Radiation‐induced lung toxicity remains a challenge in thoracic radiotherapy. This systematic review focuses on radiation dose‐volume histogram parameters for lung and heart as predictors for changes in FEV1 and diffusion capacity after radiotherapy for lung or esophageal cancer.
Keywords: Lung cancer, Esophageal cancer, Radiotherapy, Pulmonary function tests, Dose‐volume parameters
Abstract
Background.
Despite technical developments in treatment delivery, radiation‐induced lung toxicity (RILT) remains a crucial problem in thoracic radiotherapy. Clinically based RILT scores have their limitations, and more objective measures such as pulmonary functions tests (PFTs) might help to improve treatment strategies.
Purpose.
To summarize the available evidence about the effect of dose to the lung in thoracic radiotherapy on forced expiratory volume in one second (FEV1) and diffusion capacity (DLCO) in patients with lung and esophageal cancer treated with curative intent.
Material and methods.
A systematic review following the PRISMA guidelines was performed, using MEDLINE and including clinical studies using (chemo)radiotherapy (CRT) or stereotactic ablative radiotherapy (SABR) for lung or CRT for esophageal cancer that reported both lung dose‐volume histogram (DVH) parameters and changes in PFT results. Search terms included lung and esophageal neoplasms, respiratory function tests, and radiotherapy.
Results.
Fifteen studies met the inclusion criteria. Seven out of 13 studies on lung cancer reported significant declines (defined as a p value < .05) in PFT results. Both esophageal studies reported significant DLCO declines. One SABR study found a correlation between low lung‐dose parameters and FEV1 decline. Relations between decline of FEV1 (three studies) or decline of DLCO (five studies), respectively, and DVH parameters were found in eight studies analyzing CRT. Furthermore, a heterogeneous range of clinical risk factors for pulmonary function changes were reported in the selected studies.
Conclusions.
There is evidence that pulmonary function declines after RT in a dose‐dependent manner, but solid data about lung DVH parameters predicting changes in PFT results are scarce. A major disadvantage was the wide variety of methods used, frequently lacking multivariable analyses. Studies using prospective high‐quality data, analyzed with appropriate statistical methods, are needed. The Oncologist 2017;22:1257–1264
Implications for Practice.
Radiation‐induced lung toxicity remains crucial in thoracic radiotherapy. To prevent this toxicity in the future and individualize patient treatment, objective measures of pulmonary toxicity are needed. Pulmonary function tests may provide such objective measures. This systematic review, included all available clinical studies using external beam radiotherapy for lung or esophageal cancer reporting pulmonary function combined with dose‐volume histogram parameters. There is preliminary evidence that pulmonary function declines post radiotherapy in a dose‐dependent manner. Data quality and analyses were generally limited. Analyses of high‐quality data are therefore urgently needed to improve individualization of advanced radiation therapy.
Introduction
Lung and esophageal cancer are among the most common cancers with high mortality rates [1]. For both tumors, radiotherapy is an important treatment modality, having witnessed major developments improving patient outcomes. The introduction of stereotactic ablative radiotherapy (SABR) has become a standard option in early‐stage lung cancer, especially for elderly patients and patients with comorbidity [2], [3], [4], while locally advanced stages are preferably treated with chemoradiotherapy (CRT) [5]. For esophageal cancer, neoadjuvant CRT followed by resection is now the treatment of choice [6]. However, despite improved treatment strategies such as the introduction of intensity‐modulated radiotherapy (IMRT) [7], [8], [9], [10], [11], survival remains poor, and there is a serious risk of pulmonary and cardiac toxicity.
Radiation‐induced lung toxicity (RILT), including but not limited to classic radiation pneumonitis, is typically scored using the Radiation Therapy Oncology Group (RTOG) scoring system or the Common Terminology Criteria for Adverse Events (CTCAE) [12]. Both are clinically based and have their limitations: symptoms and radiographic changes do not necessarily progress in parallel as suggested in the RTOG scoring system, differentiation between pneumonitis and local tumor progression may be challenging, and physicians inclined to prescribe steroids more often may, by definition, score higher grade pneumonitis more frequently [12], [13].
