Abstract
Aim
The aim of this study was to evaluate clinically and radiographically, extraction socket healing using autologous platelet rich fibrin (PRF).
Materials and methods
Twenty-four subjects needing single tooth simple extractions were selected. Twenty-four extraction sockets were divided into test group (PRF, n = 12) and control group (blood clot, n = 12). PRF was prepared with blood drawn from individuals after extraction using standard technique. PRF was placed in test group sockets followed by pressure application and figure 8 sutures. Sockets in control group were allowed to heal in the presence of blood clot and received a figure 8 suture. Ridge width was assessed using cast analysis with the help of acrylic stent and a pair of calipers. Radiographic analysis of socket surface area was performed using computer graphic software program. The clinical follow up assessments were performed at 1, 4 and 8 weeks. Collected data was assessed using ANOVA and multiple comparisons test.
Results
Subjects were aged between 25 and 50 (mean 37.8) years, including 15 females. The mean horizontal ridge width for sockets in the test group were 11.70 ± 2.37 mm, 11.33 ± 2.30 mm and 10.97 ± 2.33 mm at 1, 4 and 8 weeks respectively. Ridge width proportions were significantly higher among test group as compared to control group between baseline to 4 and 8 weeks respectively. The mean radiographic bone fill (RBF) percentage in the test group, was 74.05 ± 1.66%, 81.54 ± 3.33% and 88.81 ± 1.53% at 1, 4 and 8 weeks respectively. The mean RBF was significantly higher in the test group than control group at all time intervals.
Conclusion
The study outcomes demonstrate that the use of PRF accelerate socket wound healing after tooth extraction as noticed by increased bone fill and reduced alveolar bone width resorption using clinical and radiographic methods.
Keywords: Platelet rich fibrin, Extraction socket, Alveolar bone width, Bone resorption
1. Introduction
Tooth extraction is a common dental procedure in the management of tooth decay, complicated fractures, periodontal disease, infections and orthodontic space creation (Buchwald and Kocher, 2013, Gonda and MacEntee, 2013). Physiologic healing of the post-extraction socket involves a complex process of bone cells migration and maturation leading to selective bone resorption and apposition (Cardaropoli and Araujo, 2003, Araujo and Lindhe, 2005). These post extraction events result in dimensional loss in both horizontal and vertical planes of the residual alveolar ridge. Replacement of lost teeth is further complicated, specially in case of implant therapy, due to loss of bone volume required for successful implant treatment. In addition, post extraction bone loss necessitates bone-grafting procedures for implant placement to predictably restore function and esthetic (Penarrocha-Diago and Aloy-Prosper, 2013).
Multiple procedures are employed for prevention of post-extraction bone loss and predictable implant placements after extraction, including socket preservation with grafts (biomaterials), and immediate or early implant placements. While the clinician has a number of graft materials to choose from, some bone graft materials need longer healing time to achieve even a small amount of new bone incorporation into the graft site (Norton and Wilson, 2002). In addition, immediate implant placements to avoid subsequent bone resorption often result in buccal bone defects requiring simultaneous grafts, showing lower success rates compared to non graft implant placements (Le and Borzabadi-Farahani, 2014). Early implant placement is another possible alternative for avoiding post extraction bone loss, however, at 4 weeks bone formation is slow and bone density is suboptimal (Hammerle and Chen, 2004).
Socket preservation using biomaterials has been proposed and autologous platelet concentrates including platelet rich plasma (PRP) with growth factors and platelet rich fibrin (PRF) are employed (Rutherford and Niekrash, 1992, Zhang and Wang, 2007). PRF is a second-generation of autologous growth factors, which encourages healing and is proposed to be associated with effective and early organization of bone substance and bone volume percentage (Dohan and Choukroun, 2006, Kutkut and Andreana, 2012). In addition, PRF is a platelet concentrate with leukocytes in dense fibrin matrix, which can be conveniently prepared from autogenous non anti-coagulated blood when centrifuged (Choukroun and Diss, 2006). Reports with regards to the clinical efficacy of using platelet concentrates (like PRF) in the healing of extraction sockets have been controversial. With studies showing significant and comparable outcomes among control and test groups for assessing the effect of platelet concentrates on post extraction socket preservation (Simonpieri and Del Corso, 2009, Simonpieri and Del Corso, 2012). It is hypothesized that PRF will accelerate socket wound healing after tooth extraction, noticed by increased bone fill and reduced bone resorption. Therefore the aim of this study was to evaluate clinically and radiographically extraction socket healing using autologous platelet rich fibrin (PRF) membrane.
