Immune checkpoint inhibitors are a novel class of immunotherapeutic agents being used in clinical practice for many advanced malignancies. Checkpoint blockade targeting the programmed cell death 1 receptor or its primary ligand with pembrolizumab, nivolumab, or atezolizumab has received U.S. Food and Drug Administration approval for the treatment ofmetastatic melanoma, non‐small cell lung cancer, renal cell carcinoma, Hodgkin's lymphoma, head and neck squamous cell carcinoma, and urothelial carcinoma. Considering that checkpoint blockade treatments now extend to tumor types beyond melanoma, a study was conducted to evaluate whether the development of immune‐related adverse events correlates with treatment response in other cancer subtypes.
Keywords: Programmed cell death 1 inhibitors, Nivolumab, Pembrolizumab, Checkpoint inhibitors, Immunotherapy, Immune‐related adverse events
Abstract
Background.
The programmed death 1 (PD‐1) checkpoint inhibitors (CKIs) can lead to immune‐related adverse events (irAEs). We sought to evaluate whether the development of irAEs correlates with treatment response in non‐melanoma malignancies.
Materials and Methods.
We conducted a retrospective study of patients who received anti‐PD‐1 CKI monotherapy at Fox Chase Cancer Center. Endpoints included overall response rate (ORR), time to next therapy or death (TTNTD), and overall survival (OS). Fisher's exact tests and logistic regression models were used to determine the association between irAE incidence and ORR, and Kaplan‐Meier curves with log‐rank tests and Cox regression models were used for the comparison of TTNTD and OS.
Results.
Between November 2011 and November 2016, 160 patients were treated with >1 dose of an anti‐PD‐1 CKI. Seventy‐three (46%) were treated on a clinical trial. Immune‐related adverse events were noted in 64 patients (40%), with steroids required in 36 (23%). Of the 142 patients evaluable for clinical response, 28 patients (20%) achieved a partial response at first scan. An association between irAEs and ORR was seen in clinical trial patients (p = .007), but not in non‐trial patients (p = .13). When controlling for clinical trial participation and cancer type using multivariate analysis, low‐grade irAEs had higher ORR (p = .017) and longer TTNTD (p = .008). No association between irAE incidence and OS was seen (p = .827). Immune‐related adverse events that required steroid treatment were marginally associated with increased TTNTD (p = .05, hazard ratio 0.62) but were not associated with OS (p = .13).
Conclusion.
We demonstrate several positive associations between the development of irAEs and clinical outcomes in non‐melanoma patients treated with PD‐1 CKIs, for which further validation is required.
Implications for Practice.
This study evaluated whether the development of immune‐related adverse events in non‐melanoma patients treated with programmed cell death 1 checkpoint inhibitors correlates with improved clinical outcomes. The results indicate that for a subset of patients, in particular those with low‐grade immune‐related adverse events, immune‐related adverse events predicted for an improved response rate and longer time to next therapy or death.
Introduction
Immune checkpoint inhibitors (CKI) are a novel class of immunotherapeutic agents now being used in clinical practice for many advanced malignancies. Their mechanism of action is based on relieving the immune system's innate stop signal for maintaining self‐tolerance [1]. Checkpoint blockade targeting the programmed cell death 1 (PD‐1) receptor, or its primary ligand PD‐L1, with pembrolizumab, nivolumab, or atezolizumab, has received U.S. Food and Drug Administration (FDA) approval for the treatment of metastatic melanoma, non‐small cell lung cancer (NSCLC), renal cell carcinoma (RCC), Hodgkin's lymphoma, head and neck squamous cell carcinoma (HNSCC), and urothelial carcinoma (UC) [2], [3], [4], [5], [6], [7].
The biology and kinetics of response to immune CKIs can differ from traditional anticancer therapies. Atypical responses marked by initial tumor growth and appearance of new lesions, followed by subsequent regression, have been well described and may be a result of pseudoprogression from tumor‐infiltrating immune cells [8], [9]. Therefore, traditional measures of tumor growth such as Response Evaluation Criteria in Solid Tumors (RECIST) may underestimate the benefit provided by immunotherapy. Novel measures of assessment such as the immune‐related response criteria (irRC) have been proposed [10] and recent studies have shown subsequent tumor regression when patients with RCC continued treatment beyond first RECIST disease progression in the setting of clinical benefit [11]. However, irRC are difficult to implement in daily practice; thus, additional predictive markers of clinical benefit would be useful to guide decision‐making and mitigate premature treatment termination.