Pulmonary function tests (PFTs), including forced expiratory volume in one second (FEV1) and hemoglobin‐level‐adjusted carbon monoxide diffusing capacity (DLCO), measure patients’ respiratory functional state and are well established in assessing functionally relevant lung damage in numerous diseases, including chronic obstructive pulmonary disease (COPD) and lung fibrosis [14], [15], [16]. Lung function has been postulated to change due to radiation injury; however, results are equivocal [17], [18], [19]. Mehta et al. published an overview of changes of FEV1 and DLCO after radiotherapy and concluded that FEV1 reduction was 12% or less after radiotherapy and DLCO reduction was usually 13% or more from baseline [20]. Furthermore, changes in PFT results may be used as surrogate parameters to assess RILT, especially if changes can be predicted based on radiation dose‐volume histogram (DVH) parameters, as these DVH parameters are adjustable and could guide radiation treatment planning. Such guidance might include choosing between photon‐ and proton‐based therapy, as IMRT is able to considerably redistribute the dose between lungs and heart, whereas proton therapy reduces both pulmonary and cardiac doses [21].
This systematic review focuses on radiation DVH parameters for lung (and heart) as predictors for changes in FEV1 and DLCO after radiotherapy for lung or esophageal cancer. Both stereotactic and conventionally fractionated treatment schedules were eligible in order to include a wide range of dose distributions.
Materials and Methods
A literature search was performed in MEDLINE according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [22] using the following keywords: (synonyms for lung cancer and esophageal cancer) AND (synonyms for radiotherapy) AND (synonyms for respiratory function tests). The complete search strategy was as follows: ([“Lung Neoplasms” {Mesh} OR “Lung neoplasma” OR “lung cancer” OR “Esophageal Neoplasms” {Mesh} OR “esophageal cancer”] AND [“Respiratory Function Tests” {Mesh} OR “pulmonary function” OR “Pulmonary Diffusing Capacity” {Mesh} OR “Pulmonary Diffusing Capacity”] AND [“Radiotherapy” {Mesh} OR “radiotherapy” OR “irradiation” OR “radiation”]). The search was completed on July 22, 2015. Studies in languages other than English, Dutch, or German, studies published before 1990, and studies only available in abstract form were excluded from this review.
Studies had to fulfill the following eligibility criteria: (a) clinical studies, (b) including patients with lung or esophageal cancer, (c) primary curative treatment with external beam radiotherapy (no brachytherapy or surgery alone), (d) reporting PFTs (at least FEV1 and DLCO) at baseline and during follow‐up and, especially, (e) presenting data on lung and—optional—heart DVH parameters.
The first selection, based on title and abstract, was performed independently by two researchers (AN and RdJ) and any disagreement was resolved by discussion after reading the full text. In addition, study references were screened by hand in order to retrieve additional relevant studies and a definitive list was assembled by agreement (available from the corresponding author upon request). Overviews of methodology, basic patient and treatment characteristics, baseline and percentage changes of PFTs results with p values (statistical significance was defined as a p value of <.05), lung and heart DVH parameters, and other possible risk factors influencing post‐radiotherapy pulmonary function were summarized and tabulated by one author (A.N.) and checked and discussed with two other reviewers (R.D.J. and J.W.).
Results
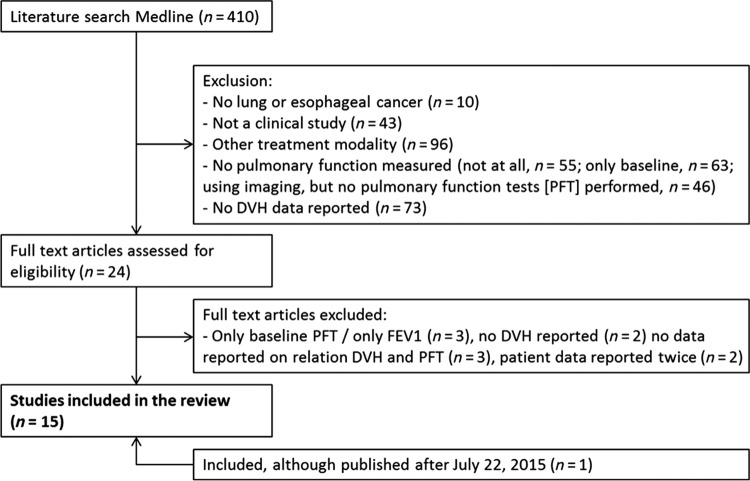
The search retrieved 410 studies. After the selection process described above, 15 studies were included in this review. Figure 1 shows the flow chart of the literature search. Initially, 24 studies were selected based on the eligibility criteria. However, after full‐text review, 10 studies were excluded because they (a) only presented data on baseline PFT results or only on FEV1 (n = 3), (b) reported no DVH parameters (n = 2) and no data on the relationship between DVH parameters and changes of PFT results (n = 3), or (c) reported data on identical cohorts (n = 2). Finally, one study was added, although published after July 22, 2015 [23], bringing the total number of studies to 15.
