Abstract
Potentially useful in the treatment of neurodegenerative disorders, Kaempferia parviflora and Myristica fragrans have been shown to possess a wide spectrum of neuropharmacological activities and neuroprotective effects in vivo and in vitro. In this study, we determined whether and how K. parviflora ethanolic extract and M. fragrans volatile oil could influence the levels of neurotransmitters and the whole proteomic profile in the hippocampus of Sprague Dawley (SD) rats. The effects of K. parviflora and M. fragrans on protein changes were analyzed by two-dimensional gel electrophoresis (2D-gel), and proteins were identified by liquid chromatography tandem mass spectrometry (LC-MS/MS). The target proteins were then confirmed by Western blot. The levels of neurotransmitters were evaluated by reversed-phase high-performance liquid chromatography (RP-HPLC). The results showed that K. parviflora, M. fragrans and fluoxetine (the control drug for this study) increased serotonin, norepinephrine and dopamine in the rat hippocampus compared to that of the vehicle-treated group. Our proteomic data showed that 37 proteins in the K. parviflora group were up-regulated, while 14 were down-regulated, and 27 proteins in the M. fragrans group were up-regulated, while 16 were down-regulated. In the fluoxetine treatment group, we found 29 proteins up-regulated, whereas 14 proteins were down-regulated. In line with the proteomic data, the levels of GFAP, PDIA3, DPYSL2 and p-DPYSL2 were modified in the SD rat groups treated with K. parviflora, M. fragrans and fluoxetine as confirmed by Western blot. K. parviflora and M. fragrans mediated not only the levels of monoamine neurotransmitters but also the proteomic profiles in the rat hippocampus, thus shedding light on the mechanisms targeting neurodegenerative diseases.
Keywords: Kaempferia parviflora, Myristica fragrans, Neurotransmitters, Rat hippocampal proteomics, Neuroprotection
Abbreviations: GFAP, glial fibrillary acidic protein; PDIA3, protein disulfide-isomerase A3; DPYSL2, dihydropyrimidinase-related protein 2; p-DPYSL2, dihydropyrimidinase-related protein 2; NE, norepinephrine; DA, dopamine; 5-HT, serotonin
Graphical abstract
1. Introduction
The World Health Organization (WHO) predicted that by 2040, neurodegenerative diseases (NDs) will become the second leading cause of death in the world.1, 2, 3, 4 NDs, such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington disease (HD), belong to a heterogeneous group of disorders that are characterized by progressive degeneration of the structure and function of the central nervous system or peripheral nervous system. Common NDs include psychiatric disorders such as depression and bipolar disorder. In addition, depression is the most prevalent symptom in PD and HD, occurring in approximately 40–60% of patients.5 The hippocampus is considered to be one of the most important brain regions for mood regulation. The monoamine neurotransmitters, including dopamine (DA), norepinephrine (NE), epinephrine (E), and serotonin (5-hydroxytryptamine, 5-HT) are produced from neurons both in the brain and peripheral nervous system.6, 7 The functions of monoamine neurotransmitters are considered to play crucial roles in arousal, emotion, and cognition. Drugs that augment the effects of monoamines on their target tissue are used to treat psychiatric disorders, including depression, anxiety, and schizophrenia.8, 9 For these reasons, the measurement of monoamine neurotransmitters in the rat hippocampus is important for understanding the effects of herbal treatments on the secretion of neurotransmitters. Recently, proteomics is an important tool for identifying the expression of proteins that are important for a comprehensive understanding of the pharmacological role of a drug.10, 11, 12 Many medicinal plants have been identified and used for neuroprotective and neurotrophic agents that promote neuronal survival, differentiation, neuritogenesis and synaptic plasticity, both in in vitro and in vivo models.13, 14, 15 Kaempferia parviflora Wall Ex. Baker, or black ginger with the local name of “Kra-chai-dam”, is a plant from the family Zingiberaceae used for health promotion in traditional Thai medicine. The phytochemical studies revealed that the rhizomes of K. parviflora contained volatile oil,16 chalcones,17 phenolic glycosides18 and many flavonoids such as 5-hydroxy-7-methoxyflavone, 5,7-dimethoxyflavone and 3,5,7-trimethoxyflavone.19, 20 5,7-Dimethoxyflavone is a major active constituent of K. parviflora20 that showed high potential inhibitory activity toward acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).21, 22 Moreover, polymethoxyflavones from black ginger (K. parviflora) were discovered to be potential inhibitors of β-secretase (BACE1).23 The rhizomes of this plant have been traditionally used for leucorrhea, oral diseases, abdominal pain, and health promotion as well as an aphrodisiac.24 In addition, the ethanolic extract of K. parviflora has been shown to induce relaxation of both the aortic rings and the ileum precontracted with phenylephrine and acetylcholine.25 It has been reported that the alcoholic extract of K. parviflora rhizomes contained numerous flavonoids20 that were previously reported to possess antioxidant activity and neuroprotective and cognitive enhancing effects.26 A recent finding showed that the alcoholic extract of K. parviflora rhizome could mitigate depression-like behavior in aged rats27 and displays antidepressant activity in aged rats. We hypothesized that alcoholic extract of K. parviflora rhizome might also possess other neuropharmacological activities. Myristica fragrans Houtt. (nutmeg, a tropical evergreen dioecious tree with a narrow range of distribution) is a source of high-value medicinal spices, nutmeg (endosperm) and mace (the reddish aril) with immense phytochemical diversity.28 It contains volatile oils that include myristicin, elemicin, eugenol, isoeugenol, geraniol, pinese, cineole, borneol, and safrole. Its reported antibacterial, antiviral, antidiabetic, and antileukemic effects as well as its other biological activities28, 29, 30 indicate its enormous therapeutic potential. Medicinally, eugenol and isoeugenol from nutmeg are known for their anti-inflammatory and antithrombotic,31 as well as anti-rheumatic, carminative and stimulant properties.32 The psychoactive effects of nutmeg have been reported to cause hallucinations, feelings of unreality, euphoria, and delusions. Both myristicin and elemicin have been shown to be metabolized into amphetamine-related compounds that have effects on the serotonergic systems and possibly an antidepressant effect,33 but this has not yet been verified. These compounds were shown to have an antidepressant effect in the forced swimming test in male rats.34 The main bioactive compounds of M. fragrans seed essential oil were identified as including camphene, elemicin, eugenol, isoelemicin, isoeugenol and methoxyeugenol.29 Sabinene, α-pinene, β-pinene, terpine-4-ol, limonene, safrole and myristicin were also reported to be included in the essential oil of nutmeg.35, 36 In this study, we aim to investigate the effects of K. parviflora and M. fragrans on the levels of monoamine neurotransmitters (norepinephrine, serotonin, and dopamine), as well as on the proteomic profiles in the rat hippocampus. Discoveries from this study could help us to better understand the molecular mechanisms of these compounds.
2. Material and methods
2.1. Plant material and extraction
K. parviflora was collected from the Loei province in northeast Thailand, and M. fragrans was from the Nakhonpathom province in central Thailand. Plants were identified by an expert Ass. Prof. Dr. Nijsiri Ruangrungsi. K. parviflora rhizomes were shade dried and agitated by using a blender, and the powder was extracted with 95% ethanol by maceration for 4 days (1 kg sample: 3 L) with occasional stirring. The extract was dissolved in 2% Tween-80 in water to a final concentration of 200 mg/ml.17 Crude extracts of K. parviflora were standardized by reversed-phase HPLC (Agilent 1260 Infinity, Austria). 5,7-Dimethoxyflavone was used for the standard. For M. fragrans, 1 kg of powder from dry seeds was extracted by stream distillation for 12 h. Then, the volatile oil was dissolved in 2% Tween-80 in water to a final concentration 300 mg/ml.34 The M. fragrans nutmeg volatile oil was analyzed by GC-MS on gas chromatograph with a model 7890A GC and 5978C MSD mass selective detector (EIMS, electron energy, 70 eV) and an Agilent ChemStation data system. Fluoxetine (20 mg tablet) was dissolved in 2% Tween-80 in water.17, 34
2.2. Experimental protocol for animals
Two-month-old healthy male Sprague Dawley rats (300–400 g) were obtained from and approved by the National Laboratory Animal Center Animal Care and Use Committee (NLAC-ACUC), Mahidol University, Salaya, Nakornpathom, Thailand. Rats were housed individually (one per cage) in standard conditions (NLAC-MU; SOP-VM.VCP-01.10.) at 22 ± 1 °C on a standard fluorescent 12:12 h light:dark cycle, with changing of bedding once a week. Rats were given access to a standard diet and water (hyperchorinate 10–12 ppm) ad libitum.
All rats were randomly divided into 4 groups, each group containing 6 rats.
Group 1: The control group or vehicle-treated group was treated with 2% Tween-80, 1 mL/kg BW/day via oral route for 12 days.
Group 2: The fluoxetine group was treated with 20 mg/kg BW via oral route for 12 days.
Group 3: The K. parviflora group was treated with ethanolic extract (200 mg/kg BW via oral route once per day for 12 days).
Group 4: The M. fragrans group was treated with seed oil (300 mg/kg BW via oral route once per day for 12 days).
2.3. Tissue preparation and protein extraction
Dissected brains were frozen in liquid nitrogen at −80 °C until extraction. Hippocampal tissue was powderized in liquid nitrogen, and proteins were extracted with buffer containing 40 mM Tris, 7 M Urea, 2 M Thiourea, 4% CHAPS, 65 mM DTT, 0.3 mg/ml EDTA, 35 μg/ml PMSF and 1 tablet of protease inhibitor cocktail. The protein was precipitated with 20% trichloroacetic acid (TCA) in acetone with 20 mM dithiothreitol (DTT). The protein was re-suspended in lysis buffer, and the protein concentration was measured by Bradford assay.
