Abstract
Zanthoxylum armatum fruits are used traditionally as a spice in various food preparations. The aim of this study was analysis of antimicrobial, cytotoxic, phytotoxic, insecticidal, and anti-leishmanial activity. The crude extract showed 86 ± 10% antifungal activity (Agar tube dilution method) against Trichophyton longifusis while n-hexane, chloroform, and aqueous-methanol fractions inhibited this pathogen by 90 ± 7, 85 ± 10 and 70 ± 9% respectively. The n-hexane and aqueous-methanol fraction also, respectively, showed 40 ± 10 and 87 ± 9% inhibition of Microsporum canis. Chloroform fraction also displayed antifungal activity against Aspergillus flavus (60 ± 10%) and aqueous-methanol fraction against F. solani (40 ± 8%). The crude ethanolic extract and its chloroform and aqueous-methanol fraction exhibited significant toxicity (Brine shrimps lethality assay) against brine shrimps having LC50 value of 6.66 ± 1.1, 21.4 ± 3.3 and 29.6 ± 3.9 μg/ml, respectively. The crude ethanolic extract and its n-hexane soluble portion exhibited good anti-leishmanial activity (well serial dilution method) each having IC50 values of 50 ± 5 μg/ml. The crude extract and various fractions possessed excellent herbicidal activity (Lemna minor assay), and caused more than 90% inhibition of the plant growth at 1000 μg/mL. The ethanolic extract, n-hexane and chloroform soluble portions caused 90% mortality in insecticidal activity (direct contact method) of Rhyzopertha dominica. The ethanolic extract and its n-hexane soluble portion, respectively, caused 80 and 90% mortality of Callosobruchus analis. The present study showed that the tested fruit extracts of Z. armatum exhibited strong antifungal, cytotoxic, phytotoxic, insecticidal, and anti-leishmanial effects.
Keywords: Zanthoxylum armatum, Antimicrobial, Cytotoxic, Phytotoxic, Insecticidal, Anti-leishmanial
Graphical abstract
1. Introduction
The foods obtained from plants are valuable source of vitamins, minerals, fibers and so on and are therefore provide many important component of a healthy diet. In addition to diet component, plants are chemo preventive agents. There is always need to search for natural cytotoxic, antimicrobial, and other chemotherapeutic agents due to overwhelming toxicity reports of synthetic compounds.1
Zanthoxylum armatum (Roxb.) (Rutaceae) is a common plant in Southeast Asia. It grows wildly in Dir, Hazara, and Muree hills of Pakistan.2 The seeds of this plants are particularly part of many food ingredients and also used in many traditional medicine. The seeds and the bark of this plant are used as in the treatment of fevers, toothache, indigestion/heartburn, stomachic, carminative, cholera and as tonic.3 Z. armatum branchlets is used as miswak (tooth brush) (Tejbal) for cleaning the teeth while fruit powder is applied in toothache.4 Various species of Zanthoxylum genus possess antimicrobial, larvicidal and cytotoxic activity.5, 6, 7, 8 The different classes of chemical constituents reported from Z. armatum include terpenes, sterols, flavonoids, alkaloids and coumarins.2, 9, 10, 11
The current study was designed to extend the search for potential novel agents that may be of therapeutic applications. Here, we report the in vitro study of this plant which include antifungal, antibacterial, cytotoxic, phytotoxic, insecticidal and anti-leishmanial activities in order to rationalize some traditional uses and to explore the presence of these bioactivities in Z. armatum.
2. Materials and methods
2.1. Plant material
Z. armatum berries were collected from the hilly area of Tanawal, District Haripur, and N.W.F.P. Pakistan in the month of June–July. The identification of the plant was confirmed by plant taxonomist Manzoor Hussain and specimen voucher (PG/B/ZA, 2014) was deposited in the herbarium of Botany Department, Government Postgraduate College, Abbottabad.
2.2. Extraction and fractionation
The fresh berries (800 g) were ground and macerated with ethanol for 72 h. It was filtered and the filtrate was evaporated under reduced pressure at 40 °C which yielded the crude extract (81 g). A portion of the crude extract (60 g) was successively fractioned to yield n-hexane (16 g), chloroform (20.2 g), and aqueous-methanol (23 g) fractions.
