An increasing number of cancer patients appears to seek a second opinion about diagnosis or treatment. This systematic review examines the available empirical evidence on patient‐initiated second opinions in oncology and provides recommendations to clinicians for optimal communication about second opinions.
Keywords: Cancer, Review, Second opinion, Referral and consultation, Quality of care, Physician‐patient relations
Abstract
Background.
Although patient‐driven second opinions are increasingly sought in oncology, the desirability of this trend remains unknown. Therefore, this systematic review assesses evidence on the motivation for and frequency of requests for second opinions and examines how they evolve and their consequences for oncological practice.
Materials and Methods.
Relevant databases were sought using the terms “cancer,” “second opinion,” and “self‐initiated.” Included were peer‐reviewed articles that reported on patient‐initiated second opinions within oncology. Selection, data extraction, and quality assessment were performed and discussed by two researchers.
Results.
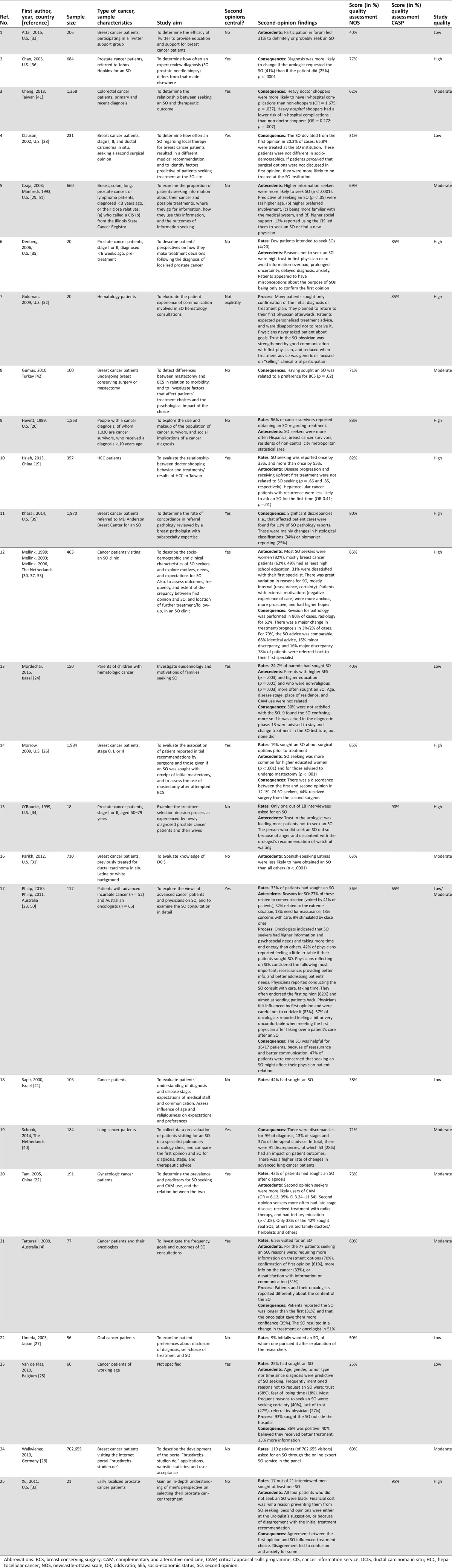
Of the 25 included studies, the methodological designs were qualitative (n = 4), mixed (n = 1), or quantitative (n = 20). Study quality was rated high for 10 studies, moderate for eight, and low for seven studies. Reported rates of second opinion seeking ranged from 1%–88%. Higher education was most consistently related to seeking a second opinion. Patients’ primary motivations were a perceived need for certainty or confirmation, a lack of trust, dissatisfaction with communication, and/or a need for more (personalized) information. Reported rates of diagnostic or therapeutic discrepancies between the first and second opinions ranged from 2%–51%.
Discussion.
Additional studies are required to further examine the medical, practical, and psychological consequences of second opinions for patients and oncologists. Future studies could compare the potential advantages and disadvantages of second opinion seeking, and might offer guidance to patients and physicians to better facilitate the second opinion process. Some practical recommendations are provided for oncologists to optimally discuss and conduct second opinions with their patients. The Oncologist 2017;22:1197–1211
Implications for Practice.
Although cancer patients increasingly seek a second opinion, the benefits of this process remain unclear. Results of this systematic review suggest that the available studies on this topic are highly variable in both methodology and quality. Moreover, reported rates for a second opinion (1%–88%) as well as for disagreement between the first and second opinion (2%–51%) range widely. The primary motivations of patients are a need for certainty, lack of trust, dissatisfaction with communication, and/or a need for more (personalized) information. Additional research should evaluate how unnecessary second opinions might be avoided. Practical suggestions are provided for oncologists to optimize second opinions.
Introduction
Because cancer has a considerable distressing impact on a patient's life, these patients need to feel especially confident about the care received from their medical professional(s). For a variety of reasons, a patient may feel the need to seek the opinion of an oncology professional other than their own, in the form of a second opinion. The term “second opinion” has been defined in various ways [1], [2], [3], [4]. However, based on previous work, for the present study we propose the following definition [3], [4]: a second opinion is when a patient, or a physician or a “payer” (i.e., a health insurer, or a hospital) solicits the assessment of a diagnosis or treatment proposal by a second, independent physician within the same specialty as the physician who gave the first opinion. The second opinion (hereafter referred to as SO) is sought with the intention of returning to the first physician; otherwise, it is called a “tertiary referral” [3]. Based on this definition, SOs are not always patient‐initiated. For example, payers (such as health insurers) may mandate SO programs to improve efficiency and reduce medical costs [5]. Physicians may refer a patient to another colleague to gain advice or to introduce standard SO pathology or radiology programs [1], [6]. In contrast, patient‐initiated SOs are not part of standard care and are based solely on the patient's initiative.
Patient‐initiated SOs have become an increasingly regular phenomenon within health care [7] and, not surprisingly, the field of oncology has particularly high rates of SOs. For patients with cancer, the diagnosis, prognosis, and treatment plans are frequently (and literally) a matter of life and death. Moreover, because medical information in this field is complex and characterized by uncertainty [8], this may increase a patient's need for an SO. Nevertheless, the frequency of actually requesting an SO in oncology remains unclear [3], [9].