2.4. Determination of the concentration of monoamine neurotransmitters including norepinephrine, serotonin (5-hydroxytryptamine, 5-HT), and dopamine by reversed-phase high-performance liquid chromatography (HPLC)
The hippocampal tissue was extracted with 0.1 M perchloric acid and sonicated for 30 min at an ice-cold temperature. Homogenates were centrifuged at 12,000 g for 15 min at 4 °C, while clear supernatants were decanted and filtered through a 0.45-μm filter. The concentration of monoamine neurotransmitters was determined from this filtrate. The HPLC system consisted of the analytical C18 reversed-phase column (ODS3 C18, 4.6 × 250 mm i.d., 5-micrometer particle size) and UV detector (best condition at 220 nm). The mobile phase consists of 0.02 M sodium acetate, buffered to a pH of 4 with 0.0125 M citric acid, containing 0.042 M methanesulfonic acid and 0.1 mM EDTA. The flow rate was set at 1 mL/min. The working standard solutions were freshly prepared in 0.05 M perchloric acid containing 0.1 mM Na2EDTA on ice and stored at −20 °C before using. Peaks were identified by comparing the retention time of each peak in the sample solution, where each individual peak was further compared to the standard solution of norepinephrine, serotonin (5-hydroxytryptamine, 5-HT), dopamine and caffeine (Sigma-Aldrich, USA) served as an internal standard.
2.5. Determination of protein changes in the SD rat hippocampus by two-dimensional gel electrophoresis (2-DE)
Here, 400 μg of protein was mixed with 340 μl of rehydration buffer (8 M urea, 4% CHAPS, 0.001% bromphenol blue and 3 mM dithiothreitol) containing 1% IPG buffer, pH gradients 3–10. The sample was loaded onto 13-cm IPG strips with a pH range of 3–10 using an isoelectric focusing system (Ettan IPGphore III, GE Healthcare Biosciences, USA). Samples were run through the steps of strip rehydration (20 °C, 16 h) and isoelectric focusing (500 V for 500 V−h, 1,000 V for 800 V−h, and 10,000 V to reach 27,000 V−h). The maximum current was maintained at 75 μA per strip. After the completion of the process, the strips were equilibrated twice (15 min each) in equilibration buffer supplemented with 65 mM DTT and 135 mM iodoacetamide. Each strip was subjected to a second dimensional separation (PROTEAN II xi cell, Bio-Rad, USA) using an SDS-polyacrylamide gel (12.5%). Separation of protein was executed under the applied voltage of 300 volts with 16 mA to 24 mA per gel at 20 °C until the bromophenol blue dye front reached 0.5 cm from the bottom of the gel. The gels were stained with colloidal Coomassie blue staining according to the standard recommendation. After staining, gel images were acquired using an Image scanner III (GE Healthcare Biosciences). Differential analysis was performed by Image Master 2D Platinum version 7.0 (GE Healthcare Biosciences) software tool, and spots of interest were excised for identification by mass spectrometry.
2.6. Protein identification with liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The spots of interest were carefully cut and destained in a solution containing 50% ACN in 100 μL of 25 mM ammonium bicarbonate ((NH4)HCO3) and then submitted to an in-gel trypsin digestion overnight at 37 °C in 25 mM ((NH4)HCO3). Peptides were extracted in 50 µL of 5% formic acid/50% ACN and then put into the ultrasonic bath for 15 min and dried in a speed-vac. Peptide samples were dissolved in 98% H2O, 2% ACN and 0.1% formic acid. The LC–MS/MS system consists of a liquid chromatography part (Dionex Ultimate 3000, Thermo Scientific) in combination with an electrospray ionization (ESI)-ion trap mass spectrometer (amaZon SL, Bruker, Germany). The LC separation was performed on a reversed-phase column (Hypersil GoLD 50 × 0.5 mm, 5 μm C18), protected by a guard column, eluted at a flow rate of 100 μl/min under gradient conditions of 5–80% B over 50 min. Mobile phase A consists of water/formic acid (99.9:0.1, v/v), and B consists of acetonitrile (100, v). Mass spectral data from 150 to 1500 m/z was collected in the positive ionization mode. For protein identification, MS/MS data were analyzed by the Mascot search engine against the SwissProt database. The Mascot score was taken into consideration and reported after manual verification of the fragmentation spectrum. Protein–protein interaction and signaling pathway analysis, using the Ingenuity Pathways Analysis (IPA), offered us some additional valuable clues about the complex interactive links between the various identified proteins within their commonly known interactive protein networks also obtained from other cellular metabolic information.
2.7. Determination of protein expression by Western blot analysis
Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride (PVDF). Membranes were blocked with non-fat dried milk in Tris-buffered saline containing 0.01% Tween-20 and incubated in the following primary antibodies at 1:8000: Anti-PIA3 (ERp57), Anti-GFAP, Anti-DPYSL2 (CRMP2), Anti-Phospho-DPYSL2 (Phospho-CRMP2) and Anti-β-actin (cell signaling, USA). After secondary antibody treatment (HRP159 linked antibody), the signal was developed with a chemiluminescence reagent (GE healthcare, USA), and band images were detected with an X-ray film.
2.8. Data analysis and statistical methods
Raw data are presented as the mean and standard deviation, and the data were analyzed to find significant P-values <0.05 and 0.01. Differences between the groups were established by using an unpaired Student's t-test, while within-group comparisons were performed using the paired Student's t-test. The spot densities were further compared to control using an ANOVA.
3. Results
3.1. Standardized crude extract of K. parviflora and M. fragrans nutmeg volatile oil
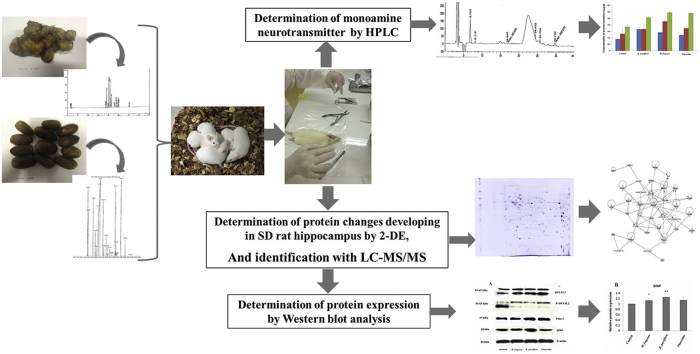
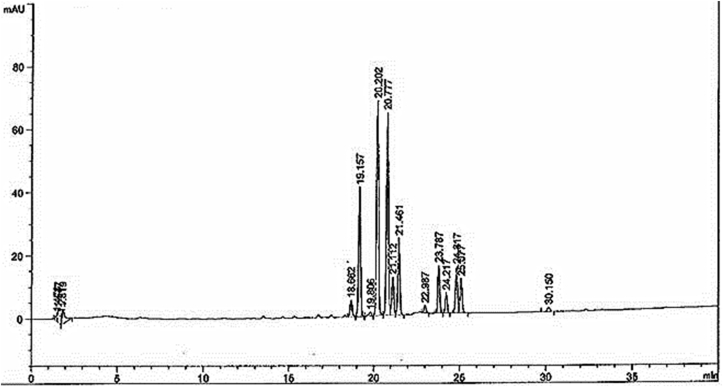
The content of 5,7-dimethoxyflavone in K. parviflora extract was analyzed by reversed-phase HPLC. The sample contained 13.4618 ± 0.0145 mg/g (R2 = 0.9997) of 5,7-dimethoxyflavone in the dry sample, with a representative HPLC fingerprint as shown in Fig. 1. The chemical composition of M. fragrans nutmeg volatile oil was analyzed by using the GC–MS technique (Fig. 2). A total of 26 volatile compounds were tentatively identified and reported in the nutmeg oil. The data describing all compounds are shown in Table 1. This research demonstrated that the crude extract of K. parviflora and nutmeg volatile oil are of good quality with a high potential.
Fig. 1.
Representative HPLC fingerprint of K. parviflora ethanolic extract.
Fig. 2.
GC–MS chromatogram of M. fragrans nutmeg volatile oil.
Table 1.
Chemical composition of nutmeg volatile oil obtained by GC–MS.