Several prior reports have suggested that the development of immune‐related adverse events (irAEs) in patients with melanoma treated with CKIs may correlate with clinical response [12], [13], [14], [15], [16], [17]. For example, the development of any irAE in a population of patients with melanoma being treated with ipilimumab, with or without peptide vaccinations, was associated with a statistically significant increase in the probability of antitumor response, and all patients with a complete response (CR) had grade 3/4 irAEs [12]. In another study, the development of cutaneous irAEs in a population of predominantly melanoma patients being treated with pembrolizumab correlated with a statistically significant improvement in progression free survival [14]. However, in a recent study in which 85% of patients with melanoma treated with ipilimumab developed an irAE of any grade, there was no association between irAEs or steroid use and overall survival (OS) or time to treatment failure [18]. These studies included patients with melanoma, a highly mutated disease with strong immunogenic potential. Given that checkpoint blockade treatments now extend to tumor types beyond melanoma, we sought to evaluate whether the development of irAEs correlates with treatment response in other cancer subtypes.
Materials and Methods
We conducted a retrospective study of advanced stage non‐melanoma patients who initiated PD‐1 inhibitor monotherapy at Fox Chase Cancer Center. Thirteen patients who only received 1 dose of the drug were excluded. Patients treated both on and off clinical trials were included. Electronic medical records were reviewed to obtain patient‐specific information including the following: (a) patient demographics, (b) cancer type, (c) prior systemic therapy, (d) number of anti‐PD‐1 drug doses received, (e) any irAEs (including endocrinopathies, dermatitis, colitis, pneumonitis, and transaminitis), (f) use of corticosteroids, (g) response at first and second restaging scans, (h) date of progression, and (i) start of new treatment or death. The precise irAE grade for clinical trial patients was extracted from patient research files. The irAE grade for non‐clinical trial patients was categorized as high‐grade (corresponding to Common Terminology Criteria for Adverse Events [CTCAE] grades 3–5 and defined as the use of high dose steroids at ≥1 mg/kg per day prednisone equivalent, use of intravenous steroids, hospitalization, death or discontinuation of therapy) or low‐grade (corresponding to CTCAE grades 1 and 2 and defined as all irAEs that did not meet the high‐grade definition) because exact grade could not always be precisely determined from chart review. The primary objective was to correlate the development of low‐ or high‐grade irAEs with clinical response. We also sought to examine the correlation of steroid use as a surrogate marker for high‐grade irAEs with outcomes in this patient population. Our hypothesis was that patients who develop an irAE are more likely to derive benefit from CKI treatment. Response was recorded at first restaging scan, and endpoints included overall response rate (ORR) per RECIST, overall survival (OS), and time to next therapy or death (TTNTD). This study was approved by the Fox Chase Cancer Center Institutional Review Board (IRB 15–9055). The need for informed consent was waived by the Fox Chase Cancer center IRB for the purpose of this study. The study was performed in accordance with the Declaration of Helsinski.
Statistical Analysis
Overall response rate was determined at first scan before 14 weeks as clinical complete or partial response (PR) per RECIST 1.1. Patients who did not undergo a scan and died before 14 weeks were considered to have progressive disease. Overall survival was defined as time from initiation of PD‐1 inhibitor treatment until death. Time to next therapy or death was defined as time from initiation of PD‐1 inhibitor until initiation of another systemic agent or death. Any irAEs and subsequent treatment with systemic corticosteroids were recorded. No patients received additional immunomodulatory medications such as infiliximab for treatment of irAEs. Fisher's exact tests were used to determine the association between irAE incidence and ORR. Multivariate analysis via logistic regression was also performed to determine the association between irAE incidence and ORR, adjusting for clinical trial participation and disease site. Survival outcomes (TTNTD and OS) were analyzed using Kaplan‐Meier curves with log‐rank tests, and also using Cox proportional hazards regression, again adjusting for clinical trial status and disease site. We also conducted a sensitivity analysis where we did not exclude patients who only received 1 dose of a PD‐1 inhibitor. We repeated all the analyses (modeling ORR, TTNTD, and OS) in this expanded patient population.
