIDs and opioids. The use of these classical medicine for long term treatment such as in case of RA, may produces severe adverse effects such as gastrointestinal disturbances, renal damage, respiratory depression, and possible dependence. Therefore, new anti-inflammatory and analgesic drugs lacking those effects are being searched all over the world as alternatives. Medicinal plant have great value to phytochemists because of their medicinal properties5 so that, the study of plants that have been traditionally used as pain killers should still be seen as a fruitful and logical research strategy in the search for new analgesic drugs and pain mechanisms.6
Bridelia retusa Spreng. Syn:Bridelia airy shawii (Family: Euphorbiaceae) is a small to moderate sized deciduous tree, spinous when young with the grey bark, found throughout India up to altitude of 1000 m except in very dry regions. Previous study reported that the bark of B. retusa exhibited anti-viral, hypoglycemic and hypotensive properties.7 According to Ayurveda, the bark is good for removal of urinary concretions, useful in lumbago and hemiplegia. The bark is also used as liniment with gingelly oil in rheumatism8 (Kirtikar and Basu, 1999). The bark is documented to be used ethno botanically to promote antifertility.
The presence of triterpene ketone [4-desmethyl eupha 7, 24 (28)-diene-3-one] in the bark has been reported. The bark contains 16–40% of tannins.9 Literature survey revealed that there is no phytopharmacological approach has been made on the B. retusa bark. Acute inflammatory effect of B. retusa bark has been preliminary investigated by carrageenan-induced hind paw edema.10 Species of Bridelia have been widely used in folk medicine for their rheumatic property.
In general, phytosterols, triterpenoid and tannins have been reported to display anti-inflammatory, anti-ulcer, anti-nociceptive and antiarthritic properties. The objective of the present work was to investigate the analgesic and anti-inflammatory effects of B. retusa bark and to elucidate its possible mechanisms of action.
2. Materials and methods
2.1. Plant material and preparation of extracts
The plant material of B. retusa bark was collected from Toranmal region of Satpuda hills, India and it was identified by Dr. D.A.Patil, Taxonomist, SSVPS Science College, Dhule, MS, India. A voucher specimen (B-12) of plant was deposited at the RCPCOP herbarium for reference purpose.
The shade-dried bark powder (1 kg) was successively extracted with petroleum ether (60–80 °C) (PE), mixture of methanol: dichloro methane (1:1) and acetone: water mixture (70:30) (ACE). Further residue obtained from methanol: dichloro methane (1:1) was again partitioned with ethyl acetate (EA; 4 × 500 ml). The yields of PE, EA and ACE extracts were 0.7, 1.1 and 9.2% (w/w), respectively. Proximate chemical analysis and pharmacological activity of all the extracts was carried out according to standard method.11
2.2. GC-MS analysis
GC-MS analysis of EA was carried out using a gas chromatography with mass (Perkin Elmer USA model auto system XL GC interfaced to a API 20 NL) equipped with a split/split less injector inlet and a flame ionization detector (FID). HP-5MS capillary columns (30 m × 0.25 μm film thickness). The column temperature was programmed at 60 °C (6 min), increasing to 240 °C at a rate of 5 °C/min, carrier gas (helium) was set at a flow rate of 0.9 μL/min; ionization energy 70 eV, and scan mode EI. One μL of sample was injected and the compounds were identified by matching their mass fragmentation pattern and retention time. The compounds were identified by comparison with the mass fragmentation pattern of compounds available in NIST library, USA.
2.3. Separation and isolation of phytoconstituents
The unsaponifiable fraction of PE (5 g) was subjected to column chromatography on a silica gel (60–120 mesh)with gradient elution using petroleum ether: ethyl acetate [(90:10,82:18 v/v) yielded compound 1 (28 mg) and compound 2 (22 mg). Similarly EA fraction was chromatographed over silica gel (60–120 mesh) column, eluted with methanol and chloroform (80:20 v/v) in order of increasing polarity to gave compound 3. All the compounds were identified and characterized by melting point, UV, FT-IR, NMR, HPLC, HPTLC and GC-MS analysis.
2.4. HPLC analysis
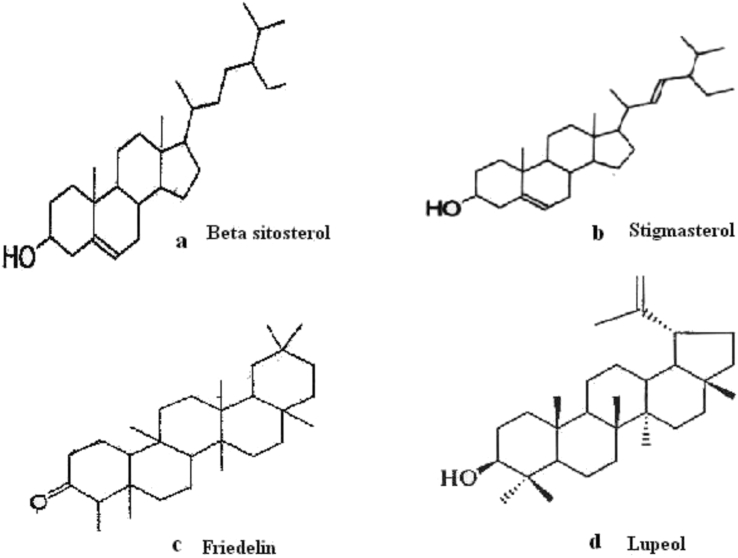
The HPLC system of Agilent 1200 series system (Agilent Technologies, Waldbronn, Germany) with photodiode array detector, ODS C18 (5 μm) column was used (150 mm × 4.6 mm) interfaced with an IBM Pentium 4 personal computer. Elution of the phyconstituents with isocratic of two solvents denoted as A and B was employed (Solvent A: acetonitrile and B: water (60:40%), flow rate: 1 ml/min, injection volume: 20 μl, wavelength: 286 nm. The HPLC profile of isolated compounds was compared with reference compound at a specific wavelength. Identification of phytoconstituents was done on the basis of the retention time and identical spectra of standards. The chemical structures of the isolated compounds are given in Fig. 2a–d.
Fig. 2.
Probable structure of Phytoconstituents. 2a: ß-sitosterol; 2b: stigmasterol; 2c: friedelin; 2d: lupeol.
2.5. Experimental animals
Adult albino Wistar rats and Swiss albino mice of either sex weighing between 150–230 and 30–40 g, respectively were used for the study. The animals were housed in well ventilated cages in the air conditioned animal house at 12 h light & dark conditions and fed with standard pellet diet and water ad libitum. All the experimental procedure and protocols used in the study were approved by Institutional animal Ethical committee (Protocol number 045/2015) under North Maharashtra University, Jalgaon, India in accordance with Committee for the purpose of Control and supervision on experiments on Animals (CPCSEA), guidelines, Chennai, India.
2.6. Acute oral toxicity study
Acute oral toxicity studies were performed according to OECD-423 guidelines (acute toxic class method). Swiss mice of either sex used in this study. Mortality was observed in 2/3 or 3/3 animals and then the dose was fixed.
