This article describes the case of a 52‐year‐old male who was diagnosed with anaplastic thyroid cancer. The patient harbored a V600E mutation in BRAF and a PD‐L1 positivity in both the tumor and the tumor infiltrating lymphocytes. Diagnosis and treatment details are reported.
Abstract
Chemotherapy with or without radiation is the standard therapy for anaplastic thyroid cancer (ATC), although the response rate is not high and not durable. We describe a 62‐year‐old male who was diagnosed with ATC and initially treated with a thyroidectomy and lymph node dissection, followed by chemotherapy. Next generation sequencing was then performed to guide therapy and the tumor was found to have BRAF and programmed death‐ligand 1 (PD‐L1) positivity that was subsequently treated with vemurafenib and nivolumab. This led to substantial regression of tumor nodules. Genomic sequencing‐based approaches to identify therapeutic targets has potential for improving outcomes. Currently, the patient continues to be in complete radiographic and clinical remission 20 months after beginning treatment with nivolumab.
Key Points.
Programmed death‐1 (PD‐1)/PD‐L1 immunotherapy has shown evidence of durable responses in certain malignancies such as melanoma, lung cancer, and renal cell carcinoma.
PD‐L1 positive tumors promote autoimmunity against the tumor; therefore, PD‐1/PD‐L1 blockade may be beneficial.
Molecular profiling could possibly result in improved targeted therapy for certain malignancies.
Introduction
Anaplastic thyroid cancer (ATC) is a rare disease that affects one per million persons annually, accounting for less than 10% of all thyroid cancers, and is responsible for half of thyroid cancer deaths each year [1]. Patients with ATC have a median survival of 3–5 months, and there is currently no standardized treatment [1], [2]. Prior data have suggested that the combination of cisplatin with doxorubicin is superior to doxorubicin alone, making these commonly used agents. Despite this, the overall response rates are typically short and the various therapies probably do not impact overall survival at the population level.
Because of the poor prognosis and paucity of therapies, molecular profiling of tumors may provide additional opportunities for therapeutic targets. We describe a dramatic response to nivolumab in a 62‐year‐old man with programmed death‐ligand 1 (PD‐L1) positive ATC.
Patient History
A 62‐year‐old man with a past medical history of ulcerative colitis (UC) and psoriasis presented to an ear, nose, and throat physician for an enlarging right‐sided neck mass. The UC was diagnosed about 3 decades ago and, after intermittent therapies, was in remission. The psoriasis had been treated with intermittent phototherapy and was also well controlled. The patient underwent a fine needle aspiration of the right thyroid mass and this was consistent with thyroiditis. Two months later, he underwent a partial right thyroidectomy due to continued growth of the neck mass. The pathology revealed a 3.8 cm partially encapsulated T2 N0 M0 well‐differentiated papillary carcinoma with no lymphovascular or perineural invasion. He proceeded to have a left thyroidectomy 1 month later and pathology showed only Hashimoto's thyroiditis. His thyroglobulin levels, which were elevated before surgery, decreased but did not normalize postoperatively. A whole‐body radioiodine scan showed activity in the thyroid bed and thus the patient subsequently received a 151.9 mCi oral dose of iodine‐131 for thyroid ablation.
Nine months after the initial presentation, the patient palpated a recurrent enlarging neck mass and underwent a right modified neck dissection. The patient was found to have 1 out of the 23 lymph nodes positive for poorly differentiated adenocarcinoma with extranodal extension. Immunohistochemical studies showed the neoplastic cells to be negative for TTF1 and PAX 8 and positive for p63, CK 7, CK 5/6, pancytokeratin (weak), TTF1 (focal), and EMA (weak), supporting the diagnosis of ATC. Staging was performed with a positron emission tomography (PET)/computed tomography fusion image that showed a 3.4 × 3.0 cm nodule at the level of thyroid with an elevated SUV value of 24, a second focus of activity in the right supraclavicular region, and nodules in the right and left upper lobes of the lung consistent with metastatic disease.
The patient was initially treated with doxorubicin (60 mg/m2) and cisplatin (60 mg/m2) every 3 weeks for two cycles. Unfortunately, the patient tolerated therapy poorly and had evidence of radiographic progression in the lungs. The patient was then treated with second‐line paclitaxel (175 mg/m2) and simultaneously underwent next generation sequencing to guide therapy.
Molecular Tumor Board
Genotyping Results and General Interpretation
Molecular profiling was performed in a Clinical Laboratory Improvement Amendments‐certified laboratory via Paradigm Diagnostics (Paradigm Diagnostics, Phoenix, AZ, http://www.paradigmdx.com). Sequencing results revealed a BRAF V600E mutation, mRNA overexpression of tubulin beta 3, thymidine phosphorylase, and survivin. Additionally, immunohistochemistry (IHC) was performed and the tumor was PD‐L1 3+, PD‐L1 tumor infiltrating lymphocytes (TILs) 1+, PD‐1 tumor 1+, and PD‐1 TILs 2+.
