Abstract
Noise exposure that causes a temporary threshold shift but no permanent threshold shift can cause degeneration of synaptic ribbons and afferent nerve fibers, with a corresponding reduction in wave I amplitude of the auditory brainstem response (ABR) in animals. This form of underlying damage, hypothesized to also occur in humans, has been termed synaptopathy , and it has been hypothesized that there will be a hidden hearing loss consisting of functional deficits at suprathreshold stimulus levels. This study assessed whether recreational noise exposure history was associated with smaller ABR wave I amplitude and poorer performance on suprathreshold auditory test measures. Noise exposure histories were collected from 26 men and 34 women with hearing thresholds ≤ 25 dB hearing loss (HL; 250 Hz to 8 kHz), and a variety of functional suprathreshold hearing tests were performed. Wave I amplitudes of click-evoked ABR were obtained at 70, 80, 90, and 99 dB (nHL) and tone-burst evoked ABR were obtained at 90 dB nHL. Speech recognition performance was measured in quiet and in competing noise, using the Words in Noise test, and the NU-6 word list in broadband noise (BBN). In addition, temporal summation to tonal stimuli was assessed in quiet and in competing BBN. To control for the effects of subclinical conventional hearing loss, distortion product otoacoustic emission amplitude, an indirect measure of outer hair cell integrity, was measured. There was no statistically significant relationship between noise exposure history scores and ABR wave I amplitude in either men or women for any of the ABR conditions. ABR wave I amplitude and noise exposure history were not reliably correlated with suprathreshold functional hearing tests. Taken together, this study found no evidence of noise-induced decreases in ABR wave I amplitude or signal processing in noise in a cohort of subjects with a history of recreational noise exposure.
Keywords: Hidden hearing loss, speech in noise, noise exposure history, ABR wave I amplitude, and temporal summation
Learning Outcomes: As a result of this activity, the participant will be able to (1) identify the primary differences in hidden hearing loss research in animal studies versus human studies and (2) discuss how the theoretical premise of hidden hearing loss may be applicable to relationships among recreational noise exposures, auditory brainstem response wave I amplitude, and suprathreshold measures in the current human study.
Recreational and occupational noise exposures have the potential to permanently damage the inner ear. The risk for injury and the amount of potential damage are related to the duration and level of the exposure; thus, guidelines have been established in an effort to reduce the likelihood of permanent hearing loss as a consequence of workplace noise exposure. 1 2 Whereas occupational noise exposure is often experienced as chronic exposure to elevated sound levels multiple hours per day and multiple days per week, recreational noise is likely to fluctuate in sound pressure level (SPL) and frequency and is likely to be shorter in duration. These factors make it difficult to assess risk for hearing loss as a function of recreational noise. Despite these challenges, there has been significant interest in researching the potential for hearing loss associated with personal listening device use, 3 4 5 6 concert music exposures, 7 8 9 and other nonoccupational exposures. 10 Although many studies suggest relatively low risk for permanent hearing loss as a function of typical recreational exposures, some studies have reported small deficits (i.e., on the order of 3 to 6 dB) in the extended high-frequency (EHF) range. 3 5 More recently, there have been suggestions that recreational noise exposure could result in functional deficits (such as hearing in noise or in environments with competing sound sources) that are hidden behind a normal audiogram. 11 12
Suggestions that recreational noise may result in hidden hearing loss are based on several studies in which animals were exposed to acute hazardous noise events. The exposures are typically 2 to 4 hours in duration and they result in a robust temporary threshold shift (TTS), described as a 30 to 50 dB increase in threshold sensitivity at the most affected frequencies when measured 24 hours post–noise exposure. Following the exposure, no outer hair cell (OHC) loss or permanent threshold shift is observed; however, electrophysiologic deficits in the form of reduced auditory brainstem response (ABR) wave I amplitudes at suprathreshold sound levels suggest permanent afferent synaptic damage despite the complete threshold recovery. 13 14 15 16 Threshold is typically measured using both ABR and distortion product otoacoustic emission (DPOAE) tests. The ABR and DPOAE threshold shifts recover, but the suprathreshold ABR wave I amplitude remains reduced in mice and guinea pigs. 13 14 15 16 The reduction in ABR wave I amplitude observed at the higher test frequencies (where the largest TTSs were observed) suggests an underlying permanent noise-induced neural pathology. Consistent with selective neural pathology, postmortem histologic analysis reveals changes in inner hair cells (IHCs) and pre- and postsynaptic neural elements, with no reported damage to the OHCs. 14 Kujawa and Liberman have suggested that a likely functional correlate of this neural injury is difficulty understanding speech in noisy environments, 17 which has stimulated significant interest in speech-in-noise tests for use in clinical trials and other studies of hidden hearing loss. 18 19 20
More recent animal studies have shown that lower-level exposures resulting in smaller TTS deficits (<30 dB) do not result in neural pathology, 12 21 22 even when the TTS-inducing exposure has been repeated weekly over a 6-week period. 23 Although specific dose–response relationships remain to be determined, when the duration of an acute exposure at a lower, previously nonpathologic, sound level was extended from 2 hours to 8 hours, neural pathology emerged with the longer duration exposure, demonstrating that both noise level and exposure duration influence the risk of synaptopathic injury. 22 Taken together, the findings from animal studies indicate that recovery of TTS cannot be interpreted as necessarily reflecting complete recovery of the auditory system and that there is a relationship between time and intensity with respect to risk of synaptopathic damage. These data have led to efforts to translate the data to humans, including an effort to identify potential at-risk populations. A major issue related to the translation to humans is the extent to which the kinds of acute TTS changes that appear to be required in rodents are experienced in humans in occupational or recreational settings (for discussion, see Dobie and Humes 24 ).
Although several studies have attempted to determine associations among noise exposure history, reduced ABR wave I amplitude, and poorer speech-in-noise performance in human participants (see for example, Bramhall et al, 25 Liberman MC et al, 26 and Stamper and Johnson 27 ), the results are not convincing. For example, Stamper and Johnson reported a relationship in which greater recreational noise exposure within the past 12 months was significantly associated with smaller ABR wave I amplitude. 27 However, based on their subsequent analyses controlling for sex differences, Stamper and Johnson later reported decreasing ABR wave I amplitude values as a function of increasing noise exposure history were limited to females, with no evidence of such a relationship in males. 28 Bramhall et al reported a correlation between ABR wave I amplitude and speech-in-noise performance, 25 consistent with hypotheses regarding speech-in-noise deficits, however, the relationship was only evident with overt hearing loss; the relationship was not observed in normal hearing participants. More recently, Liberman et al reported significant differences in EHF thresholds, word recognition scores, summating potential (SP) amplitudes, and ratio of SP to action potential (AP) in college students at risk for noise injury (largely those studying music) relative to those at low risk (largely those enrolled in a communication sciences program). 26 However, AP amplitude was not reliably different between the high- and low-risk groups.
In two other recent studies assessing potential relationships between recreational noise history and ABR wave I amplitude, there were no statistically significant relationships between recreational noise exposure history and ABR wave I amplitude in either healthy young adults or young adults with diabetes mellitus. 29 30 In contrast to these studies, when Bramhall et al recruited participants with high levels of noise exposure during military service and civilian recreational firearm use, they observed reduced ABR wave I amplitudes in these groups relative to nonveteran participants with no history of firearm use and veterans categorized as having low noise exposure. 31 Taken together, although some of these retrospective analyses provide preliminary evidence that is consistent with a potential noise-induced synaptopathic injury in humans, other data clearly raise questions about where risk begins, which populations may be at risk, and whether there is a selective neural injury in humans or a relatively more mixed pathology. 32 At this time, there are no data from human participants directly demonstrating causal relationships between: (1) noise exposure history and ABR wave I amplitude reductions; or (2) noise-induced reductions in ABR wave I amplitude and poorer speech-in-noise performance or degraded temporal processing. The current investigation was therefore designed to further assess the potential relationships between noise exposure history, ABR wave I amplitude, the processing of signals in noise, and temporal processing.