2.7. Carrageenan and histamine induced rat paw edema
According to a modification of method Winter et al.,12 male wistar rats (150–200 g each) divided into 6 animals in each groups used for study, induced by subcutaneous injection of a 0.1 ml of 1% freshly prepared carrageenan in saline in the right hind paw of rats and sub-plantar injection of histamine at dose of 0.1 ml of 0.1%). Paw volume of the injected rats was measured every hour for 6 h using a plethysmometer (Ugo Basile, Italy). The percentage increase in paw volume was calculated according to formula.13
2.8. Acetic acid induced vascular permeability
The method of Whittle14 was used to evaluate the effect of B. retusa on vascular permeability in adult albino mice of both sexes. One hour after oral administration of the extract, 0.1 ml/10 g b.w. of 1% Evans blue solution was intravenously administered through the tail vein into mice (n = 6), immediately followed by an intra peritoneal injection of, 0.1 ml/10 g of 0.7% acetic acid. Mice were killed by cervical dislocation after 30 min of administration. The peritoneal cavity washed with normal saline (10 ml) into heparinized tubes and centrifuged. The concentration of Evans blue in the peritoneal cavity was measured by absorbance at 630 nm using an ELISA Analyzer (Biotek, USA).15
2.9. Granulomatous tissue induction
The effect of B. retusa fractions on chronic inflammation was evaluated using cotton-pellet granuloma test in rats.12 On day 1, adult albino rats of either sex received orally 200 & 400 mg/kg of PE, EA, ACE fractions. Control group received vehicle only (1%,w/v,carboxymethylcellulose, 5 ml/kg). Thirty minutes later, one autoclaved cotton pellets 15 ± 1.0 mg were aseptically implanted under the previously depilated back of rats anaesthetized with diethyl ether. Test materials were administered once daily for the next 7 days. Animals were killed by overdose of ether on day 8. The pellets were dissected out and dried in the oven overnight at 60 °C. The mean weight of the granuloma tissue formed around each pellet was weighed. The level of inhibition of granuloma tissue development was calculated.
2.10. Acetic acid induced writhing method
Male wistar albino mice (n = 6) weighing 30–35 g were used. The writhings response was induced by intra peritoneal administration of acetic acid solution (1%, 10 mL/kg). The mice were treated with PE, EA and ACE of B. retusa (200 &400 mg/kg), 30 min before administration of acetic acid. The numbers of abdominal writhing were counted for 20 min observation beginning at 5 min after the injection. Writhings inhibitions was calculated by using the following ratio: (control mean treated−mean) × 100/control mean.16, 17
2.11. Formalin induced nociception
The antinociceptive activity of the B. retusa fractions were evaluated using the formalin test according to Hunskaar et al.18 One hour before testing, the rat was placed in a standard cage that served as an observation chamber. The dried extract (PE, EA, ACE) and ethyl morphine hydrochloride (10 mg/kg) were administered i.p. in a volume of 1.5 ml control group received only vehicle (2 ml). 50 μl 2.5% formalin was injected to the dorsal surface of the left hind paw after 15 min. The rat was observed for 30 min after the injection of formalin, and duration of paw licking was measured for 0–5 min (early phase) and 15–30 min (late phase). The percentage inhibition of licking was calculated.
2.12. Freund's complete adjuvant-induced arthritis
Freund's complete adjuvant (CFA, Sigma, USA) induced Arthritis was used to assess the anti-arthritic activity of bark. Animals were divided into groups of six animals in each (n = 6) group. Group I served as control received 1% tween 80, Group II received dexamethasone (5 mg/kg, p.o.) served as reference standard and Group III, IV and V received the crude fractions of PE, EA and ACE of B. retusa at the dose of 200 & 400 mg/kg, b.w. (p.o.) respectively. Arthritis was induced by injecting a 0.1 ml (0.5% w/v) suspension of killed Mycobacterium tuberculosis bacteria homogenized in liquid paraffin into the sub plantar region of left hind paw.19, 20 Drug treatment was started from the day 1 i.e. from the day of adjuvant injection, 30 min before adjuvant injection and continued till 21st day. Paw volume was measured on every three days after the induction of arthritis till day 21 using plethysmometer (Ugo Basile 7140, Italy). The % inhibition of paw edema in the test (extract) treated groups was calculated with respect to untreated group I. On day 21st, the animals were sacrificed by cervical decapitation and blood was collected and separated plasma for assaying the hematological & biochemical parameters.21 Arthritis was also assessed by body and spleen weight of rats.
Hematological parameters blood samples were collected from the retro-orbital plexus for laboratory tests. The hematological parameters like Red Blood Cell (RBC), White Blood Cell (WBC), and Erythrocyte Sedimentation Rate (ESR) were determined.22 RA and CRP Test Rheumatoid arthritis (RA) testing was carried out by utilizing the agglutination reaction between immunoglobulin and rheumatoid factor. C-reactive protein (CRP) factor based on the immunologic reaction between CRP and latex particles. Both the test was measured in serum using a commercial kit.
2.13. Determination of tissue marker enzymes
The marker enzymes glutamate oxalo acetate transaminase/aspartate aminotransferase (GOT/AST) and glutamate pyruvate transaminase/alanine aminotransferase (GPT/ALT) and alkaline phosphatase (ALP) were analyzed in serum. A lysosomal enzyme such as acid phosphatase (ACP) was estimated in plasma. Spleen were dissected out, washed and transferred to an ice-cold saline solution. The organs were weighed.
2.14. Leukocyte migration assay in mice
After oral administration of test samples of B. retusa, animals received an intraperitonial injection of 1% CMC-Na solution in normal saline after 1 h. Four hours later, mice were sacrificed and the peritoneal cavities were washed with 5 ml of the normal saline Hong-Yue Ma et al.15 Twenty micro liter of peritoneal fluid was mixed with 0.38 ml of Turk's solution (0.01% crystal violet in 3% acetic acid) and the number of leukocytes was counted under a light microscope.
2.15. Heat induced haemolysis
Preparation of erythrocyte suspension: Fresh rat blood was collected and transferred to heparinized centrifuge tubes. The tubes were centrifuged at 3000 rpm for 10 min, and washed three times with equal volume of normal saline. The blood volume was measured and reconstituted as a 40% v/v suspension with isotonic buffer solution (10 mM sodium phosphate buffer pH 7.4). The inhibitory effect of test samples on rat erythrocyte haemolysis was assayed by the method described by Perez et al.23 The percent inhibition of haemolysis was calculated using following formula,24, 25
| Percent inhibition = A 540 Control − A 540 Sample × 100/A 540 Control. |
2.16. Inhibition of protein denaturation
The reaction mixture consisted of bovine serum albumin (5% aqueous solution) and test extract. pH was adjusted at 6.3 using a small amount of 1N HCl. The samples were incubated at 370c for 20 min and then heated at 570c for 3 min, after cooling; phosphate buffer saline (pH 6.3) was added to each tube. Turbidity was measured spectrophotometrically at 660 nm. For control tests distilled water was used instead of extracts while product control tests lacked bovine serum albumin.26 The percentage inhibition of protein denaturation was calculated as:
| Percentage inhibition = 100 − (O.D. of test − O.D. of product control)/O.D. of control × 100. |
2.17. Statistical analysis
Data are reported as Mean ± SD. and were analyzed statistically by analysis of variance (ANOVA) followed by Dunnett's test. Results with P < 0.05 were considered significant.