Figure 1.
Flow chart of the literature search.
Abbreviations: DLCO, carbon monoxide diffusing capacity; DVH, dose‐volume histogram; FEV1, forced expiratory volume in 1 second; n, number of studies; PFTs, pulmonary function tests
Patient and Treatment Characteristics
Of the 15 selected studies, 13 included patients with lung cancer (six using SABR, seven using CRT) and two with esophageal cancer. The study cohorts ranged from 15 to 250 patients (median n = 70, mean n = 89). In the six studies on SABR, the radiotherapy dose ranged from 45–64 Gy administered in three to 15 fractions. Two out of six studies on SABR included patients both with early‐stage lung cancer and lung metastases [23], [24], [25], [26], [27], [28]. Seven studies combined radiotherapy (three‐dimensional conformal radiation therapy [3DCRT], IMRT, or proton therapy) with induction, concomitant, and/or sequential chemotherapy. The dose ranged from 54–103 Gy (one study included patients from a dose escalation study), given in 28 to 39 fractions [29], [30], [31], [32], [33], [34], [35]. Two of the studies compared two fractions a day with one fraction a day. The two studies reporting on esophageal cancer included treatment with CRT using 3DCRT, followed by surgery in most patients. In one study, a dose of 45 Gy (and in selected patients an additional boost of 5.4 Gy) was administered in 28 fractions, the other reported on a 30 Gy (20 × 1.5 Gy twice daily) or 45 Gy (30 × 1.5 Gy twice daily, split course) CRT regimen [36], [37].
PFT Timing
All studies reported on FEV1 and DLCO at varying time points during follow‐up (Table 1), at least at baseline and at one time point after radiotherapy. For the lung cancer studies, the follow‐up time points ranged from 2 weeks to 2 years. The two studies on esophageal cancer evaluated PFTs at one time point shortly after RT (2 and 4 weeks after RT, respectively).
Table 1. Changes in pulmonary function tests.
Abbreviations: CI, confidence interval; DLCO, diffusion capacity; FEV1, forced expiratory volume in one second; FU, follow up time for pulmonary function test; NR, not reported; NS, no significant difference
Pulmonary Function Changes
Baseline FEV1 and DLCO as well as changes in PFT results including p values after radiotherapy are presented in Table 1. For all studies that included lung cancer patients, both FEV1 and DLCO declined after radiotherapy. However, the decline in DLCO was more pronounced than the decline in FEV1. Of note, the study by Ohashi et al. was the only one to observe an increase in DLCO 12 months after radiotherapy [28]. If reported, there was a progressive decline in FEV1 and DLCO over time in most studies. For the lung SABR studies, four out of six reported a slight but statistically significant decline of FEV1 or DLCO at different time points after RT. Three out of seven studies investigating pulmonary function after CRT described significant changes of PFT results. In two of those, the decline was limited to patients with pneumonitis [29], [33], while one study reported an overall linear decrease of FEV1 and DLCO [30]. In the studies including patients with esophageal cancer, the FEV1 did not change significantly, while DLCO decreased after CRT.
For all studies that included lung cancer patients, both FEV1 and DLCO declined after radiotherapy. However, the decline in DLCO was more pronounced than the decline in FEV1.
Risk Factors for Pulmonary Function Changes
A diversity of parameters that might influence changes in pulmonary function testing after treatment were investigated in the studies. The results are tabulated in Table 2. In general, the investigated parameters can be categorized into patient and tumor characteristics, treatment parameters including, but not limited to, DVH parameters, and clinical pulmonary toxicity. For the treatment of lung cancer with SABR, DLCO improved in smokers based on both univariable and multivariable analysis in one study [28]. Risk factors for FEV1 decline were conformality index of the treatment plan, the lung‐V5, and V10 (percentage of lung‐volume receiving more than 5 or 10 Gy, respectively), all based on univariable analysis and in one study only [27]. After CRT, univariable analyses showed a considerably heterogeneous spectrum of parameters influencing PFT results (Table 2). Most studies did not present data based on multivariable analyses. In the two studies that did, the following risk factors for decrease in DLCO and FEV1 were found: Gopal et al. identified age >60 years, nodal status (N2–3 vs. N0–1), and concurrent chemotherapy increasing the risk for DLCO decline [33]. Lopez Guerra et al. found baseline DLCO, gross tumor volume (GTV), lung DVH parameters (mean lung dose [MLD], V20), heart DVH parameters (mean heart dose, heart‐V40) and total prescription dose as predictors of DLCO decline at different time points after radiation (Table 2) [31]. In the esophageal cancer cohorts, using univariable analyses, the lung‐V7–V10 as well as the total prescription dose were detected as risk factors for DLCO decrease shortly after radiotherapy [36], [37].