| NO. | RT | Area% | Compound |
|---|---|---|---|
| 1 | 14.428 | 1.97 | z1R-2,6,6-Trimethylbicyclo [3.1.1] hept-2-ene |
| 2 | 15.434 | 6.17 | Bicyclo [3.1.1] hex-2-ene, 2-methyl-5(1-methylethyl) |
| 3 | 15.729 | 2.68 | 1R-2,6,6-Trimethylbicyclo [3.1.1] hept-2-ene |
| 4 | 16.522 | 0.12 | Camphene |
| 5 | 18.462 | 5.13 | Cyclohexene, 4-methylene-(1-methylethyl) |
| 6 | 19.066 | 0.52 | Beta-Myrcene |
| 7 | 20.646 | 1.59 | 4-Carene |
| 8 | 21.266 | 7.09 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl) |
| 9 | 22.024 | 14.89 | o-cymene |
| 10 | 22.702 | 10.04 | Limonene |
| 11 | 23.574 | 1.95 | Gamma-terpinene |
| 12 | 24.059 | 5.28 | Gamma-terpinene |
| 13 | 25.396 | 0.46 | Cyclohexene, 1-methyl-4-(1-methylethylidene) |
| 14 | 28.767 | 0.03 | (+)-2-Bornanone |
| 15 | 30.696 | 5.67 | Terpinen-4-ol |
| 16 | 30.885 | 6.20 | 3-Cyclohexene-1-ol, 4-methyl-1-(1-methylethyl) |
| 17 | 31.051 | 0.16 | Benzenemethanol, alpha., 4-trimethyl |
| 18 | 31.228 | 0.61 | Alpha-terpineol |
| 19 | 31.217 | 0.78 | Alpha-terpineol |
| 20 | 31.347 | 0.15 | 2-Cyclohexen-1-ol, 3-methyl-6-(1-methylethyl), cis |
| 21 | 31.761 | 0.23 | 2-Cyclohexen-1-ol, 3-methyl-6-(1-methylethyl), cis |
| 22 | 33.961 | 8.88 | Safrole |
| 23 | 35.369 | 0.18 | Eugenol |
| 24 | 36.334 | 0.78 | Methyleugenol |
| 25 | 36.695 | 0.01 | Caryophyllene |
| 26 | 38.168 | 0.05 | Benzene, 1,2-dimethoxy-4-(1-propenyl) |
| 27 | 38.665 | 0.90 | Meristicin |
| 28 | 41.320 | 0.03 | Ar-turmerone |
| 29 | 43.024 | 0.06 | Tetradecanoic |
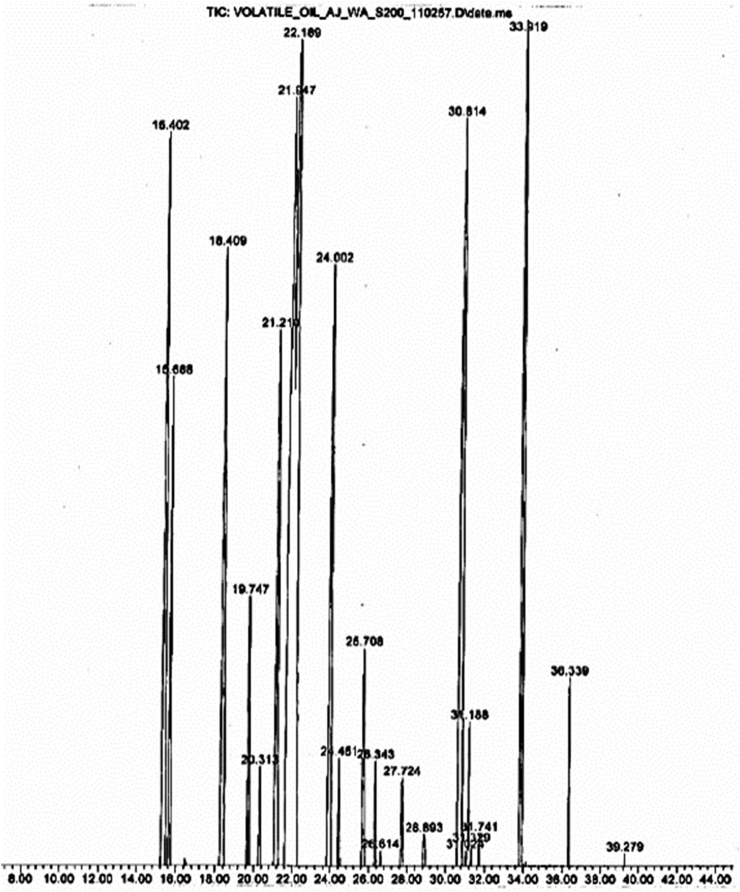
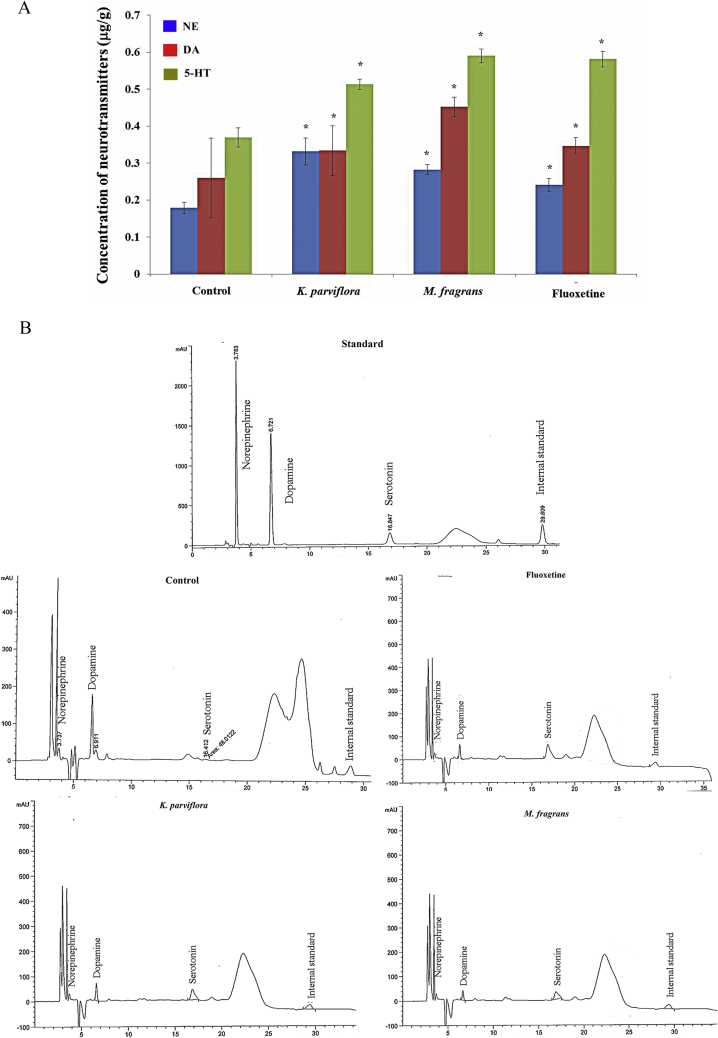
3.2. Effects of K. parviflora rhizome ethanolic extract and M. fragrans nutmeg volatile oil on the levels of monoamine neurotransmitters including norepinephrine, serotonin (5-hydroxytryptamine, 5-HT), and dopamine
Reversed-phase high performance liquid chromatography (HPLC) is a commonly used method that can accurately determine the content of neurotransmitters upon treatment with K. parviflora rhizome ethanolic extract (200 mg/kg BW), M. fragrans nutmeg volatile oil (300 mg/kg BW) or fluoxetine (20 mg/kg BW) for 12 days compared with that of the control group (2% tween 80, 1 mL/kg BW). The concentration of monoamine neurotransmitters is reported in Table 2, and the representative HPLC fingerprints are show in Fig. 3B. Administration of K. parviflora group and M. fragrans via the oral route once per day for 12 days led to a significantly higher level of serotonin (5-HT), norepinephrine and dopamine than the control treatment, as shown in Fig. 3A (P < 0.01). However, M. fragrans-treated rats exhibited a non-significant increase in serotonin compared with that of the fluoxetine-treated group, as shown in Fig. 3A. All results were expressed as the mean ± SD, and a student's paired t-test was used to compare the different groups. Results were considered statistically significant at a P-value less than 0.01.
Table 2.
Concentration of neurotransmitters (μg/g wet weight) in the hippocampal homogenates from control and treated rats.
| Group | Neurotransmitter (μg/g wet weight) |
||
|---|---|---|---|
| NE (Mean ± SD) | DA (Mean ± SD) | 5-HT (Mean ± SD) | |
| Control (n = 6) | 0.1789 ± 0.0153 | 0.2594 ± 0.1077 | 0.3695 ± 0.0262 |
| K. parviflora (n = 6) | 0.3316 ± 0.0365 | 0.3337 ± 0.0674 | 0.5132 ± 0.0137 |
| M. fragrans (n = 6) | 0.2825 ± 0.0133 | 0.4517 ± 0.0259 | 0.5897 ± 0.0189 |
| Fluoxetine (n = 5)* | 0.2408 ± 0.0171 | 0.3463 ± 0.0221 | 0.5811 ± 0.0208 |
* On the fifth day, one rat in the fluoxetine treatment group died. Data from the liver and kidney function and histology in the dead rat did not show indications of toxicity, but the data are not shown. NE, norepinephrine; DA, dopamine; 5-HT, serotonin.
Fig. 3.
(A) The increase in the level of monoamine neurotransmitters including norepinephrine (NE), serotonin (5-hydroxytryptamine, 5-HT) and dopamine (DA) in the rat hippocampus when treated with K. parviflora rhizome ethanolic extract, M. fragrans nutmeg volatile oil and fluoxetine. The statistical analysis showed a significant difference between the control and each of the treatment groups at P < 0.05 (control: n = 6, K. parviflora: n = 6, M. fragrans: n = 6, fluoxetine: n = 5). Data are expressed as the mean ± standard deviation (S.D.). (B) HPLC chromatograms of monoamine neurotransmitters.