To address the relationship among noise-exposure history, ABR wave I amplitude, and functional measures of hearing, this study included measures of ABR wave I amplitude, noise-exposure history, hearing in noise and hearing in babble, and temporal processing. Subjects included college-aged students with a history of exposure to varying amounts of common recreational noise. Based on the existing data, it was hypothesized that increased levels of recreational noise may be reliably associated with both reduced ABR wave I amplitude and poorer speech-in-noise performance and temporal processing. Multiple functional tests were used to identify potential deficits in speech processing in noise backgrounds both with and without informational masking (i.e., babble background versus broadband noise (BBN) background; for recent discussion of test differences, see Le Prell and Clavier 20 ). Some evidence suggests noise exposure has the potential to affect temporal processing, 33 34 35 and thus the design also included tasks that assessed temporal summation in quiet and in a noise background, in an effort to assess potential temporal processing deficits for signals in quiet and in noise.
Methods
Participants
All testing was performed on the University of Florida Campus in Gainesville, Florida. Procedures were approved by the University of Florida's Institutional Review Board. Participation was voluntary; and participants who completed the study were compensated monetarily and with possible extra credit in specified courses. Inclusion criteria required that participants be aged between 18 and 30, have good general health, and provide written informed consent. Participants were required to have at least 50% of each ear canal unoccluded, and both tympanic membranes had to be visible and intact. Investigations of hidden hearing loss, by definition, require a population in which there is no overt hearing loss; therefore, hearing thresholds were required to be ≤25 dB hearing loss (HL) at octave frequencies between 250 and 8,000 Hz, as well as 3,000 and 6,000 Hz. To determine eligibility, otoscopic and audiometric evaluations were completed after participants provided written informed consent. Sixty-five potential participants contacted the research team and were evaluated; 5 participants were excluded because of obstructive cerumen or audiometric thresholds greater than 25 dB HL at one or more frequencies. Of the 60 participants that completed the study, 26 identified as men (43.3%; 18 to 29 years old, mean = 21.1 years, standard deviation [SD] = 2.7 years) and 34 identified as women (56.7%; 18 to 23 years old, mean = 20.4 years, SD = 1.0). Following the methods of Stamper and Johnson, 27 participants were persons with varying noise exposure; they were not solicited or selected based on a specific noise exposure history.
Study Procedures
Otoscopy
Otoscopy was performed to ensure that ear canals were clear, showed no signs of abnormality, and were unobstructed with both tympanic membranes visible. A diagnostic otoscope (Welch Allyn, Inc., Skaneateles Falls, NY) with a halogen bulb and standard lens magnification of 2.2 times was used. A new disposable tip was used for each participant.
Audiometric Testing
Acoustic stimuli (tones, 250 to 8,000 Hz) were presented using a GSI 61 audiometer (Grason-Stadler, Viasys Healthcare, Madison, WI) and ER-3A insert earphones (Etymotic Research, Inc., Elk Grove Village, IL) calibrated in accordance with American National Standards Institute S3.6–1996. 36 A series of three brief pulsed tones was presented at 30 dB HL, with subsequent levels selected using the modified 5-up 10-down Hughson-Westlake method. 37 Participants were instructed to respond as soon as they heard the acoustic stimulus, and threshold was then defined as the lowest level where two out of three responses were obtained on an ascending series.
Words in Noise
The Words in Noise (WIN) test was administered using the protocol and two 35-word lists described by Wilson and Burks. 38 Specifically, monosyllabic, Northwestern University Auditory Test #6 (NU-6) words were presented monaurally at seven signal-to-babble ratios. Babble consisted of multitalker background at 80 dB SPL; speech target levels varied from 104 to 80 dB SPL in 4 dB decrements. Participants were instructed to ignore the background noise and repeat the target words. Participants were encouraged to guess when they were unsure of the target word. The WIN test provides insight into whether participants demonstrate difficulty encoding acoustic information at suprathreshold levels (80 to 104 dB SPL) in the presence of loud (80 dB SPL) multitalker babble. It has been suggested that deficits will be observed for difficult speech-in-noise tests as a function of IHC dysfunction and/or neural loss in otherwise normal hearing persons.
Words in Broadband Noise
The Words in Broadband Noise (WIBBN) test was performed separately for both ears using recorded NU-6 words played monaurally at 80-dB HL. A competing BBN was presented using the GSI 61 audiometer white noise function. For the first 25 NU-6 words, BBN was fixed at 50 dB HL. During the second 25 NU-6 words, the BBN level was fixed at 60 dB HL. Participants were instructed to ignore the background noise and repeat the target words. If unsure about the target word, participants were encouraged to guess the correct word. The WIBBN test is a potentially easier speech-in-noise task than the WIN task as there is no informational masking of the speech targets by the background noise. Thus, the WIBBN was used as a complement to the WIN to determine if any observed deficits would extend to difficulty at either signal-to-noise ratio with masking stimuli unrelated to linguistic content.
Distortion Product Otoacoustic Emission
DPOAEs were recorded from both ears of all participants using an Intelligent Hearing Systems, IHS 4820, Version 3.69 and Smart-OAE software (Intelligent Hearing Systems Corporation Miami, FL). The probe assembly was coupled to the subject's ear with an appropriately sized soft ear tip. Both the DPOAE response and the noise floor data were recorded. Responses were elicited by two simultaneously presented primary tones, with frequencies f2/f1 = 1.2, and levels L1/L2 at 65/55 (following Lee et al 39 ) and 53/35 dB SPL (following Oswald and Janssen 40 and Wagner et al 41 ). The f2 frequencies were at 2, 3, 4, 6, and 8 kHz. DPOAE amplitudes (2f1 - f2) and adjacent noise floors were averaged and analyzed. DPOAE amplitude was measured to assess potential OHC involvement in any reduced ABR wave I amplitudes or reduced performance on suprathreshold functional tests.
Auditory Brainstem Response
Testing was performed using an Intelligent Hearing System, IHS 4820, Version 3.69 and SmartEP software (Intelligent Hearing Systems Corporation, Miami, FL). Surface electrodes were placed on the high and low forehead and the ipsilateral earlobe in a conventional electrode montage. All participants had hearing within normal limits (≤ 25 dB HL); thus, all ABR stimulus levels were at suprathreshold intensities. In animal models, the diagnostic gold standard for hidden hearing loss is reduced ABR wave I amplitude for stimuli presented at suprathreshold intensities. All electrode impedances were less than 5 kΩ. 100 microsecond rarefaction clicks, at a rate of 21.1/s, were presented at 70, 80, 90, and 99 dB normal hearing level (nHL) through ER-3A (300-ohm) (Etymotic Research, Inc., Elk Grove Village, IL) insert earphones.
Tone-burst testing was performed twice at 90 dB nHL. During the first run, Tiptrode electrodes (Etymotic Research, Inc., Elk Grove Village, IL) were used, and during the second run, earlobe electrodes were used. Tiptrode electrodes were placed in the ear and a stimulus at 4,000 Hz was presented, at a rate of 27.1/s with an intensity level of 90 dB nHL and alternating polarity with a duration of 1,250 microseconds. A minimum of 2,000 accepted trials per run were obtained, averaged, and stored. The same parameters were used for the earlobe electrodes. A completed ABR test therefore consisted of 12 ABR stimulus conditions being evaluated (i.e., four click conditions and two tone-burst conditions per ear). Latencies for wave I, III, and V were calculated and peak-to-trough amplitudes of wave I were recorded, measured, and analyzed at each intensity level for clicks and tone-bursts.