2.3. Antifungal activity
Antifungal activity of the ethanolic extract and subsequent fractions was measured by employing the agar tube dilution method against the selected human, animal and plant pathogens.12 The molds and yeasts used in this study included Trichophyton longifusis (clinical isolate, human pathogen), Microsporum canis (ATCC11622, human/animal pathogen), Aspergillus flavus (ATCC32611, human pathogen), Fusarium solani (ATCC11712, plant pathogen), Candida albicans (ATCC2091, human pathogen) and Candida glabrata (ATCC90030, human pathogen).
Dilutions were made as to make the final concentration of the extracts, subsequent fractions and the standard drug to 200 μg/ml of SDA (Sabouraud Dextrose Agar). Miconazole was used as the standard drug and the experiments were run in triplicates.
2.4. Antibacterial activity
Antibacterial activity of the crude extract and subsequent factions was assessed against various Gram positive and Gram negative human pathogens by the agar well diffusion protocol.12 Gram positive bacteria included Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923) while Gram negative organisms included Escherichia coli (ATCC 25922), Shigella flexenari (clinical isolate), Pseudomonas aeruginosa (ATCC 27853) and Salmonella typhi (ATCC 19430). Imipenum was used as the standard antibacterial agent.
2.5. Cytotoxicity
Brine shrimp lethality of the ethanolic extract and subsequent fractions of Z. armatum was assessed against Artemia salina (brine shrimp larvae) in three different concentrations (5, 50, and 500 μg/mL).12 The data were analyzed using with the SPSS (Version 19) statistics software to calculate LD50 values with a 95% CI (confidence interval). The etoposide was used as standard cytotoxic drug. Prior to experiment approval was taken from the institute ethical committee for use of brine shrimps in bioassay, the approval number allotted was; PHM 0028/EC/B-4-5-15.
2.6. Phytotoxicity
Phytotoxic activity of the crude extract and subsequent fractions were evaluated against Lemna minor L.13 in different concentrations (1000, 100 and 10 μg/mL). Paraquat (0.9025 μg/mL) was used as positive control. The results were interpreted as percentages by analyzing the growth regulation calculated with reference to negative control by using the following expression:
2.7. Insecticidal activity
Insecticidal activity of the ethanolic extract and various fractions was evaluated against Trogoderma granarium, Rhyzopertha dominica and Callosobruchus analis by direct contact method using filter paper (90 mm diameter).14 Permethrin (235.71 μg/cm2) was used as the standard insecticidal agent. The percent mortality was calculated after 36 h using the following formula:
2.8. Anti-leishmanial activity
Anti-leishmanial activity of the ethanolic extract and subsequent fractions was assessed against Leishmania major using well serial dilution protocol as reported previously.12 The results were calculated with the SPSS (Version 19) statistics software as the concentrations inhibiting parasite growth by 50% (IC50) after a three-day incubation period. Amphotericin B was used as the reference.
3. Results
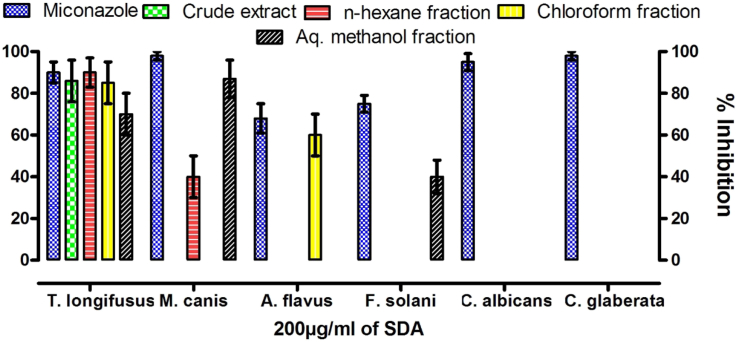
The results obtained for the antifungal activity of the extract and subsequent fractions derived from Z. armatum against the tested pathogens are summarized in Fig. 1. The most prominent antifungal activity was observed against T. longifusus. The crude extract showed 86% inhibition against this pathogen while n-hexane, chloroform and aqueous-methanol fractions inhibited the growth of T. longifusus by 90 ± 10, 85 ± 10 and 70 ± 9% respectively. The n-hexane and aqueous-methanol fraction showed antifungal activity against M. canis and inhibited its growth by 40 ± 10 and 87 ± 9%, respectively. The other active fractions in this bioactivity were chloroform against A. flavus (60 ± 10%) and aqueous-methanol against F. solani (40 ± 8%).