3.3. Proteomic identification of proteins modulated by K. parviflora, M. fragrans and fluoxetine in the SD rat hippocampus
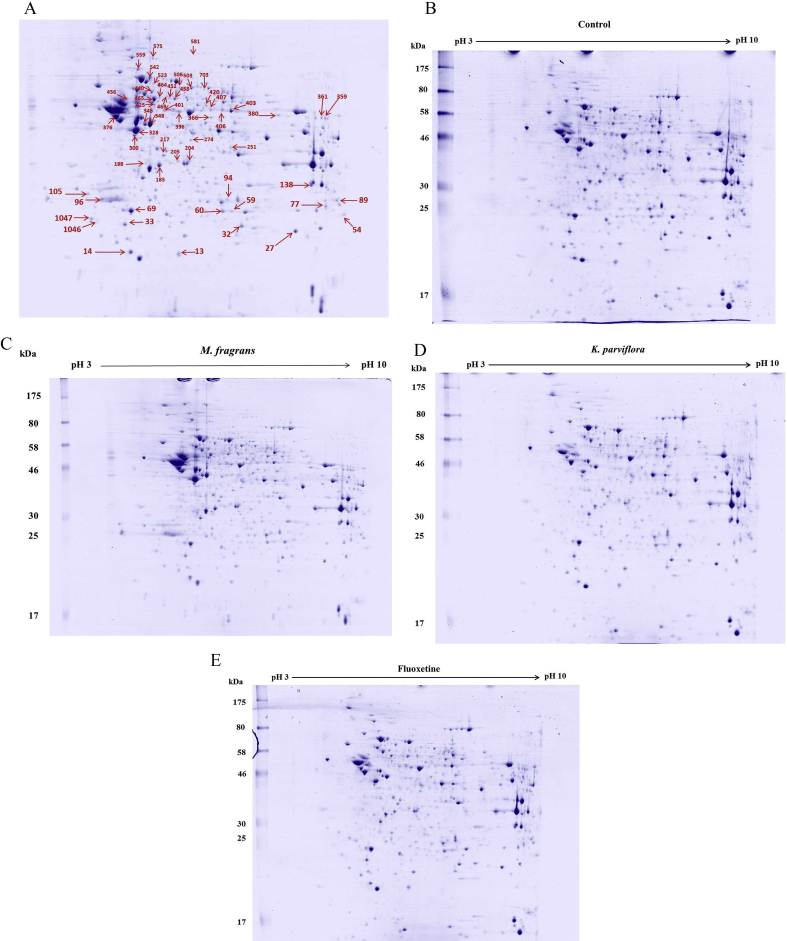
We have extended our study to identify possible molecular targets for K. parviflora rhizome ethanolic extract (200 mg/kg BW), M. fragrans nutmeg volatile oil (300 mg/kg BW) and fluoxetine (20 mg/kg BW) treatment on hippocampal proteomic profiles in SD rats. The hippocampal protein extracts of rats from the control and treated groups were analyzed by 2-DE. For each experimental group, gels were made in triplicate (12 gels in total). The representative gels are presented in Fig. 4. The image analysis of the gels demonstrated a similar number and pattern distribution of spots in the twelve 2D gels by using Image Master 2D Platinum version 7. Approximately 1050 spots were detected on each gel (as shown in Fig. 4A).
Fig. 4.
Representative gels showing the two-dimensional gel analysis profile of the hippocampal protein from control (B), fluoxetine (E), K. parviflora (D) and M. fragrans (C) -treated rats. Gels were made in triplicate (12 gels in total). The gels were stained with Coomassie blue and analyzed by HPLC-ESI-IT-MS/MS. The spots were identified by MASCOT, and the names of the proteins are presented in Table 3 (Not all listed proteins are shown in Fig. 4).
We focused our analysis on the proteins in the three replicates of each treatment group that showed a fold change ≥1.5 compared with that in the control group (P < 0.05). Table 3 presents the functional classification and regulation of the differentially expressed proteins in each group. Ninety proteins were accepted in total as significantly different and were analyzed with LC-MS/MS. Mass spectrometry gave identified 79 of the 90 spots (87.78% success rate). However, we found the presence of the same proteins in different spots, which our data does not display. After that each group was compared with the control group, the analysis indicated that 37 proteins were up-regulated and 14 were down-regulated in the K. parviflora group. The M. fragrans group had 27 proteins up-regulated and 16 down-regulated. In the fluoxetine-treated group, we found 29 proteins that were up-regulated, and 14 that were down-regulated.
Table 3.
Functional classification and regulation of differentially expressed proteins in treated rat hippocampal tissue.
| Spot No. | Gene symbol | Proteins name | pI | MW (Da) | localization | Molecular function | Regulated |
||
|---|---|---|---|---|---|---|---|---|---|
| KP | MP | Flu | |||||||
| 12 | Tagln3 | Transgelin-3 | 8.96 | 24887 | Mt (matrix) | Development of central nervous system. | ↑ | ↓ | ↑ |
| 13 | Atp5H | ATP synthase subunit d | 6.79 | 18491 | Mt | Enzyme, produces ATP from ADP. | ↑ | ↓ | ↑ |
| 14 | Prdx2 | Peroxiredoxin-2 | 5.34 | 21941 | Cp | Enzyme, redox regulation of the cell. | ↑ | ↑ | ↑ |
| 15 | Aprt | Adenine phospho-ribosyltransferase | 6.17 | 19761 | Cp | Enzyme, catalyzes a salvage reaction in the formation of AMP. | ↓ | – | ↑ |
| 27 | Sod2 | Superoxide dismutase [Mn] | 8.96 | 24887 | Mt (matrix) | Enzyme, destroys superoxide anion radicals. | ↑ | – | – |
| 32 | Uqcrfs1 | Cytochrome b-c1 complex subunit Rieske | 9.04 | 29712 | Mt (Inner membrane) | Enzyme, generates an electrochemical potential coupled to ATP synthesis. | ↑ | ↓ | ↑ |
| 33 | Ddir1 | Rho GDP-dissociation inhibitor 1 | 5.12 | 23450 | Cp | Controls the homeostasis of Rho proteins. | – | ↓ | – |
| 59 | Tpi1 | Triosephosphate isomerase | 6.89 | 27345 | Cp | Enzyme, involved in the gluconeogenesis pathway and carbohydrate biosynthesis. | ↑ | ↓ | ↑ |
| 69 | Uchl1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 5.14 | 25165 | Cp, ER (membrane) | Enzyme, involved both in the processing of ubiquitin precursors and of ubiquitinated proteins. | – | ↑ | ↑ |
| 77 | Gstm | Glutathione S-transferase | 8.78 | 25360 | Cp | Enzyme, conjugation of reduced glutathione to exogenous and endogenous hydrophobic electrophiles. | ↑ | ↑ | ↑ |
| 89 | Map | Microtubule-associated protein | 4.87 | 300831 | Cp, Csk | Bind to the tubulin subunits that regulate microtubule stability. | ↑ | – | – |
| 94 | Hagh | Hydroxyacyl glutathione hydrolase | 7.64 | 34544 | Cp | Enzyme, catalyzes the hydrolysis of S-D-lactoyl-glutathione to form glutathione and d-lactic acid. | ↑ | – | – |
| 96 | Ywhag | Tryptophan 5-monooxygenase activation protein, gamma (14-3-3 protein gamma) | 4.80 | 28456 | Cp | Intracellular signaling and cell cycle. | ↓ | ↑ | – |
| 105 | Ank3 | Ankyrin-3 | 7.94 | 285692 | Cp, Csk, CM | Maintenance/targeting of ion channels and cell adhesion molecules at axonal initial segments and the nodes of Ranvier. | ↑ | – | ↑ |
| 138 | Vdac1 | Voltage-dependent anion-selective channel protein 1 | 8.62 | 30851 | Mt (outer membrane) | Forms a channel through the mitochondrial outer membrane and the plasma membrane. | ↑ | ↓ | ↓ |
| 169 | Hnrnpa1 | Heterogeneous nuclear ribonucleoprotein A1 | 9.20 | 34362 | Cp, Nu | Involved in the packaging of pre-mRNA into hnRNP particles, transport of poly(A) mRNA from the nucleus to the cytoplasm. | ↑ | ↑ | ↑ |
| 185 | Ldhb | l-lactate dehydrogenase B chain | 5.70 | 36874 | Cp | Involved in the subpathway that synthesizes (S)-lactate from pyruvate. | ↓ | – | ↓ |
| 194 | Ppp1B | Serine/threonine-protein phosphatase PP1-beta catalytic subunit | 5.84 | 37961 | Cp, Nu | Cell division, role in the control of chromatin structure and cell cycle progression during the transition from mitosis into interphase. | ↑ | ↑ | ↑ |
| 204 | Syt1 | Synaptotagmin-1 | 9.43 | 43385 | Cp (vesicle) | A regulatory role in the membrane interactions of synaptic vesicles at the active synapse zone. | ↑ | ↑ | – |
| 205 | Akt | Serine/threonine-protein phosphatase | 5.94 | 38229 | Cp, Nu | Regulates many processes including metabolism, proliferation, cell survival, growth and angiogenesis. | ↑ | ↓ | ↑ |
| 217 | Ddah1 | N(G)-dimethylarginine dimethylaminohydrolase | 5.75 | 31805 | Cp | A role in the regulation of nitric oxide generation, inhibits NOS | – | ↑ | – |
| 227 | Ark72 | Acetyl-CoA carboxylase 1 | 5.97 | 266678 | Cp | Role in controlling fatty acid metabolism. | ↑ | ↑ | ↑ |
| 245 | Ak1A1 | Alcohol dehydrogenase 1 | 6.84 | 36711 | Cp, Nu | Catalyzes the oxidation of long-chain primary alcohols and the oxidation of S-(hydroxymethyl) glutathione. | ↑ | ↓ | ↑ |
| 251 | LOC102548564 | Peptidyl-prolyl cis-trans isomerase D | 6.73 | 41139 | Cp, Nu | Catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides. | ↑ | – | ↑ |
| 262 | Tmod2 | Tropomodulin 2 | 5.34 | 39468 | Cp, Csk | Blocks the elongation and depolymerization of the actin filaments at the pointed end. | – | ↑ | ↑ |
| 274 | Pura | Transcriptional activator protein Pur-alpha (Fragments) | 4.69 | 15370 | Nu | Role in the initiation of DNA replication and in recombination. | ↑ | ↓ | – |
| 300 | Sh3gl2 | Endophilin-A1 | 5.26 | 40045 | Cp, CM | Implicated in synaptic vesicle endocytosis. | ↓ | – | ↓ |
| 317 | Pgk1 | Phosphoglycerate kinase 1 | 8.02 | 44909 | Cp | Role as a glycolytic enzyme. | ↑ | ↑ | ↑ |