2. Materials and methods
2.1. Patient selection
A total of twenty-four subjects who required tooth extraction and future implant therapy were included in the study. Patients were selected from Periodontics Clinic, of a dental school in Riyadh, Saudi Arabia, Riyadh College of Pharmacy and Dentistry. The protocol for the investigation was approved and registered by the institutional review board of the research center (FPGRP- 43431004/138). The present study was performed in accordance with the declaration of Helsinki. All participants have been informed about the procedure and informed consents in english and Arabic (based on patient background) were obtained.
Inclusion criteria consisted of patients with an unremarkable medical history, subjects with at least one site bordered by minimum of one tooth, nonsmokers, teeth with root fracture, patients having teeth with hopeless periodontal prognosis, teeth with failed endodontic therapy or advanced carious lesion. Patients with systemic diseases, with presence or history of osteonecrosis of the jaws, with use of bisphosphonates, exposure to head and neck radiation, chemotherapy, and patients with distinct peri-apical pathology were excluded. A sample size of minimum of 12 subjects in each group was identified using power calculation, incorporating means and standard deviations from previous studies (Hauser and Gaydarov, 2013).
The patients fulfilling the criteria were randomly allocated into two groups:
Group I (test group-n = 12): Extraction sockets which received platelet rich fibrin.
Group II (control group-n = 12): Eight extraction sockets left for normal healing (blood clot).
2.2. PRF preparation
Immediately after surgical procedure, 20 ml of blood was drawn from each patient in test group without adding anticoagulant. Following blood collection each sample was centrifuged at 3000 rpm (approximately 400 g) for 10 min using compact centrifuge (Hermle labortechnik, Germany). This results in a fibrin clot formation, containing platelets located in the middle of the tube, just between the red blood cell layer at the bottom and acellular plasma at the top. This clot is removed from the tube using sterilized tweezers and the attached red blood cells scraped off and discarded. The PRF clot was then placed on the grid in the PRF Box (Process Ltd., Nice, France), and covered with the compressor and lid (Fig. 1). This produces an inexpensive autogenous fibrin membrane.
Fig. 1.
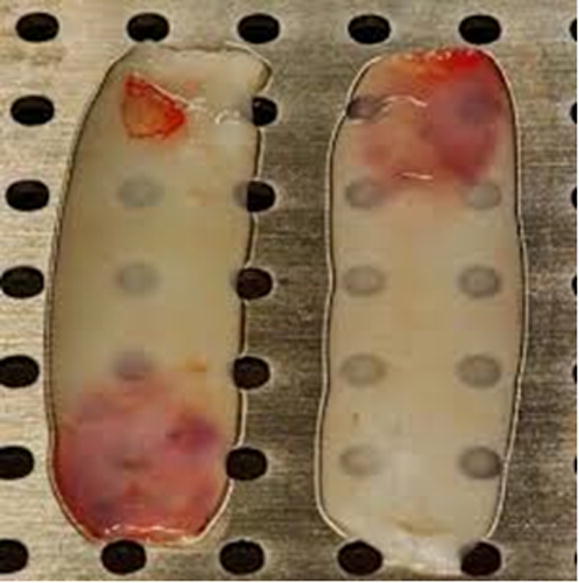
PRF membrane after compression by using PRF box.