The desirability of the increasing rate of patient‐initiated SOs is currently being debated. SOs may entail potential advantages and/or disadvantages for patients, physicians, and society in general. Seeking an SO may benefit patients medically, resulting in improved diagnosis or better treatment, or may benefit them psychologically by enabling them to act more autonomously and exercise some control and freedom of choice [10]. Second opinions may also offer reassurance and more certainty for both patients and their physicians [1]. However, these possible benefits are counteracted by the idea that the vast majority of SOs do not yield medical benefits for patients and may critically delay their treatment. Moreover, SOs may be physically and psychologically demanding for patients, resulting in disappointment and/or increased uncertainty, or may harm the relationship with their initial physician [1], [10], [11]. For physicians, SOs may increase the workload and might be perceived as signaling a patient's lack of trust [10]. On a societal level, SOs may be costly if they involve additional consultations and diagnostic testing [10], [12], [13]. In contrast, others have argued that SOs may save costs by preventing unnecessary treatment [1].
However, whether and to what extent these proposed advantages and drawbacks of SOs in oncology actually occur is currently unknown; therefore, it is not possible to compare the possible advantages/disadvantages of seeking an SO. Moreover, data are lacking on the frequency of occurrence of SOs and the motivation of the patients requesting them. Also, there is a lack of consensus among the few available reports. For example, a systematic review assessing whether SOs result in better health care for cancer patients in Western countries led to the conclusion that the evidence is unsubstantial [14]. Thus, more insight is required into the rates (frequency of occurrence), antecedents (predictive factors), processes (content and characteristics of the consultation), and consequences (medical, practical, and psychological outcomes) of SOs to inform future research and, eventually, clinical practice and policy.
A systematic overview of the empirical literature would enable researchers to systematically address the topic of SOs and assess their desirability. On a societal level, this would enhance discussion on whether SO seeking should be further stimulated, and/or whether alternatives should be developed. Within health care, strategies could be developed to enable optimal use of SOs: for example, avoiding unnecessary or enhancing necessary utilization. Moreover, interventions targeted at patients and physicians could aim to improve the quality of SO consultations.
Therefore, to acquire a comprehensive overview of the literature, this systematic review examines the available empirical evidence on patient‐initiated SOs in oncology.
Materials and Methods
Search Strategy
A search was made in the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Medline, and PsycINFO (as of September 1, 2015) for empirical studies presenting data on (suspected) cancer patients or their physicians in relation to patient‐driven SOs. A scoping search (including checking of references and cited articles) in PubMed and Google Scholar identified key references (“golden bullets”) that had to be retrieved in the final systematic search strategy.
The following search concepts were combined into a systematic search strategy: (([second opinion AND self‐initiated]) and ([cancer] OR [meta analysis] OR [review as publication type])) OR ([SO or hospital or doctor] adjacent [request or search]).
The search was formulated in Medline (see supplemental online Appendix A for the full electronic search strategy) and then translated to the other databases. Table 1 provides an overview of all relevant search terms, including adjacency operators if applicable.
Table 1. Key search terms and variations.
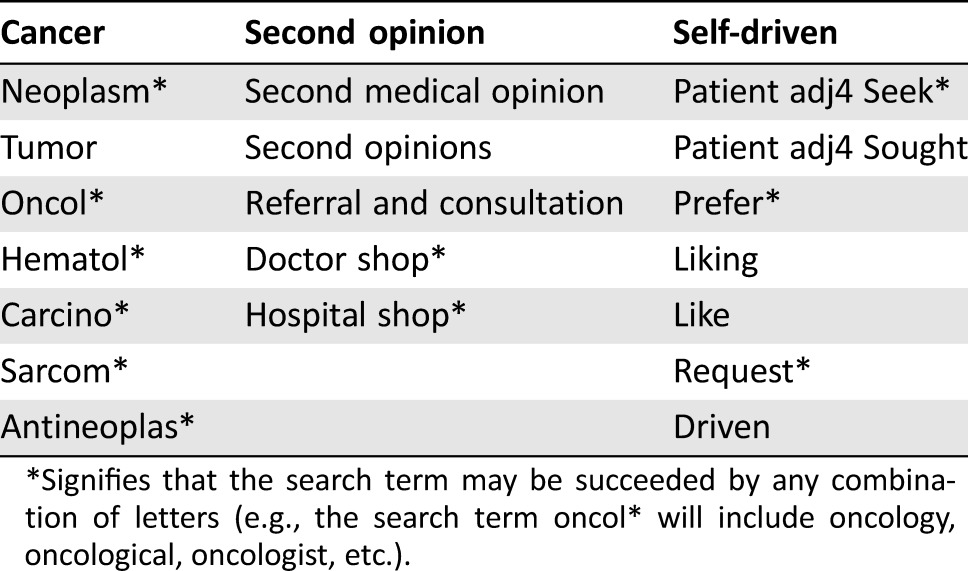
*Signifies that the search term may be succeeded by any combination of letters (e.g., the search term oncol* will include oncology, oncological, oncologist, etc.).
Article Selection and Exclusion Criteria
First, two reviewers (M.H. and N.M.) screened the titles and abstracts of all articles for eligibility. The following were excluded: (a) non‐empirical articles, (b) conference abstracts, (c) articles not sampling cancer patients or their physicians, (d) articles not dealing with SOs, and (e) articles not in English, Dutch, or German. Second, a forward and backward search was performed for the reference lists of the included abstracts. Third, for all resulting articles, the full text was read by two reviewers (M.H. and N.M.).
Also excluded were papers fulfilling the following criteria: (a) no full text retrievable, (b) case reports, (c) literature reviews, (d) reporting on “doctor‐shopping” (if the term was used for the process of repeatedly changing physicians without referral), (e) including both patient‐ and physician‐initiated SOs but not reporting results specifically for patient‐driven SOs, (f) including cancer patients and other patients but not reporting results on SOs specifically for cancer patients, and (g) describing SOs hypothetically. In case of disagreement, a third reviewer (E.S.) read the full text and made a final decision regarding inclusion.