Results
Between November 2011 and November 2016, we identified 173 non‐melanoma patients treated with nivolumab or pembrolizumab. We excluded 13 patients who received just 1 dose of the drug. A total of 160 patients were evaluable for any outcome (159 evaluable for survival, 142 evaluable for response). Of these patients, 73 (46%) were treated on a clinical trial. The average age was 65 years, and 101 (64%) were male. The majority of patients received nivolumab (123, 77%). Most of the patients treated with nivolumab were not on a clinical trial (84, 68%) while the majority of patients treated with pembrolizumab were on a clinical trial (34, 92%). The number of patients included by cancer subtype was as follows: NSCLC (71, 44%), RCC (43, 27%), HNSCC (23, 14%), UC (20, 13%), other (3, 2%). When comparing patients treated on versus patients treated off clinical trials, there was an imbalance in baseline characteristics with respect to which PD‐1 inhibitor was administered, cancer subtype, prior systemic therapy, and gender. There was no difference in the number of PD‐1 inhibitor doses received, irAEs observed, treatment response at first scan, patient age, or steroid use (Table 1).
Table 1. Comparison of clinical trial patients versus non‐clinical trial patients.
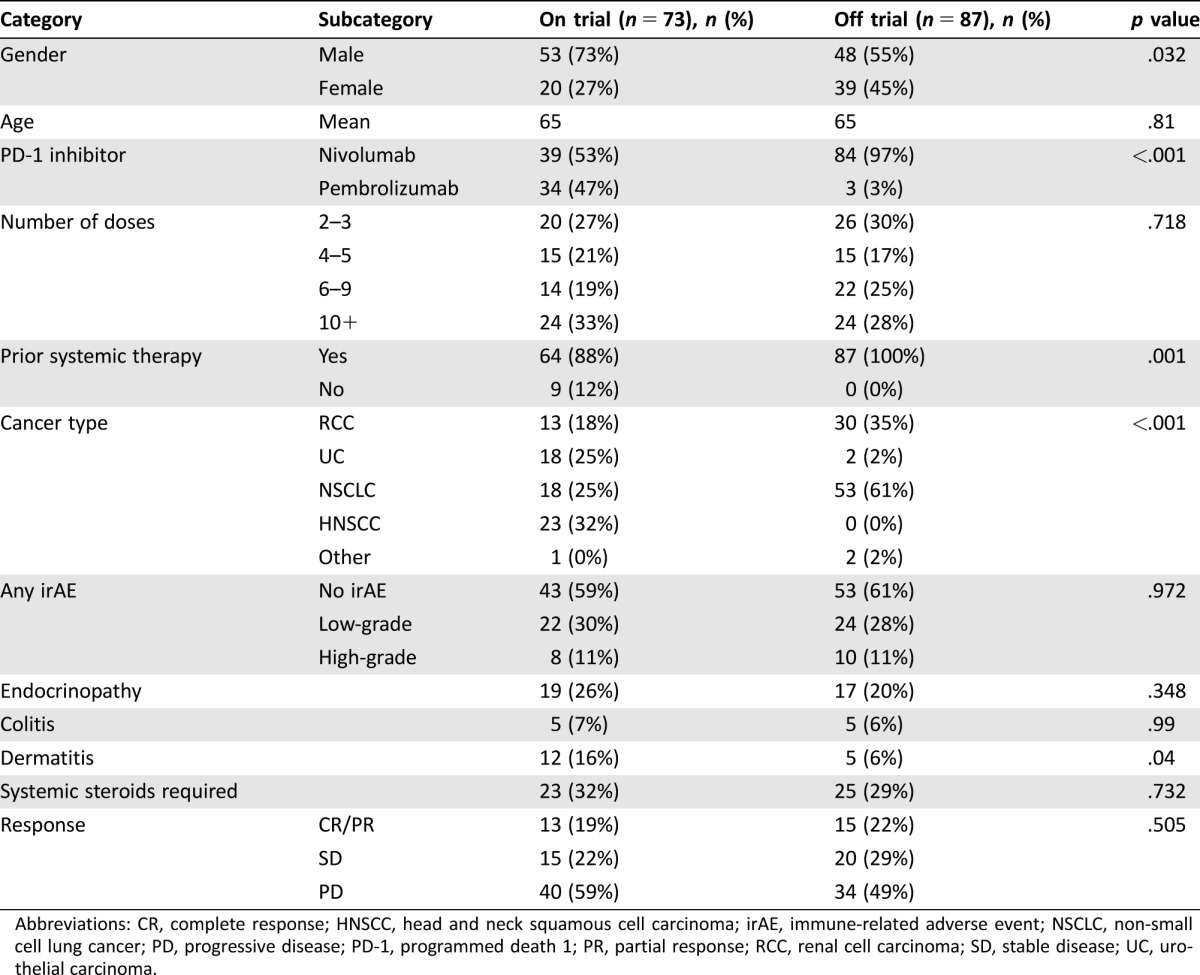
Abbreviations: CR, complete response; HNSCC, head and neck squamous cell carcinoma; irAE, immune‐related adverse event; NSCLC, non‐small cell lung cancer; PD, progressive disease; PD‐1, programmed death 1; PR, partial response; RCC, renal cell carcinoma; SD, stable disease; UC, urothelial carcinoma.