Table 2. Risk factors influencing pulmonary function tests.
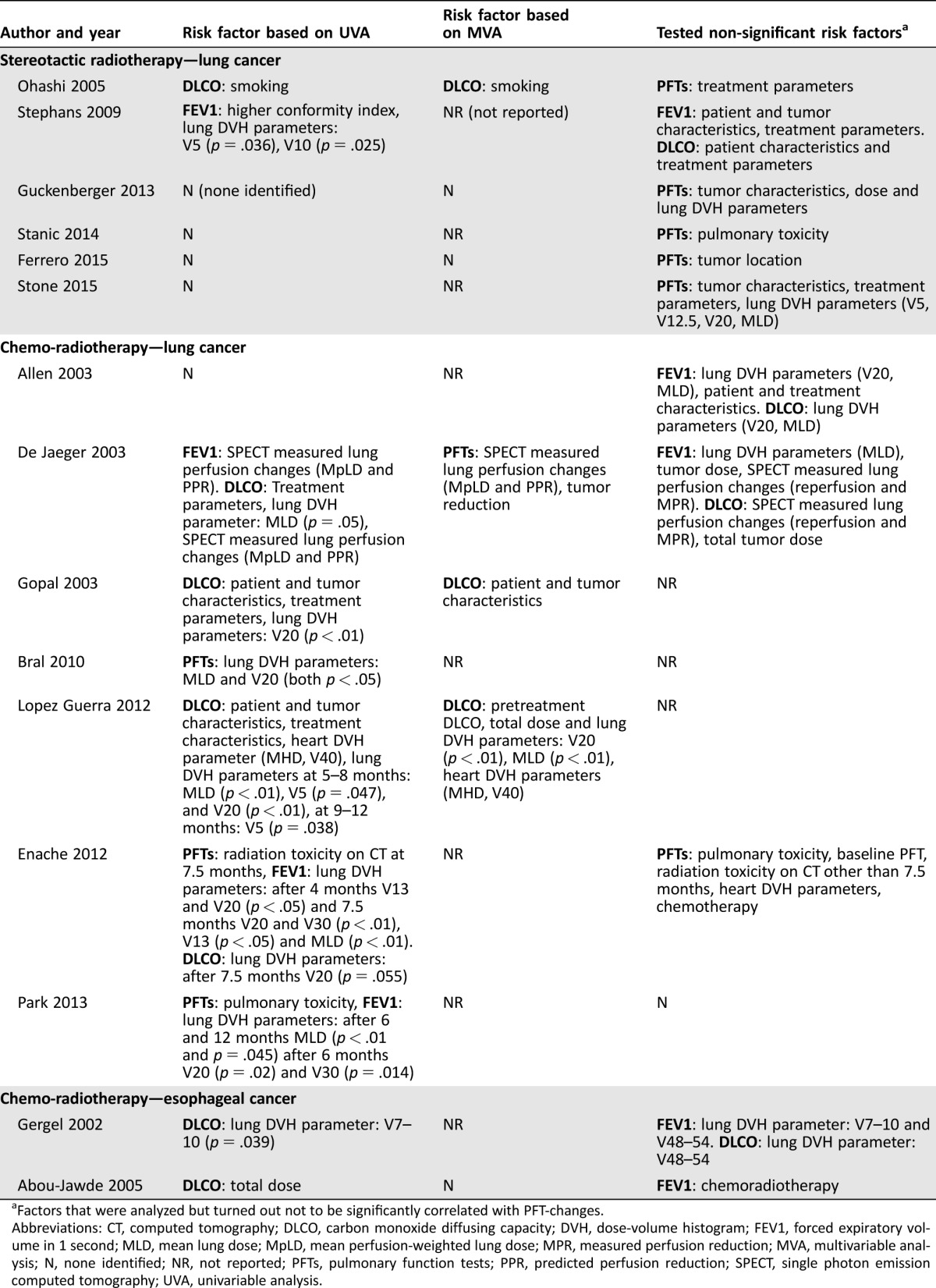
Factors that were analyzed but turned out not to be significantly correlated with PFT‐changes.