Temporal Summation in Quiet and Noise
In normal hearing individuals, tone detection thresholds increase (get worse) when the duration of a target signal is reduced from 300 milliseconds to 3 milliseconds. 42 Monaural thresholds were measured for a 4,000 Hz tone at 3, 30, and 300 milliseconds, and then the difference in thresholds was calculated for 300 milliseconds versus 30 milliseconds stimuli, and for 30 milliseconds stimuli versus 3 milliseconds stimuli for each ear. If the history of noise exposure affects the detection of short-duration signals in people with otherwise normal audiograms, then the difference thresholds should be larger in those with a history of noise exposure.
The tone stimuli (Audacity software 1.3.5, β version 2008) were played through the audiometer, using an RCA external cable, and delivered using Telephonic Dynamic Headphones (TDH-39; Telephonics Corporation, Farmingdale, NY). Tones were presented monaurally in quiet, using the modified 5-up 10-down Hughson-Westlake method, with a burst of three tones at each presentation level. If a response was obtained, the level was decreased by 10 dB and a burst of three tones was presented again until a threshold level was determined. This procedure was repeated for each signal duration with no masking noise present (temporal summation in quiet). The test protocol was then repeated in the presence of BBN (white noise on the GSI 61 audiometer). The BBN was fixed at 50 dB HL for each signal duration.
Noise Exposure Questionnaire
The noise questionnaire was a modified version of the noise exposure questionnaire used by Spankovich; 43 the primary modification was that the section of the questionnaire specific to diabetes was deleted. Noise exposure scores based on the survey were derived from the A-weighted equivalent continuous noise level (L Aeq ) formula used by Megerson, 44 which is derived from the National Institute of Occupational Safety and Health (NIOSH) recommended exposure limit (REL) of 85 A-weighted decibels for 8 hours with a 3 dB exchange rate used to adjust for exposure at other sound levels. 1 The number of hours worked annually, given an 8-hour work day, is modeled as 2,000 hours (8 h/d × 5 d/wk × 50 wk/y). Work-related noise exposure during the 2,000 annual work hours are then represented as L Aeq2000 , 45 but work hours are assumed to be only a portion of the total hours in a year during which noise exposure occurs (24 h/d × 365 d/y = 8,760 h/y). Therefore, to estimate total annual exposure (L Aeq8760 ), a REL is calculated for the combined occupational and nonoccupational exposures for 8,760 h/y. Nonoccupational exposures were divided into nine potentially hazardous noise exposure categories (power tools, heavy equipment/machinery, commercial sporting/entertainment events, motorized vehicles, small/private aircraft, musical instrument playing, music listening via personal earphones, music listening via audio speakers, and occupational noise) and one category for routine environmental sounds. Occupational exposure was queried during the survey, but most of the students were not regularly employed, or were unemployed. Thus occupational noise exposure was only reported by a few students.
Per NIOSH, 1 for every doubling of time, the allowed exposure level decreases by 3 dB. Megerson suggests the increase from 2,000 work hours to 8,760 total hours equates to approximately doubling the time twice, requiring a 6 dB decrease in the REL. 44 Thus, given an allowable 8-hour exposure limit of 85 dB (2,000 h/y), the REL for total annual exposure (8,760 h/y) is suggested to be 79 dB (i.e., a 6-dB reduction from 85 dB based on the 3-dB decrease in level per doubling of time, for two doublings of time). The lowest possible L Aeq8760 score is 64, representing exposure to normal environmental sound levels at all times with no exposure to noise from the nine potentially hazardous categories. The maximum L Aeq8760 score is 95.5 representing the sum of the highest scores possible in each noise activity category (based on duration) measured on this noise exposure history scale. Noise exposure (L Aeq8760 ) was also used by Stamper and Johnson to calculate routine recreational and occupational noise in normal hearing participants. 27 28 Their selection of 79 dB as a boundary for high and low noise was specifically based on the premise that a 79 dB exposure over 8,760 h/y is approximately equivalent to the NIOSH limit of 85 dB exposure over 2,000 h/y. We used the same criterion to maintain consistency with this previous work.
Data Analysis
All statistical analyses were conducted using Statistical Package for the Social Sciences software version 22 (IBM, Armonk, NY) and SigmaPlot software (Systat Software, San Jose, CA). Pearson bivariate correlation was used to describe the relationships observed between ABR wave I amplitude and noise exposure history, speech-in-noise tests, and the temporal summation test. Unpaired t tests were used to determine the significance of mean differences between men and women for ABR wave I amplitudes and noise exposure history, and paired samples t tests were used to determine if there were significant differences between the right and left ears for threshold data and functional measures. Finally, a two-way analysis of variance (ANOVA) was performed to assess whether there were significant differences between men and women suspected of having a history of TTS. During initial analyses, Bonferroni corrections were applied, however, this correction was found to be too conservative and unacceptably increased the likelihood of a type II error, in which a real difference might be incorrectly rejected based on the very small α value allocated for each comparison. To minimize the possibility of type I and type II errors, the significance level was therefore set at α = 0.01 for all comparisons.
Results
Noise Exposure History
Male and female cohorts were compared for potential differences across variables. As shown in Fig. 1 , women had a mean 1-year noise exposure score (L Aeq8760 ) of 78 dB (SD = 3.7; range = 64 to 84), and men had a mean 1-year noise exposure score (L Aeq8760 ) of 79 dB (SD = 4.7, range = 68 to 87). One-year noise exposure scores (L Aeq8760 ) were higher than 79 dB for 54% of the male participants ( n = 14) and 50% of the female participants ( n = 17). An unpaired t test revealed no statistically significant differences between noise exposure scores for men and women ( t [58] = 0.803, p = 0.426).
Figure 1.
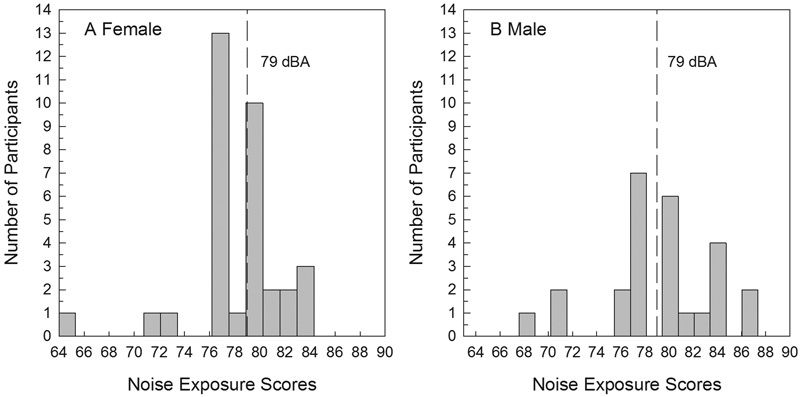
One-year L Aeq8760 noise exposure score distribution for women (A) and men (B). The dotted line in each figure represents the 79 dB exposure line, separating high exposure scores from low exposure scores.
Threshold Data
Female participants had lower hearing thresholds than male participants across the frequency range of 250 to 8,000 Hz; these differences were statistically significant at 1,000 Hz ( t [58] = − 2.819, p < 0.01) and 2,000 Hz ( t [54.6] = − 4.221, p < 0.01 [ Fig. 2 ]). Although statistically reliable, these threshold differences were small (i.e., ∼ 2 dB) and were not clinically significant. These differences replicate those reported for several other cohorts of normal hearing young adults, with men having slightly poorer hearing at a small number of frequencies across cohorts. 46 47 48 The relationship between threshold at 4,000 Hz and noise exposure score (L Aeq8760 ) was evaluated for men ( Figs. 3A , 3B ) and women ( Figs. 3C , 3D ), using Pearson bivariate correlation, as 4,000 Hz is the frequency at which noise is most likely to affect thresholds. Thresholds at 4,000 Hz were evaluated for the right ( Figs. 3A , 3C ) and left ( Figs. 3B , 3D ) ears given the potential for different exposures at each ear; however, there was not a statistically significant association between thresholds and L Aeq8760 noise exposure scores and there were no consistent trends observed across conditions ( Figs. 3A to 3D ).
Figure 2.