Fig. 1.
Antifunagal activity as % inhibition of crude extract and subsequent fractions of Z. armatum berries against various pathogens fungi.
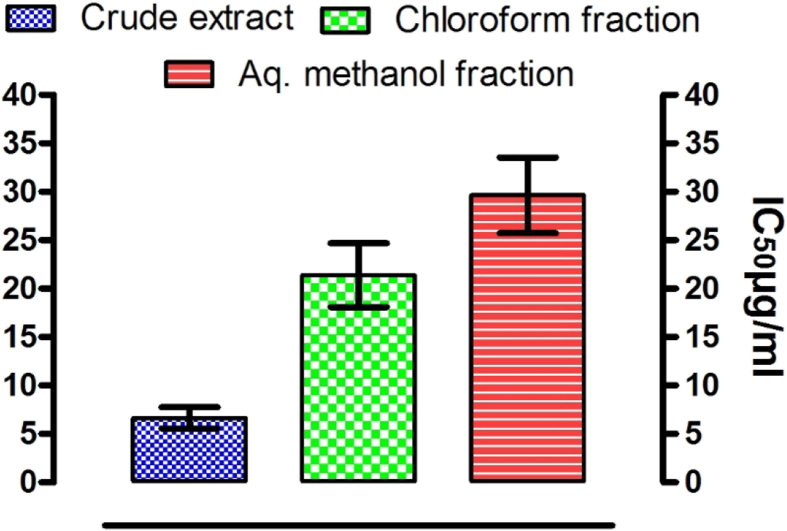
The extract and subsequent fractions derived from Z. armatum displayed a week antibacterial activity against the tested organisms. The crude extract, n-hexane, and chloroform soluble portions inhibited the growth of B. subtilis only by 30% as compared to standard drug (Imipenum). The crude extract and subsequent fractions from Z. armatum were screened for brine shrimp lethality studies and the LC50 values are reported in Fig. 2. The crude extract derived from the berries of Z. armatum exhibited significant toxicity against brine shrimps having LC50 value of 6.66 ± 1.1 μg/mL, which probably indicates the distribution of synergistic activity of compounds within these two fractions. Similarly, the chloroform and aqueous-methanol fraction also displayed promising cytotoxic activity showing LC50 values of 21.4 ± 3.3 and 29.6 ± 3.9 μg/ml. The n-hexane fraction, however, showed LC50 values greater than 1000 μg/mL, and thus could be considered to have a low general toxicity. The brine shrimp toxicity bioassay is a useful indicator of general toxicity and also a guide for the detection of antitumor and pesticidal constituents.
Fig. 2.
Cytotoxic effects of crude extract and subsequent fraction of Z. armatum berries against brine shrimps larvae.
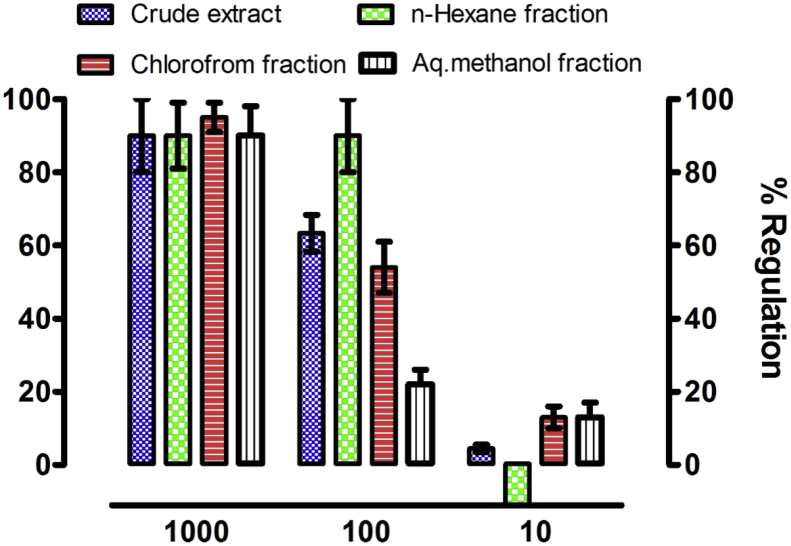
The herbicidal potentials of the ethanolic extract and subsequent fractions obtained from Z. armatum were evaluated against L. minor L. and the results are exhibited in Fig. 3. The crude extract obtained from Z. armatum and various fractions have excellent herbicidal activity at the highest concentration (1000 μg/mL) and caused complete inhibition of the plant growth. At 100 μg/mL, the n-hexane fraction caused a complete inhibition of L. minor while a moderate phytotoxic activity was observed for the crude extract and chloroform fraction and exhibited 63.3 ± 5 and 54.54 ± 7% inhibition, respectively. At this concentration, however, only a weak inhibitory activity (22.72 ± 4%) was observed for the aqueous-methanol fraction. Neither the crude extract nor the fractions exhibited any significant herbicide activity at further lower concentration (10 μg/mL). Rather, the n-hexane fraction acted as a plant growth promoter at this concentration Fig. 3.