| 331 | Gfap | Glial fibrillary acidic protein | 5.35 | 49984 | Cp | Role in astrocyte-neuron and many important processes in CNS such as development of CNS. | ↑ | ↑ | ↑ |
| 345 | Ckb | Creatine kinase B-type | 5.39 | 42983 | Cp | Catalyzes the transfer of phosphate between ATP and various phosphagens. | ↓ | ↑ | ↓ |
| 354 | Eftu | Elongation factor Tu, mitochondrial | 7.23 | 49890 | Mt | Promotes the GTP-dependent binding of aminoacyl-tRNA to the A-site of ribosomes in protein synthesis. | – | ↓ | ↓ |
| 359 | Sept7 | Septin-7 | 8.82 | 50818 | Nu, Cp | Normalizes the organization of the actin cytoskeleton, role in ciliogenesis and collective cell movements. | ↑ | – | ↑ |
| 361 | Sept11 | Septin-11 | 6.24 | 50005 | Cp, Csk | Plays important role in cytokinesis, cytoarchitecture of neurons (dendritic arborization and dendritic spines), and in GABAergic synaptic connectivity. | ↑ | ↓ | ↑ |
| 366 | Aldh5a1 | Succinate-semialdehyde dehydrogenase | 8.35 | 56723 | Mt | Catalyzes the degradation of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). | ↓ | ↓ | ↓ |
| 396 | Eno1 | Alpha-enolase | 6.16 | 47440 | Cp, CM | Plays role in glycolysis. | ↑ | – | ↑ |
| 401 | Dlst | dihydrolipoyllysine-residue succinyltransferase | 8.76 | 67637 | Mt (matrix) | Converts 2-oxoglutarate to succinyl-CoA and CO2. | ↑ | ↑ | ↑ |
| 403 | Glud1 | Glutamate dehydrogenase 1 | 8.05 | 61719 | Mt (matrix) | Converts l-glutamate into alpha-ketoglutarate and increases the turnover of the excitatory neurotransmitter glutamate | ↑ | – | ↑ |
| 442 | Tba4a | Tubulin alpha-4A chain | 4.95 | 50634 | Cp, Csk | A major constituent of microtubules | ↓ | ↑ | ↓ |
| 450 | Cap1 | Adenylyl cyclase-associated protein 1 | 7.61 | 51899 | CM | Regulates filament dynamics and plays role in a number of complex developmental and morphological processes, (mRNA localization and cell polarity). | ↑ | ↑ | ↑ |
| 452 | Pdia3 | Protein disulfide-isomerase A3 | 4.74 | 64400 | ER | Plays a role in protein folding by promoting the formation of disulfide bonds. | ↑ | ↑ | ↑ |
| 456 | Vim | Vimentin | 5.06 | 53757 | Nu (matrix) | The major IF protein in mesenchymal cells. | – | ↑ | ↓ |
| 464 | Hspd1 | 60-kDa heat-shock protein | 5.91 | 61088 | Mt (matrix) | Prevents misfolding and promotes the refolding and proper assembly of unfolded polypeptides. | – | ↑ | ↑ |
| 465 | Dpysl2 | dihydropyrimidinase-related protein 2 | 5.95 | 62638 | Cp, Csk, CM | Involved in neuronal differentiation and axonal guidance. | ↑ | ↑ | ↑ |
| 490 | Cct5 | T-complex protein 1 subunit epsilon | 5.51 | 59955 | Cp, Csk, MT | Molecular chaperone, helps the folding of proteins upon ATP hydrolysis. | ↓ | ↑ | ↓ |
| 559 | Ndufs1 | NADH-ubiquinone oxidoreductase 75 kDa subunit | 8.51 | 51499 | Mt (Inner membrane) | Role in the transfer of electrons from NADH to the respiratory chain. | ↓ | – | ↓ |
| 574 | Gsn | Gelsolin | 5.76 | 86413 | Cp, Csk | Promotes the assembly of monomers into filaments. | ↓ | ↓ | ↓ |
| 581 | Pdcd6ip | Programmed cell death 6-interacting protein | 6.15 | 97141 | MT | Plays a role in the regulation of both apoptosis and cell proliferation. | ↓ | – | – |
| 641 | Pura | Transcriptomal activator protein Pur-alpha | 4.69 | 15370 | Nu | Plays important role in the initiation of DNA replication and recombination. | ↑ | ↓ | ↓ |
| 687 | Sept8 | Septin 8 | 5.74 | 51562 | Cp, Csk | Role in cytokinesis | ↑ | ↑ | ↑ |
| 703 | Me1 | NADP-dependent malic enzyme | 6.49 | 64589 | Cp | Generates NADPH for fatty acid biosynthesis and reverses malate to pyruvate. | – | – | ↓ |
| 819 | Stag3 | Cohesin subunit SA-3 | 5.81 | 143628 | Nu | A component of cohesin complex. | ↑ | ↑ | ↑ |
| 846 | Hyou1 | Hypoxia up-regulated protein 1 | 8.73 | 63702 | Cp, Nu | Role in cytoprotective, mechanisms triggered by oxygen deprivation. | – | – | ↓ |
| 848 | Nsf | Vesicle-fusing ATPase | 6.55 | 83170 | Cp | Catalyzes the fusion of transport vesicles within the Golgi cisternae. | ↓ | ↓ | ↓ |
| 849 | Dldh | Dihydrolipoyl dehydrogenase | 7.96 | 54574 | Mt (matrix) | A component of the glycine cleavage system and alpha-ketoacid dehydrogenase complexes. | ↑ | ↑ | ↓ |
| 873 | Hnrpm | Heterogeneous nuclear ribonucleoprotein M | 8.90 | 74076 | Nu (matrix) | Important role in p53/TP53 response to DNA damage. | ↓ | – | ↓ |
| 882 | Tkt | Transketolase | 7.23 | 68342 | Ly | Catalyzes the transfer of a two-carbon ketol group from a ketose donor to an aldose acceptor. | ↑ | ↑ | _ |
| 890 | Arhgef28 | Rho guanine nucleotide exchange factor 28 | 5.47 | 192748 | Cp, CM | Functions in axonal branching, synapse formation and dendritic morphogenesis. Regulates numerous cellular responses such as proliferation, differentiation and movement. | ↑ | ↑ | ↓ |
| 918 | Bin1 | Myc box-dependent interacting protein 1 | 4.95 | 64721 | Cp, Nu | Role in the regulation of synaptic vesicle endocytosis. | ↑ | ↑ | – |
| 960 | Sptn1 | Spectrin alpha chain | 5.20 | 285261 | Cp, Csk | Role in secretion and interaction of calmodulin in a calcium-dependent manner, candidate for the calcium-dependent movement of the cytoskeleton. | ↓ | ↓ | ↓ |
| 1046 | Snap25 | Synaptosomal-associated protein 25 | 4.66 | 23528 | Cp, CM | Important role in synaptic function including molecular regulation of neurotransmitter release, vesicle docking and membrane fusion. | ↑ | – | – |
The table reports the list of spots, SwissProt protein, full name, theoretical pI and MW (Da), molecular function and protein regulation. The main localization: Cp, Cytoplasm; Nu, Nucleus; Csk, Cytoskeleton; CM, Cell Membrane; Mt, Mitochondrion; Ly, Lysosome; ER, Endoplasmic Reticulum.
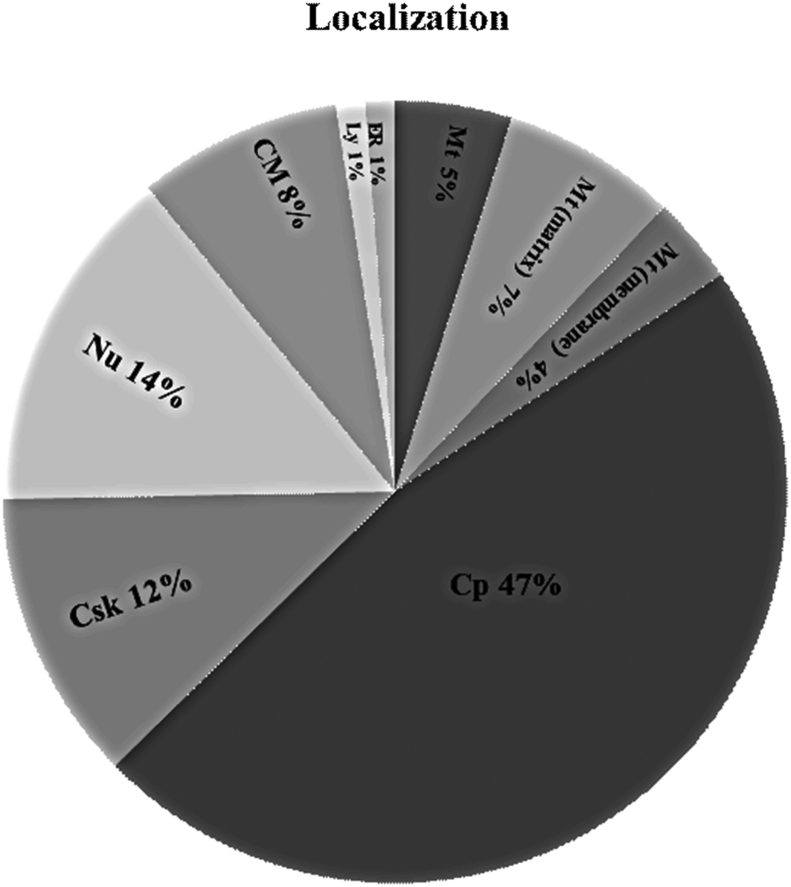
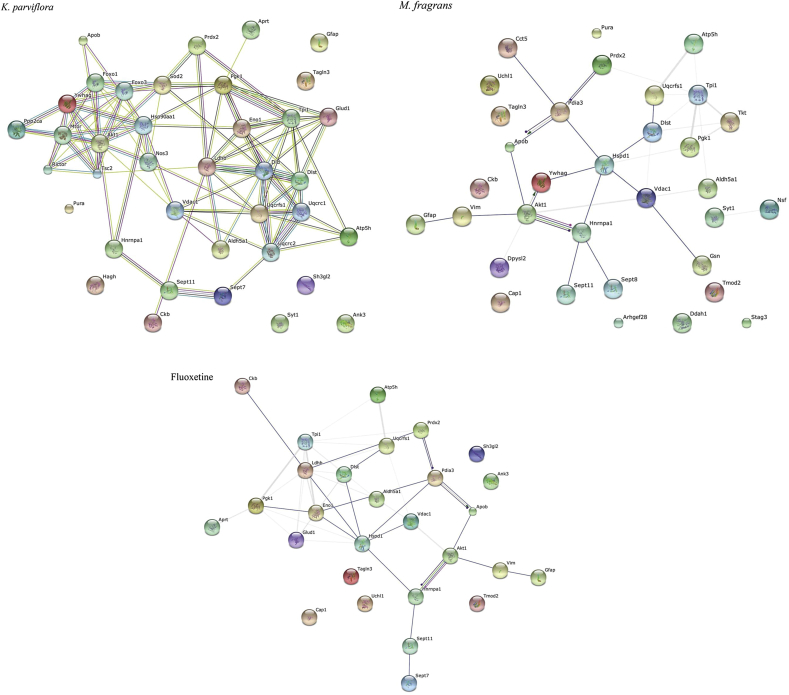
Bioinformatics analysis (SwisProt, UniProtKB, IPA and STRING-10.0) has been used for pathway analysis, the functional classification and regulation of the differentially expressed proteins (see Table 3 and Supplementary data). Considering their biological function, the proteins were classified by their functional process into nine different cellular or subcellular localizations. This analysis revealed that most of the entries were localized in the cytoplasm (47% of total), and others were localized to the nucleus (14%), cytoskeleton (12%), cell membrane (8%), mitochondrial matrix (7%), mitochondrion (5%), and mitochondrial membrane (4%) (see Fig. 6). Moreover, the IPA analysis showed that the numerous proteins participate in many pathways important to nervous system development and function and neurological disease (see Tables 1–5 in Supplementary data). The protein-protein interaction data (STRING and IPA) integrates the information and provides an understanding of the cellular physical and functional interactions. The IPA analysis demonstrated the involvement of the identified proteins and their interactive pathways within a neuronal network especially important for neuro-regenerative processes such as neural plasticity (Supplementary data). In addition, proteins that have a potential antioxidative role, which is important for neuronal protection in the brain, were up-regulated, while proteins that are known for their inhibitory effects on neuro-regenerative processes were down-regulated as shown in Table 3 (see Fig. 7).