Data Extraction
Data extraction was performed by two authors (M.H. and N.M.) using an extraction instrument (see supplemental online Appendix B) based on the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines [15] and the RAMESES publication standards for realist syntheses [16].
Quality Assessment
The validity of the included studies was assessed using the Critical Appraisal Skills Programme (CASP) for qualitative studies [17], and the Newcastle‐Ottawa Scale (NOS) [18] adapted for cross‐sectional studies and quantitative studies (see supplemental online Appendix C).
The CASP has 10 items, scored with maximally two points each. The adapted NOS has eight items, scored with maximally two points each for study aim (one item), subject selection (four items), comparability (one item), and outcome (two items). Total scores on the CASP or NOS were divided by the total attainable score. Studies with scores >75% were considered “high quality,” scores >50% “moderate quality,” and scores ≤50% were considered “low quality.”
Results
Article Selection
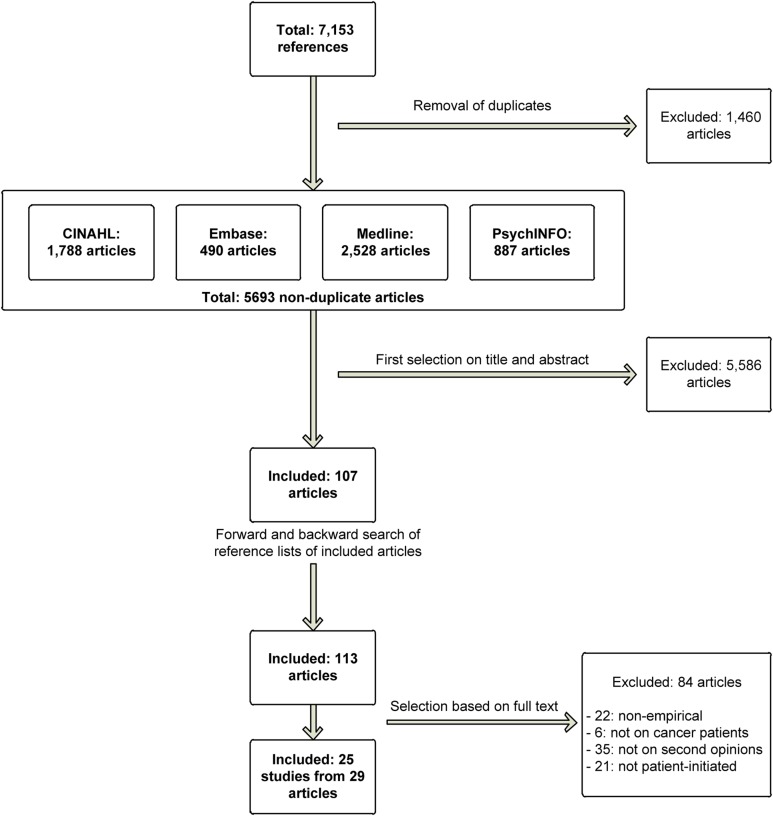
Figure 1 shows the selection process for article inclusion. The search yielded 5,693 non‐duplicate references, of which 107 remained after screening of titles and abstracts. Forward and backward searching of the literature yielded six additional articles. Of the 113 articles included based on title and abstract, 29 remained (after selection based on their full text), reporting on 25 unique studies.
Figure 1.
Flowchart of the article selection process.
Abbreviation: CINAHL, cumulative index to nursing and allied health literature.
Characteristics of Included Studies
Study Characteristics.
The 25 studies had a qualitative (n = 4), quantitative (n = 20), or mixed (n = 1) design (Table 2). Qualitative studies consisted of individual, semi‐structured interviews. Quantitative studies consisted of either surveys (n = 12), retrospective analysis of SO outcomes (n = 7), or a combination of both (n = 1). There were no experimental or intervention studies. Studies were performed between 1993 and 2015 in the U.S. (n = 12), Australia (n = 2), The Netherlands (n = 2), Israel (n = 2), China (n = 2), and Japan, Taiwan, Turkey, Belgium, and Germany (all n = 1).
Table 2. Overview of study characteristics and quality assessment scores.
Abbreviations: BCS, breast conserving surgery; CAM, complementary and alternative medicine; CASP, critical appraisal skills programme; CIS, cancer information service; DCIS, ductal carcinoma in situ; HCC, hepatocellular cancer; NOS, newcastle‐ottawa scale; OR, odds ratio; SES, socio‐economic status; SO, second opinion.
Study Quality.
For quantitative studies, the NOS scores ranged from 25%–86%; for qualitative studies, the CASP scores ranged from 65%–90%. Of the 25 studies, 10 were considered to be of high quality, 8 moderate, and 6 of low quality; one study using mixed methods scored moderate and low on the CASP and the NOS, respectively.
Sample Characteristics.
All studies included either cancer patients, their parents (n = 1), or cancer survivors (n = 1). Six studies additionally included patients’ oncologists (n = 2), patients’ spouse/caregivers (n = 2), or small proportions of patients with non‐malignant disease (n = 2). Sample sizes ranged from 18–1,984 participants.
Outcome Assessment.
Studies addressed SO rates (n = 14), antecedents (n = 15), processes (n = 4), and/or consequences (n = 13). Antecedents included sociodemographic characteristics, medical characteristics, and psychosocial factors. Process‐related factors included content, patients’ experience, and characteristics of the consultations. Outcomes relating to consequences of the SO were medical or assessed patients’ evaluation, treatment preference, and well‐being.
Results on SOs
SO Rates.
Twelve studies (of which nine were quantitative) assessed self‐reported SO rates, which varied strongly. In a study on “doctor‐shopping behavior” (the term in this case used as equivalent to SO seeking) among Taiwanese colorectal cancer patients, 88% reported having sought multiple doctors at any point [19]. High rates (56%) were also reported by cancer survivors in the U.S., although the authors of this study acknowledged that the term “second opinion” might have been misunderstood by patients [20]. Similarly, high SO seeking rates were reported by Israeli cancer patients (44%) [21] and Chinese gynecologic patients (42%) [22]. Among Australian patients with advanced cancer, rates of 33% were reported [23]. Two studies, one in the Israeli pediatric hematology setting and one among Belgian cancer patients, reported rates of 25% [24], [25]. Among a large sample of U.S. breast cancer patients, 19% reported having sought an SO about surgical options [26]. Conversely, much lower rates of SO seeking (7%) were reported in a study of Australian cancer patients (7%) [4] and among Japanese oral cancer patients (<1%) [27]. Finally, of 702,655 German patients visiting an internet portal, 119 requested an SO [28].