Abbreviations: CT, computed tomography; DLCO, carbon monoxide diffusing capacity; DVH, dose‐volume histogram; FEV1, forced expiratory volume in 1 second; MLD, mean lung dose; MpLD, mean perfusion‐weighted lung dose; MPR, measured perfusion reduction; MVA, multivariable analysis; N, none identified; NR, not reported; PFTs, pulmonary function tests; PPR, predicted perfusion reduction; SPECT, single photon emission computed tomography; UVA, univariable analysis.
Lung DVH Parameters Predicting PFT Changes
Most studies used the following lung DVH parameters for their analyses: V10, V20, V30, and MLD. After SABR for lung cancer, four out of five studies that evaluated the influence of DVH parameters on pulmonary function did not find any correlations. However, Stephans et al. found lung‐V5/V10 to predict FEV1 decline (p = .036 and p = .025, respectively) [27]. For lung cancer patients treated with CRT, three out of seven studies found DVH parameters as predictors of FEV1 decline after treatment, and five identified DVH parameters predicting decline in DLCO. The MLD or lung‐V20 were most frequently associated with declines in PFT results (Table 2). The study by Gergel et al. comprised the only cohort of esophageal cancer patients in which the relationship between DVH parameters and changes in DLCO were evaluated. They reported lung‐V7–10 as predictors of DLCO decline (p = .039) [37].
Discussion
Almost 20 years ago the first paper was published establishing lung DVH parameters as predictors of lung toxicity (radiation pneumonitis) in patients irradiated for non‐small cell lung cancer [38]. Moreover, there is strong evidence that radiation to lungs (and heart) causes lung function impairment in a dose‐volume‐dependent manner in an animal model [39], [40]. However, clinical models quantitatively predicting lung function impairment after radiotherapy are still not available.
In the era of personalized medicine, more insight into the relationship between radiation dose‐volume parameters and ensuing toxicity and function loss is needed. One of the challenges is to define and objectively measure lung toxicity. Several clinical and subclinical endpoints or combinations can be used: (a) overt pneumonitis requiring medication or ventilatory support (that carries even a risk of mortality), (b) bronchial stricture or shortness of breath as clinical endpoints, and (c) clinical surrogates such as radiologic abnormalities, changes in PFT results, six‐minute walk tests, blood gases, or exercise capacity [12]. PFT changes might be particularly promising surrogate endpoints, as they are more objectively assessable than clinical endpoints (RTOG scoring system or CTCAE), noninvasive, and still ubiquitously available. Disadvantages of using PFTs may be some remaining operator dependence [41], as well as treatment‐induced tumor shrinkage potentially resulting in improved overall lung function, which might eventually confound the interpretation of lung function measurements [42]. Radiation‐induced improvement of high‐grade COPD in nonfunctional pulmonary volume suggests dependence of RILT on baseline lung function and may further complicate interpretation of post‐treatment PFT results [43]. Changes in PFT results should be related to changes in clinical symptoms to be useful in clinical practice. Symptomatic relevance of PFT declines after radiotherapy has not been reported on, but some data are available about minimal clinically important differences (MCID) in patients with asthma or COPD. The MCID in FEV1 for patients with asthma and COPD is a 10% and 4% change, respectively [14]. Because pulmonary comorbidity was not reported in all the studies, it is not possible to draw a definitive conclusion here, but, as shown in Table 1, in most studies, FEV1 declines were more than 4%. To our knowledge, there are no data available on MCID for DLCO decline. As shown in Table 1, the decline in DLCO was more pronounced than the decline in FEV1, which is in line with the overview of Mehta [20]. Given that low radiation doses to large lung volumes lead to pulmonary microvascular damage in rats [40], it is tempting to speculate that more pronounced diffusion capacity loss compared with less pronounced increase in obstructive lung disease (i.e., FEV1 decline) might be due to pulmonary microvascular damage.
Given that low radiation doses to large lung volumes lead to pulmonary microvascular damage in rats, it is tempting to speculate that more pronounced diffusion capacity loss compared with less pronounced increase in obstructive lung disease (i.e., FEV1 decline) might be due to pulmonary microvascular damage.