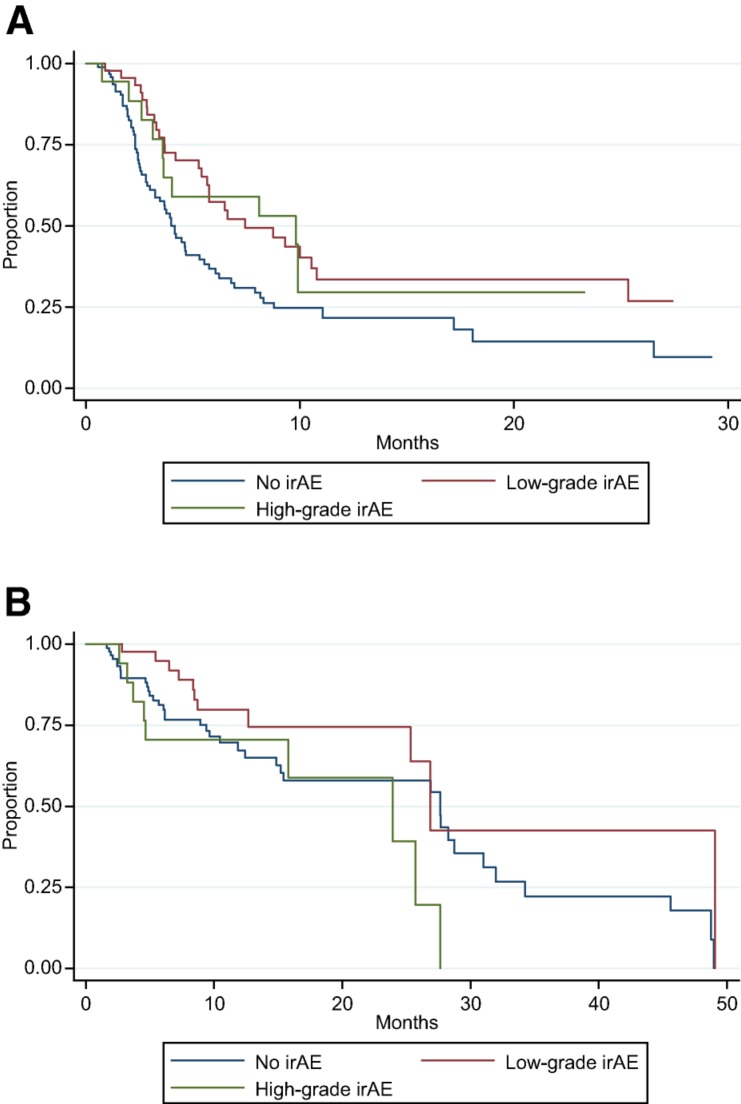
Overall, irAEs were noted in 64 patients (40%). Steroids were used to treat an irAE in 36 patients (23%). An additional 12 patients were prescribed steroids to treat other conditions (e.g., chronic obstructive pulmonary disease exacerbation or cerebral edema due to brain metastases), but not for a documented irAE. Endocrinopathies, dermatitis, and colitis were the most common, occurring in 23%, 11%, and 6% of patients, respectively (Table 1). Of the 142 patients evaluable for clinical response at first scan, 28 patients (20%) achieved PR (no CRs were observed). The ORR was 14% in patients with no irAEs, 32% in patients with low‐grade irAEs, and 20% in patients with high‐grade irAEs. Although this was not statistically significant overall, (p = .09), there was a statistically significant association in the clinical trial patient subgroup, with any irAE associated with improved ORR (p = .007; Table 2). There also was a statistically significant association between low‐grade irAEs and improved ORR when controlling for trial participation status and cancer type using multivariate analysis (p = .017; Table 3). In addition, when controlling for trial participation status and cancer type, there was a statistically significant relationship between any irAEs and TTNTD (p = .006; Fig. 1A). The hazard ratios (HR) for low‐ and high‐grade irAEs were similar (0.529 and 0.629, respectively); however, only