Patient Characteristics Associated with SO Seeking
Sociodemographic Characteristics.
Higher age was related to more frequent SO seeking in one study [29], but not in two others [24], [25]. SO seekers were more often female in one study [30], but another study reported no gender differences [25]. Three studies examined education level as a predictor, all of which found that SO seekers were higher educated [22], [24], [26]; one of these also established a relation with higher socioeconomic status [24]. Results with regard to ethnicity were mixed: whereas one study found that Spanish‐speaking Hispanic breast cancer patients less often sought SOs [31], another found higher rates among Hispanics compared with non‐Latina white women [20]. A third study found that SO seekers were more often non‐blacks than blacks [32]. Place of residence was not predictive of SO seeking in one study [24], whereas another found that SO seekers were more often residents of non‐central city metropolitan areas [20]. Finally, SO seeking was associated with higher familiarity with the medical system, more social support [29], and being non‐religious [24].
Medical Characteristics.
Two studies found high SO seeking rates among breast cancer patients compared with other tumor types [20], [30], whereas a third found no such relation [25]. The latter study also failed to find a relation with time since diagnosis. Results with regard to disease stage or progression were mixed: one study reported higher rates of SO seeking among patients with a later disease stage [22], whereas a second study found no such relation but did report that having recurrent disease was related to less SO seeking [19]. A third study found no relation with disease stage [24]. Use of complementary and alternative medicine was related to more SO seeking among Chinese gynecological patients [22] but not among parents of Israeli pediatric patients [24]. Other medical characteristics correlated with SO seeking were treatment advice (breast cancer patients advised to have a mastectomy vs. breast‐conserving therapy more likely sought an SO) [26], and previous treatments undergone (gynecological cancer patients seeking an SO more likely had received radiotherapy) [22].
Personality/Psychosocial Characteristics.
Only one study examined the relationship between personality characteristics and SO seeking, reporting that SO seekers were more likely high information seekers with higher preferred involvement in their medical care (p < .001) [29].
Forum/Online Platform Participation.
Two studies report that using patient information services may stimulate patients to seek SOs. One study reported that use of a telephone cancer information service led 12% of patients to seek an SO or find a new physician [33]. The other found that participation in an online forum led 31% of breast cancer patients to seek an SO [29].
Motivations to Seek or Not Seek an SO
A wide variety of reasons for seeking an SO were reported in six different studies. The most frequently reported reasons related to acquiring more certainty. Cancer patients visiting a Dutch SO clinic reported mostly that they were seeking certainty and reassurance [30], as did 61% of SO seekers in Australia [4] and 40% of Belgian SO seekers [25].
A wide variety of reasons for seeking an SO were reported in six different studies. The most frequently reported reasons related to acquiring more certainty. Cancer patients visiting a Dutch SO clinic reported mostly that they were seeking certainty and reassurance, as did 61% of SO seekers in Australia and 40% of Belgian SO seekers.
A lack of trust or dissatisfaction with the (communication by the) first specialist was reported as a motivation by 31%, 27%, 31%, and 27% of patients, respectively, in four quantitative studies [4], [23], [25], [30], and additionally reported in two qualitative studies [32], [34].
Other reasons frequently reported by patients were (a) requiring more information on treatment options (70%) or on the cancer itself (33%) [4], (b) because of the severity of their disease (32%), (c) having concerns with care (13%), or (d) being influenced by their close ones (9%) [23].
Trust in the physician was reported in three studies as an important reason not to seek an SO [25], [34], [35]. In another study, 47% of patients expressed concern that seeking an SO might affect the relationship with their physician [23]. Other reasons to abandon the wish to seek an SO were fear of losing precious time (18%) [25], [35] and information overload, prolonged uncertainty, or anxiety [35]. In a qualitative study, prostate cancer patients reported that financial cost did not prevent them from seeking an SO [32].
Process of SOs
Four studies reported about the content, or process, of SOs. One of these addressed the perspective of oncologists, who reported conducting the SO consult with care and taking time. Many of them (83%) felt influenced by the first opinion and avoided criticizing it [23]. Results of a second study revealed significant discrepancies between patients’ and oncologists’ recollections of the topics discussed during the SO consultation [4]. In a third, quantitative study, 93% of SO seekers did so in a different hospital than that of their first opinion. In a fourth study, physicians did not ask patients about their consultation goals in any of the observed hematological SO consultations [25].
Consequences
Thirteen studies reported on the consequences of SO seeking, mostly related to medical outcomes.
Medical Outcomes.
Among prostate cancer patients, diagnosis was less likely to change if the patient had requested the SO (in 25% of cases) than if the urologist had (in 41% of cases; p < .0001) [36]. Five other studies reported on changes in diagnosis or treatment advice, based on the SO. The SO resulted in a major treatment or diagnosis change in 2%–3% of Dutch cancer patients visiting an SO clinic [37]. The advice was identical in 68%, there were minor discrepancies for 16%, and major discrepancies for another 16%. A 20% rate of discrepancies between the first opinion and SO was found among breast cancer patients seeking SOs [38]. Another analysis among breast cancer patients reported significant discrepancies in 11% of SO pathology reports, mainly in histological classifications (34%) or biomarker reporting (25%) [39]. Among lung patients seeking an SO in a tertiary pulmonary clinic, discrepancies were found for 9% of diagnoses, 13% for staging, and 37% of treatment advices [40]. Of the discrepancies, 28% significantly affected patient outcomes. The SO was found to result in a change of treatment or oncologist in 51% in a sample of Australian cancer patients [4]. Finally, among Taiwanese colorectal cancer patients, in‐hospital complications more frequently occurred among patients who consulted several different physicians (“heavy doctor shoppers”; odds ratio = 1.675: p = .037) [41].
Treatment Location.