The present systematic review shows that data about the relationship between lung DVH parameters and pulmonary function as well as additional risk factors for decrease in PFT results post radiotherapy are scarce and heterogeneous. Although gradually declining FEV1 and DLCO post‐SABR was reported in half of the studies, only one of the SABR studies found a correlation between lung DVH parameters (V5 and V10) and decline in FEV1 and DLCO [27], which might suggest that underlying pulmonary pathology rather than radiation may be the dominant driver of such decline. In the non‐stereotactic studies, a relation between DVH and pulmonary function was found in six out of seven studies, but there was little consistency on the relevant parameters, and four out of the seven studies did not perform a multivariable analysis (Table 2), which limits the interpretation of the findings. The most frequently used parameters in clinical practice—MLD and V20—were most often reported as predictors of decreasing pulmonary function.
Both univariable and multivariable analyses showed high heterogeneity in the evaluated risk factors, which resulted in a broad range of risk factors for pulmonary function changes. Most of these factors cannot be influenced by adapting therapy. Only one of the studies described lung DVH parameters as risk factors for decreasing lung function based on multivariable analysis [31]. Although an influence of heart dose on lung toxicity has been strongly suggested [7], [10], [44], only three studies reported on heart DVH parameters, two of which included them in the analysis [30], [31]. None of the studies reported on patient outcome measures such as dyspnea, self‐reported physical condition, or exercise capacity in addition to PFT results.
The results of this review should be interpreted with caution due to several limitations in the included studies. Some studies included small numbers of patients (five based their analyses on fewer than 50 patients; only four included more than 100 patients) and a broad range of follow‐up time points were used to measure pulmonary function (Table 1). Besides that the statistical methods employed differ among studies, for some it was not entirely clear how the analyses were performed, and a substantial part performed no multivariable analyses [23], [24], [25], [27], [29], [30], [32], [35], [37].
The prediction of pulmonary function decline using multivariable models could be a useful tool to tailor therapy. Rationally guiding radiation treatment planning, including the selection of patients for intensity‐modulated photon‐based or proton‐based treatment using models, might help to improve the overall outcome of radiotherapy for intrathoracic cancers. Such models should include clinical, treatment‐related, and DVH parameters (Table 2). However, no such models predicting pulmonary function decline have been published so far.
Conclusion
There is evidence that pulmonary function declines after RT, especially after (chemo)radiation using 3DCRT, IMRT, or volumetric modulated arc therapy (VMAT), and to a lesser degree after SABR. Declines of FEV1 seem to be considerably less pronounced than declines of DLCO. Lung DVH parameters do not seem to predict post‐treatment pulmonary function changes for patients irradiated with SABR, but in non‐stereotactic treatment, decreasing lung function depends on lung DVH parameters, especially on MLD and V20. Only one study found lung DVH parameters as risk factors using multivariable analysis, but, overall, there is a severe lack of appropriate multifactorial models predicting post‐radiotherapy lung function based on clinical factors combined with DVH parameters.
Data based on larger cohorts and employing suitable methods of multivariable analysis are urgently needed given rapid technological progress in RT, where cardio‐pulmonary dose can be redistributed using intensity‐modulated photon therapy and can be reduced using proton‐based therapy.
Footnotes
For Further Reading: Gary R. Epler, Eileen M. Kelly. Systematic Review of Postradiotherapy Bronchiolitis Obliterans Organizing Pneumonia in Women With Breast Cancer. The Oncologist 2014;19:1216–1226; first published on October 31, 2014.
Implications for Practice: Bronchiolitis obliterans organizing pneumonia (BOOP) is a rare but potentially serious complication of radiation therapy for breast cancer. Pulmonary symptoms occur within several weeks to a year or more after completion of radiation. Initial signs and symptoms suggest pneumonia, but symptoms and lung involvement progress despite antibiotic therapy. The pulmonary infiltrates may be in the opposite lung from the radiation field. Patients without symptoms can be monitored, and those with moderate to severe BOOP usually require corticosteroid therapy. Clinical oncologists and radiation oncologists managing these patients need to consider obtaining diagnostic chest radiographic studies for women who report new respiratory symptoms during the late radiation or postradiation period.