the relationship with low‐grade irAEs was statistically significant (p = .008 and p = .184, respectively). There was no significant association between irAE incidence and OS when controlling for trial status and cancer type (p = .827; Fig. 1B). There was an association between steroid use and severity of irAE (p < .001). Two patients may have had unconfirmed treatment‐related deaths from pneumonitis. Immune‐related adverse events that required steroid treatment were marginally associated with TTNTD when controlling for trial participation status and cancer type (p = .05, HR 0.62), but were not associated with OS (p = .13). In the sensitivity analysis, including patients who only received 1 dose of the PD‐1 inhibitor, the above results were consistent in direction and significance. The adjusted OR for CR/PR for low‐grade irAE = 3.17 (p = .014); adjusted HR for TTNTD for low‐grade irAE = 0.49 (p = .002); and adjusted HR for OS for low‐grade irAE = 0.48 (p = .055).
Table 2. Univariate analysis of the association between irAEs and treatment response in clinical trial patients versus non‐clinical trial patients.
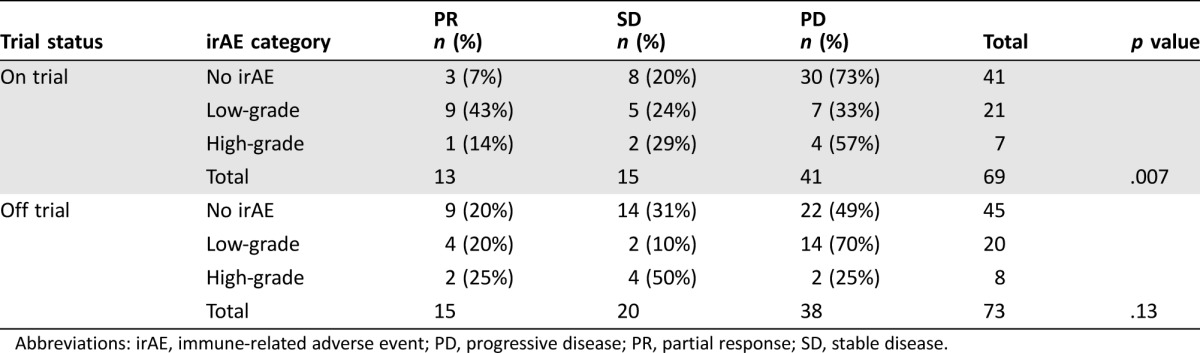
Abbreviations: irAE, immune‐related adverse event; PD, progressive disease; PR, partial response; SD, stable disease.
Table 3. Multivariate analysis of the association between irAEs and treatment response controlled for cancer type and clinical trial participation.
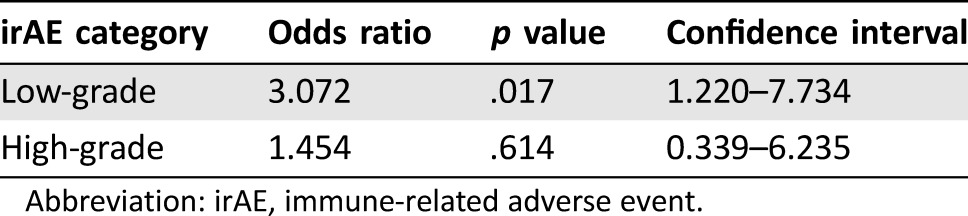
Abbreviation: irAE, immune‐related adverse event.