2.3. Clinical procedure
All patients were given buccal and lingual/palatal infiltration anesthesia of lidocaine HCl 2% with epinephrine 1:100,000 (Cook-Waite, Rochester, NY). The teeth were extracted with minimal trauma and without flap elevation, using periotomes by single experienced periodontist. The periotome was inserted around as much of the circumference of the root and the socket was dilated. The final delivery of the tooth was performed with forceps. For molars, root separation was performed using surgical bur before the use of periotomes.
The PRF treatment sites (group I) were treated immediately post extraction by placement of PRF, pressure application and figure-8 suture (3-0 chromic gut) (Fig. 2). After removal of the tooth, the control group (II) extraction sites were treated immediately by pressure application and figure-8 suture. Post operative instructions included prevention of wound disturbance. Avoid excessive rinsing and spitting for 48 h. Tongue and fingers should not be used to apply pressure at wound site. No smoking and pulling or lifting of lips.
Fig. 2.
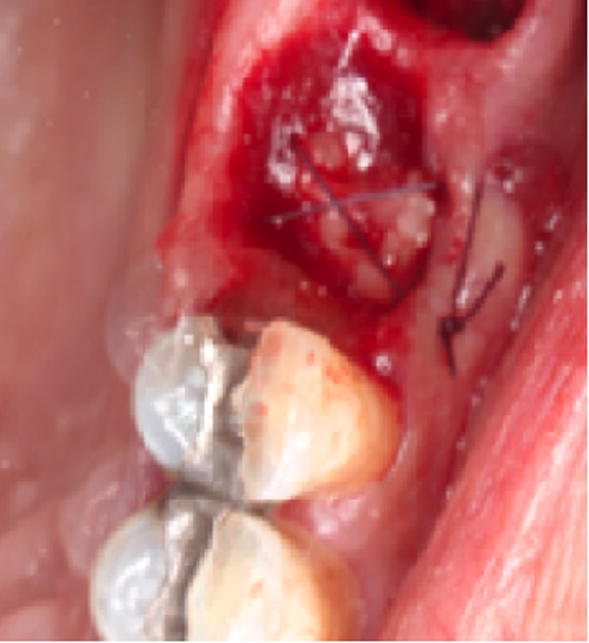
PRF membrane filled in socket and figure 8 suture placed.
2.4. Cast analysis
Patients were seen for postoperative appointments at 1 week, 4 weeks and 8 weeks. Alginate impression for study cast construction was taken after extraction and at each interval. Rigid acrylic stents were made of 3 mm thick light cured resin, based on the cast model prepared after surgical procedure. Two holes at 5 mm from mid-buccal and mid-lingual sites apical to crest were made in the acrylic to create reference points to ensure that the follow-up measurements would be standardized and reproducible. Reference marks were made on the cast at the point of these holes. A digital caliper (accuracy to 1/1000 of an inch) was used to measure alveolar ridge width at these points after each appointment (Fig. 3). The methodology was adopted from previous studies (Simon and Von Hagen, 2000).
Fig. 3.
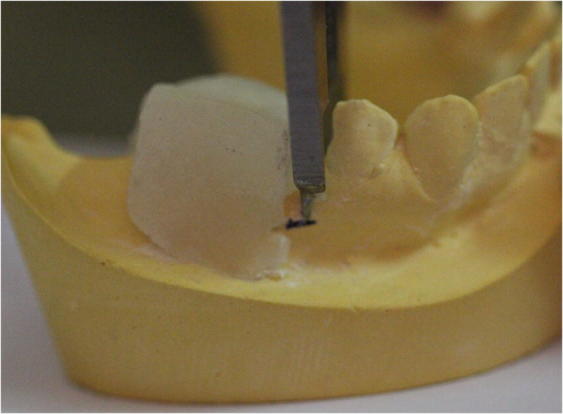
Measurements were taken at the reference marks on the cast at the place of the two holes.