3. Results
3.1. Phytochemical analysis of Bridelia retusa
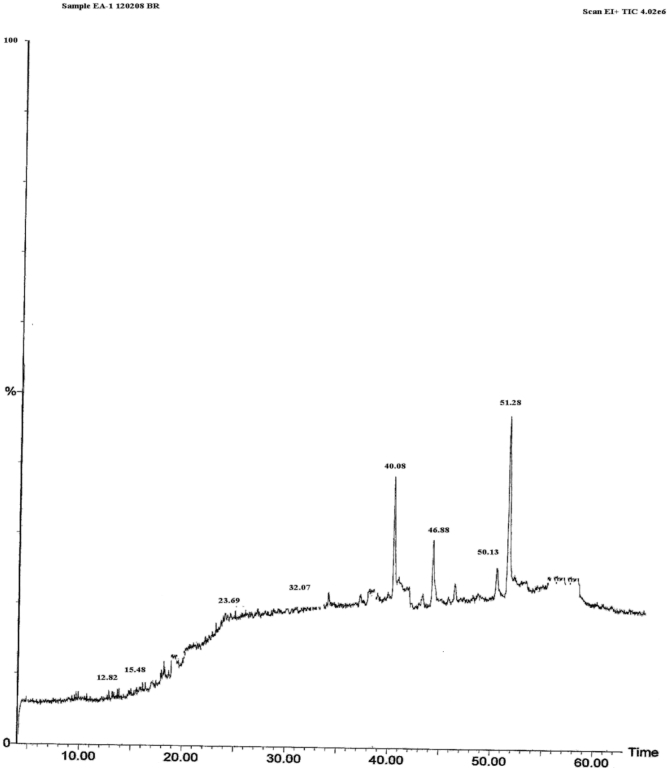
Extracts of B. retusa gave positive test for steroidal/triterpenoidal glycosides and tannins etc. The gas chromatography-mass spectroscopy analysis of B. retusa extract resulted for the presence of ß–sitosterol, stigmasterol, friedelin & Lupeol as displayed in Fig. 1. The melting point of Compound 1 and 2 were 134° and 176 °C respectively and both of them showed positive Lieberman–Burchared chemical test.
Fig. 1.
GC-MS chromatograph profile of EA of Bridelia retusa stem bark, Time (min) 40.08: ß-sitosterol; 46.88: stigmasterol; 50.13: lupeol; 51.28 friedelin.
Mass spectrum of compound 1 & 2 showed a parent molecular ion [M+H]+ peak at m/z 414 and 412, respectively which corresponds to C29H50O and C29H48O. Further evidence in support of the structure of compound 1 & 2 was provided by its IR, 1H-NMR spectral data. The 1H-NMR spectra of compound 1 & 2 was similar to those of a known compound ß-sitosterol and stigmasterol respectively, thus, it was as assigned as ß-sitosterol and stigmasterol. Similarly the melting point of compound 3 was 215–217 °C molecular ion [M+H]+ peak at m/z 426, which corresponds to C30H50O. Besides the molecular ion peak at 426, EI mass spectrum also showed other fragment ion peak at m/z 411 (M-CH3), 218 (M-C14; H28), 207 (M-16; H-27) which are characteristic for pentacyclic triterpenoid. The 1H-NMR, 13C-NMR spectra of compound 3 was similar to those of a known compound Lupeol, thus the structure of compound 3 was assigned as Lupeol. The structures of chemical compound are shown in Fig. 2a–d.
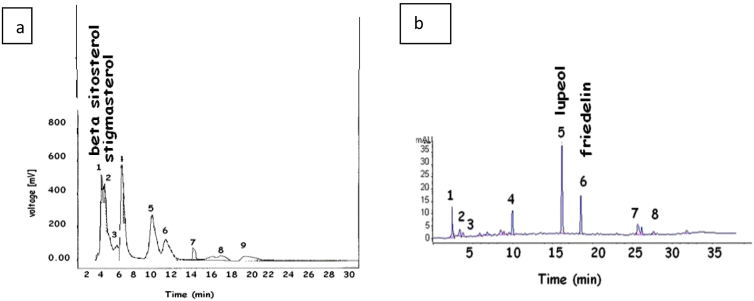
In this paper, reversed phase HPLC chemical fingerprinting method was utilized and developed for identifying and determining the phytoconstituents of B. retusa extract. A total of 9 characteristic peaks in the chromatogram 1 and 8 peaks in chromatogram 2 were observed. ß-sitosterol and stigmasterol were identified in Chromatogram 1 whereas lupeol was identified in chromatogram 2 according to corresponding standards (Fig. 3).
Fig. 3.
HPLC chromatogram of petroleum ether (a) and ethyl acetate extract (b) of Bridelia retusa.
3.2. Carrageenan and histamine-induced rat paw edema
PE, EA and ACE extracts of B. retusa (200 & 400 mg/kg, p.o.) significantly (P < 0.01) inhibited the mean paw volume at 3 h after carrageenan injection. The % inhibition of rat paw edema was gradually increases in PE, ACE and EA at higher dose study. Anti-inflammatory activity of PE, EA and ACE (400 mg/kg, p.o.) produced statistically significant (P < 0.01 and P < 0.001) inhibition of the edema induced by histamine at 2nd & 3rd h., when compared to the vehicle treated control groups (Table 1).
Table 1.
Effect of Bridelia retusa bark on Carrageenan and histamine induced rat paw edema.
| Test group & dose (mg/kg) | Mean (±SD) increases in paw volume in ml |
|||||
|---|---|---|---|---|---|---|
| Carrageenan |
Histamine |
|||||
| 3 h | 5 h | 1 h | 2 h | 3 h | ||
| Control | 0.533 ± 0.024 | 0.721 ± 0.014 | 0.383 ± 0.063 | 0.333 ± 0.054 | 0.283 ± 0.074 | |
| Diclofenac | 13.5 | 0.252 ± 0.011** (52.72) |
0.282 ± 0.016** (60.88) |
0.237 ± 0.064** (38.12) |
0.225 ± 0.038* (32.43) |
0.195 ± 0.033* (31.09) |
| PE | 200 | 0.266 ± 0.036** (50.09) |
0.339 ± 0.011** (52.98) |
0.170 ± 0.079*** (55.61) |
0.208 ± 0.073** (37.53) |
0.123 ± 0.020** (56.53) |
| PE | 400 | 0.255 ± 0.017** (52.15) |
0.310 ± 0.012** (57.00) |
0.260 ± 0.066** (32.11) |
0.287 ± 0.067 (13.81) |
0.243 ± 0.068 (14.13) |
| EA | 200 | 0.327 ± 0.022** (38.64) |
0.423 ± 0.016** (41.33) |
0.216 ± 0.029*** (43.60) |
0.230 ± 0.043* (30.93) |
0.131 ± 0.049** (53.71) |
| EA | 400 | 0.292 ± 0.014** (45.21) |
0.383 ± 0.015** (46.87) |
0.221 ± 0.058** (42.29) |
0.233 ± 0.072* (30.03) |
0.247 ± 0.088 (12.72) |
| ACE | 200 | 0.380 ± 0.010** (28.70) |
0.471 ± 0.017** (34.67) |
0.203 ± 0.042*** (46.99) |
0.173 ± 0.050** (48.04) |
0.137 ± 0.035** (51.59) |
| ACE | 400 | 0.282 ± 0.007** (47.09) |
0.342 ± 0.011** (52.56) |
0.146 ± 0.048*** (61.87) |
0.115 ± 0.047** (65.46) |
0.106 ± 0.045** (62.55) |
Values are expressed as Mean ± S.D. (n = 6), while those in parenthesis represent percentage inhibition of paw edema. *P < 0.05, **P < 0.01, ***P < 0.001. Compared with vehicle control (ANOVA followed by multiple Dunnet's test).
3.3. Acetic acid induced vascular permeability
Administration of B. retusa (200 & 400 mg/kg; p.o) extract evoked a significant (P < 0.001) dose-related inhibition of vascular permeability induced by acetic acid in mice (Table 5).
Table 5.