Figure 1.
Kaplan‐Meier survival estimates. (A): Time to next therapy or death (TTNTD) for patients with low‐grade, high‐grade, or no irAEs. There is a statistically significant relationship between irAEs and TTNTD when controlling for trial status and cancer subtype (p = .006). (B): Overall survival (OS) for patients with low‐grade, high‐grade, or no irAEs. There is no relationship between irAEs and OS when controlling for trial status and cancer subtype (p = .827).
Abbreviation: irAE, immune‐related adverse event.
Discussion
We retrospectively analyzed 160 non‐melanoma patients who received nivolumab or pembrolizumab over a period of 5 years, of which 142 were evaluable for clinical response at first scan. Sensitivity analysis determined that the patients excluded from the study, based on having received only 1 PD‐1 inhibitor dose, did not change ORR, TTNTD, or OS results. The ORR in this mixed cohort was comparable to prior studies of single‐agent PD‐1 inhibitors in these individual diseases. In addition, the percentage of patients who required systemic steroids to treat irAEs is comparable to recently reported studies with single agent PD‐1 inhibitors [19], [20].
In the years considered for this study, rates of treatment on or off trial differed by cancer type. The majority of urothelial carcinoma and HNSCC patients were treated on trial, while the majority of NSCLC patients were treated off trial, which could be expected based on the lag in FDA approval of PD‐1 inhibitor treatment across cancer subtypes. Similarly, pembrolizumab was primarily used to treat clinical trial patients, while nivolumab was mostly used to treat patients off trial, consistent with the commercial availability of these drugs in the diseases analyzed. In addition, more patients off clinical trial had received previous systemic therapy, which can be explained by the approval of nivolumab as second‐line therapy for NSCLC and RCC when the majority of data were collected. There was an increased incidence of dermatitis in trial patients, which may be due to more rigorous reporting of clinically benign irAEs that required no treatment in the trial setting versus the non‐trial setting.
We did find a statistically significant association between low‐grade irAEs and improved ORR, with the difference primarily driven by clinical trial patients. We also found a statistically significant relationship between irAEs and TTNTD when controlling for trial status and cancer subtype.
The lack of strong effect in non‐trial patients may have been due to factors such as sampling error, differences in tumor type, and varying rates and intensity of follow up and response determination between trial and non‐trial patients. However, rates of irAEs and response rates were very similar in trial versus non‐trial patients. Therefore, the lack of association and differences in outcomes could be in part due to selection for better performance status and overall health, as well as closer follow‐up and monitoring for on‐trial patients. As such, the association between irAEs and outcomes needs to be verified in a larger and more homogeneous population. It is also possible that irAEs are a clinical indicator of response to treatment with PD‐1 inhibitors in some cancer subtypes but not in others.
Surprisingly, we found stronger associations between clinical outcomes and low‐ grade irAEs than with high‐grade irAEs. As the incidence of high‐grade irAEs is lower, our study may not have had adequate power to detect an association. Additionally, severe irAEs can be dangerous and even life‐threatening despite immunosuppressive treatment and may prevent clinicians from adequately assessing tumor response. These findings are supported by a recent, large, retrospective analysis of melanoma patients who received nivolumab monotherapy as part of a clinical trial, which found a significantly better ORR in patients who experience any grade irAEs, but no significant difference in patients with grade 3 or 4 irAEs [17].
Immune‐related adverse events that required steroid treatment were marginally associated with TTNTD when controlling for trial participation status and cancer type (p = .05, HR 0.62), but were not associated with OS (p = .13). There was a statistically significant association between need for systemic steroid treatment and irAE grade (p < .001), as would be expected based on irAE management algorithms. Thus, the association between steroid use and TTNTD may be explained in part by patients stopping immunotherapy in the face of irAEs, but not necessitating further treatment. Alternatively, use of steroids could be an indicator of grade 2 or higher irAEs. Most of the irAEs we observed required medical intervention at grade 2 by CTCAE, and grade 2 irAEs appear to be the driver behind the association between low‐grade irAEs and ORR as well as TTNTD.