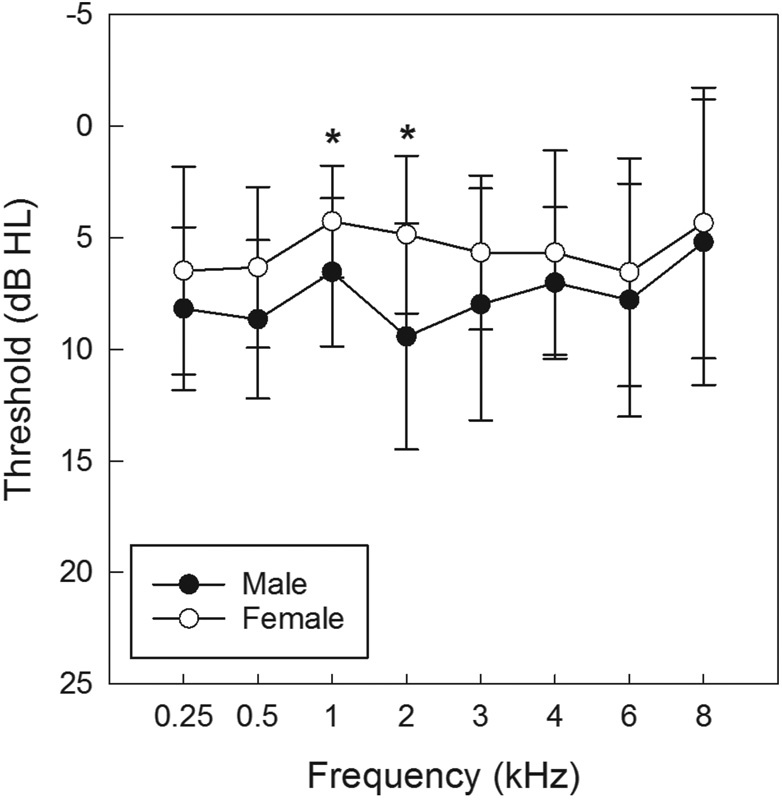
Average threshold sensitivity for men and women at frequencies from 250 to 8,000 Hz (mean ± standard deviation). HL, hearing loss.
Figure 3.
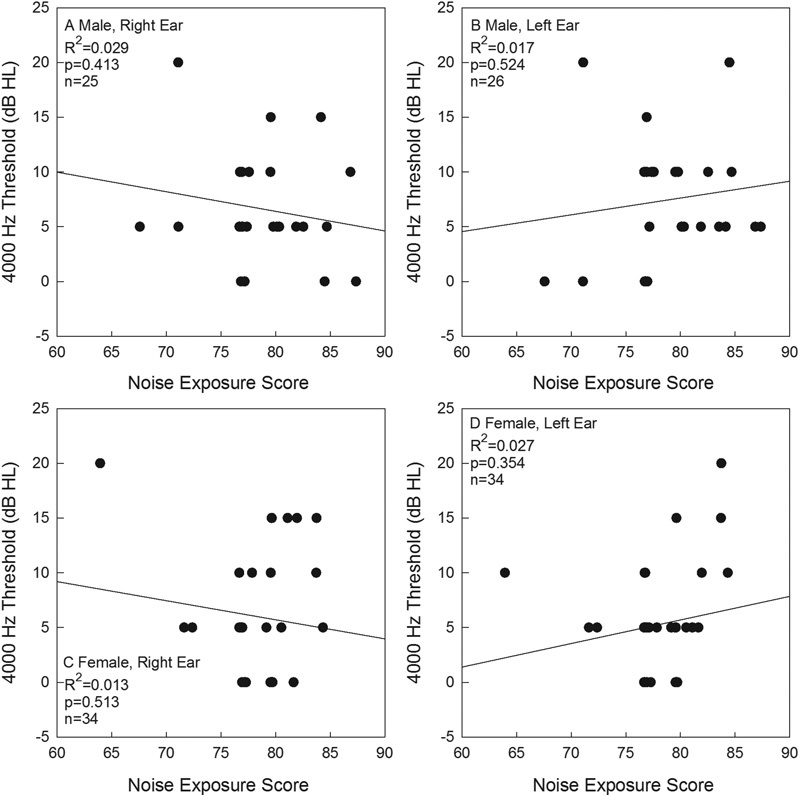
Male (A, B) and female (C, D) thresholds at 4,000 Hz shown as a function of noise exposure history. Data are shown for right ears (A, C) and left ears (B, D). HL, hearing loss.
Distortion Product Otoacoustic Emission Amplitude
Women had significantly larger DPOAE amplitudes than men in the right and left ear at 2,000, 4,000, and 6,000 Hz, for the tests completed at L1 = 65. For the tests completed at L1 = 53, DPOAE amplitudes were significantly larger in women in the right ear at 3,000, 4,000, and 6,000 Hz. There were no statistically significant differences between men and women for DPOAE amplitude in the left ear at L1 = 53. As shown in Table 1 , there were no statistically significant relationships between noise exposure score (L Aeq8760 ) and DPOAE amplitude revealed within the correlation analysis completed at each frequency (2,210, 2,782, 3,506, 4,416, 5,565, 7,013, 8,837 Hz) at test levels of 53/35 or 65/55. A small subset of the correlation analyses had p values less than 0.05, although none of these relationships were statistically significant at the 0.01 criterion established a priori ( Table 1 ). Interestingly, in all three conditions that approached significance, DPOAE amplitude was observed as increasing (improving) with increasing noise exposure (L Aeq8760 ). However, given the small number of frequency × level conditions in which the relationship approached statistical significance, it seems unlikely that these relationships represent a significant effect of recreational noise history on DPOAE amplitude.
Table 1. Relationship between Distortion Product Otoacoustic Emission (DPOAE) Amplitude and Noise Exposure Score in Males and Females.
| Sex | Level | DPOAE Frequency | RE vs One Year Noise Exp. | LE vs One Year Noise Exp. |
|---|---|---|---|---|
| r ( p ) | r ( p ) | |||
| Female | 53/35 | 2210 Hz | −0.057 (0.78) | −0.030 (0.87) |
| Female | 53/35 | 2782 Hz | −0.014 (0.94) | 0.040 (0.82) |
| Female | 53/35 | 3506 Hz | 0.218 (0.22) | −0.167 (0.35) |
| Female | 53/35 | 4416 Hz | 0.237 (0.18) | 0.013 (0.94) |
| Female | 53/35 | 5565 Hz | 0.242 (0.17) | 0.095 (0.59) |
| Female | 53/35 | 7013 Hz | 0.152 (0.39) | 0.007 (0.97) |
| Female | 53/35 | 8837 Hz | −0.012 (0.95) | 0.234 (0.18) |
| Female | 65/55 | 2210 Hz | −0.032 (0.86) | −0.102 (0.57) |
| Female | 65/55 | 2782 Hz | −0.028 (0.88) | −0.099 (0.58) |
| Female | 65/55 | 3506 Hz | 0.202 (0.25) | −0.099 (0.58) |
| Female | 65/55 | 4416 Hz | 0.252 (0.15) | −0.082 (0.64) |
| Female | 65/55 | 5565 Hz | 0.375 (0.03) | 0.043 (0.81) |
| Female | 65/55 | 7013 Hz | 0.224 (0.20) | 0.147 (0.41) |
| Female | 65/55 | 8837 Hz | 0.158 (0.37) | 0.220 (0.21) |
| Male | 53/35 | 2210 Hz | 0.233 (0.26) | −0.095 (0.64) |
| Male | 53/35 | 2782 Hz | 0.130 (0.53) | −0.185 (0.37) |
| Male | 53/35 | 3506 Hz | 0.373 (0.07) | −0.071 (0.73) |
| Male | 53/35 | 4416 Hz | 0.004 (0.98) | −0.125 (0.54) |
| Male | 53/35 | 5565 Hz | 0.072 (0.73) | −0.167 (0.42) |
| Male | 53/35 | 7013 Hz | 0.205 (0.33) | 0.096 (0.64) |
| Male | 53/35 | 8837 Hz | 0.252 (0.23) | 0.003 (0.99) |
| Male | 65/55 | 2210 Hz | 0.286 (0.16) | −0.142 (0.49) |
| Male | 65/55 | 2782 Hz | 0.456 (0.02) | −0.053 (0.80) |
| Male | 65/55 | 3506 Hz | 0.220 (0.29) | −0.024 (0.91) |
| Male | 65/55 | 4416 Hz | 0.185 (0.38) | −0.096 (0.64) |
| Male | 65/55 | 5565 Hz | 0.046 (0.82) | −0.195 (0.34) |
| Male | 65/55 | 7013 Hz | 0.422 (0.04) | 0.007 (0.97) |
| Male | 65/55 | 8837 Hz | 0.221 (0.29) | 0.265 (0.19) |
Figures in bold indicate trends approaching statistical significance (0.01 < p < 0.05). LE, left ear; RE, right ear.