Effects of B.retusa on leucocytes migration induced by Na-CMC and vascular permeability induced by acetic acid.
| Treatments | Dose (mg/kg) | Leucocytes migration assay |
Vascular permeability assay |
||
|---|---|---|---|---|---|
| Total leucocytes (×103) | % Inhibition of leucocytes migration | Absorbance | Inhibition (%) | ||
| Normal | – | 3.31 ± 0.23 | – | ||
| Control | – | 5.49 ± 0.09 | – | 2.019 ± 0.033 | – |
| PE | 200 | 4.93 ± 0.16* | 10.20 | 0.490 ± 0.089** | 75.73 |
| 400 | 3.74 ± 0.13** | 31.87 | 0.359 ± 0.023** | 82.21 | |
| EA | 200 | 4.92 ± 0.65* | 10.38 | 0.394 ± 0.046** | 80.48 |
| 400 | 3.85 ± 0.61** | 29.87 | 0.289 ± 0.010** | 85.68 | |
| ACE | 200 | 5.06 ± 0.11* | 7.83 | 0.541 ± 0.056** | 73.20 |
| 400 | 4.14 ± 0.09** | 24.59 | 0.416 ± 0.020** | 79.39 | |
| Diclofenac | 10 | 3.23 ± 0.11** | 41.16 | 0.325 ± 0.012** | 83.90 |
Each data represents the Mean ± SD.n = 5. *P < 0.01, **P < 0.001 significantly different from control group. (ANOVA followed by multiple Dunnet's test).
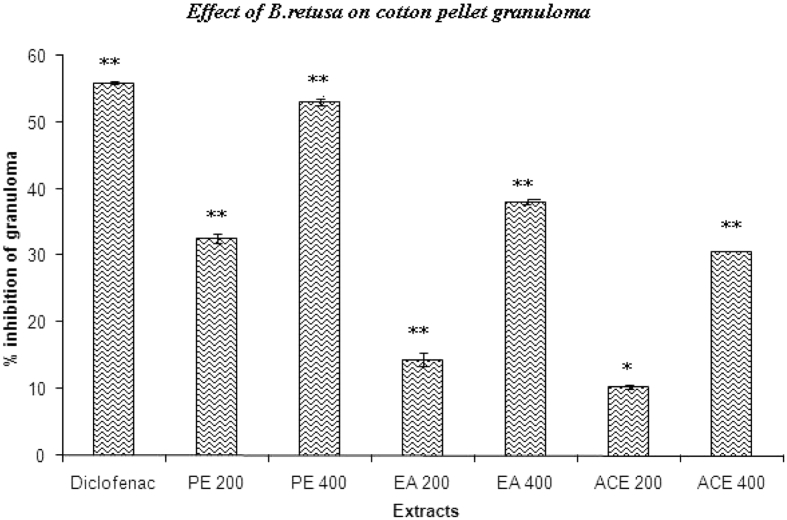
3.4. Cotton pellet-induced granuloma formation
Fig. 4 shows the effect of B. retusa bark extracts on cotton pellet-induced granuloma formation in rats. The results indicate that PE, EA, and ACE at an oral dose of 400 mg/kg significantly inhibited the transudative weight and granuloma formation. Diclofenac elicited significant reduction.
Fig. 4.
Effects of B. retusa bark on cotton pellet granuloma. *P < 0.05, **P < 0.01, as compared with the control group. (One-way ANOVA followed by Dunnett's test).
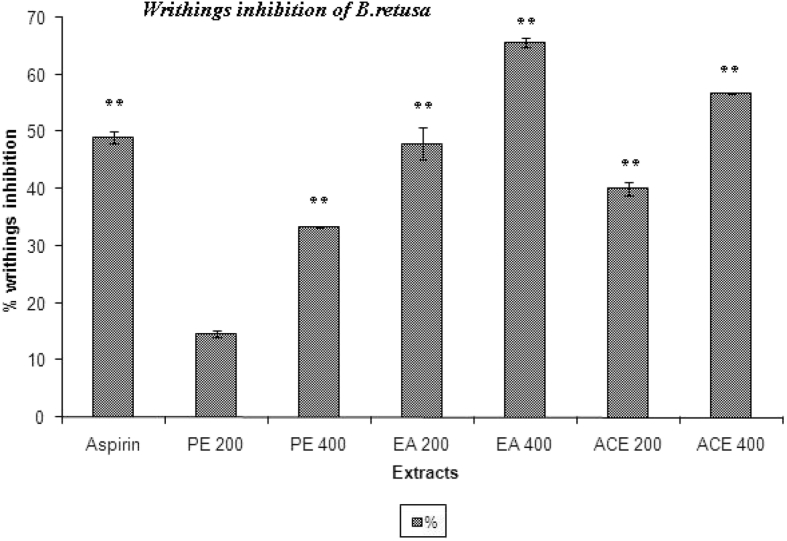
3.5. Writhing test
The peripheral analgesic action of EA (400 mg/kg) was found to be comparable to that of morphine (10 mg/kg). The peripheral analgesic activities of B. retusa and its extracts seem to be related to the inhibition of the synthesis of the arachidonic acid metabolite.
Maximum percentage of inhibition of writhing response was observed with EA (400 mg/kg) as 65.62%. PE, EA and ACE extract significantly (P < 0.01) reduced number writhings at dose dependent manner (200 & 400 mg/kg) (Fig. 5).
Fig. 5.
Effects of PE, EA and ACE fraction of Bridelia retusa on acetic acid-induced writhing response in mice. **P < 0.01, significant as compared to the control by Dunnett's t-test.
3.6. Formalin induced nociception method in rat
The time spent for licking of injured paw was significantly reduced in PE, EA and ACE extract treated animals at 400 mg/kg dose in the late phase (15–30 min) when compared with formalin treated group. The results of test extracts were comparable with morphine treated group, which showed the reduction in licking response up to 51–53% in the late phase (Table 2).
Table 2.
Effect of various fractions of Bridelia retusa stem bark on the licking time of rats in formalin test.
| Treatment/dose mg/kg |
Liking time (sec) |
% Inhibition |
|||
|---|---|---|---|---|---|
| 0–15 min | 15–30 min | 1st phase | 2nd phase | ||
| Control | – | 52.08 ± 2.29 | 66.17 ± 4.24 | – | – |
| PE | 200 | 32.97 ± 1.73 | 37.01 ± 1.80 | 36.69 | 44.06 |
| PE | 400 | 21.33 ± 1.23 | 27.57 ± 1.90 | 59.04 | 58.33 |
| EA | 200 | 31.11 ± 1.05 | 36.47 ± 1.65 | 40.26 | 44.88 |
| EA | 400 | 23.44 ± 3.56 | 27.70 ± 2.27 | 54.99 | 58.13 |
| ACE | 200 | 43.46 ± 3.35 | 46.46 ± 2.76 | 16.55 | 29.78 |
| ACE | 400 | 28.45 ± 2.84 | 34.49 ± 1.92 | 45.37 | 47.87 |
| EMH | – | 25.32 ± 3.53 | 31.30 ± 1.71 | 51.38 | 52.69 |
Values are expressed as Mean ± S.D. (n = 6) compared with vehicle control (ANOVA followed by multiple Dunnet's test). All are significant at P < 0.01. PE = pet. ether, EA = ethyl acetate, ACE = acetone extracts; EMH = Ethyl morphine hydrochloride.