While several prior studies have suggested an association between the development of irAEs and clinical response in melanoma patients treated with checkpoint blockade, most were with the cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) inhibitor ipilimumab. For example, a recent study showed that melanoma patients who developed grade 3 toxicities requiring steroid treatment while being treated with ipilimumab had higher response rates and a longer median duration of response [21]. Two other studies in melanoma patients treated with PD‐1 inhibitors have had similar results demonstrating that cutaneous irAEs were associated with a better treatment response [14], [22]. In a study of metastatic RCC patients treated with ipilimumab, 33% of patients had grade 3–4 irAEs, and these patients had an ORR of 30%, compared with 0% in those without irAEs [23]. However, compared with anti‐PD‐1 therapy, ipilimumab is associated with a higher incidence of irAEs and a lower ORR, which could impact the potential correlation [24]. It is interesting that Michot et al. found improved ORR with severe irAEs, which differs from our results [24]. The target site could account for differences, as CTLA‐4 inhibitors act primarily at lymphoid organs, while PD‐1 inhibitors are believed to act predominantly in the tumor microenvironment. It is conceivable that this difference could impact the spectrum of T‐cell tolerance and self‐antigens encountered, which could in turn alter the specificity of the immune response. The more recently reported retrospective study in melanoma patients receiving nivolumab by Weber et al. seems to more closely align with our findings, albeit with a stronger association of irAEs with response, perhaps owing to the higher ORR in melanoma‐only patients all treated as part of a clinical trial [17].
Our study is not without limitations. The abovementioned studies were performed in a homogenous disease population, unlike our heterogeneous sample. We may have lost possible associations with individual cancer types due to lack of power, necessitating studies in larger, disease‐specific populations. While the total sample size analyzed may lack power to detect associations, this is, to our knowledge, the largest cohort assessed for an association between irAEs and efficacy in a non‐melanoma population, and it can serve as a hypothesis‐generating finding. Another limitation of our study is our definition of response, which was based on the first scan (before 14 weeks). Response to immunotherapy agents may be delayed; we saw three delayed responses in our patient population. However, based on the large proportion of patients without subsequent scans (50%), we were unable to reliably measure “best response.” To overcome this issue, we confirmed our findings using the alternative measure of TTNTD. Finally, we acknowledge that there are complex interrelationships between the irAEs, number of doses of immunotherapy received, and clinical outcomes. While irAEs are not the cause of better responses, the relationship between outcomes and irAEs may indicate an as‐of‐yet unknown biological mechanism that predisposes patients to both outcomes. We therefore did not require irAEs to happen at a specific timepoint. However, to ensure that the relationship was not simply an artifact of the length of treatment, we reran the analysis in the clinical trial subgroup where there were the most precise data in regard to the timing of irAEs, including only irAEs that occurred early (before the first scan), and found a similarly strong relationship between irAEs and ORR using this stricter definition.
Conclusion
Our study shows an association between low‐grade irAEs and improved ORR as well as a statistically significant relationship between irAEs and TTNTD. It is conceivable that development of irAEs in non‐melanoma patients treated with anti‐PD‐1 agents may correlate with a more robust immune response against the targeted cancer. Differences in the lack of a positive effect seen in patients with high‐grade irAEs may reflect underlying tumor biology or limitations of the power and retrospective nature of our study. Further study, either prospectively or via retrospective analysis of ongoing or recently completed large phase II and III trials, could help answer this question. As CKIs continue to gather clinical indications across disease types and settings, understanding associations, or lack thereof, between irAEs and outcomes is important. The possibility of unconventional responses and pseudoprogression complicates clinical decisions, and if early indicators of treatment response could be identified, it would help clinicians determine the most effective treatment course.
Acknowledgments
We would like to thank Michelle Collins and Deborah Kister for assisting with data acquisition. This work was supported by no outside funding. Part of this work has been previously presented at ASCO Annual Meeting in 2016.