Fig. 3.
Phytotoxic activity as % regulation of crude extract and subsequent fractions of Z. armatum against Lemna minor fronds with dose of 1000, 100 and 10 μg/mL.
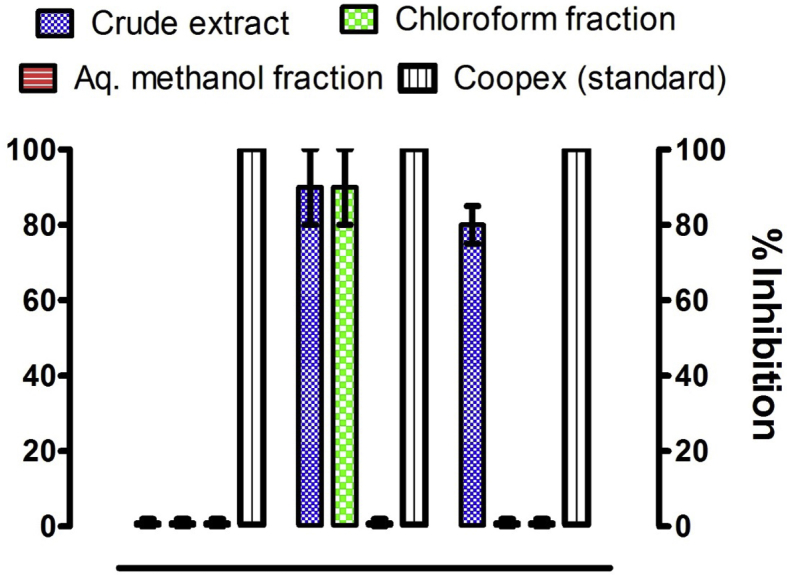
The results obtained for the insecticidal activity of the ethanolic extract and subsequent fractions obtained from Z. armatum against T. granarium, R. dominica and C. analis are given in Fig. 4. Crude extract, n-hexane and chloroform fractions showed excellent insecticidal activity against R. dominica and caused 90 ± 10% mortality of this species after 36 h of exposure. Crude extract and n-hexane fraction also displayed significant inhibitory activity against C. analis. These caused, respectively, 80 ± 5 and 90 ± 10% mortality of this specie. A 20 ± 5% insecticidal activity was also found in n-hexane fraction against T. granarium.
Fig. 4.
Insecticidal activity as % inhibition of crude extract and subsequent fractions of Z. armatum against different common stored grain pests.
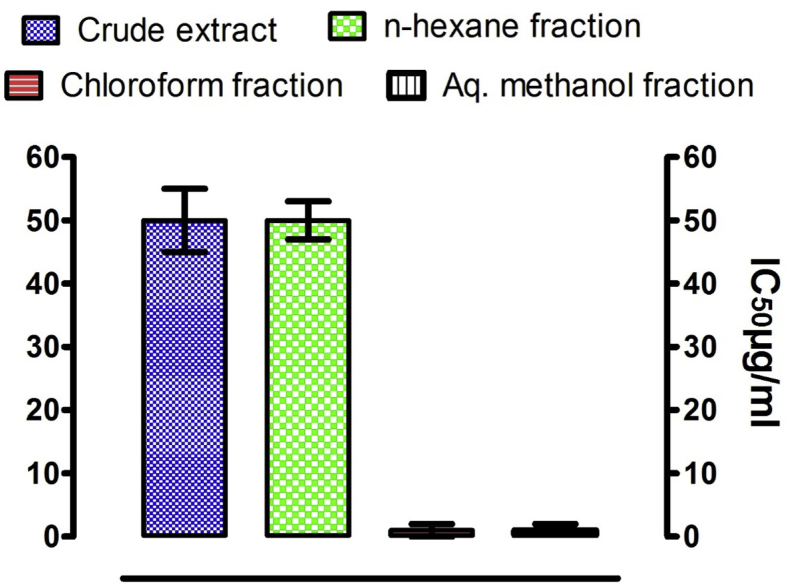
The results for the effects of the ethanolic extract and fractions of Z. armatum on the viability of L. major promastigote forms are shown in Fig. 5. The ethanolic extract and its hexane soluble portion showed activity against L. major with IC50 values of 50 ± 5 μg/ml each. The n-hexane, chloroform and aqueous-methanol fractions, however, did not show any significant anti-leishmanial activity against L. major as their IC50 values were higher than 50 μg/ml.