Three studies reported on treatment location after the SO. Among early breast cancer patients, 66% were eventually treated at the institution where they received an SO [38]. Cancer patients visiting an SO clinic were referred back to their first specialist in 78% of cases [37]. Parents of pediatric cancer patients were advised in 35% of cases to receive treatment in the SO institute, but none did so [26].
Patient Perspective and Evaluation.
Turkish women with breast cancer who had consulted another surgeon preoperatively were more likely to prefer breast‐conserving surgery than those who had not [42]; the authors concluded that the SO may influence women in their eventual decision making.
Four studies reported on patients’ evaluation and self‐reported well‐being after the SO. Among parents of pediatric cancer patients seeking an SO, 39% were not satisfied with the SO, mostly because they found it confusing [26]. In a second, qualitative, study, 16 out of 17 patients seeking an SO found it helpful, mainly because it provided more reassurance and better communication than the first opinion [23]. Of a group of patients seeking an SO, 86% rated the consultation positively; 40% believed they received better treatment, and 33% reported they received better information [25]. Finally, some patients with early localized prostate cancer indicated that disagreement between the first opinion and SO caused confusion and anxiety [32].
Discussion
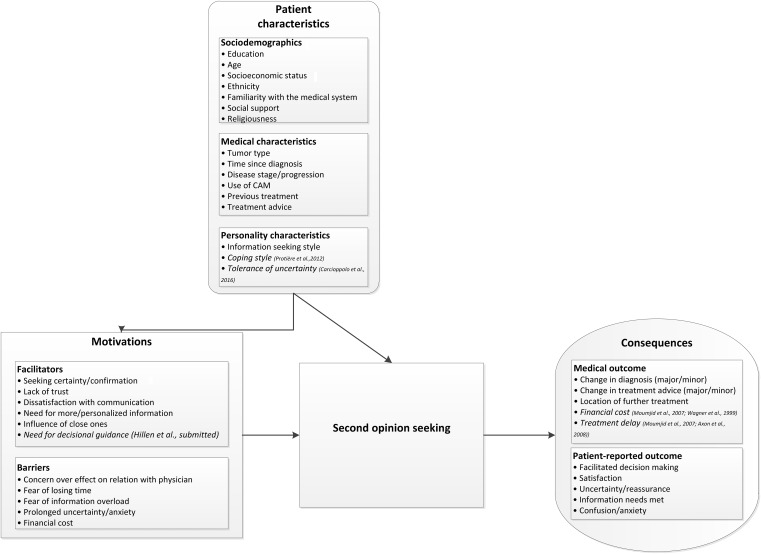
This study reviewed the empirical literature on patient‐driven SOs in oncology. Results show a diverse and incomplete picture of the SO landscape. The high heterogeneity in focus, and in research methods and quality, makes it difficult to compare the results of the different studies. Until now, most of the studies have a cross‐sectional design and only a few are considered to be of high methodological quality. Therefore, the findings reported in this review should be interpreted with caution, and few solid conclusions can be drawn regarding the benefits and drawbacks of patient‐driven SOs in oncology; this is unfortunate given the ongoing debate on SOs. Notwithstanding the lack of empirical evidence, the literature thus far does provide a picture of the potential factors that might stimulate and result from SO seeking. Figure 2 presents a model of the different factors identified in the existing studies that might play a role in the SO process; these are supplemented with other factors previously suggested in the literature.
Figure 2.
Overview of the factors, that is, patient characteristics, motivations, and consequences that may be involved in patient‐driven second opinions. Characteristics and factors displayed in italics were not identified in the articles included in the present review but were suggested as possible factors in previous publications.
Abbreviation: CAM, complementary and alternative medicine.
Most research to date concentrates on patients’ motivations for SO seeking; the main reasons are either patients’ felt need for certainty or confirmation, a lack of trust or dissatisfaction with communication, and/or a need for more (personalized) information.
Most research to date concentrates on patients’ motivations for SO seeking; the main reasons are either patients’ felt need for certainty or confirmation, a lack of trust or dissatisfaction with communication, and/or a need for more (personalized) information. Future research might identify additional motivations for SO seeking. For example, a need for more decisional guidance may be a motivating factor, because patients are increasingly expected to contribute to their own decision‐making process [43]. Particularly in settings where uncertainty is strongly felt (e.g., in prostate cancer), patients may seek an SO to acquire more decisional guidance [44]. In such settings, greater use of collaborative decision‐making models (like tumor boards) could obviate the need for SOs. Surprisingly, to date, only one study has examined the influence of personality factors in SO seeking; more work in this area is needed, as it may shed light on the origins of patients’ motivations. For example, a need for more information may indicate inadequate provision of information, or may arise from an individual patient's coping style.
Future research could also investigate the influence of external factors motivating a patient's request for an SO. Preliminary evidence indicates that, for a substantial subset of patients, family and friends primarily drive the request for an SO [45]; surprisingly, oncologists may perceive this to be the case more frequently than patients actually report [23]. Patients may also be stimulated to seek an SO through advocacy or support groups. Only one of our reviewed studies provides evidence in this area, showing that participation in a social media support group led to SO seeking among breast cancer patients [33]. However, to what extent patient advocacy groups drive patients’ SO requests remains to be elucidated.
Furthermore, we need to study how well patients who seek SOs (unbeknown to their first specialist) are able to adequately self‐refer. Extensive media coverage for specific treatments or diagnostic tests, or a particular treatment center's strong public relations image, may play an important role in creating overly optimistic expectations and driving patients to seek advice in less appropriate places.
More insight into what keeps patients from seeking SOs might also help patients to overcome unnecessary barriers, such as concern about how the SO might affect their relationship with their first physician. It may also shed light on which barriers might be justified by relating them to objective and subjective outcomes of the SO.
The present review reveals that, because studies reporting on SO‐seeking rates were conducted within highly diverse samples and used various methods, they yielded highly diverse results (ranging from 1%–56%). Moreover, all studies employed self‐report methods and used various ways of asking about SO seeking, which may not always have been interpreted as intended by the patients [20]; therefore, the present results may not be totally reliable. Moreover, because no research center has kept track of SO rates over time, it remains unclear whether the presumed/reported increase in SO seeking in oncology is in fact real.