Fig. 6.
Pie chart represents the percentage of nine different cellular/subcellular localizations by using the Protein Knowledgebase UniProtKB.
Fig. 7.
STRING analysis of fluoxetine-, K. parviflora- and M. fragrans-modulated proteins in the rat hippocampus. Different colored lines represent the types of evidence for the association.
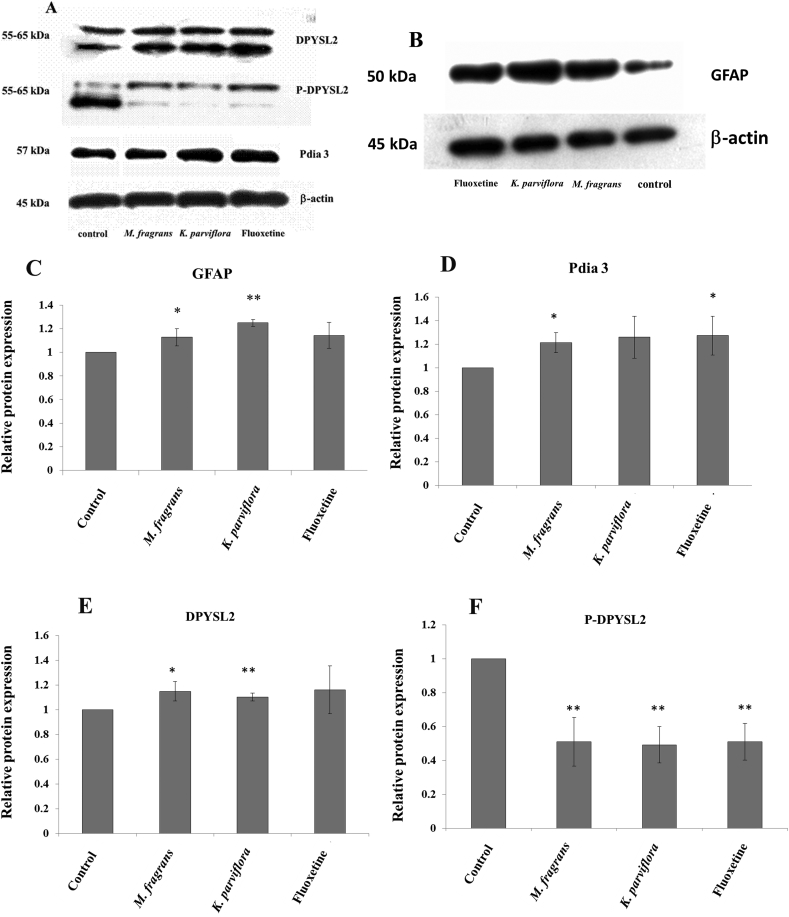
3.4. Validation of the change in protein expression from the 2D-GE results by Western blot analysis
We validated the expression level of proteins by choosing representative proteins from those that were down- and up-regulated in the 2D gels. Western blot analysis was performed with specific antibodies that detect bands of PDIA3 at 57 kDa, GFAP at 50 kDa, and DPYSL2 and p-DPYSL2 at 55–65 kDa. The intensity of the bands was normalized by β-actin, and the values were obtained in the experimental groups, which were normalized to the corresponding value in the control group. Semi-quantitative analysis of the Western blot confirmed the tendencies observed in the 2D gel image analysis. Representative Western blots of hippocampal samples are presented in Fig. 5A and B. In detail, the GFAP protein level was significantly higher in the hippocampus of the K. parviflora- and M. fragrans-treated groups but non-significantly higher in the fluoxetine-treated group than in the control group (Fig. 5C). The PDIA3 level was significantly increased in the M. fragrans and fluoxetine groups but not changed in the K. parviflora group (see Fig. 5D). The statistical analysis demonstrated significant differences in the levels of DPYSL2 and p-DPYSL2 between the experimental and control groups (see Fig. 5E and F). The expression level of DPYSL2 was higher in the two herbal groups than in the control group (Fig. 5D). However, no difference was observed in the fluoxetine-treated group. In this study, we are interested in the role of p-DPYSL2 in neuronal development. Western blotting revealed that p-DPYSL2 was significantly lower in the experimental groups than in the control group (Fig. 5E). The expression levels of the proteins were in accordance with the data from the proteomics analysis.
Fig. 5.
(A–F) Representative image of Western blots evaluating the expression of GFAP, PDIA3, DPYSL2 and p-DPYSL2 from the rat hippocampus. Data are expressed as the mean ± standard deviation (S.D.); statistically significant differences were analyzed by t-test (P < 0.05).
4. Discussion
The level of monoamine neurotransmitters in the hippocampus of the brain is involved in the regulation of cognitive processes such as mood, attention, sleep, arousal, and certain types of memory.37 Monoamine neurotransmitters have been found to play an important role in neurological disorders. Drugs or herbal remedies that augment the effects of monoamines on their target tissue are used to treat psychiatric disorders, including depression, anxiety, and schizophrenia.38 This study demonstrates, for the first time, the effect of K. parviflora and M. fragrans on the levels of NE, DA and 5-HT. Our results showed that K. parviflora, M. fragrans and fluoxetine increased the levels of serotonin (5-HT), norepinephrine and dopamine in the hippocampus of SD rats when compared with that of the control group. Disturbances in the DA, 5-HT and NA neurotransmitter systems have been suggested to be involved in the pathogenesis of mood disorders.39, 40 Moreover, we found an up-regulation of proteins that are involved in synaptic function and endocytosis, including BIN1 and SYT1, in the K. parviflora and M. fragrans group and SNAP25 only in the K. parviflora-treated group. Interestingly, a previous study demonstrated that norepinephrine triggers the release of glial ATP to increase postsynaptic efficacy,41 which is related to our proteomic results. Our results showed that K. parviflora, M. fragrans and fluoxetine regulate seven proteins involved in ATP synthesis: ATP5H, APRT, URCRFS1, CKB, ENO1, NDUFS and NSF. Several findings have been discovered in the rat model: selective serotonin reuptake inhibitors (SSRI), such as fluoxetine, increase the extracellular 5-HT levels in the rat hippocampus.37, 42, 43, 44 Our observations indicate that K. parviflora and M. fragrans increase the level of 5-HT through similar mechanisms as fluoxetine.
In the present work, we focused on the expression of GFAP, PDIA3, DPYSL2 and p-DPYSL2. Glial fibrillary acidic protein (GFAP) is the intermediate filament protein in mature astrocytes, in addition to vimentin, nestin and synemin.45, 46, 47, 48 There is mounting evidence suggesting aberrant astrocytic function in neurodegenerative diseases such as dementia, Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD).46 Moreover, the expression of both GFAP mRNA and protein was reduced in the hippocampus in major depressive disorder (MDD). Astrocyte pathology in depression is less well known in the human hippocampus, but the literature suggests the involvement of hippocampal astrocytes in the blood of depressed subjects at the time of death without antidepressant medication.48 Recently, GFAP antibodies demonstrated a protective effect on oxidative-stressed neuroretinal cells via an interaction with PDIA3.49 Our study presents an up-regulation of GFAP and PDIA3 in the rat hippocampus treated with K. parviflora and M. fragrans. Furthermore, we show that M. fragrans increases the level of vimentin (VIM), a type III intermediate filament (IF). Thus, M. fragrans may be effective in promoting neuroregeneration in plasticity. In addition, we found that the effected proteins play important roles in neuronal differentiation and morphology of neuronal cells, including microtubule-associated protein (MAP), which binds to the tubulin subunits regulating microtubule stability, tubulin alpha-4A chain (Tba4a), a major constituent of microtubules, and dihydropyrimidinase-related protein 2 (Dpysl2), which is involved in neuronal differentiation and axonal guidance.