Fig. 5.
Anti-leishmanial activity of Z. armatum crude extract and subsequent fractions against L. major. Values are given as IC50 (μg/mL).
4. Discussions
It is evident from this study that Z. armatum and subsequent fractions could be a rich source of antifungal agents especially against T. longifusus, M. canis, A. flavus and F. solani. As the literature reveals, this is first report on the antifungal potentials of Z. armatum. Several other species of Zanthoxylum have previously been reported to possess antifungal activity against a range of strains.7, 15, 16 The previous reports suggest that the presence of alkaloids in the plant may possibly responsible for antifungal activity.17, 18 This study also indicated that the extract and fractions from the berries of Z. armatum could be rich sources of valuable antitumor and pesticides constituents. A variety of compounds including alkaloids, terpenoids and coumarins are reported to have such cytotoxic effects.18 On the basis of these experimental and literature evidence the same plant's crude extract and its fractions are under study for anticancer activity on different cell lines in the author's laboratory.
Leishmaniosis, a parasitic infection, is prevalent throughout the world mostly in underdeveloped countries.19 Due to some drawbacks with synthetic drugs used against leishmaniosis the attention have been shifted to the active phytochemicals isolated from plant for the effective treatment along with minimal side effects.20 This study revealed that the ethanolic extract and its hexane fraction could be a valuable source of anti-leishmanial compounds as also evidenced from previous studies on Zanthoxylum.21 It is well known that the weeds are developing resistant and the efficiency of the conventional synthetic herbicides is decreasing against these resistant biotypes.22 These facts necessitate the need for the discovery of new herbicides. Natural herbicides are gaining more importance because they have non-toxic effect and are more suitable as compare to synthetic agrochemicals in use. The results of this study showed that the extract and fractions from Z. armatum possessed significant phytotoxic activity against L. minor and could be useful as bio-herbicides.
The synthetic insecticides possess well-known adverse effects on agro ecological systems and thus the interest in the insecticides from botanical origin has been increased during the past few decades. Consequently, the studies on plant-insect chemical interactions has increased to explore the potential of plants' secondary metabolites as pest control agents23 and the use of plant allelochemicals has been accepted as a leading strategy for pest management. In the present study, the ethanolic extract, n-hexane and chloroform soluble portions have shown considerable insecticidal activity against the most common insects which cause serious damage to wheat, rice and pulses in store condition. This activity may be due to contribution of some alkaloid components for e.g. pellitorine reported from the same genus.24
In conclusion, it can be stated that the tested Z. armatum extracts appear to be good and safe natural antimicrobial and cytotoxic agent and could also be of significance in the food industry and in the control of various human, animal and plant diseases. Further studies should be done to search for new biological active compounds from Z. armatum fruit extracts.