2.5. Radiographic analysis
The surface area of the extraction sockets was measured using computer graphic software program (Adobe Photoshop version 11, adobe system incorporation, 345 Park Avenue, san Joe, 95/10). The size of the extraction sockets were calculated by the technique described by Chiapasco and Rossi (2000). The radiographic images were transferred to software and converted to grayscale tonalities of 256. Auto-tracing of the size of the residual cavity using a magnetic tool was done for each defect. The area marked was converted into a histogram, which gave the number of pixels in the residual cavity. The surface area was calculated in millimeters. The decreasing number of millimeter in the surgical defect overtime gave us the relative bone filling in the area of the lesion. The percentage of radiographic bone fill (RBF) was then calculated. The residual cavity defect and regenerated bone density in both the test group and control group were also calculated using Radio Visio-Graphs to rule out bias (Fig. 4).
Fig. 4.
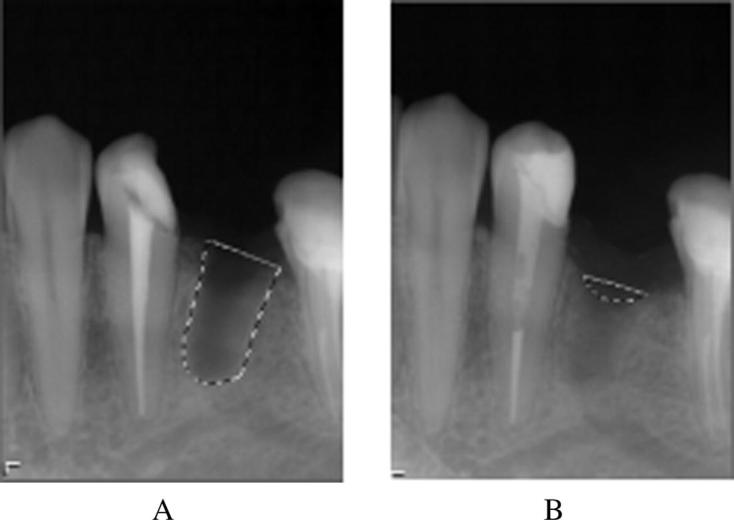
(A) The size of socket calculated by grayscale immediately after extraction. (B) Size of socket calculated in pixels after 8 weeks.
Bone regeneration results of the participants on test group and control group at 1 week, 4 weeks and 8 weeks follow up were compared and statistically analyzed. The radiographic and clinical measurements at the 1st week, 4th week and 8th week follow up appointments were compared for changes in bone fill and alveolar ridge width changes. Means and standard deviations were identified with descriptive statistics and compared using ANOVA and Mann-Whitney U Test (Graphpad-Instat).
3. Result
Twenty-four patients aged between 25 and 50 (mean 37.8) years, including 15 females and 9 males completed the study. Each patient had single tooth extraction.
3.1. Cast analysis results
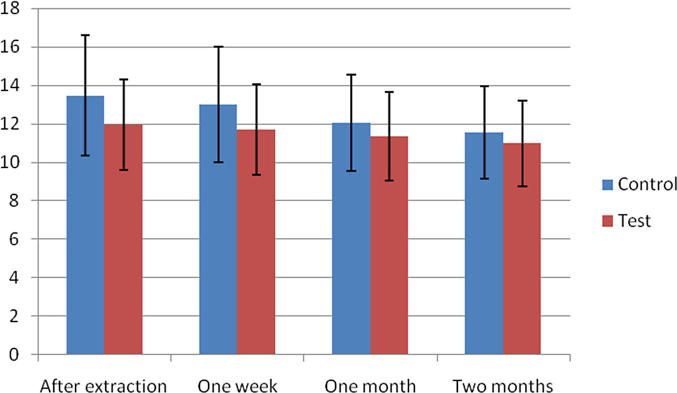
The width of the alveolar ridge was measured after extraction (at baseline) as well as 1 week, 4 weeks, and 8 weeks in both the control and the test groups. The test group presented with a mean horizontal ridge width of 11.94 ± 2.33 mm after extraction, which reduced to 11.70 ± 2.37, 11.33 ± 2.30 and 10.97 ± 2.33 mm at 1, 4 and 8 weeks respectively. For the control group the mean horizontal ridge width was 13.46 ± 3.13 mm after extraction, which reduced to 13.01 ± 3.00 mm, 12.04 ± 2.50 mm and 11.54 ± 2.42 mm at 1, 4 and 8 weeks respectively (Table 1).