The report indicates a molecular alteration in BRAF, resulting in a change in the encoded amino acid from a valine to glutamic acid at position/codon 600. Programmed death‐ligand 1 IHC is a qualitative immunohistochemical assay using monoclonal mouse anti‐PD‐L1, clone 223C intended for use in the detection of PD‐L1 protein in formalin‐fixed, paraffin‐embedded tissue. Programmed death‐ligand 1 IHC positivity has been implicated as a predictive biomarker in PD‐1/PD‐L1 checkpoint blockade immunotherapy [3]. The molecular tumor board recommendations included the two independent recommendations: target BRAF V600E with vemuarfenib or PD‐L1 IHC positivity with immunotherapy, such as nivolumab.
Significance of Specific Mutation/Expression
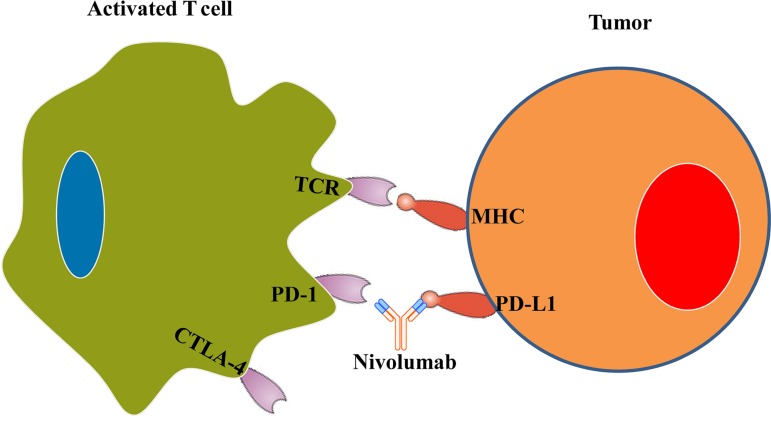
Our patient's tumor was positive for PD‐L1, which interacts with the PD‐1 receptor on activated T cells to dampen the immunologic response and decrease autoimmunity. Programmed death‐1 is part of the inhibitory B7 molecules and is classified as a T‐cell checkpoint molecule. It decreases autoimmunity by inhibiting T‐cell proliferation and interferon‐γ, tumor necrosis factor‐α, and interleukin‐2 production. Therefore, blocking PD‐1/PD‐L1 can help re‐establish T‐cell function and promote tolerance. Nivolumab (Fig. 1) and Pembrolizumab are two approved antibodies that target this PD‐1/PD‐L1 interaction. Recent studies have shown that PD‐1 blockade improved overall survival and progression‐free survival for metastatic melanoma, non‐small cell lung cancer (NSCLC), and renal cell carcinoma [4].
Figure 1.
Programmed death‐ligand 1/PD‐1 interaction mediates inhibition of T‐cell mediated tumor cell killing. Blockade of PD‐L1 with nivolumab results in reactivation of T‐cell mediated tumor cell killing.
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; MHC, major histocompatibility complex; PD‐1, programmed death 1; PD‐L1, programmed death‐ligand 1 (CD274 or B7‐H1 protein); TCR, T‐cell receptor.
BRAF is part of the mitogen‐activated protein kinase (MAPK) signaling pathway, which promotes oncogenesis. The V600E mutation seen in this patient is the most common type of BRAF mutation seen in malignancies. Sustained proliferative signaling occurs when there is continued activation of the MAPK pathway, which promotes cell growth and survival. In the V600E mutation, there is a conformational change in the G‐loop activation segment of BRAF, which allows it to bind the monomers, MEK and ERK, and remain active [5]. Blocking BRAF with inhibitors such as vemurafenib, which was used in this patient, could result in regression of the tumor. A study performed by Hyman et al. suggests that BRAF V600E may be a targetable oncogene in some nonmelanoma cancers such as NSCLC and Langerhans’‐cell histiocytosis [6].
Patient Update
Following progression of paclitaxel monotherapy, the patient was treated with the BRAF inhibitor vemurafenib. He tolerated therapy reasonably well, with the exception of joint aches and generalized body aches. Within days of starting vemurafenib, there was clinical evidence of regression of his right cervical nodal mass, but he developed a rapidly growing new palpable midline neck mass as well as enlargement of his right supraclavicular node and a PET‐avid left upper lobe nodule. Based on concurrent shrinkage of the pre‐existing mass and growth in other areas, this was felt to represent a mixed response. The midline neck mass was resected and pathology showed dense fibrous connective tissue with poorly differentiated carcinoma with squamoid features consistent with ATC.