Conflict of interest
Author declares that there is no conflict of interest to disclose.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Nowak R., Olech M., Pecio Ł. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J Sci Food Agri. 2014;94(3):560–567. doi: 10.1002/jsfa.6294. [DOI] [PubMed] [Google Scholar]
- 2.Chopra R., Nayar S., Chopra I. National Institute of Science Communication and Information Resources. CSIR; New Delhi: 2006. Glossary of Indian Medicinal Plants. [Google Scholar]
- 3.Jain S., Jain M. Antifungal studies on some indigenous volatile oils and their combinations. Planta Med. 1972;22(2):136–139. doi: 10.1055/s-0028-1099595. [DOI] [PubMed] [Google Scholar]
- 4.Dikshit A., Husain A. Antifungal action of some essential oils against animal pathogens. Fitoterapia. 1984;55:171–176. [Google Scholar]
- 5.Nair A., Nair G.A., Joshua C. Confirmation of structure of the flavonol glucoside tambuletin. Phytochemistry. 1982;21(2):483–485. [Google Scholar]
- 6.Kumar S., Müller K. Inhibition of keratinocyte growth by different Nepalese Zanthoxylum species. Phytother Res. 1999;13(3):214–217. doi: 10.1002/(SICI)1099-1573(199905)13:3<214::AID-PTR431>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Islam A., Sayeed A., Bhuiyan M., Mosaddik M., Islam M., Astaq Mondal Khan G. Antimicrobial activity and cytotoxicity of Zanthoxylum budrunga. Fitoterapia. 2001;72(4):428–430. doi: 10.1016/s0367-326x(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 8.Motsei M., Lindsey K., Van Staden J., Jäger A. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J Ethnopharmacol. 2003;86(2):235–241. doi: 10.1016/s0378-8741(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee H., Pal S., Adityachaudhury N. Occurrence of rutaecarpine in Zanthoxylum budrunga. Planta Med. 1989;(4):55. doi: 10.1055/s-2006-962049. [DOI] [PubMed] [Google Scholar]
- 10.Chen I.-S., Tsai I.-W., Teng C.-M. Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry. 1997;46(3):525–529. [Google Scholar]
- 11.Chen I.-S., Wu S.-J., Tsai I.-L. Chemical and bioactive constituents from Zanthoxylum simulans. J Nat Prod. 1994;57(9):1206–1211. doi: 10.1021/np50111a003. [DOI] [PubMed] [Google Scholar]
- 12.Atta-ur-Rehman C.M.I., William J.T. Harward Academic Press; Amsterdam: 1999. Manual of Bioassay Techniques for Natural Product Research. [Google Scholar]
- 13.Mclaughlin J.L. Crown gall tumours on potato discs and brine shrimp lethality: two simple bioassays for higher plant screening and fractionation. Methods Plant Biochem. 1991;6:1–32. [Google Scholar]
- 14.Ahn Y, Kim G, Cho K. Bioassay system for insecticidal compounds. Paper presented at: Proceedings of the third symposium on the biochemical methodology for the research and development of the bioactive substances, held at Seoul, Republic of Korea 1995.
- 15.Bafi-Yeboa N., Arnason J., Baker J., Smith M. Antifungal constituents of Northern prickly ash, Zanthoxylum americanum Mill. Phytomedicine. 2005;12(5):370–377. doi: 10.1016/j.phymed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Steenkamp V., Fernandes A.C., Van Rensburg C.E. Screening of Venda medicinal plants for antifungal activity against Candida albicans. S Afr J Bot. 2007;73(2):256–258. [Google Scholar]
- 17.Thouvenel C., Gantier J.C., Duret P. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother Res. 2003;17(6):678–680. doi: 10.1002/ptr.1137. [DOI] [PubMed] [Google Scholar]
- 18.Yang C.-H., Cheng M.-J., Chiang M.Y., Kuo Y.-H., Wang C.-J., Chen I.-S. Dihydrobenzo [c] phenanthridine alkaloids from stem bark of Zanthoxylum nitidum. J Nat Prod. 2008;71(4):669–673. doi: 10.1021/np700745f. [DOI] [PubMed] [Google Scholar]
- 19.Le Pape P. Development of new antileishmanial drugs–current knowledge and future prospects. J Enzyme Inhib Med Chem. 2008;23(5):708–718. doi: 10.1080/14756360802208137. [DOI] [PubMed] [Google Scholar]
- 20.Mendonça-Filho R.R., Rodrigues I.A., Alviano D.S. Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae) Res Microbiol. 2004;155(3):136–143. doi: 10.1016/j.resmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira M.E., Nakayama H., de Arias A.R. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on Trypanosoma cruzi-infected mice. J Ethnopharmacol. 2007;109(2):258–263. doi: 10.1016/j.jep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Bhowmik P.C. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003;22(4):661–671. [Google Scholar]
- 23.Pavela R. Insecticidal activity of certain medicinal plants. Fitoterapia. 2004;75(7):745–749. doi: 10.1016/j.fitote.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.He W., Puyvelde L.V., Kimpe N.D. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother Res. 2002;16(1):66–70. doi: 10.1002/ptr.849. [DOI] [PubMed] [Google Scholar]