Table 1.
Mean ± standard deviation of alveolar ridge width for control and test group right after extraction, 1, 4 and 8 weeks two after extraction in mm.
| Groups | Control | Test | |
|---|---|---|---|
| After extraction | Mean | 13.46 | 11.94 |
| Std. deviation | 3.13 | 2.33 | |
| One week | Mean | 13.01 | 11.70 |
| Std. deviation | 3.00 | 2.37 | |
| Four weeks | Mean | 12.04 | 11.33 |
| Std. deviation | 2.50 | 2.30 | |
| Eight weeks | Mean | 11.54 | 10.97 |
| Std. deviation | 2.42 | 2.23 | |
The mean difference in proportion of alveolar ridge width in control and test groups with regards to the time intervals (baseline, 1 week, 4 weeks and 8 weeks) are presented in Table 2. Significant differences were observed in alveolar ridge width proportions among test and control groups for observations between baseline to 4 and 8 weeks respectively (Fig. 5). Similarly significant ridge width proportion difference was also observed among test and control groups for intervals between 1 week as compared to 4 and 8 weeks respectively (Mann-Whitney U test) (Table 2).
Table 2.
Mean (SD) difference in proportion and P value of alveolar ridge width for control and test group 1, 4 and 8 weeks after extraction.
| Extraction to one week | Extraction to four weeks | Extraction to eight weeks | One week to four weeks | One week to eight weeks | Four weeks to eight weeks | ||
|---|---|---|---|---|---|---|---|
| Control group (mean difference) | 3.26 ± 2.21 | 9.79 ± 6.02 | 13.54 ± 6.57 | 6.72 ± 5.25 | 11.08 ± 6.78 | 4.20 ± 1.47 | |
| Test group (mean difference) | 2.09 ± 0.84 | 5.22 ± 0.80 | 8.58 ± 1.73 | 3.19 ± 0.77 | 6.33 ± 1.35 | 3.24 ± 1.21 | |
| P value | 0.141 | 0.012* | 0.036* | 0.012* | 0.036* | 0.37 | |
The mean difference is significant at the P < 0.05.
Fig. 5.
Mean ± standard deviation of alveolar ridge width for control and test group one week, four weeks and eight weeks after extraction in mm.
3.2. Radiographic analysis
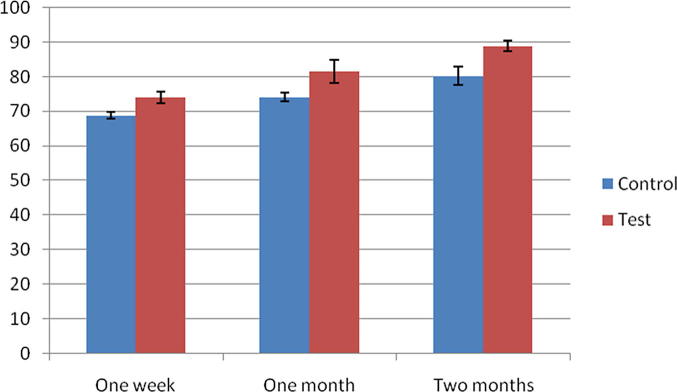
The mean radiographic bone fill (RBF) percentage in the control group at 1, 4 and 8 weeks was 68.82 ± 1.07%, 74.03 ± 1.22% and 80.35 ± 2.61% respectively. While in the test group, the mean radiographic bone fill percentage was 74.05 ± 1.66%, 81.54 ± 3.33% and 88.81 ± 1.53% at 1, 4 and 8 weeks respectively (Table 3). The mean RBF was significantly higher in the test group than control group at all time intervals (1, 4 and 8 weeks) (Table 4) (Fig. 6).
Table 3.