In addition to the role of Dpysl2 in cytoskeleton organization, Dpysl2 is also a component of the dynein complex, acting on the intracellular retrograde motility of vesicles and organelles along with microtubules at the synaptic level.50 DPYSL2 down-regulation might be associated with neuronal and synaptic loss, as the protein is involved in axon guidance and growth, neurite development, and neuronal polarization.51 The expression of DPYSL2 was significantly increased in the K. parviflora and M. fragrans groups, although in the fluoxetine group, there was no significant difference in its expression. A previous study identified a crucial function of DPYSL2 in axon formation of hippocampal neurons, thereby establishing and maintaining neuronal polarity.52 DPYSL2 interacts with tubulin heterodimers and promotes microtubule assembly in vitro. Thus, DPYSL2 seems to promote neurite elongation and axon specification by regulating microtubule assembly, endocytosis of adhesion molecules and reorganization of actin filaments.53 In addition, we found that K. parviflora up-regulated microtubule-associated protein (MAP), which plays an important role in microtubule stability. A more direct link to changes in synaptic activity suggested by our results is the up-regulation of SNAP25, which plays a key role in synaptic functioning and synaptic formation. We also found that the M. fragrans group showed an increase in the expression of tubulin alpha-4A chain (TBA4A), which is a major constituent of microtubules. Phosphorylated DPYSL1 and DPYSL2 are localized in the dendrites of cortical neurons where they regulate dendritic branch trajectories.54 A previous study in mice revealed that the abnormal phosphorylation of DPYSL1 and DPYSL2 resulted in a curling dendrite phenotype, suggesting that these two molecules are critical for normal dendrite patterning in cortical neurons.55 The most important mechanism of action of non-phosphorylated DPYSL2 is its strong binding to tubulin, which leads to microtubule formation. Phosphorylation of DPYSL2 by Rho kinase suppresses its binding to tubulin and Numb. Surprisingly, our study found that p-DPYSL2 was significantly decreased in the rat hippocampus treated with K. parviflora, M. fragrans and fluoxetine. This is the first time that the effects of these compounds on DPYSL2 and p-DPYSL2 expression have been reported.
Moreover, we found that the PDIA3 level was increased in the M. fragrans and fluoxetine group but not changed in the K. parviflora group. ERp57, also known as Pdia3, Grp58, ER60 and 1,25D3-MARRS, is a member of the protein disulfide isomerase (PDI) family and a lumenal protein of the endoplasmic reticulum (ER). To enter the secretory pathway, proteins are cotranslationally translocated across the membrane of the endoplasmic reticulum (ER) as extended polypeptide chains.56, 57 ERp57 can interact with calreticulin and calnexin to ensure the correct folding of newly synthesized glycoproteins as a molecular chaperone and is associated with a number of diseases including cystic fibrosis, prion diseases, Huntington's disease and Alzheimer's disease.57
In fact, impaired mitochondrial function is commonly observed in many types of neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, Huntington's disease, alcoholic dementia, brain ischemia-reperfusion related injury, and others. Although many of these neurological disorders contain unique etiological factors, may share common factors of increased nitroxidative stress and mitochondrial dysfunction, likely resulting from protein post-translational modifications (PTMs) including oxidation, nitration, hyperphosphorylation, acetylation, glycosylation and formation of various protein adducts.58, 59 Interestingly, K. parviflora increased the level of superoxide dismutase [Mn] or SOD2, which is an enzyme that destroys superoxide anion radicals. In addition, K. parviflora, M. fragrans and fluoxetine increased the level of glutathione S-transferase. We expect that an increase in some of these beneficial agents in the treatment of mitochondrial dysfunction-related organ damage may prevent some neurodegenerative conditions because these mitochondria-targeted antioxidants were effective in preventing mitochondrial dysfunction and nitroxidative tissue injuries in various disease models.37, 60, 61 Lastly, these findings may lead to the development of new herbal medications for neurological disorders, including neurodegenerative disease and psychiatric disorders.
5. Conclusion
In summary, we revealed that K. parviflora, M. fragrans and fluoxetine increase the level of serotonin (5-HT), norepinephrine, and dopamine in the rat hippocampus. The proteomic analysis data revealed that 37 proteins were up-regulated, while 14 were down-regulated in the K. parviflora group. In the M. fragrans group, 27 proteins were up-regulated, while 16 were down-regulated. In the fluoxetine group, we found that 29 proteins were up-regulated, and 14 were down-regulated. The levels of GFAP, PDIA3, DPYSL2 and p-DPYSL2 were up- and down-regulated in the hippocampus of K. parviflora-, M. fragrans- and fluoxetine-treated SD rats. The data suggest that K. parviflora and M. fragrans can target and regulate multiple pathways involved in the underlying molecular therapeutic mechanisms; specifically, they can change the expression levels of GFAP, PDIA3, DPYSL2 and p-DPYSL2. In this study, K. parviflora and M. fragrans are shown to exert a synergistic therapeutic effect on the prevention and treatment of neurodegenerative diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the 90th Anniversary Fund (Ratchadaphisek Somphot Endowment) of Chulalongkorn University and Aging Society Research Fund (CU-58-918-AS). We would like to extend our sincerest gratitude to the Graduate School and Department of Clinical Chemistry, Faculty of Allied Health Sciences at Chulalongkorn University for providing the Tuition Fee Scholarship, as well as to the National Laboratory Animal Centre and MT Proteomic unit at Mahidol University and the Pilot plan and Instrument center at Rangsit University for animal maintenance and instrument support. Lastly, we would like to thank Assoc. Prof. Dr. Nijsiri Ruangrungsi for guidance in plant identification and Dr. Tewarit Sarachana for his kindness and valuable advice.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jtcme.2017.01.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Halliday M., Mallucci G.R. Targeting the unfolded protein response in neurodegeneration: a new approach to therapy. Neuropharmacology. 2014;76:169–174. doi: 10.1016/j.neuropharm.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Mak TN, Caldeira S (2014) and Healthy Ageing, http://publications.jrc.ec.europa.eu/repository/bitstream/JRC90454/lbna26666enn.pdf.
- 3.Freitas-Andrade M., Naus C. Astrocytes in neuroprotection and neurodegeneration: the role of connexin43 and pannexin1. Neuroscience. 2016 May 26;323:207–221. doi: 10.1016/j.neuroscience.2015.04.035. Epub 2015 Apr 23. Review. PubMed PMID: 25913636. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Huerta P., Troncoso-Escudero P., Jerez C., Hetz C., Vidal R.L. The intersection between growth factors, autophagy and ER stress: a new target to treat neurodegenerative diseases? Brain Res. 2016 Oct 15;1649(Pt B):173–180. doi: 10.1016/j.brainres.2016.02.052. Epub 2016 Mar 18. Review. PubMed PMID: 26993573. [DOI] [PubMed] [Google Scholar]
- 5.Duff K., Paulsen J.S., Beglinger L.J., Langbehn D.R., Stout J.C., Group P-HIotHS Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Duncan J., Johnson S., Ou X.-M. Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov Ther. 2012;6:112–122. [PubMed] [Google Scholar]
- 7.Garcia-Miralles M., Ooi J., Bardile C.F. Treatment with the MAO-A inhibitor clorgyline elevates monoamine neurotransmitter levels and improves affective phenotypes in a mouse model of Huntington disease. Exp Neurol. 2016;278:4–10. doi: 10.1016/j.expneurol.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Swoboda K.J., Hyland K. Diagnosis and treatment of neurotransmitter-related disorders. Neurol Clin. 2002;20:1143–1161. doi: 10.1016/s0733-8619(02)00018-x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S., Stockmeier C.A., Meyer J.H. The reduction of R1, a novel repressor protein for monoamine oxidase A, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Liu P., Dai R. Analysis of plasma proteome from cases of the different traditional Chinese medicine syndromes in patients with chronic hepatitis B. J Pharm Biomed Anal. 2012;59:173–178. doi: 10.1016/j.jpba.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Zhang A., Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. Omics. 2012;16:414–421. doi: 10.1089/omi.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Li Z., Tang F., Ge R. Proteomics annotate therapeutic properties of a traditonal Tibetan medicine–Tsantan Sumtang targeting and regulating multiple perturbed pathways. J Ethnopharmacol. 2016 Apr 2;181:108–117. doi: 10.1016/j.jep.2015.12.023. Epub 2015 Dec 19. PubMed PMID: 26707570. [DOI] [PubMed] [Google Scholar]
- 13.Hamidpour M., Hamidpour R., Hamidpour S., Shahlari M. Chemistry, pharmacology, and medicinal property of Sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J Tradit Complement Med. 2014;4:82–88. doi: 10.4103/2225-4110.130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yende S.R., Harle U.N., Chaugule B.B. Therapeutic potential and health benefits of Sargassumspecies. Pharmacogn Rev. 2014;8:1. doi: 10.4103/0973-7847.125514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhuiyan M.M.H., Mohibbullah M., Hannan M.A. The neuritogenic and synaptogenic effects of the ethanolic extract of radix Puerariae in cultured rat hippocampal neurons. J Ethnopharmacol. 2015;173:172–182. doi: 10.1016/j.jep.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Wongsinkongman P., Mongkolchaipak N., Chansuvanich N., Techadumrongsin Y., Boonruad T. Quality evaluation of crude drugs and volatile oil of Krachai-dam rhizomes. Bull Dep Med Sci. 2003;45:1–16. [Google Scholar]