Based on a mixed response to vemurafenib and given the PD‐L1 positivity, nivolumab was added to the treatment regimen. The patient had progressive joint and body aches, dictating discontinuation of the vemurafenib, and continued on nivolumab monotherapy. Subsequently, clinical exam on demonstrated substantial regression of the right supraclavicular lymph node and imaging showed decrease in the size of the previously documented pulmonary nodules.
Two months into nivolumab therapy, the patient experienced an exacerbation of psoriasis, and this was treated successfully with topical steroids and phototherapy. Computed tomography scans demonstrated continued reduction of pulmonary lesions with complete radiographic resolution (Fig. 2). Following the 12th cycle of nivolumab, the patient experienced nausea, vomiting, and diarrhea, and was found to have acute colitis on colonoscopy deemed to be grade 2 colitis. His acute colitis responded well to a short course of prednisone taper and mesalamine. In total, the patient received 12 cycles of nivolumab, and treatment had been held since the exacerbation of his colitis. The patient continues to be in complete radiographic and clinical remission 20 months after beginning treatment with nivolumab.
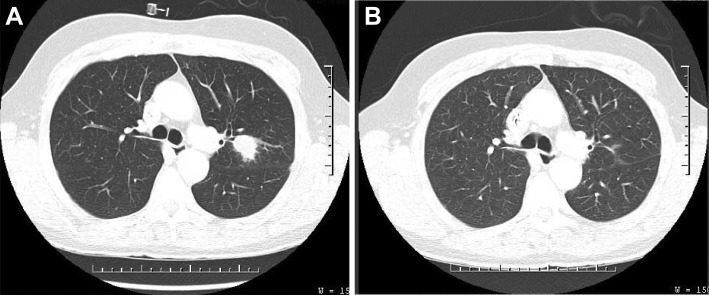
Figure 2.
Comparison chest computed tomography scans of patient with anaplastic thyroid cancer. (A): One year after diagnosis: large left lung nodule. (B): Fifteen months later: left lung nodule after therapy with vemurafenib followed by nivolumab.
Potential Strategies and Implications for Clinical Practice
The genomic landscape of thyroid cancer includes activating mutations in BRAF and RAS, which are observed in both the well‐differentiated thyroid cancers as well as anaplastic tumors. Anaplastic thyroid cancer also carries mutations in p53, TERT promoter, and phosphatidylinositol 3‐kinase, among others [7], [8]. However, limited data exist on immunotherapy, given the extreme virulence and genetic complexity of ATC.
This patient harbored an activating V600E mutation in BRAF. There was a clear clinical response indicating that the BRAF mutation was an important driver for the tumor. Unfortunately, the mixed response may have indicated genomic heterogeneity or acquired resistance of a subclone. This patient also had PD‐L1 positivity in both the tumor and the tumor‐infiltrating lymphocytes. This case suggests that immunotherapy in patients with ATC based upon PD‐L1 evaluation provides a therapeutic option. Observations in more patients will be required to determine durability of response, frequency of response, and potential resistance mechanisms.
Acknowledgments
This publication and project is supported by Indiana University Health, Indianapolis, IN with assistance from the Indiana Clinical and Translational Sciences Institute, and is funded in part by Grant # UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was published online on 04 August 2017. After online publication, minor revision was made to the text. This notice is included in the online and print versions to indicate that it has been corrected on 24 August 2017.
Author Contributions
Conception/design: Revathi Kollipara, Bryan Schneider, Milan Radovich, Sunil Babu, Patrick J. Kiel
Provision of study material or patients: Revathi Kollipara, Bryan Schneider, Milan Radovich, Sunil Babu, Patrick J. Kiel
Manuscript writing: Revathi Kollipara, Bryan Schneider, Milan Radovich, Sunil Babu, Patrick J. Kiel
Final approval of manuscript: Revathi Kollipara, Bryan Schneider, Milan Radovich, Sunil Babu, Patrick J. Kiel
Disclosures
The authors indicated no financial relationships.
References
- 1. Nagaiah G, Hossain A, Mooney CJ et al. Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J Oncol 2011;2011:542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smallridge RC, Ain KB, Asa SL et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104–1139. [DOI] [PubMed] [Google Scholar]
- 3. Meng X, Huang Z, Teng F et al. Predictive biomarkers in PD‐1/PD‐L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015;41:868–876. [DOI] [PubMed] [Google Scholar]
- 4. Buchbinder EI, Desai A. CTLA‐4 and PD‐1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall RD, Kudchadkar RR. BRAF mutations: Signaling, epidemiology, and clinical experience in multiple malignancies. Cancer Control 204;21:221–230. [DOI] [PubMed] [Google Scholar]
- 6. Hyman DM, Puzanov I, Subbiah V et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito K, Hanamura T, Murayama K et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: Improved survival in subgroups of patients with localized primary tumors. Head Neck 2012;34:230–237. [DOI] [PubMed] [Google Scholar]
- 8. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016;375:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]