Mean ± standard deviation of bone fill percentage for control and test group at 1, 4 and 8 weeks.
| Control group |
Test group |
|||||
|---|---|---|---|---|---|---|
| One week | Four weeks | Eight weeks | One week | Four weeks | Eight weeks | |
| Mean | 68.8213 | 74.0313 | 80.3488 | 74.0525 | 81.5438 | 88.8088 |
| Std. deviation | 1.07191 | 1.22187 | 2.61328 | 1.66499 | 3.33451 | 1.53355 |
| Minimum | 67.30 | 72.24 | 76.98 | 71.37 | 77.99 | 86.09 |
| Maximum | 70.11 | 75.76 | 84.96 | 76.44 | 86.93 | 90.32 |
Table 4.
Mean difference and p value of bone fill proportion for control and test group at 1, 4 and 8 weeks after extraction in%.
| One week |
Four weeks |
Eight weeks |
||||
|---|---|---|---|---|---|---|
| Control | Test | Control | Test | Control | Test | |
| Mean | 68.82 | 74.05 | 74.03 | 81.54 | 80.35 | 88.81 |
| P value | 0.012* | 0.0* | 0.017* | |||
Statistical significance.
Fig. 6.
Mean ± standard deviation of bone fill percentage for control and test group one week, four weeks and eight weeks.
4. Discussion
The aim of the study was to evaluate extraction socket healing using autologous platelet rich fibrin (PRF) both clinically and radiographically. The hypothesis that PRF will accelerate socket wound healing after tooth extraction, appreciated by increased bone fill and reduced bone resorption was accepted. The mean loss of alveolar ridge width in the test groups (PRF-0.97 mm–8.58%) was significantly less as compared to the control group (No PRF-1.92 mm–13.54%). In addition, comparison between the proportions of the ridge width among the test and control groups showed that there was a statistically significant difference from tooth extraction to 4 weeks and 8 weeks among the two groups, again signifying the impact of using PRF. It is suggested that incorporation of PRF increases the efficiency of cell proliferation. In addition, platelets in the PRF undergo degranulation (He and Lin, 2009) providing a sustained release of growth factors [platelet derived growth factors (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), thrombospondin-1 (TSP-1), transforming growth factor-beta (TGF-β)] influencing angiogenesis, epithelialization, stem cell trapping and immune control (Boyapati and Wang, 2006, Mazor and Horowitz, 2009, Gurbuzer and Pikdoken, 2010). This provides major elements for accelerated bone healing in the presence of PRF.
Traditionally, different alveolar ridge preservation techniques have been used, most of which include the placement of graft material into extraction sockets (Froum and Cho, 2002, Vance and Greenwell, 2004). Use of grafts for socket preservation increases the treatment cost as well as the risk of disease transmission. In addition, the graft is not totally incorporated into the newly formed bone and when compared to sites without graft, they show less vital bone formation (Norton and Wilson, 2002). In addition, in the present study socket occlusion with a PRF membrane was utilized in a flapless manner for ridge preservation. According to Kotsakis and Chrepa (2014), flap advancement for primary closure in ridge preservation interventions may lead to repositioning of the mucogingival junction, displacement of the keratinized mucosa, and ridge resorption. Fickl and Zuhr (2008) studied tissue alterations after tooth extraction with and without surgical trauma on beagle dogs at 4 months. The authors (Fickl and Zuhr, 2008) reported that leaving the periosteum in place decreases the resorption rate of the extraction sockets. Moreover, in a similar study, (Yelamali and Saikrishna, 2015) mean values of bone density for PRF groups were significantly higher as compared to PRP groups at four months follow up.