Wave I Amplitude of the Auditory Brainstem Response
Women had significantly larger wave I amplitudes with both clicks and 4,000 Hz tone bursts at all but one presentation condition (90 dB nHL, click, right ear). All subsequent analyses of ABR wave I amplitudes were therefore completed for each sex separately. Paired samples t tests were used to compare right and left ears within each sex. The only statistically significant difference between right and left ears was observed in women for the 80 dB nHL click response recorded with earlobe electrodes, where wave I amplitude was significantly smaller in the right ear relative to the left ear in women ( t [33] = 3.258, p < 0.01). Given that this difference was observed in only one stimulus condition and only in women, wave I amplitude was averaged for the right and left ears in subsequent analyses (unless otherwise specified). The means and SDs for right and left ear wave I amplitudes are shown in Table 2 .
Table 2. Mean and Standard Deviation of Wave-I Amplitude.
| N | Min. | Max. | Mean | SD | # Reduced | S and J, 2015 | |
|---|---|---|---|---|---|---|---|
| Females | Right Ear (dBnHL) | ||||||
| Clicks at 70 | 26 | 0.08 | 0.58 | 0.28 | 0.13 | 0 | |
| Clicks at 80 | 34 | 0.22 | 0.73 | 0.45 | 0.14 | 0 | |
| Clicks at 90 | 33 | 0.34 | 0.98 | 0.55 | 0.17 | 0 | |
| Clicks at 99 | 33 | 0.35 | 1.04 | 0.63 | 0.19 | 0 | |
| TB at 4000 Earlobe, 90 | 34 | 0.17 | 0.69 | 0.44 | 0.11 | 1 (#043) | |
| TB at 4000 © Tiptrode, 90 | 33 | 0.25 | 0.84 | 0.50 | 0.14 | 0 | |
| Females | Left Ear (dBnHL) | ||||||
| Clicks at 70 | 26 | 0.10 | 0.69 | 0.32 | 0.16 | 0 | |
| Clicks at 80 | 34 | 0.18 | 0.84 | 0.51 | 0.14 | 1 (#13) | |
| Clicks at 90 | 33 | 0.21 | 0.92 | 0.55 | 0.18 | 0 | |
| Clicks at 99 | 33 | 0.27 | 1.04 | 0.65 | 0.19 | 0 | |
| TB at 4000 Earlobe, 90 | 34 | 0.20 | 0.76 | 0.46 | 0.13 | 0 | |
| TB at 4000 © Tiptrode, 90 | 33 | 0.25 | 1.01 | 0.52 | 0.16 | 0 | |
| Males | Right Ear (dBnHL) | ||||||
| Clicks at 70 | 25 | 0.09 | 0.31 | 0.22 | 0.06 | 1 (#049) | |
| Clicks at 80 | 25 | 0.20 | 0.58 | 0.39 | 0.10 | 0 | |
| Clicks at 90 | 26 | 0.27 | 0.73 | 0.47 | 0.13 | 0 | |
| Clicks at 99 | 26 | 0.17 | 0.86 | 0.48 | 0.16 | 0 | |
| TB at 4000 Earlobe, 90 | 26 | 0.20 | 0.58 | 0.36 | 0.11 | 0 | |
| TB at 4000 © Tiptrode, 90 | 26 | 0.21 | 0.67 | 0.43 | 0.11 | 1 (#049) | |
| Males | Left Ear (dBnHL) | ||||||
| Clicks at 70 | 23 | 0.09 | 0.34 | 0.20 | 0.06 | 0 | |
| Clicks at 80 | 26 | 0.12 | 0.59 | 0.34 | 0.11 | 1 (#047) | |
| Clicks at 90 | 26 | 0.20 | 0.68 | 0.43 | 0.14 | 0 | |
| Clicks at 99 | 26 | 0.18 | 0.75 | 0.49 | 0.15 | 1 (#047) | |
| TB at 4000 Earlobe, 90 | 26 | 0.15 | 0.53 | 0.36 | 0.10 | 1 (#024) | |
| TB at 4000 © Tiptrode, 90 | 26 | 0.23 | 0.68 | 0.45 | 0.10 | 1 (#024) | |
| Average of Right and Left Ears | |||||||
| Females, Average, Clicks at 90 | 34 | 0.28 | 0.95 | 0.55 | 0.18 | 0.48 (0.14) | |
| Males, Average, Clicks at 90 | 26 | 0.24 | 0.71 | 0.45 | 0.14 | 0.36 (0.13) | |
Values marked with bold font at the bottom of the table are calculated values that are most comparable to the values from Stamper and Johnson 28 [also shown in bold font] based on stimulus type (click) and intensity (90 dB nHL) and placement of electrodes (earlobe in the current study, mastoid in the study by Stamper and Johnson). 28
The means and other descriptive statistics for ABR amplitude in the present study were similar to those for the male and female samples from Stamper and Johnson, 27 although they recorded responses to 90 dB clicks from electrodes placed on the mastoid rather than the earlobe. To assess whether any of the current participants had significantly reduced wave I ABR amplitudes relative to the rest of the current cohort, sample means and SDs of wave I amplitude were calculated for left and right ears across all stimulus conditions, and individual ears were compared with the group data from the corresponding same-sex reference group. Those participants with wave I amplitudes that were 2 SDs less than the mean had the potential to be defined as having significantly reduced responses, particularly if reduced responses were consistent across multiple stimulus conditions. As shown in Table 2 , three male participants had wave I amplitudes that were outside of 2 SDs from the mean at 2 of the 12 possible tested stimulus conditions (e.g., click at 70, 80, 90, and 99 dB nHL in right and left ear, and tone-burst at 4,000 Hz with earlobe and Tiptrode electrodes in right and left ear). In addition, two female participants had wave I amplitudes that were outside of 2 SDs from the mean at 1 of the 12 tested stimulus conditions. Although it is worth noting that these five subjects had reduced responses for one or two stimulus conditions, the rest of the amplitudes for the other sound-evoked responses were normal relative to the rest of the cohort across the remaining 10 to 11 stimulus conditions tested. The limited and sporadic observation of decreased amplitudes within any given participant decreases confidence in any hypothesis that the sporadic differences might reflect a significant underlying neural injury. Given no consistent decrease in wave I amplitude across signal conditions for any participant, none of the participants had responses that provide compelling evidence of neuropathic injury relative to the rest of the cohort.
Relationships between Threshold and Wave I Amplitude of the Auditory Brainstem Response
There was no statistically significant relationship between 4,000 Hz audiometric thresholds and 4,000 Hz tone-burst evoked ABR wave I amplitudes measured using either surface electrodes or Tiptrodes. A trend suggesting a negative association between 4,000 Hz thresholds and 4,000 Hz tone-burst ABR wave I amplitude was observed in women when recording with Tiptrode electrodes ( p = 0.048), such that smaller tone-burst amplitudes appeared to be associated with poorer thresholds at 4,000 Hz. There was a similar trend observed when data were collected using earlobe electrodes within women, but the association failed to reach statistical significance ( p = 0.06), and these trends were not observed in data from male participants. Given that all participants had normal hearing (i.e., 25 dB HL or better thresholds), it is possible that more robust relationships would emerge in populations that include participants with overt hearing loss.