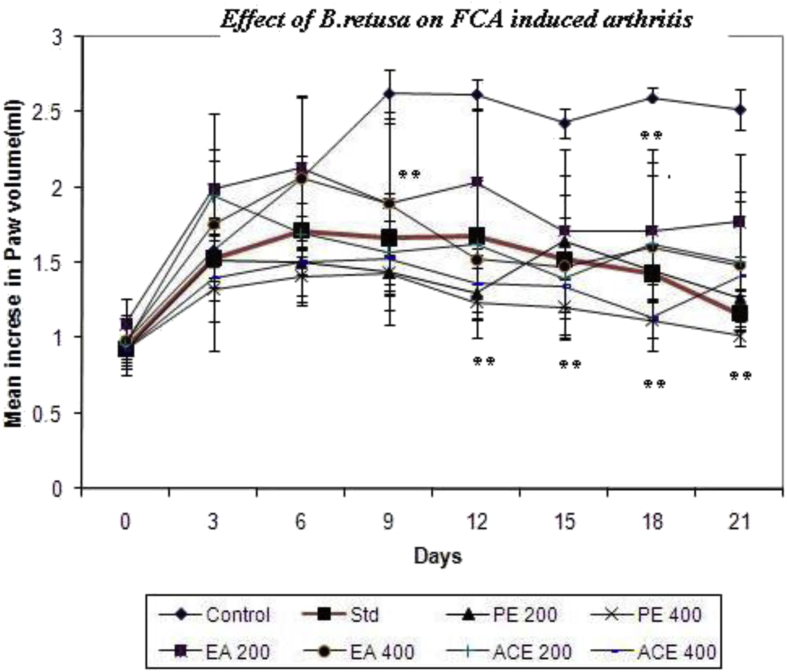
3.7. Freund's complete adjuvant-induced arthritis in rats
The inflammatory effect of B. retusa bark on a chronic arthritis model was evaluated using CFA-induced arthritis in rats. Fig. 6 showed that extracts of B. retusa was capable of reducing the severity of arthritic lesions and a statistically significant (P < 0.05) inhibition of the paw edema as compared to the control group. The 400 mg kg−1 of PE, EA and ACE samples of B. retusa showed significantly potent inhibition from day 6–21 at 31–60%, 27–41% and 26–44% respectively. Swelling and redness developed over a 24 h period in the foot injected with adjuvant. In rats treated from the day of adjuvant injection, the paw swelling was completely suppressed and no secondary sign was seen. The drug treatment for 14 d from the day of adjuvant injection suppressed the secondary increase in swelling of the injected foot that occurred with the appearance of polyarthritis.
Fig. 6.
Effects of B. retusa bark on CFA induced arthritis rat. 0.1 ml of Freund's complete adjuvant (CFA) into the sub plantar region in the right hind paw. The adjuvant contained 10 mg heat killed Mycobacterium tuberculosis in 1 ml paraffin oil. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control group. (One-way ANOVA followed by multiple Dunnett's test).
3.8. Hematology and biochemical
Table 3 represents the hematological changes associated with arthritic condition. Levels of RBC were decreased in arthritic rats while increases in WBC, platelet count and ESR. These changes were observed to near normal levels in B. retusa treated animals. ESR of test group decreased up to 4 as compared to control 5.54 mm/h arthritis induced rats with B. retusa test samples exhibited potent inhibitory effects on RA and CRP factors.
Table 3.
Effect of B.retusa extract on hematological parameter of rats treated with Freund's complete adjuvant.
| Group | Dose (mg/kg) | WBC count (103/mm3) | RBC | Platelet count (105/mm3) | ESR (mm/hr) |
|---|---|---|---|---|---|
| Normal | – | 8.50 ± 0.165 | 6.02 ± 0.529 | 2.35 ± 0.223 | 3.78 ± 0.185 |
| Control | – | 12.21 ± 0.288 | 4.25 ± 0.232 | 3.46 ± 0.180 | 5.54 ± 0.490 |
| PE | 200 | 11.32 ± 0.460 | 4.74 ± 0.320 | 2.89 ± 0.151 | 4.99 ± 0.209∗ |
| 400 | 8.98 ± 0.417∗∗∗ | 5.87 ± 0.198** | 2.58 ± 0.077 | 4.06 ± 0.439∗∗∗ | |
| EA | 200 | 11.29 ± 0.718 | 4.60 ± 0.035 | 2.94 ± 0.219 | 4.87 ± 0.496 |
| 400 | 9.25 ± 0.563∗∗∗ | 5.66 ± 0.340** | 2.61 ± 0.217 | 4.25 ± 0.323∗∗ | |
| ACE | 200 | 11.04 ± 0.659∗ | 4.57 ± 0.385 | 2.57 ± 0.201 | 5.15 ± 0.447 |
| 400 | 10.45 ± 1.228∗∗∗ | 5.22 ± 0.057** | 2.70 ± 0.144 | 4.16 ± 0.167∗∗∗ | |
| Diclofenac | 13.5 | 9.28 ± 0.320∗∗∗ | 5.63 ± 0.415** | 2.48 ± 0.283 | 4.44 ± 0.468∗∗ |
The observations are given as Mean SD. n = 6; *P < 0.05, **P < 0.01 ***P < 0.001. Compared with saline treated control group (ANOVA followed by multiple Dunnet's test).
In this AIA rat model, both ESR and CRP were found to be markedly associated with the development of the disease. A marked increase in AST, ALT and alkaline phosphatase was observed in serum of arthritic rat. The level of Aminotransferase and alkaline phosphatase was significantly reduced in arthritic rat after the administration of PE and EA at 400 mg/kg test drugs when compared to the arthritic control rat. It also showed the significant effect of PE (P < 0.01) and EA (P < 0.05) on lysosomal enzymes in the plasma of control and experimental animals (Table 4).
Table 4.
Effect of B.retusa extract on biochemical parameter of rats treated with Freund's complete adjuvant.
| Group | Dose (mg/kg) | Acid phosphatase (IU/L) | Alkaline phosphatase (IU/L) | SGOT (AST) (U/L) | SGPT (ALT) (U/L) | Spleen weight (mg) |
|---|---|---|---|---|---|---|
| Normal | – | 10.70 ± 1.002 | 35.81 ± 2.734 | 147.3 ± 27.34 | 51.91 ± 6.438 | 433.3 ± 32.49 |
| Control | – | 17.35 ± 2.857 | 64.30 ± 5.372 | 206.2 ± 56.35 | 68.99 ± 8.656 | 529.5 ± 23.61 |
| PE | 200 | 14.50 ± 0.834 | 45.47 ± 2.357 | 200.3 ± 36.32 | 66.29 ± 8.937 | 496.3 ± 60.76 |
| 400 | 12.86 ± 1.718** | 39.15 ± 1.024 | 147.7 ± 17.20* | 54.25 ± 5.267* | 441.3 ± 13.15** | |
| EA | 200 | 14.50 ± 1.901 | 45.25 ± 4.873 | 178.2 ± 44.79 | 64.26 ± 3.451 | 499.5 ± 14.82 |
| 400 | 13.16 ± 1.866* | 40.43 ± 3.591 | 145.0 ± 20.79* | 53.59 ± 5.408* | 454.5 ± 31.55** | |
| ACE | 200 | 16.90 ± 2.281 | 50.78 ± 6.593 | 197.8 ± 26.28 | 66.88 ± 8.092 | 533.3 ± 22.38 |
| 400 | 14.68 ± 1.041 | 39.98 ± 0.627 | 177.0 ± 4.848 | 65.31 ± 8.710 | 461.5 ± 10.15* | |
| Diclofenac | 13.5 | 11.68 ± 1.322** | 77.00 ± 4.149 | 209.8 ± 13.85 | 61.81 ± 12.95 | 439.3 ± 16.13** |
The observations are given as Mean SD, *P < 0.05, **P < 0.01. Compared with saline treated control group. (ANOVA followed by multiple Dunnet's test).