The present study showed the efficacy of autologous PRF in the healing of extraction sockets. These results are consistent with study by Hauser and Gaydarov (2013) who reported (0.48%) of alveolar bone loss in extraction sockets with PRF without flap elevation compared with (3.68%) in control group at 8 weeks follow up. The authors also reported that micro computed tomographic analysis showed significantly improved microarchitecture and significantly higher bone quality in the PRF group. Similarly in the present study, radiographic data showed statistically significant difference between test and control groups at one, four and eight weeks respectively, with a significant advantage in the test (PRF) group. Interestingly in the present study, significant differences were observed in alveolar ridge width proportions among test and control groups for observations between baseline to 4 and 8 weeks respectively. Similar findings were reported in the study by Simon et al., (Simon and Gupta, 2011) showing a mean width socket resorption of 0.57 mm (7.38%) with PRF after 4 months and confirmed a significant advantage in the preservation of post extraction alveolar ridge dimensions with the use of PRF. Choukroun and Diss (2006) indicated that when a PRF membrane is used, new blood vessels are generated and epithelialization is promoted. Consequently, this facilitates more rapid wound coverage. Also, after a cystic lesion is removed and filled with PRF, the time it takes to be replaced naturally with new bone was after 2.5 months. Similarly, in a study by Simon and Von Hagen (2000) during morphometric tissue experiment in which they planned a socket preservation surgery showed new bone generated in only 3 weeks when the preservation procedure was conducted by using PRF only.
Recently, studies have compared the efficacy of multiple graft materials along with bioabsorbable membranes on alveolar bone healing (Iasella and Greenwell, 2003). A study using freeze-dried bone allografts and collagen membrane showed a mean net loss of 1.2 mm (13.04%) of preoperative alveolar width at 4 months follow up (Iasella and Greenwell, 2003). Similarly Lekovic and Camargo (1998) reported 1.31 mm (17.79%) mean net loss of alveolar width after 4 months of healing when polygalactide/polylactide membrane was used for ridge preservation. These findings are comparable to the present study findings, however the use of available bioabsorbable membranes is associated with a high rate of (upto 25%) membrane exposure, impacting the amount of bone infill within the socket. Therefore it is recommended that further studies with improved materials and techniques comparing the efficacy of PRF and bioabsorbable membranes are undertaken to asses their comparative clinical efficacy in extraction socket preservations. In addition, a possible limitation of the study was the short follow-up of the socket healing, which was only 8 weeks. Therefore, further long-term studies with standardized methodology are warranted. From a clinical perspective, the use of autologous PRF in the healing sockets (extraction sites) and surgical sites is recommended to improve bone healing and minimize resorption.
5. Conclusion
The study outcomes demonstrate that the use of PRF accelerates socket wound healing after tooth extraction as noticed by increased bone fill and reduced alveolar bone width resorption using clinical and radiographic methods.
Acknowledgments
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Araujo M.G., Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005;32(2):212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Boyapati L., Wang H.L. The role of platelet-rich plasma in sinus augmentation: a critical review. Implant Dent. 2006;15(2):160–170. doi: 10.1097/01.id.0000217791.74343.60. [DOI] [PubMed] [Google Scholar]
- Buchwald S., Kocher T. Tooth loss and periodontitis by socio economic status and inflammation in a longitudinal population-based study. J. Clin. Periodontol. 2013;40(3):203–211. doi: 10.1111/jcpe.12056. [DOI] [PubMed] [Google Scholar]
- Cardaropoli G., Araujo M. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003;30(9):809–818. doi: 10.1034/j.1600-051x.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- Chiapasco M., Rossi A. Spontaneous bone regeneration after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. J. Oral Maxillofac. Surg. 2000;58(9):942–948. doi: 10.1053/joms.2000.8732. [DOI] [PubMed] [Google Scholar]
- Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101(3):299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Dohan D.M., Choukroun J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101(3):e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Fickl S., Zuhr O. Tissue alterations after tooth extraction with and without surgical trauma: a volumetric study in the beagle dog. J. Clin. Periodontol. 2008;35(4):356–363. doi: 10.1111/j.1600-051X.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- Froum S., Cho S.-C. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: a pilot study. J. Periodontol. 2002;73(1):94–102. doi: 10.1902/jop.2002.73.1.94. [DOI] [PubMed] [Google Scholar]
- Gonda T., MacEntee M.I. Predictors of multiple tooth loss among socioculturally diverse elderly subjects. Int. J. Prosthodont. 2013;26(2):127–134. doi: 10.11607/ijp.2893. [DOI] [PubMed] [Google Scholar]
- Gurbuzer B., Pikdoken L. Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J. Oral. Maxillofac. Surg. 2010;68(5):980–989. doi: 10.1016/j.joms.2009.09.092. [DOI] [PubMed] [Google Scholar]
- Hammerle C.H., Chen S.T. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int. J. Oral Maxillofac. Implants. 2004;19(Suppl.):26–28. [PubMed] [Google Scholar]
- Hauser F., Gaydarov N. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent. 2013;22(3):295–303. doi: 10.1097/ID.0b013e3182906eb3. [DOI] [PubMed] [Google Scholar]
- He L., Lin Y. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009;108(5):707–713. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Iasella J.M., Greenwell H. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J. Periodontol. 2003;74(7):990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- Kotsakis G., Chrepa V. Flapless alveolar ridge preservation utilizing the “socket-plug” technique: clinical technique and review of the literature. J. Oral Implantol. 2014;40(6):690–698. doi: 10.1563/AAID-JOI-D-12-00028. [DOI] [PubMed] [Google Scholar]
- Kutkut A., Andreana S. Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet-rich plasma: a clinical and histomorphometric study in humans. J. Periodontol. 2012;83(4):401–409. doi: 10.1902/jop.2011.110237. [DOI] [PubMed] [Google Scholar]
- Le B.T., Borzabadi-Farahani A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J. Craniomaxillofac. Surg. 2014;42(5):552–559. doi: 10.1016/j.jcms.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Lekovic V., Camargo P.M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J. Periodontol. 1998;69(9):1044–1049. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- Mazor Z., Horowitz R.A. Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J. Periodontol. 2009;80(12):2056–2064. doi: 10.1902/jop.2009.090252. [DOI] [PubMed] [Google Scholar]
- Norton M.R., Wilson J. Dental implants placed in extraction sites implanted with bioactive glass: human histology and clinical outcome. Int. J. Oral Maxillofac. Implants. 2002;17(2):249–257. [PubMed] [Google Scholar]
- Penarrocha-Diago M., Aloy-Prosper A. Localized lateral alveolar ridge augmentation with block bone grafts: simultaneous versus delayed implant placement: a clinical and radiographic retrospective study. Int. J. Oral Maxillofac. Implants. 2013;28(3):846–853. doi: 10.11607/jomi.2964. [DOI] [PubMed] [Google Scholar]
- Rutherford R.B., Niekrash C.E. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys.“. J. Periodontal. Res. 1992;27(4 Pt 1):285–290. doi: 10.1111/j.1600-0765.1992.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Simon B.I., Von Hagen S. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J. Periodontol. 2000;71(11):1774–1791. doi: 10.1902/jop.2000.71.11.1774. [DOI] [PubMed] [Google Scholar]
- Simonpieri A., Del Corso M. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part II: implant surgery, prosthodontics, and survival. Implant Dent. 2009;18(3):220–229. doi: 10.1097/ID.0b013e31819b5e3f. [DOI] [PubMed] [Google Scholar]
- Simonpieri A., Del Corso M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery Part 2: bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012;13(7):1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- Vance G.S., Greenwell H. Comparison of an allograft in an experimental putty carrier and a bovine-derived xenograft used in ridge preservation: a clinical and histologic study in humans. Int. J. Oral Maxillofac. Implants. 2004;19(4):491–497. [PubMed] [Google Scholar]
- Yelamali T., Saikrishna D. Role of platelet rich fibrin and platelet rich plasma in wound healing of extracted third molar sockets: a comparative study. J. Maxillof. Oral Surg. 2015;14(2):410–416. doi: 10.1007/s12663-014-0638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang Y. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials. 2007;28(8):1515–1522. doi: 10.1016/j.biomaterials.2006.11.040. [DOI] [PubMed] [Google Scholar]