Relationships between Noise Exposure (L Aeq8760 ) and Wave I Amplitude of the Auditory Brainstem Response
The relationship between noise exposure (L Aeq8760 ) and ABR wave I amplitude for clicks and tone bursts is shown for women in Fig. 4 and men in Fig. 5 . As shown in Figs. 4 and 5 , noise exposure history, measured using L Aeq8760, was not significantly related to any of the wave I amplitudes for men or women, with all p values were greater than 0.10, as reported in the individual panels.
Figure 4.
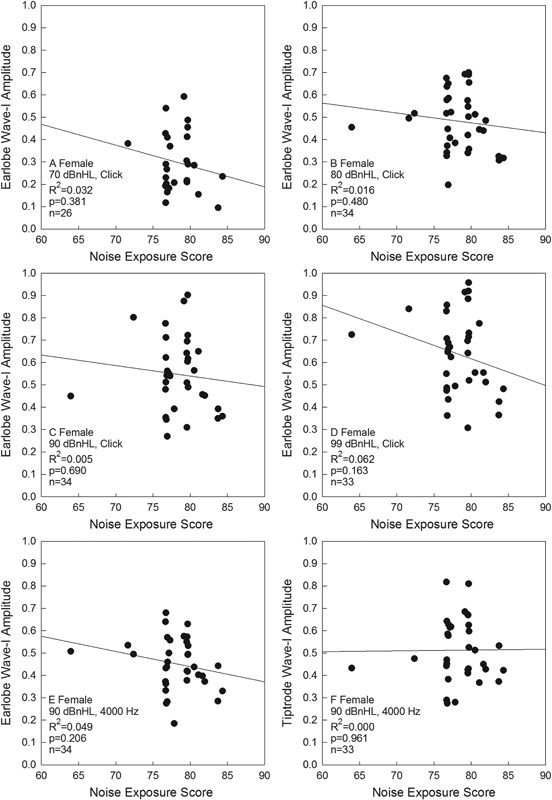
Click-evoked wave I amplitude as a function of 1-year L Aeq8760 noise exposure scores in women is shown, for responses measured using earlobe electrode placement. Click level was 70 dB nHL (A), 80 dB nHL (B), 90 dB nHL (C), or 99 dB nHL (D). A 4,000 Hz tone-evoked wave I amplitude as a function of 1-year L Aeq8760 noise exposure scores in women is also shown, with responses measured using earlobe electrode placement (E) or Tiptrode electrode placement (F). Abbreviation: nHL, normal hearing level.
Figure 5.
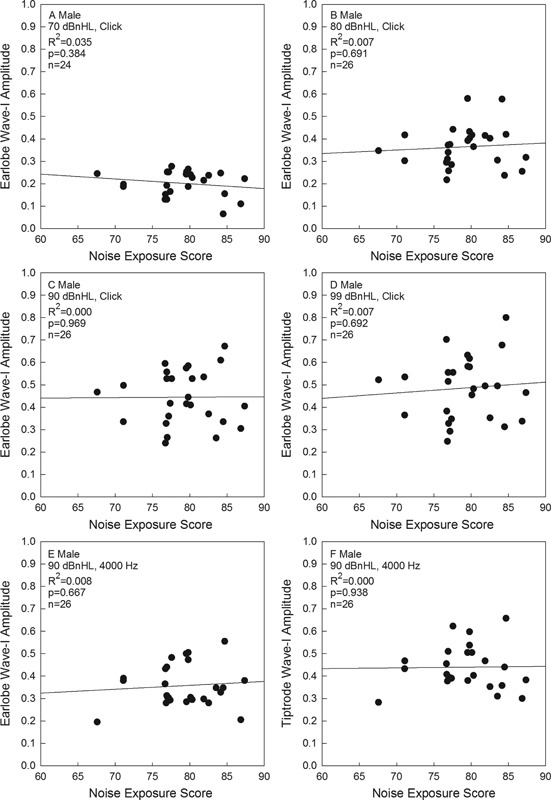
Click-evoked wave I amplitude as a function of 1-year L Aeq8760 noise exposure scores in men is shown, for responses measured using earlobe electrode placement. Click level was 70 dB nHL (A), 80 dB nHL (B), 90 dB nHL (C), or 99 dB nHL (D). A 4,000 Hz-tone-evoked wave I amplitude as a function of 1-year L Aeq8760 noise exposure scores in men is also shown, with responses measured using earlobe electrode placement (E) or Tiptrode electrode placement (F). Abbreviation: nHL, normal hearing level.
Low-Risk Temporary Threshold Shifts versus High-Risk Temporary Threshold Shifts
The data plots shown in Figs. 4 and 5 do not reveal any compelling relationship between L Aeq8760 and ABR wave I amplitude, and there was no evidence of a break point in the data such that the 79 dB L Aeq8760 provided a boundary between safe recreational noise and hazardous recreational noise. Therefore, in a final post hoc analysis, participants were grouped into low- and high-risk groups for TTS. The selection of these two groups was directly based on self-reported experience of auditory symptoms after noise exposure. The low-risk group (18 men and 21 women) consisted of participants who reported never or rarely having any auditory symptoms following exposure to noise; those reporting sometimes, often, or always having auditory symptoms following exposure to noise were included in the high-risk group (8 men and 13 women). Those in the low-risk group had L Aeq8760 noise scores ranging from 63.9 to 86.8 dB (mean = 78.35 ± 4.16), and those in the high-risk group had L Aeq8760 noise scores ranging from 67.6 to 87.4 dB (mean = 79.01 ± 4.29). A Kruskal-Wallis one-way ANOVA on ranks revealed no significant difference for L Aeq8760 noise scores between the two risk groups, indicating relatively similar exposure to noise in the previous 12-month period. However, the difference in self-reported symptoms could indicate differences in vulnerability to those exposures.
The high-risk TTS group and low-risk TTS group were compared with respect to pure-tone thresholds, DPOAE amplitudes, wave I amplitudes, and functional measures. Pure-tone thresholds were similar in the two groups from 250 to 8,000 Hz; however, low-level (L1/L2 = 53/35) DPOAE amplitudes in the left ear were significantly different between the low- and high-risk TTS groups at 2,782 Hz. These amplitudes were ∼5 dB higher (better) in the low-risk TTS group ( t [57.05] = 2.779, p < 0.01). There was a trend for the high-risk TTS group to have lower wave I amplitudes for 4,000 Hz tone bursts when response amplitudes were collected with a Tiptrode in the left ear, when compared with the low-risk TTS group ( Fig. 6 ), but this difference did not reach statistical significance ( t [57] = 2.243, p = 0.03). Additionally, this finding was specific to the Tiptrode electrode condition; differences were not statistically significant within the earlobe electrode measurements. It is interesting that differences were perhaps emerging with the Tiptrode electrode and not the earlobe electrode, given the previous report by Stamper and Johnson, in which differences were observed with mastoid (surface) electrodes, but not with electrodes in the ear canal. 27 The potential for spurious findings should be considered, as per the discussion that follows.
Figure 6.