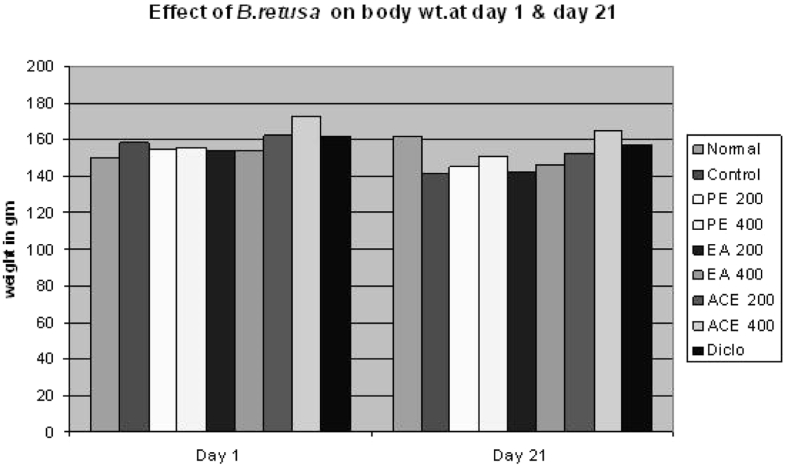
The administration of test drugs at 400 mg/kg to arthritic rat significantly reversed the biochemical changes to a normal level. The swelling of spleen weight was significantly covered up in rats treated with B. retusa test samples at higher dose. A change in the body weight is a useful index to assess the anti-inflammatory activity of bark extracts (Fig. 7).
Fig. 7.
Effect of B. retusa on body weight of CFA induced arthritis rat at day 1 & 21.
Membrane stabilizing activity B. retusa at 400 mg/kg significantly increased % inhibition of leucocytes migration from 24–32%. The positive control, dexamethasone showed (41.16%) a remarkable inhibition (Table 5). B. retusa test samples inhibited heat-induced haemolysis of RBCs to varying degrees. It evoked conc. related inhibition of haemolysis.
Denaturation of protein as a one the cause of inflammation. Effect of B. retusa extracts on inhibition of protein denaturation at different concentrations (200 and 400 μg/ml) provided significant protection against denaturation of proteins (Table 6).
Table 6.
Effects of B.retusa extracts on heat induced erythrocytes haemolysis and protein denaturation.
| Conc. (μg/ml) | % Inhibition of heat induced |
|||||||
|---|---|---|---|---|---|---|---|---|
| Erythrocytes hemolysis |
% Inhibition of protein denaturation |
|||||||
| AA | PE | EA | ACE | PE | EA | ACE | AA | |
| 200 | 29.19 ± 2.89 | 08.07 ± 0.89 | 03.10 ± 0.05 | 16.77 ± 1.80 | 37.77 ± 2.32 | 42.22 ± 3.364 | 30.00 ± 2.23 | 63.33 ± 4.52 |
| 250 | 37.26 ± 2.36 | 15.52 ± 1.00 | 12.42 ± 1.20 | 20.49 ± 1.99 | 46.66 ± 3.64 | 51.11 ± 6.310 | 40.00 ± 2.87 | 80.00 ± 5.20 |
| 300 | 42.85 ± 3.36 | 29.81 ± 2.10 | 21.73 ± 2.25 | 32.29 ± 2.90 | 61.11 ± 4.52 | 63.33 ± 5.21 | 54.44 ± 4.52 | 86.66 ± 5.98 |
| 350 | 54.65 ± 3.87 | 36.02 ± 2.89 | 30.43 ± 2.52 | 37.88 ± 3.31 | 72.22 ± 5.20 | 75.55 ± 5.00 | 68.88 ± 5.210 | 88.88 ± 5.98 |
| 400 | 63.97 ± 4.52 | 44.72 ± 4.20 | 42.23 ± 3.96 | 50.31 ± 4.50 | 80.00 ± 5.11 | 83.33 ± 6.28 | 77.77 ± 5.21 | 94.44 ± 5.69 |
PE = Pet. ether, EA = ethyl acetate, ACE = acetone extract.
4. Discussion and conclusions
The present study establishes the mechanism of analgesic and anti-inflammatory activity of B. retusa and their mechanism of action. The phytochemical study showed the presence of therapeutically effective phytoconstituents such as phytosterols, triterpenoid and tannins. Carrageenan-induced paw edema has been often used to assess the anti-inflammatory effect of natural products. It thought to show biphasic effect; the early phase is due to release, while late phase mediated through release of prostaglandins, proteases and lysosomes.27, 28 The results showed that B. retusa extracts significantly inhibited the formation of the rat paw edema, in the early and late phases, in fact somewhat more active in the late phase. It has been reported that the second phase of edema is sensitive to most clinically effective anti-inflammatory agents.29
All the extract showed significant (P < 0.01) dose-dependent inhibitory effect on peritoneal capillary permeability produced by acetic acid in mice. The PE and EA extracts (82.21 and 85.68%) at higher concentration shown the results almost equal to standard drug indomethacin (83.90%). The inflammatory response is a physiological characteristic of vascularized tissues.30 Chemical-induced vascular permeability causes an immediate sustained reaction that is prolonged over 24 h and its inhibition suggests that the extract may effectively suppress the exudative phase of acute inflammation.31
Administration of B. retusa extracts appears to be effective in inhibiting the dry weight of cotton pellet. The effect of PE (500 mg, 52.82 %) on dry weight of the cotton pellet was almost near to that of diclofenac (55.95%). This decrease in the granuloma weight indicates a suppression of the proliferative phase being effectively inhibited by the extracts.
The intraperitoneal injection of acetic acid produces peritoneal inflammation characterized as writhing16 associated with increase in concentration of PGE2 and PGF2a in the peritoneal fluid.32, 33 All the extract found to inhibit the writhing effect; amongst them the EA (400 mg/kg) shown to have maximum inhibition (65.62%). On these results, preliminary can predict the B. retusa acting through inhibition of lipooxygenase and/or cyclooxygenase in peripheral tissues, thereby reducing PGE2 synthesis and interfering with the mechanism of transduction in primary afferent nociceptor.34
In the nopciception study, the effect recorded in two phase of nociceptive behavior. The early phase initiated immediately after formalin injection and lasted about 3–5 min, resulting from chemical stimulation of nociceptors and mediated through bradykinin. The late phase initiated 15–20 min after formalin injection, lasted about 20–40 min and involves release of histamine, 5-hydroxytryptamine (5-HT), prostaglandins and bradykinin.35 B. retusa extracts showed analgesic effects in the both phase of formalin induced nociception. These results suggests that the anti-nociceptive might be due present of active analgesic principles acting both centrally and peripherally.
The CFA is an antigenic solution containing attenuated M. tuberculosis. It mobilizes the immune system by stimulating cellular mediators and potentiating the production of certain immunoglobulins.36 RA induced by CFA results in a localized inflammation of the joints associated with hypertrophy of the synovial membrane and the progressive and irreversible destruction of the cartilage.37 The CFA induced chronic inflammation is a biphasic process. The first and acute phase (0–10 days) is caused by various mediators such as histamine, serotonin, kinins, and prostaglandins, released by leukocytes that migrate to the affected region and provoked a vasculo-exsudatifs phenomena responsible for edema.38
The second and chronic phase (10–21 days) is due to cellular inflammatory mediators such as cytokines (interleukin-1β [IL-1β], IL-6, tumor necrosis factor [TNF-α]), granulocyte-macrophage colony-stimulating factor), interferon-g and prostaglandins.