For both hospitals and insurers, it would be valuable to more systematically register rates of SO seeking, together with who (e.g., the patient or physician) requested it [9]. This is challenging, especially when extending across various hospitals. Because SOs are not always systematically registered within hospital or medical insurer files [3], they must be deduced from other data: for example, two registered consecutive visits to physicians of the same specialty in a different hospital. More systematic registering would not only yield insight into the popularity of the phenomenon over time but also allow for systematic assessment of the medical and psychological benefits of SO seeking.
The literature on the effects of SOs consists mostly of retrospective evaluations of their medical impact for both diagnosis and treatment. Reported discrepancy rates vary substantially and, moreover, do not always distinguish between minor and major discrepancies, whereas these may range from a small refinement of the diagnosis to a change from benign to malignant. More uniformity is needed in reporting discrepancies between first opinions and SOs and their diagnostic and therapeutic consequences [46]. Nevertheless, the limited evidence suggests that 2%–51% of SOs yield a major change in diagnosis or treatment, indicating that they may at least offer benefit for some patients.
Importantly, however, neither the first opinion nor the SO is an “absolute truth” [10], [46]. Therefore, subjective and more long‐term outcomes of patients’ SO seeking need to be taken into account and related to objective consequences [12]. Preliminary evidence suggests that patients’ motivations are more often based on impairments of the physician‐patient relation than on patients’ doubts about their physician's medical competence [46], [47]. Thus, SOs may benefit patients, even if they do not result in medical changes, for example, by yielding more certainty [46]. On the other hand, if the SO diverges from the first opinion, patients may experience distress resulting from increased uncertainty [44]. These situations may induce third and even further opinions, at high costs to health care.
Other questions that remain include the following: how do physicians deal with and communicate about disagreements between the first opinion and SO? How fundamental are the changes resulting from the SO for the patient's treatment and health? To what extent are patients referred back to their initial physician? Is the relationship with their initial physician influenced by their SO seeking? How do patients reflect on their decision to seek SOs? These questions need to be addressed in future research.
Practical Recommendations
More systematic research is needed to provide additional insight into the benefits and drawbacks of patients’ SO seeking. Studies should focus not only on patients’ motivations and medical consequences but also on psychological and communicative factors and the oncologists’ perspectives. First, qualitative work is needed to explore patients’ internal motivations/external factors stimulating their SO seeking and how they perceive the content/outcomes of the SO consultation; on the other hand, physicians’ experiences/perceptions should be explored qualitatively to acquire insight into their opinions/emotions related to SOs. Second, longitudinal observational studies involving all stakeholders (patients, relatives, and both physicians) could elucidate how patients’ motivations and psychological characteristics relate to communication about SOs, as well as their outcomes. Third, more epidemiologic evidence is needed that registers SO rates and the effects of SO use. The resulting data can be used to better assess cost‐effectiveness of the SO process. Finally, interventions could be developed to optimize SO and/or create alternative processes.
Because of their potentially diverse effects on patients’ well‐being, SOs should be used judiciously; they may benefit some patients but should not be viewed as a solution to current limitations in the organization of care. Patients’ need for an SO may be obviated by addressing their motivation to seek it within the initial consultation. For example, more structural establishment of multidisciplinary team‐based approaches may help patients feel better cared for and help guide them through the treatment‐decision process. In addition, a number of communicative issues can be derived from the results of the present review, for which we suggest the practical recommendations described below. These suggestions are not yet sufficiently evidence based and should be seen only as a point of departure; their effectiveness needs to be established through rigorous empirical testing.
Responding to an SO Request
Various motivations can drive patients’ requests for an SO, which demand different responses. By openly responding to, and specifically asking about, a patient's motivation, oncologists might be able to optimally respond to their request. In their response to patients’ requests, oncologists should actively manage patients’ expectations of the possible benefit of seeking an SO. By preparing patients for the various possible positive/negative outcomes of an SO, oncologists can help them deliberately decide whether they actually want to pursue this and, if so, how and where. In particular cases, physicians may want to advise against SO seeking, such as when patients have already seen multiple specialists or when it may lead to harmful and/or unnecessary treatment [10].
Need for Certainty or Confirmation.
If patients report a need for more certainty, oncologists could make explicit to what extent diagnosis and treatment have already been discussed in multidisciplinary tumor boards. This may reduce some patients’ need to seek an SO, whereas others will still feel the need to personally speak to a different oncologist.
Checking the Availability of Additional Treatments.
If patients want to know about possible additional treatments, the oncologist could refer them to a treatment center that is most likely to offer such treatments, or the oncologist could offer to arrange a consultation with another colleague with similar expertise.
Need for More (Personalized) Information.
If patients report they need more information, oncologists may respond by offering extra time to provide more information themselves.
Lack of Trust or Dissatisfaction.
Patients may be hesitant to admit that a lack of trust or dissatisfaction underlies their wish for an SO. If oncologists feel that this may be the case, they can subtly explore whether the patient has perceived any difficulties or shortcomings [48]. A willingness to be open and vulnerable is a prerequisite for this conversation. If the treatment relationship is structurally damaged, oncologists may refer the patient to a colleague within the same hospital rather than the patient seeking an SO elsewhere.
Conducting an SO Consultation
Agenda‐setting is crucial for oncologists providing an SO [48], [49]. Similar to referring oncologists, SO providers should deliberately explore patients’ motivation for seeking it and their expectations of the SO. By doing this at the onset of the consultation, oncologists can tailor their consultation to the patient's needs [1], [49]. For patients who primarily need more information about their disease and/or treatment, this may imply providing (more) detailed explanations. Other motivations, such as perceptions of inadequate communication, require a listening approach, thereby exploring the patient's experiences. Before finishing the SO consultation, explicitly checking whether the patient's expectations have been met will enable oncologists to assess whether any additional action is required.