- 17.Hawiset T., Muchimapura S., Wattanathorn J., Sripanidkulchai B. Screening neuropharmacological activities of Kaempferia parviflora (Krachai Dam) in healthy adult male rats. Am J Appl Sci. 2011;8:695. [Google Scholar]
- 18.Azuma T., Tanaka Y., Kikuzaki H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry. 2008;69:2743–2748. doi: 10.1016/j.phytochem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Yenjai C., Prasanphen K., Daodee S., Wongpanich V., Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75:89–92. doi: 10.1016/j.fitote.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Patanasethanont D., Nagai J., Yumoto R. Effects of Kaempferia parviflora extracts and their flavone constituents on P-glycoprotein function. J Pharm Sci. 2007;96:223–233. doi: 10.1002/jps.20769. [DOI] [PubMed] [Google Scholar]
- 21.Jung M., Park M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules. 2007;12:2130–2139. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawasdee P., Sabphon C., Sitthiwongwanit D., Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother Res. 2009;23:1792–1794. doi: 10.1002/ptr.2858. [DOI] [PubMed] [Google Scholar]
- 23.Youn K., Lee J., Ho C.-T., Jun M. Discovery of polymethoxyflavones from black ginger (Kaempferia parviflora) as potential β-secretase (BACE1) inhibitors. J Funct Foods. 2016;20:567–574. [Google Scholar]
- 24.Wutythamawech W. OS Printing 365; Bangkok: 1997. Encyclopedia of Thai Herbs. [Google Scholar]
- 25.Wattanapitayakul S.K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Chansuvanich N. Vasorelaxation and antispasmodic effects of Kaempferia parviflora ethanolic extract in isolated rat organ studies. Fitoterapia. 2008;79:214–216. doi: 10.1016/j.fitote.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Spencer J.P. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wattanathorn J., Pangpookiew P., Sripanidkulchai K., Muchimapura S., Sripanidkuchai B. Evaluation of the anxiolytic and antidepressant effects of alcoholic extract of Kaempferia parviflora in aged rats. Am J Agric Biol Sci. 2007;2:94–98. [Google Scholar]
- 28.Iyer R.I., Jayaraman G., Ramesh A. In vitro responses and production of phytochemicals of potential medicinal value in nutmeg, Myristica fragrans Houtt. Indian J Sci Technol. 2009;2:65–70. [Google Scholar]
- 29.Sa-nguanmoo P., Poovorawan Y. Myristica fragrans Houtt. methanolic extract induces apoptosis in a human leukemia cell line through SIRT1 mRNA downregulation. J Med Assoc Thai. 2007;90:2422–2428. [PubMed] [Google Scholar]
- 30.Latha P., Sindhu P., Suja S., Geetha B., Pushpangadan P., Rajasekharan S. Pharmacology and chemistry of Myristica fragrans Houtt.-a review. J Spices Aromat Crop. 2012;14 [Google Scholar]
- 31.Olajide O.A., Ajayi F.F., Ekhelar A.I., Awe S.O., Makinde J.M., Alada A. Biological effects of Myristica fragrans (nutmeg) extract. Phytother Res. 1999;13:344–345. doi: 10.1002/(SICI)1099-1573(199906)13:4<344::AID-PTR436>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:1. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrester M.B. Nutmeg intoxication in Texas, 1998–2004. Hum Exp Toxicol. 2005;24:563–566. doi: 10.1191/0960327105ht567oa. [DOI] [PubMed] [Google Scholar]
- 34.Moinuddin G., Devi K., Khajuria D.K. Evaluation of the anti–depressant activity of Myristica fragrans (Nutmeg) in male rats. Avicenna J Phytomed. 2012;2:72. [PMC free article] [PubMed] [Google Scholar]
- 35.Pal M., Srivastava M., Soni D., Kumar A., Tewari S. Composition and anti-microbial activity of essential oil of Myristica fragrans from Andaman Nicobar Island. Int J Pharm Life Sci. 2011;2:1115–1117. [Google Scholar]
- 36.Zachariah T.J., Leela N., Maya K. Chemical composition of leaf oils of Myristica beddomeii (King), Myristica fragrans (Houtt.) and Myristica malabarica (Lamk.) J Spices Aromat Crop. 2011;17 [Google Scholar]
- 37.Ng J., Papandreou A., Heales S.J., Kurian M.A. Monoamine neurotransmitter disorders [mdash] clinical advances and future perspectives. Nat Rev Neurol. 2015;11:567–584. doi: 10.1038/nrneurol.2015.172. [DOI] [PubMed] [Google Scholar]
- 38.Lin S.-H., Chang H.-C., Chen P.-J., Hsieh C.-L., Su K.-P., Sheen L.-Y. The antidepressant-like effect of ethanol extract of daylily flowers (金針花 Jīn Zhēn Huā) in rats. J Tradit Complement Med. 2013;3:53–61. doi: 10.4103/2225-4110.106548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar R., Palit G., Dhawan B.N. Comparative behavioural effects of typical and atypical antipsychotic drugs in rhesus monkey. Eur J Pharmacol. 2003;462:133–138. doi: 10.1016/s0014-2999(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 40.Kamińska K., Gołembiowska K., Rogóż Z. Effect of risperidone on the fluoxetine-induced changes in extracellular dopamine, serotonin and noradrenaline in the rat frontal cortex. Pharmacol Rep. 2013;65:1144–1151. doi: 10.1016/s1734-1140(13)71472-5. [DOI] [PubMed] [Google Scholar]
- 41.Gordon G.R., Baimoukhametova D.V., Hewitt S.A., Rajapaksha W.K.J., Fisher T.E., Bains J.S. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 42.Robert A., Monsinjon T., Delbecque J.-P. Neuroendocrine disruption in the shore crab Carcinus maenas: effects of serotonin and fluoxetine on chh- and mih-gene expression, glycaemia and ecdysteroid levels. Aquat Toxicol. 2016 Jun;175:192–204. doi: 10.1016/j.aquatox.2016.03.025. Epub 2016 Mar 31. PubMed PMID: 27060239. [DOI] [PubMed] [Google Scholar]
- 43.Malagié I., David D.J., Jolliet P., Hen R., Bourin M., Gardier A.M. Improved efficacy of fluoxetine in increasing hippocampal 5-hydroxytryptamine outflow in 5-HT1B receptor knock-out mice. Eur J Pharmacol. 2002;443:99–104. doi: 10.1016/s0014-2999(02)01604-7. [DOI] [PubMed] [Google Scholar]
- 44.Brooks B.W., Foran C.M., Richards S.M. Aquatic ecotoxicology of fluoxetine. Toxicol Lett. 2003;142:169–183. doi: 10.1016/s0378-4274(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 45.Pekny M., Wilhelmsson U., Bogestål Y.R., Pekna M. International Review of Neurobiology. vol. 82. Academic Press; 2007. The role of astrocytes and complement system in neural plasticity; pp. 95–111. [DOI] [PubMed] [Google Scholar]
- 46.Middeldorp J., Hol E. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Hol E.M., Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Cobb J., O'Neill K., Milner J. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilding C., Bell K., Funke S., Beck S., Pfeiffer N., Grus F.H. GFAP antibodies show protective effect on oxidatively stressed neuroretinal cells via interaction with ERP57. J Pharmacol Sci. 2015;127:298–304. doi: 10.1016/j.jphs.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg R., Hoogenraad C.C. Synaptic Plasticity. Springer; 2012. Molecular motors in cargo trafficking and synapse assembly; pp. 173–196. [DOI] [PubMed] [Google Scholar]
- 51.Ciavardelli D., Silvestri E., Del Viscovo A. Alterations of brain and cerebellar proteomes linked to Aβ and tau pathology in a female triple-transgenic murine model of Alzheimer's disease. Cell Death Dis. 2010;1:e90. doi: 10.1038/cddis.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koide T., Aleksic B., Ito Y. A two-stage case–control association study of the dihydropyrimidinase-like 2 gene (DPYSL2) with schizophrenia in Japanese subjects. J Hum Genet. 2010;55:469–472. doi: 10.1038/jhg.2010.38. [DOI] [PubMed] [Google Scholar]
- 53.Arimura N., Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 54.Quach T., Honnorat J., Kolattukudy P., Khanna R., Duchemin A. CRMPs: critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol Psychiatry. 2015;20:1037–1045. doi: 10.1038/mp.2015.77. [DOI] [PubMed] [Google Scholar]
- 55.Niisato E., Nagai J., Yamashita N., Nakamura F., Goshima Y., Ohshima T. Phosphorylation of CRMP2 is involved in proper bifurcation of the apical dendrite of hippocampal CA1 pyramidal neurons. Dev Neurobiol. 2013;73:142–151. doi: 10.1002/dneu.22048. [DOI] [PubMed] [Google Scholar]
- 56.Erickson R.R., Dunning L.M., Olson D.A. In cerebrospinal fluid ER chaperones ERp57 and calreticulin bind β-amyloid. Biochem Biophys Res Commun. 2005;332:50–57. doi: 10.1016/j.bbrc.2005.04.090. [DOI] [PubMed] [Google Scholar]
- 57.He J., Shi W., Guo Y., Chai Z. ERp57 modulates mitochondrial calcium uptake through the MCU. FEBS Lett. 2014;588:2087–2094. doi: 10.1016/j.febslet.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 58.Narayan P.J., Lill C., Faull R., Curtis M.A., Dragunow M. Increased acetyl and total histone levels in post-mortem Alzheimer's disease brain. Neurobiol Dis. 2015;74:281–294. doi: 10.1016/j.nbd.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Akbar M., Essa M.M., Daradkeh G. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016;1637:34–55. doi: 10.1016/j.brainres.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subash S., Essa M.M., Al-Asmi A. Pomegranate from Oman alleviates the brain oxidative damage in transgenic mouse model of Alzheimer's disease. J Tradit Complement Med. 2014;4:232–238. doi: 10.4103/2225-4110.139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C.-L., Tsai W.-H., Chen C.-J., Pan T.-M. Centella asiatica extract protects against amyloid β1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J Tradit Complement Med. 2016;6:362–369. doi: 10.1016/j.jtcme.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.