In this study, animals were treated from day 10 to day 21, corresponding to the second phase of the arthritis and B. retusa extract-treated animals showed significant inhibition of joint diameter inflammation (paw volume) as well as arthritic scores compared to control rats. On these main parameters, the B. retusa bark extracts found to be efficient to inhibit the arthritis; amongst all extracts PE at 400 mg/kg was more than that of diclofenac, the positive control drug used in our study strongly suggests the possible anti-inflammatory effect of the extract. It has shown that the terpenoids and tannin containing fraction of the extract produced marked anti-inflammatory effect.
Another significant finding of the present study is the effect of CFA treatment on body weight changes of treated rats. While CFA-treated rats were observed to have reduced weight gain pattern over the treatment period which is in concordance with that previously reported by Roubenoff et al.,39 Pretreatment with B. retusa bark extract at 200–400 mg/kg caused significant weight gain changes in the treated rats reversing the cachexic effect of CFA treatment. It is well documented in the literature that weight loss and cachexia are common features in rheumatoid arthritis and these are thought to be due to reduced absorption of glucose and leucine in the rat intestine and increased cytokine production which increases resting metabolic rate and protein breakdown as well as poor appetite.40, 41, 42 Due to failure in adequately meeting the nutritional needs especially the increased calorie and protein needs, weight loss usually ensue.41, 43 Splenomegaly occurs as a result of profound induction of extramedullary hematopoiesis in the extracts conjunction with pyogranulomatous inflammation. Ideally, an agent active in adjuvant disease should restore the spleen weights and morphology to normal as is the case with both of herbal drugs.44 The fact that oral treatment with B. retusa bark extract improved and or maintained the spleen weight and body weight pattern in the treated arthritic rats as observed in this study suggest that this could be due to improvement in the arthritic state of the rats. However, this remains an assertion until validated.
The elevated levels of WBC, RBC, platelet count and ESR are manifestation of CFA induced arthritis45 (Cai et al., 2006). All the extracts were stabilizes the hematological changes to normal levels, indicative of antiarthritic potential. These protective effects are due to suppression of leucocytes towards inflamed area, stabilization of reticulo-endothelial system and inhibition of release of inflammatory cytokines and TNF.46
The CFA administration caused significant elevations in the serum acid phosphatase, alkaline phasphatase, AST and ALT. The profound elevation in the serum biochemical parameters in the arthritic rats strongly points to the establishment of an active bone lesion such as arthritis in the CFA-treated rats which is in complete agreement with results of other studies.47, 48, 49, 50 The stabilization of elevated serum levels of acid phosphatase, alkaline phasphatase, AST and ALT caused by oral treatment with B. retusa bark extracts (100–200 mg/kg) may be due to anti-edematous effect in the CFA-induced arthritic rats. The decreased enzyme levels on herbal drugs treatments emphasize the decreased bone loss and organ protective role of B. retusa in AIA rats. The enhanced protective effect of sample might be due to the combined effect of phytosterol and triterpenoid along with tannins. It has been well documented that sitosterol and gallic acid have antioxidant/anti-lipidperoxidative activities as they prevent the lipid per-oxidation51, 52 and decreases the inflammatory cytokines.53
A characteristic feature of adjuvant-induced arthritis in rats is the correlation between the development of inflammation and the release of lysosomal enzymes into the extra cellular compartment. Reduction of the release of lysosomal enzymes would prove beneficial and this indirectly confirms the protective effect of the drug. B. retusa administration decreases the lysosomal enzyme release in adjuvant-induced arthritic rat which indicate its anti-inflammatory effect.54, 55 Beneficial action attributed to phytosterols, their ability to reduce pain, swelling and inflammatory joint diseases with ageing and stress.56 Phytosterols and triterpenoid are necessary to maintain normal biological function as an immune modulation, anti-inflammatory and antipyretic activity.53
Membrane stabilization study was performed for the mechanism of anti-inflammatory action of B. retusa, inhibited both heat-induced lysis and protein denaturation. PE, EA and ACE significantly inhibited heat-induced erythrocytes lysis. These provide evidence for membrane stabilization as an additional mechanism of their anti-inflammatory effect. Denaturation of protein as a one the cause of inflammation.
To the best of our knowledge, this is the first study evaluating the antinociceptive, anti-inflammatory and membrane stabilizing activity of B. retusa. The study may indicate the ethno pharmacological basis of the use of B. retusa in traditional medicine for treating pain, swellings, rheumatoid arthritis etc. There is a need for find out the mechanism of action of the active fraction and phytochemical.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are thankful to SICART, Vallbh Vidhyanagar, Anand, Gujarat, for carry out analytical work. The authors are also thankful to Dept.of Pharmacology, RCPIPER, Shirpur for providing good animal facility for project work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Maldini M., Sosa S., Montoro P. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J Ethnopharmacol. 2009;122(3):430–433. doi: 10.1016/j.jep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Hossain M., Alam M., Chowhdury N. Antioxidant, analgesic and anti-inflammatory activities of the herb Eclipta prostrata. J Pharmacol Toxicol. 2011;6(5):468–480. [Google Scholar]
- 3.Butler S.H., Godefroy F., Besson J.-M., Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48(1):73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q., Kuang H., Su Y. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146(1):9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Oladosu I., Ogundajo A., Alyalaagbe O., Emenyonu N. Phytochemical and antituberculosis activity of Coffea brivipes, hiern exracts. Res J Phytochem. 2011;5:130–135. [Google Scholar]
- 6.Calixto J.B., Cabrini D.A., Ferreira J., Campos M.M. Kinins in pain and inflammation. Pain. 2000;87(1):1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous . CSIR, Publication and Information Directorate; New Delhi: 1988. The Wealth of India: Raw Materials: 2(B) [Google Scholar]
- 8.Basu B.D., Kirtikar K. International Book Distributors; 1999. Indian Medicinal Plants. [Google Scholar]
- 9.Chopra R.N., Nayar S. CSIR, Publication and Information Directorate; New Delhi: 1992. Glossary of Indian Medicinal Plants. [Google Scholar]
- 10.Mehare I.D., Hatapakki B.C. Anti-inflammatory activity of bark of Bridelia retusa Spreng. Indian J Pharm Sci. 2003;65(4):410–411. [Google Scholar]
- 11.Trease G., Evans W. Balliere Tindall; United Kingdom: 1983. Drugs of Biological Origin; pp. 309–540. [Google Scholar]
- 12.Winter C.A., Porter C.C. Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharm Assoc. 1957;46(9):515–519. doi: 10.1002/jps.3030460902. [DOI] [PubMed] [Google Scholar]
- 13.Vasudevan M., Gunnam K.K., Parle M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol. 2007;109(2):264–270. doi: 10.1016/j.jep.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Whittle B. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br J Pharmacol Chemother. 1964;22(2):246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma H.-Y., Kou J.-P., Wang J.-R., Yu B.-Y. Evaluation of the anti-inflammatory and analgesic activities of Liu-Shen-Wan and its individual fractions. J Ethnopharmacol. 2007;112(1):108–114. doi: 10.1016/j.jep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Koster R., Anderson M., De Beer E. 1959. Acetic Acid-induced Analgesic Screening. [Google Scholar]