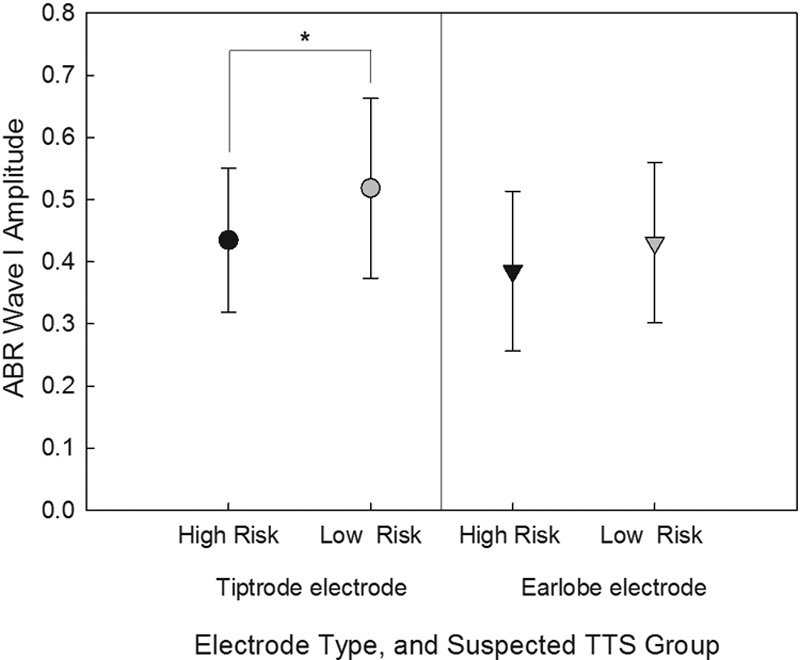
Wave I amplitude evoked by 4,000 Hz tone burst was reduced in high-risk TTS group (those reporting “sometimes,” “often,” or “always” having auditory symptoms following exposure to noise) compared with low-risk group (participants who reported “never” or “rarely” having auditory symptoms following exposure to noise) when assessed using Tiptrode electrodes. There were no reliable differences when the responses were assessed using the earlobe electrode placements. ABR, auditory brainstem response; TTS, temporary threshold shifts.
Relationships between Threshold, Wave I Amplitude, and Functional Measures
Before assessing the relationship between ABR amplitudes and functional measures, it was necessary to confirm whether or not hearing thresholds were associated with performance on the WIN and WIBBN. Pearson bivariate correlations were calculated for men and women, and no significant correlations were found, suggesting that performance was independent of hearing thresholds. The same type of analysis was then used to analyze the relationship between ABR wave I amplitude and each word test (WIN and WIBBN) and the temporal summation task in quiet and BBN. Data were averaged for the right and left ears. The data from men and women were analyzed separately, however, due to the differences in wave I amplitude for men and women. The association between ABR wave I and each of the additional functional measures follows.
Words in Noise Test
Women had significantly higher signal-to-babble ratio thresholds on the WIN test ( t [59] = 11.01, p <0.01), indicating poorer performance than men. The mean score on the WIN test (right and left ear averaged) was 4.9 dB (SD = 1.7) signal-to-babble for men and 6.2 dB (SD = 1.5) signal-to-babble for women. The mean WIN score for women was borderline normal, as mild deficits are scored as beginning at 6.8 dB signal-to-babble ratio thresholds. The WIN test was not significantly associated with any of the wave I amplitude measurements, whether the ABR was elicited with click stimuli at 70 to 99 dB nHL, or with 4,000 Hz tone-burst stimuli at 90 dB nHL, and regardless of whether responses were recorded using surface electrodes on the earlobe or Tiptrode electrodes. In summary, wave I amplitude was not significantly associated with speech-in-noise performance using the WIN test in either men or women.
The relationship between the WIN test and noise exposure history (L Aeq8760 ) also was examined based on previous studies suggesting that speech in noise may be poorer in those with a history of noise exposure. 33 49 There was no significant association between noise exposure and WIN threshold in men or in women.
Words in Broadband Noise Test
There were no significant associations between the WIBBN test outcomes and ABR wave I amplitudes elicited by clicks or tone-burst stimuli in either men or women, with earlobe electrode measurements or when using Tiptrodes (all p values greater than 0.01). Additional analyses examined the relationship between noise exposure (L Aeq8760 ) and WIBBN performance in men and women. As observed for the WIN test, lower WIBBN performance was not significantly related to noise exposure (L Aeq8760 ) for men or women.
Temporal Summation in Quiet
The relationship between the 4,000 Hz threshold difference at 300, 30, and 3 milliseconds and ABR wave I amplitude was not statistically significant for either men or women. However, when male and female participants were assigned to low-risk or high-risk TTS groups, temporal summation performance was significantly different between the two groups for a small number of comparisons. The high-risk TTS group had significantly greater changes in detection thresholds as stimulus duration was decreased from 300 milliseconds to 3 milliseconds in the left ear ( t [52] = 2.677, p ≤ 0.01). A two-way ANOVA was performed to evaluate a potential main effect for sex in addition to the observed main effect of risk of TTS, as well as the potential interaction of sex × TTS. Risk of TTS (high versus low) had a statistically significant main effect at the conventional α level of 0.05 ( F [1,50] = 6.809, p < 0.05) but the main effect of sex was not significant ( F [1,50] = 0.078, p > 0.05) and there was no reliable interaction effect ( F [1,50] = 0.225, p > 0.05). With only one out of six possible temporal summation in quiet conditions showing a statistically reliable effect of TTS risk, caution against overinterpretation of the difference is warranted.
Temporal Summation in Broadband Noise
The analysis of the relationship between temporal summation in BBN and wave I amplitude for clicks and tone bursts at 4,000 Hz revealed statistically significant negative associations ( p < 0.01) between ABR wave I amplitudes at 90 and 99 dB nHL for clicks and 90 dB nHL for tone bursts at 4,000 Hz recorded with surface electrodes and tone in noise difference thresholds, but only for women. In brief, female participants with larger threshold differences between 300 milliseconds versus 3 milliseconds appeared to have smaller wave I amplitudes.
Discussion
In this study, normal hearing young adults with varying histories of recreational noise exposure were evaluated to determine whether there was an association between self-reported noise exposures (estimated as L Aeq8760 ) and performance on several electrophysiologic metrics and functional tasks at threshold and suprathreshold levels. There was no evidence of noise-induced decreases in suprathreshold ABR wave I amplitude (obtained with clicks and tone bursts, using earlobe and Tiptrode electrodes, from 70 to 99 dB nHL), and there was no evidence of dysfunction on measures including the WIN, WIBBN, tones in noise, or temporal summation tests. The results are discussed in detail in the following sections.
Relationship between Noise and Auditory Brainstem Response Wave I Amplitude
The current results contrast with those of Stamper and Johnson, who initially reported that ABR wave I amplitude is related to noise exposure (L Aeq8760 ), 27 although they later adjusted their report to reflect that the relationship was limited to females, 28 after reanalyzing their data to control for the potential confound associated with well-known sex differences in the amplitude of the ABR. 50 However, the current data are consistent with the more recent reports by Prendergast et al and Spankovich et al, who reported no statistically significant relationship between noise exposure history and ABR amplitude for other similar young adult populations with varied recreational noise exposure histories. 29 30 The current data do not preclude the possibility that noise-induced synaptopathy may occur in humans that experience higher noise doses, however. For example, when Bramhall et al recruited participants with high levels of noise exposure during military service and civilian recreational firearm use, they observed reduced ABR wave I amplitudes in these groups relative to nonveteran participants with no history of firearm use and veterans categorized as having low noise exposure. 31 Careful consideration of multiple key variables is necessary in these types of investigations. Liberman et al, 26 for example, recently reported that student participants who were enrolled in a music conservatory had smaller ratios for the AP relative to the SP, but this finding was largely driven by statistically significant differences in the SP amplitude in the absence of reliable differences in the amplitude of the AP, and there were reliable threshold differences during EHF tests. Although more significant noise exposure may ultimately be shown to be reliably associated with smaller ABR amplitude, the relationship between these variables clearly requires additional investigation to determine where risk begins and how risk grows (see also the commentary by Dobie and Humes 24 ).