Author Contributions
Conception/design: Anne G. H. Niezink, Renske A. de Jong, Christina T. Muijs, Johannes A. Langendijk, Joachim Widder
Provision of study material or patients: Anne G. H. Niezink, Renske A. de Jong
Collection and/or assembly of data: Anne G. H. Niezink, Renske A. de Jong
Data analysis and interpretation: Anne G. H. Niezink, Renske A. de Jong, Christina T. Muijs, Johannes A. Langendijk, Joachim Widder
Manuscript writing: Anne G. H. Niezink, Renske A. de Jong, Christina T. Muijs, Johannes A. Langendijk, Joachim Widder
Final approval of manuscript: Anne G. H. Niezink, Renske A. de Jong, Christina T. Muijs, Johannes A. Langendijk, Joachim Widder
Disclosures
Johannes A. Langendijk: Ion Beam Applications (H), Elekta, Philips, RaySearch, Miranda (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. De Ruysscher D, Faivre‐Finn C, Nestle U et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high‐dose, high‐precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301–5310. [DOI] [PubMed] [Google Scholar]
- 3. Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Berg LL, Klinkenberg TJ, Groen HJ et al. Patterns of recurrence and survival after surgery or stereotactic radiotherapy for early stage NSCLC. J Thorac Oncol 2015;10:826–831. [DOI] [PubMed] [Google Scholar]
- 5. Auperin A, Le Pechoux C, Rolland E et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol 2010;28:2181–2190. [DOI] [PubMed] [Google Scholar]
- 6. van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 7. Beukema JC, van Luijk P, Widder J et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85–90. [DOI] [PubMed] [Google Scholar]
- 8. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 9. Palma DA, Senan S, Tsujino K et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta‐analysis. Int J Radiat Oncol Biol Phys 2013;85:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley JD, Paulus R, Komaki R et al. Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): A randomised, two‐by‐two factorial phase 3 study. Lancet Oncol 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Movsas B, Hu C, Sloan J et al. Quality of life analysis of a radiation dose‐escalation study of patients with non‐small‐cell lung cancer: A secondary analysis of the radiation therapy oncology group 0617 randomized clinical trial. JAMA Oncol 2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marks LB, Bentzen SM, Deasy JO et al. Radiation dose‐volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76(suppl 3):S70–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faria SL, Aslani M, Tafazoli FS et al. The challenge of scoring radiation‐induced lung toxicity. Clin Oncol (R Coll Radiol) 2009;21:371–375. [DOI] [PubMed] [Google Scholar]
- 14. Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2:111–124. [DOI] [PubMed] [Google Scholar]
- 15. Li X, Peng S, Wei L et al. Relevance analysis of clinical and lung function parameters changing and prognosis of idiopathic pulmonary fibrosis. Int J Clin Exp Med 2014;7:4759–4769. [PMC free article] [PubMed] [Google Scholar]
- 16. Weinreich UM, Thomsen LP, Brock C et al. Diffusion capacity of the lung for carbon monoxide ‐ A potential marker of impaired gas exchange or of systemic deconditioning in chronic obstructive lung disease? Chron Respir Dis 2015;12:357–364. [DOI] [PubMed] [Google Scholar]
- 17. Groen HJ, van der Mark TW, van der Leest AH et al. Pulmonary function changes in lung‐cancer patients treated with radiation with or without carboplatin. Am J Respir Crit Care Med 1995;152:2044–2048. [DOI] [PubMed] [Google Scholar]
- 18. Marks LB, Fan M, Clough R et al. Radiation‐induced pulmonary injury: Symptomatic versus subclinical endpoints. Int J Radiat Biol 2000;76:469–475. [DOI] [PubMed] [Google Scholar]
- 19. Farr KP, Moller DS, Khalil AA et al. Loss of lung function after chemo‐radiotherapy for NSCLC measured by perfusion SPECT/CT: Correlation with radiation dose and clinical morbidity. Acta Oncol 2015;54:1350–1354. [DOI] [PubMed] [Google Scholar]
- 20. Mehta V. Radiation pneumonitis and pulmonary fibrosis in non‐small‐cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5–24. [DOI] [PubMed] [Google Scholar]
- 21. Stuschke M, Kaiser A, Pottgen C et al. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother Oncol 2012;104:45–51. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. Open Med 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 23. Stone B, Mangona VS, Johnson MD et al. Changes in pulmonary function following image‐guided stereotactic lung radiotherapy: Neither lower baseline nor post‐SBRT pulmonary function are associated with worse overall survival. J Thorac Oncol 2015;10:1762–1769. [DOI] [PubMed] [Google Scholar]