Similar to referring oncologists, SO providers should deliberately explore patients’ motivation for seeking it and their expectations of the SO. By doing this at the onset of the consultation, oncologists can tailor their consultation to the patient's needs
Interprofessional Communication
Communication between the providers of the first opinion and the SO may be particularly delicate, as our review suggests [50]. Because most patients are referred back after the SO, they should be able to continue an optimal treatment relationship with their initial oncologist. Hence, the person providing the SO needs to enable them to maintain a positive view of their oncologist, whenever possible [49]. When an oncologist who provides the SO disagrees only slightly with the first professional, rather than emphasizing the differences, they could explain how the referring oncologist may have come to a slightly different conclusion. In case their conclusion is substantially different from the first professional, the oncologist could personally contact the referring oncologist to discuss how this discrepancy arose. By negotiating a treatment plan that is acceptable to all parties, patients may be spared the confusion associated with two different viewpoints. Eventually, this may lead to more satisfactory outcomes of the SO for all parties involved.
Conclusion
These practical recommendations may help to facilitate the process of discussing SOs, as well as improving how they evolve and their outcomes. Further empirical research may yield more evidence‐based conclusions and advice for clinicians. The results of such studies may provide valuable data for the ongoing debate on the desirability of SOs, as well as how best to organize SOs in practice.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by the Dutch Cancer Society (KWF Kankerbestrijding) [Grant number UVA 2014‐6671].
Author Contributions
Conception/design: Marij A. Hillen, Joost G. Daams, Ellen M. Smets
Provision of study material or patients: Marij A. Hillen
Collection and/or assembly of data: Marij A. Hillen, Niki M. Medendorp, Joost G. Daams, Ellen M. Smets
Data analysis and interpretation: Marij A. Hillen, Niki M. Medendorp, Joost G. Daams, Ellen M. Smets
Manuscript writing: Marij A. Hillen, Niki M. Medendorp, Joost G. Daams, Ellen M. Smets
Final approval of manuscript: Marij A. Hillen, Niki M. Medendorp, Joost G. Daams, Ellen M. Smets
Disclosures
The authors indicated no financial relationships.
References
- 1. Moumjid N, Gafni A, Bremond A et al. Seeking a second opinion: Do patients need a second opinion when practice guidelines exist? Health Policy 2007;80:43–50. [DOI] [PubMed] [Google Scholar]
- 2.Heeft een patiënt recht op verwijzing voor een second opinion?: Available at https://www.knmg.nl/advies-richtlijnen/artseninfolijn/praktijkdilemmas-1/praktijkdilemma/heeft-een-patient-recht-op-verwijzing-voor-een-second-opinion.htm. Accessed May 25, 2017.
- 3.Zorginstituut Nederland. Second opinion in de zorgverzekeringswet, 2015. Available at https://www.zorginstituutnederland.nl/publicaties/standpunten/2015/01/06/second-opinion-in-de-zorgverzekeringswet. Accessed May 25, 2017.
- 4. Tattersall MH, Dear RF, Jansen J et al. Second opinions in oncology: The experiences of patients attending the Sydney Cancer Centre. Med J Aust 2009;191:209–212. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SN, Gorman SA, Snitzer S et al. Patients' reactions and physician‐patient communication in a mandatory surgical second‐opinion program. Med Care 1989;27:466–477. [DOI] [PubMed] [Google Scholar]
- 6. Epstein JI, Walsh PC, Sanfilippo F. Clinical and cost impact of second‐opinion pathology. Review of prostate biopsies prior to radical prostatectomy. Am J Surg Pathol 1996;20:851–857. [DOI] [PubMed] [Google Scholar]
- 7. Sutherland LR, Verhoef MJ. Why do patients seek a second opinion or alternative medicine? J Clin Gastroenterol 1994;19:194–197. [DOI] [PubMed] [Google Scholar]
- 8.BMJ Clinical Evidence [online]. Available at http://www.clinicalevidence.com/ceweb/about/guide.jsp. Accessed May 8, 2017.
- 9. Tattersall MH. Can a second medical opinion in a patient with cancer be truly independent? Asia Pac J Clin Oncol 2011;7:1–3. [DOI] [PubMed] [Google Scholar]
- 10. Axon A, Hassan M, Niv Y et al. Ethical and legal implications in seeking and providing a second medical opinion. Dig Dis 2008;26:11–17. [DOI] [PubMed] [Google Scholar]
- 11. Sikora K. Second opinions for patients with cancer. Br Med J 1995;311:1179–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mustafa M, Bijl M, Gans R. What is the value of patient‐sought second opinions? Eur J Intern Med 2002;13:445–447. [DOI] [PubMed] [Google Scholar]
- 13. Wagner TH, Wagner LS. Who gets second opinions? Health Aff (Millwood) 1999;18:137–145. [DOI] [PubMed] [Google Scholar]
- 14. Ruetters D, Keinki C, Schroth S et al. Is there evidence for a better health care for cancer patients after a second opinion? A systematic review. J Cancer Res Clin Oncol 2016;142:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Ann Intern Med 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 16. Wong G, Greenhalgh T, Westhorp G et al. RAMESES publication standards: Realist syntheses. BMC Med 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Critical Appraisal Skills Programme (CASP), 2014. Available at http://www.casp-uk.net/#!checklists/cb36. Accessed May 8, 2017.