Author Contributions
Conception/design: Matthew Zibelman, Hossein Borghaei, Elizabeth R. Plimack, Ranee Mehra, Daniel M. Geynisman
Collection and/or assembly of data: Julia Judd, Matthew Zibelman, John O'Neill, Chethan Ramamurthy, Sasini Bentota, Jamie Doyle
Data analysis and interpretation: Julia Judd, Matthew Zibelman, Elizabeth Handorf, Chethan Ramamurthy, Robert G. Uzzo, Jessica Bauman, Daniel M. Geynisman
Manuscript writing: Julia Judd, Matthew Zibelman, Elizabeth Handorf, John O'Neill, Chethan Ramamurthy, Sasini Bentota, Jamie Doyle, Robert G. Uzzo, Jessica Bauman, Hossein Borghaei, Elizabeth R. Plimack, Ranee Mehra, Daniel M. Geynisman
Final approval of manuscript: Julia Judd, Matthew Zibelman, Elizabeth Handorf, John O'Neill, Chethan Ramamurthy, Sasini Bentota, Jamie Doyle, Robert G. Uzzo, Jessica Bauman, Hossein Borghaei, Elizabeth R. Plimack, Ranee Mehra, Daniel M. Geynisman
Disclosures
Matthew Zibelman: EMD Serono (C/A), Horizon Pharma (RF); Elizabeth Handorf: Pfizer (RF); Hossein Borghaei: Bristol‐Meyers Squibb, Eli Lilly & Co., Pfizer, Celgene, Boehringer‐Ingelheim, Genentech, Trovogene (C/A), Celgene (H); Elizabeth R. Plimack: AstraZeneca, Bristol‐Myers Squibb, Eli Lilly & Co., Exelexis, Genentech, Novartis, Pfizer, Roche, Synergene (C/A), Acceleron, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly & Co., Novartis, Merck, Peloton, Pfizer (RF); Ranee Mehra: Novartis, Bristol‐Meyers Squibb, Genentech (C/A), Genentech (RF); Daniel M. Geynisman: Pfizer, Novartis (C/A), Pfizer, Merck, Millenium, Genentech (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seiwert TY, Haddad RI, Gupta S et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE‐012 expansion cohort. Abstract presented at: ASCO Annual Meeting Proceedings; 2015; Chicago, IL.
- 7. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015;33:3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodi FS, Hwu WJ, Kefford R et al. Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolchok JD, Hoos A, O'Day S et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 11. George S, Motzer RJ, Hammers HJ et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: A subgroup analysis of a randomized clinical trial. JAMA Oncol 2016;2:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Downey SG, Klapper JA, Smith FO et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL‐associated antigen‐4 blockade. Clin Cancer Res 2007;13:6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teulings HE, Limpens J, Jansen SN et al. Vitiligo‐like depigmentation in patients with stage III‐IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta‐analysis. J Clin Oncol 2015;33:773–781. [DOI] [PubMed] [Google Scholar]
- 14. Sanlorenzo M, Vujic I, Daud A et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eigentler TK, Schlaak M, Hassel JC et al. Effectiveness and tolerability of ipilimumab: Experiences from 198 patients included in a named‐patient program in various daily‐practice settings and multiple institutions. J Immunother 2014;37:374–381. [DOI] [PubMed] [Google Scholar]
- 16. Mian I, Yang M, Zhao H et al. Immune‐related adverse events and survival in elderly patients with melanoma treated with ipilimumab J Clin Oncol 2016;34:3047a. [Google Scholar]
- 17. Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–792. [DOI] [PubMed] [Google Scholar]
- 18. Horvat TZ, Adel NG, Dang TO et al. Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weber JS, Antonia SJ, Topalian SL et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. J Clin Oncol 2015;33:9018a. [DOI] [PubMed] [Google Scholar]
- 20. McDermott DF, Drake CG, Sznol M et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prior LM, Harrold E, O'Leary CG et al. Toxicities in immunotherapy: Can they predict response? J Clin Oncol 2016;34:e14534a. [Google Scholar]
- 22. Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang JC, Hughes M, Kammula U et al. Ipilimumab (anti‐CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michot JM, Bigenwald C, Champiat S et al. Immune‐related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016;54:139–148. [DOI] [PubMed] [Google Scholar]