- 17.Franzotti E., Santos C., Rodrigues H., Mourao R., Andrade M., Antoniolli A. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca) J Ethnopharmacol. 2000;72(1):273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 18.Hunskaar S., Fasmer O.B., Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14(1):69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 19.Newbould B. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother. 1963;21(1):127–136. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbier A., Breliere J., Remandet B., Roncucci R. Studies on the chronic phase of adjuvant arthritis: effect of SR 41319, a new diphosphonate. Ann Rheum Dis. 1986;45(1):67–74. doi: 10.1136/ard.45.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay P., Besra S., Gomes A. Anti-inflammatory activity of tea (Camellia sinensis) root extract. Life Sci. 2004;74(15):1839–1849. doi: 10.1016/j.lfs.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 22.Gokhale A., Damre A., Kulkarni K., Saraf M. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine. 2002;9(5):433–437. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- 23.Perez H., Weissmann G. 1st ed. WB Saunders; Philadelphia: 1981. Lysozymes as Mediators of Inflammation. Textbook of Rheumatology; p. 179. [Google Scholar]
- 24.Shinde U., Phadke A., Nair A., Mungantiwar A., Dikshit V., Saraf M. Membrane stabilizing activity—a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70(3):251–257. [Google Scholar]
- 25.Dharmasiri M., Jayakody J., Galhena G., Liyanage S., Ratnasooriya W. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87(2):199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S., Das S. Anti-arthritic and anti-inflammatory effect of a poly-herbal drug (EASE): its mechanism of action. Indian J Pharmacol. 1996;28(2):116. [Google Scholar]
- 27.Di Rosa M., Giroud J., Willoughby D. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 28.Antonio M., Brito A.S. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae) J Ethnopharmacol. 1998;61(3):215–228. doi: 10.1016/s0378-8741(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 29.Suba V., Murugesan T., Kumaravelrajan R., Mandal S.C., Saha B. Antiinflammatory, analgesic and antiperoxidative efficacy of Barleria lupulina Lindl. extract. Phytother Res. 2005;19(8):695–699. doi: 10.1002/ptr.1734. [DOI] [PubMed] [Google Scholar]
- 30.Rang H.P., Ritter J.M., Flower R.J., Henderson G. Elsevier Health Sciences; 2014. Rang & Dale's Pharmacology: With Student Consult Online Access. [Google Scholar]
- 31.Okoli C., Akah P., Nwafor S., Anisiobi A., Ibegbunam I., Erojikwe O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana CD Adams. J Ethnopharmacol. 2007;109(2):219–225. doi: 10.1016/j.jep.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Collier H., Dinneen L., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bose A., Mondal S., Gupta J.K., Ghosh T., Dash G.K., Si S. Analgesic, anti-inflammatory and antipyretic activities of the ethanolic extract and its fractions of Cleome rutidosperma. Fitoterapia. 2007;78(7):515–520. doi: 10.1016/j.fitote.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Sulaiman M.R., Hussain M., Zakaria Z.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79(7):557–561. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Tjølsen A., Berge O.-G., Hunskaar S., Rosland J.H., Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 36.Mythilypriya R., Shanthi P., Sachdanandam P. Salubrious effect of Kalpaamruthaa, a modified indigenous preparation in adjuvant-induced arthritis in rats—a biochemical approach. Chem-Biol Interact. 2008;173(2):148–158. doi: 10.1016/j.cbi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Klinkhoff A. Biological agents for rheumatoid arthritis. Drugs. 2004;64(12):1267–1283. doi: 10.2165/00003495-200464120-00001. [DOI] [PubMed] [Google Scholar]
- 38.Tripathy S., Sahoo S., Pradhan D., Sahoo S., Satapathy D. Evaluation of anti arthritic potential of Hybanthus enneaspermus. Afr J Pharm Pharmacol. 2009;3(12):611–614. [Google Scholar]
- 39.Roubenoff R., Freeman L.M., Smith D.E., Abad L.W., Dinarello C.A., Kehayias J.J. Adjuvant arthritis as a model of inflammatory cachexia. Arthritis Rheum. 1997;40(3):534–539. doi: 10.1002/art.1780400320. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizaki K, Nishimoto N, Mihara M, Kishimoto T. Therapy of rheumatoid arthritis by blocking IL-6 signal transduction with a humanized anti-IL-6 receptor antibody. Paper presented at: Springer seminars in immunopathology 1998. [DOI] [PubMed]
- 41.Rall L., Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology. 2004;43(10):1219–1223. doi: 10.1093/rheumatology/keh321. [DOI] [PubMed] [Google Scholar]
- 42.Somasundaram S., Sadique J., Subramoniam A. In vitro absorption of [14 C] leucine during inflammation and the effect of antiinflammatory drugs in the jejunum of rats. Biochem Med. 1983;29(2):259–264. doi: 10.1016/0006-2944(83)90046-7. [DOI] [PubMed] [Google Scholar]
- 43.Gartlehner G., Hansen R.A., Jonas B.L., Thieda P., Lohr K.N. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatology. 2006;33(12):2398–2408. [PubMed] [Google Scholar]
- 44.Bendele A. Animal models of rheumatoid arthritis. J Musculoskelet Neuronal Interact. 2001;1(4):377–385. [PubMed] [Google Scholar]
- 45.Cai X., Wong Y., Zhou H. The comparative study of Sprague–Dawley and Lewis rats in adjuvant-induced arthritis. Naunyn Schmiedeberg's Arch Pharmacol. 2006;373(2):140–147. doi: 10.1007/s00210-006-0062-5. [DOI] [PubMed] [Google Scholar]
- 46.Rani H.S., Madhavi G., Srikanth B., Jharna P., Rao U., Jyothy A. Serum ADA and C-reactive protein in rheumatoid arthritis. Int J Hum Genet. 2006;6(3):195. [Google Scholar]
- 47.Price C., Sammons H. The nature of the serum alkaline phosphatases in liver diseases. J Clin Pathol. 1974;27(5):392–398. doi: 10.1136/jcp.27.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosalki S., Foo A., Tanner P. Serum gamma-glutamyltransferase and alkaline phosphatase in rheumatoid arthritis. J Clin Pathol. 1982;35(12):1395. doi: 10.1136/jcp.35.12.1395-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cimmino M., Accardo S. Changes in the isoenzyme pattern of alkaline phosphatase in patients with rheumatoid arthritis. Clin Chem. 1990;36(7):1376–1377. [PubMed] [Google Scholar]
- 50.Cimmino M., Buffrini L., Barisone G., Bruzzone M., Accardo S. Alkaline phosphatase activity in the serum of patients with rheumatoid arthritis. Z Rheumatol. 1989;49(3):143–146. [PubMed] [Google Scholar]
- 51.Yokota J., Takuma D., Hamada A. Scavenging of reactive oxygen species by Eriobotrya japonica seed extract. Biol Pharm Bull. 2006;29(3):467–471. doi: 10.1248/bpb.29.467. [DOI] [PubMed] [Google Scholar]
- 52.Bahorun T., Trotin F., Pommery J., Vasseur J., Pinkas M. Antioxidant activities of Crataegus monogyna extracts. Planta Med. 1994;60(04):323–328. doi: 10.1055/s-2006-959493. [DOI] [PubMed] [Google Scholar]
- 53.Gupta M., Nath R., Srivastava N., Shanker K., Kishor K., Bhargava K. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 1980;39(06):157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 54.Gupta O., Sharma N., Chand D. A sensitive and relevant model for evaluating anti-inflammatory activity—papaya latex-induced rat paw inflammation. J Pharmacol Toxicol Methods. 1992;28(1):15–19. doi: 10.1016/1056-8719(92)90060-e. [DOI] [PubMed] [Google Scholar]
- 55.Amresh G., Singh P., Rao C.V. Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J Ethnopharmacol. 2007;111(3):531–536. doi: 10.1016/j.jep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 56.Bouic P.J. Sterols and sterolins: new drugs for the immune system? Drug Discov Today. 2002;7(14):775–778. doi: 10.1016/s1359-6446(02)02343-7. [DOI] [PubMed] [Google Scholar]