- 18. Wells G, Shea B, O'Connell D et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Eur J Epidemiol 2000;25:603–605. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh CI, Chung KP, Yang MC et al. Association of treatment and outcomes of doctor‐shopping behavior in patients with hepatocellular carcinoma. Patient Prefer Adherence 2013;7:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: Analyses of the 1992 National Health Interview Survey. J Natl Cancer Inst 1999;91:1480–1486. [DOI] [PubMed] [Google Scholar]
- 21. Sapir R, Catane R, Kaufman B et al. Cancer patient expectations of and communication with oncologists and oncology nurses: The experience of an integrated oncology and palliative care service. Support Care Cancer 2000;8:458–463. [DOI] [PubMed] [Google Scholar]
- 22. Tam KF, Cheng DK, Ng TY et al. The behaviors of seeking a second opinion from other health‐care professionals and the utilization of complementary and alternative medicine in gynecologic cancer patients. Support Care Cancer 2005;13:679–684. [DOI] [PubMed] [Google Scholar]
- 23. Philip J, Gold M, Schwarz M et al. Second medical opinions: The views of oncology patients and their physicians. Support Care Cancer 2010;18:1199–1205. [DOI] [PubMed] [Google Scholar]
- 24. Mordechai O, Tamir S, Weyl‐Ben‐Arush M. Seeking a second opinion in pediatric oncology. Pediatr Hematol Oncol 2015;32:284–289. [DOI] [PubMed] [Google Scholar]
- 25. Van De Plas J, Buntinx F, De Vadder I et al. Cancer patients looking for a second opinion. [dutch]. Tijdschrift voor Geneeskunde 2010;66:770–774. [Google Scholar]
- 26. Morrow M, Jagsi R, Alderman AK et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 2009;302:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Umeda M, Komatsubara H, Minamikawa T et al. A questionnaire on requests for disclosure of diagnosis, self‐choice of treatment, and second opinion of patients with oral cancer in Japan. J Palliat Care 2003;19:206–208. [PubMed] [Google Scholar]
- 28. Wallwiener M, Wallwiener CW, Brucker SY et al. The Brustkrebs‐Studien.de website for breast cancer patients: User acceptance of a German internet portal offering information on the disease and treatment options, and a clinical trials matching service. BMC Cancer 2010;10:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Czaja R, Manfredi C, Price J. The determinants and consequences of information seeking among cancer patients. J Health Commun 2003;8:529–562. [DOI] [PubMed] [Google Scholar]
- 30. Mellink WA, Dulmen AM, Wiggers T et al. Cancer patients seeking a second surgical opinion: Results of a study on motives, needs, and expectations. J Clin Oncol 2003;21:1492–1497. [DOI] [PubMed] [Google Scholar]
- 31. Parikh AR, Kaplan CP, Burke NJ et al. Ductal carcinoma in situ: Knowledge of associated risks and prognosis among Latina and non‐Latina white women. Breast Cancer Res Treat 2013;141:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu J, Dailey RK, Eggly S et al. Men's perspectives on selecting their prostate cancer treatment. J Natl Med Assoc 2011;103:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Attai DJ, Cowher MS, Al‐Hamadani M et al. Twitter social media is an effective tool for breast cancer patient education and support: Patient‐reported outcomes by survey. J Med Internet Res 2015;17:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Rourke ME. Narrowing the options: The process of deciding on prostate cancer treatment. Cancer Invest 1999;17:349–359. [DOI] [PubMed] [Google Scholar]
- 35. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: The influence of emotion, misconception, and anecdote. Cancer 2006;107:620–630. [DOI] [PubMed] [Google Scholar]
- 36. Chan TY, Epstein JI. Patient and urologist driven second opinion of prostate needle biopsies. J Urol 2005;174:1390–1394; discussion 1394; author reply 1394 [DOI] [PubMed] [Google Scholar]
- 37. Mellink WA, Henzen‐Logmans SC, Bongaerts AH et al. Discrepancy between second and first opinion in surgical oncological patients. Eur J Surg Oncol 2006;32:108–112. [DOI] [PubMed] [Google Scholar]
- 38. Clauson J, Hsieh YC, Acharya S et al. Results of the Lynn Sage second‐opinion program for local therapy in patients with breast carcinoma. Changes in management and determinants of where care is delivered. Cancer 2002;94:889–894. [DOI] [PubMed] [Google Scholar]
- 39. Khazai L, Middleton LP, Goktepe N et al. Breast pathology second review identifies clinically significant discrepancies in over 10% of patients. J Surg Oncol 2015;111:192–197. [DOI] [PubMed] [Google Scholar]
- 40. Schook RM, ter Avest MJ, van Setten CH et al. Lung cancer patients benefit from second opinions by improvement of diagnosis and therapy. Cancer Clin Oncol 2014;3:43–57. [Google Scholar]
- 41. Chang HR, Yang MC, Chung KP. Can cancer patients seeking a second opinion get better care? Am J Manag Care 2013;19:380–387. [PubMed] [Google Scholar]
- 42. Gumus M, Ustaalioglu BO, Garip M et al. Factors that affect patients' decision‐making about mastectomy or breast conserving surgery, and the psychological effect of this choice on breast cancer patients. Breast Care (Basel) 2010;5:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stiggelbout AM, Van der Weijden T, De Wit MP et al. Shared decision making: Really putting patients at the centre of healthcare. BMJ 2012;344;e256. [DOI] [PubMed] [Google Scholar]
- 44.Hillen MA, Gutheil CM, Smets EMA et al. The evolution of uncertainty in second opinions about prostate cancer treatment. Health Expect 2017. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radhakrishnan A, Grande D, Mitra N et al. Second opinions from urologists for prostate cancer: Who gets them, why, and their link to treatment. Cancer 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Payne VL, Singh H, Meyer AN et al. Patient‐initiated second opinions: Systematic review of characteristics and impact on diagnosis, treatment, and satisfaction. Paper presented at: Mayo Clinic Proceedings, 2014. [DOI] [PubMed] [Google Scholar]
- 47. van Dalen I, Groothoff J, Stewart R et al. Motives for seeking a second opinion in orthopaedic surgery. J Health Serv Res Policy 2001;6:195–201. [DOI] [PubMed] [Google Scholar]
- 48. Back AL, Arnold RM, Baile WF et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin 2005;55:164–177. [DOI] [PubMed] [Google Scholar]
- 49. Links M, Aghmesheh M. Second opinions: Agendas and ego. Acta Oncol 2009;48:1210–1213. [DOI] [PubMed] [Google Scholar]
- 50. Philip J, Gold M, Schwarz M et al. An exploration of the dynamics and influences upon second medical opinion consultations in cancer care. Asia Pac J Clin Oncol 2011;7:41–46. [DOI] [PubMed] [Google Scholar]
- 51. Manfredi C, Czaja R, Buis M et al. Patient use of treatment‐related information received from the Cancer Information Service. Cancer 1993;71:1326–1337. [DOI] [PubMed] [Google Scholar]
- 52. Goldman RE, Sullivan A, Back AL et al. Patients' reflections on communication in the second‐opinion hematology‐oncology consultation. Patient Educ Couns 2009;76:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mellink WA, Henzen‐Logmans SC, Bongaerts AH et al. Second Opinion Consult Clinic for Surgical Oncology in the Daniel den Hoed Clinic: Analysis of the first 245 patients [in Dutch]. Ned Tijdschr Geneeskd 1999;143:2471–2475. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.