- 24. Ferrero C, Badellino S, Filippi AR et al. Pulmonary function and quality of life after VMAT‐based stereotactic ablative radiotherapy for early stage inoperable NSCLC: A prospective study. Lung Cancer 2015;89:350–356. [DOI] [PubMed] [Google Scholar]
- 25. Stanic S, Paulus R, Timmerman RD et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early‐stage peripheral non‐small cell lung cancer: An analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guckenberger M, Klement RJ, Kestin LL et al. Lack of a dose‐effect relationship for pulmonary function changes after stereotactic body radiation therapy for early‐stage non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1074–1081. [DOI] [PubMed] [Google Scholar]
- 27. Stephans KL, Djemil T, Reddy CA et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838–844. [DOI] [PubMed] [Google Scholar]
- 28. Ohashi T, Takeda A, Shigematsu N et al. Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. Int J Radiat Oncol Biol Phys 2005;62:1003–1008. [DOI] [PubMed] [Google Scholar]
- 29. Park YH, Kim JS. Predictors of radiation pneumonitis and pulmonary function changes after concurrent chemoradiotherapy of non‐small cell lung cancer. Radiat Oncol J 2013;31:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enache I, Noel G, Jeung MY et al. Impact of 3D conformal radiotherapy on lung function of patients with lung cancer: A prospective study. Respiration 2013;86:100–108. [DOI] [PubMed] [Google Scholar]
- 31. Lopez Guerra JL, Gomez DR, Zhuang Y et al. Changes in pulmonary function after three‐dimensional conformal radiotherapy, intensity‐modulated radiotherapy, or proton beam therapy for non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e537–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bral S, Duchateau M, Versmessen H et al. Toxicity and outcome results of a class solution with moderately hypofractionated radiotherapy in inoperable Stage III non‐small cell lung cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys 2010;77:1352–1359. [DOI] [PubMed] [Google Scholar]
- 33. Gopal R, Starkschall G, Tucker SL et al. Effects of radiotherapy and chemotherapy on lung function in patients with non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:114–120. [DOI] [PubMed] [Google Scholar]
- 34. De Jaeger K, Seppenwoolde Y, Boersma LJ et al. Pulmonary function following high‐dose radiotherapy of non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2003;55:1331–1340. [DOI] [PubMed] [Google Scholar]
- 35. Allen AM, Henning GT, Ten Haken RK et al. Do dose‐volume metrics predict pulmonary function changes in lung irradiation? Int J Radiat Oncol Biol Phys 2003;55:921–929. [DOI] [PubMed] [Google Scholar]
- 36. Abou‐Jawde RM, Mekhail T, Adelstein DJ et al. Impact of induction concurrent chemoradiotherapy on pulmonary function and postoperative acute respiratory complications in esophageal cancer. Chest 2005;128:250–255. [DOI] [PubMed] [Google Scholar]
- 37. Gergel TJ, Leichman L, Nava HR et al. Effect of concurrent radiation therapy and chemotherapy on pulmonary function in patients with esophageal cancer: Dose‐volume histogram analysis. Cancer J 2002;8:451–460. [DOI] [PubMed] [Google Scholar]
- 38. Graham MV, Purdy JA, Emami B et al. Clinical dose‐volume histogram analysis for pneumonitis after 3D treatment for non‐small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–329. [DOI] [PubMed] [Google Scholar]
- 39. van der Veen SJ, Faber H, Ghobadi G et al. Decreasing irradiated rat lung volume changes dose‐limiting toxicity from early to late effects. Int J Radiat Oncol Biol Phys 2016;94:163–171. [DOI] [PubMed] [Google Scholar]
- 40. Ghobadi G, Bartelds B, van der Veen SJ et al. Lung irradiation induces pulmonary vascular remodelling resembling pulmonary arterial hypertension. Thorax 2012;67:334–341. [DOI] [PubMed] [Google Scholar]
- 41. Mahler DA, Weinberg DH, Wells CK et al. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85:751–758. [DOI] [PubMed] [Google Scholar]
- 42. Ghobadi G, Wiegman EM, Langendijk JA et al. A new CT‐based method to quantify radiation‐induced lung damage in patients. Radiother Oncol 2015;117:4–8. [DOI] [PubMed] [Google Scholar]
- 43. Kimura T, Matsuura K, Murakami Y et al. CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: Are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 2006;66:483–491. [DOI] [PubMed] [Google Scholar]
- 44. Ghobadi G, van der Veen S, Bartelds B et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 2012;84:e639–e646. [DOI] [PubMed] [Google Scholar]