With respect to L Aeq8760 noise exposures for participants in the current study, the range of values (64 to 84 dB for women; 68 to 87 dB for men) was generally consistent with those reported by Stamper and Johnson (67 to 83 dB for females; 70 to 82 dB for males) and Megerson (64 to 84 dB for females; 64 to 88 dB for males). 27 44 Although Stamper and Johnson recorded from the mastoid and data described here were recorded from the earlobe, the measured amplitudes were similar across studies. 27 28 Comparison of their ABR wave I amplitudes with the data measured here, using (unpaired) t tests, revealed no statistically significant differences within men ( t [34] = 1.879, p = 0.07) or women ( t [50] = 1.594, p = 0.12). Therefore, in a final exploratory analysis, the 90 dBnHL click data from Stamper and Johnson 28 were combined with the current 90 dB nHL click data to increase the total sample size, which increases the power to detect subtle relationships. When the bivariate relationship between wave I amplitude and noise exposure (L Aeq8760 ) was reassessed within the combined sample, there were no statistically significant associations between noise exposure (L Aeq8760 ) and wave I amplitude elicited by clicks at 90 dB nHL within men or women even with the larger combined sample. The obtained regression lines were generally flat, with no statistically significant relationship observed in men or women ( Fig. 7 ). Although studies with animals provide compelling evidence of synaptopathic injury after some (but not all) TTS-inducing noise exposures, the current data did not provide any evidence that would suggest that common recreational exposures experienced by these young adult participants were neuropathic.
Figure 7.
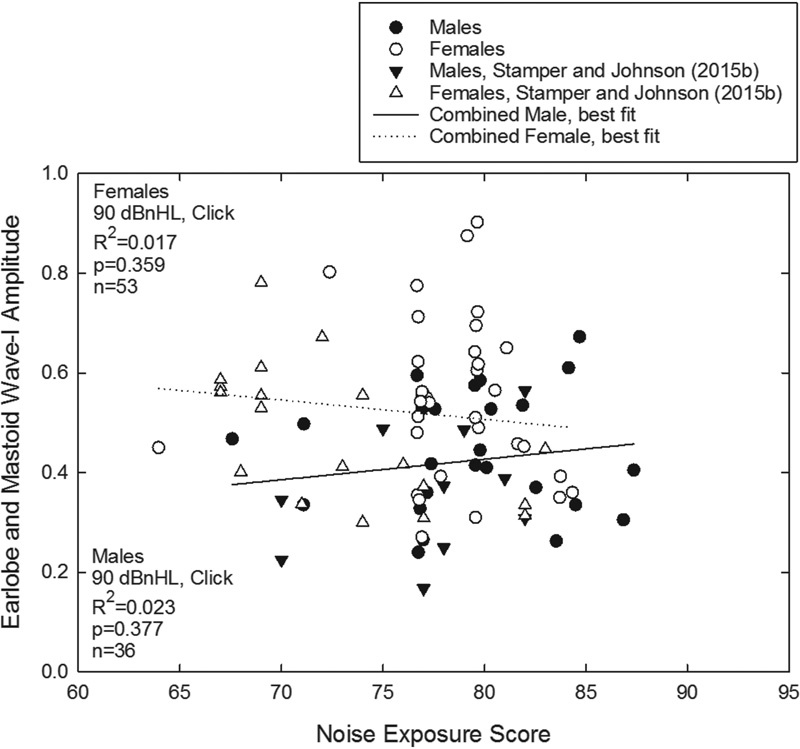
Click-evoked wave I amplitude as a function of 1-year L Aeq8760 noise exposure scores was measured using earlobe electrode placement (current data set, circles) or mastoid electrode placement (triangles), for click signals presented at 90 dB nHL. Data from both men (filled symbols) and women (open symbols) are plotted, with no significant associations revealed when the current data were analyzed in combination with the data from Stamper and Johnson. 28 Abbreviation: nHL, normal hearing level.
Because this was a retrospective study, there are no data documenting the presence or magnitude of any previous TTSs. However, the kinds of noise exposures that participants reported here have resulted in relatively small TTSs in other reports. For example, data collected from concert goers in several other studies revealed average TTSs of ∼8 to 10 dB immediately postconcert, and it is reasonable to hypothesize that participants who reported previous concert attendance likely would have had similar deficits after the concerts they attended (i.e., averaging 8 to 10 dB). 7 8 9 Data from rodents and data that are emerging from primate models suggest it is unlikely that there would be a significant risk of synaptic damage in humans from concerts or other equivalent recreational exposures that result in small TTSs. 12 21 22 51 However, any inference related to the potential hazard associated with a single loud recreational event must be made with caution. Unlike animals tested in laboratory models that include a single acute exposure, humans experience noise exposure on a repeat basis, and they may experience noise exposure in combination with other hazards (such as during chemical exposure in the workplace). Thus, it is possible that a smaller TTS repeated on a more frequent basis or experienced in combination with another risk factor for hearing loss (such as a chemical hazard) could be more hazardous than a single smaller TTS in the absence of any additional exposure, as implemented in most animal models. The limited repeated exposure data from animal models are mixed, with recent animal data showing that repeat exposure to octave band noise of 85 dB SPL for 6 hours a day for 3 months resulted in a statistically significant loss of spiral ganglion neurons and structural damage to the cell bodies and dendrites of the remaining neurons, 52 whereas shorter but more intense exposures of rats to BBN for 90 minutes every 6 weeks did not result in any wave I decrements beyond those expected as a function of aging, suggesting there was no noise-induced neural pathology despite the repeated exposures. 23
Although we did not observe a systematic reduction in wave I amplitude as a function of noise exposure (L Aeq8760 ), those participants that self-reported a higher risk of having experienced a TTS had reduced wave I amplitudes to 4,000 Hz tone-bursts at 90 dB nHL in the left ear, for the waveforms recorded with Tiptrode electrodes. Further investigation will be necessary to determine the repeatability of this observation as this may be a spurious finding given that this difference was detected only for the left ear, only with a Tiptrode electrode, only at 4,000 Hz, and only for the 90 dB nHL stimulus level. No other differences at other sound levels, with other electrode configurations, or for the opposite (right) ear were observed. The lack of any broad trends reduces confidence in the validity of this observed “difference.” However, these findings might guide future investigations in that the data are perhaps consistent with the possibility that careful grouping of subjects based on a significant history of TTS experiences might allow detection of ABR wave I amplitude differences in future studies, if a significant TTS history in fact reliably results in a neural pathology in humans as hypothesized based on the data from rodent models.
Relationship between Wave I Amplitude and Functional Measures
There were no consistent statistically significant relationships between wave I amplitude and the WIN, WIBBN, and temporal summation in quiet and noise. Similarly, in the high-risk TTS versus low-risk TTS group analysis, there were no observed significant differences in performance on functional measures with speech, and there was only one statistically significant finding in the temporal summation comparisons. Taken together, the current data suggest that ABR wave I amplitude may have a minimal relationship with speech-in-noise performance in normal hearing persons. However, as discussed previously, the current study did not include any participants with wave I amplitudes that were consistently significantly reduced (defined as 2 SDs from the mean) relative to other participants within this sample. This suggests that there may not have been any subjects with ABR amplitudes that were “reduced enough” to drive a functional deficit. This interpretation is consistent with Bramhall et al, 25 who reported that there was no statistically significant relationship between speech-in-noise test performance and ABR amplitude among participants with normal hearing, whereas there was a reliable relationship between ABR amplitude and speech-in-noise test performance among participants with overt hearing loss. Whether noise-induced functional deficits can be reliably measured in the absence of threshold shift remains an open question.
The recent data from Liberman et al highlight the importance of including EHF thresholds in any study attempting to assess hidden hearing loss, as they recently reported that high-risk participants (primarily college students studying music performance) had poorer EHF thresholds, and poorer word recognition performance in noise at 0 dB and 5 dB SNR conditions, with increasing group differences in the most difficult reverberation conditions, relative to low-risk participants (primarily college students studying communication sciences and disorders). 26 Those data suggest that, in humans with a history of noise exposure, changes in threshold data may be present in hearing regions not traditionally tested, which has the potential to confound the interpretation of functional deficits as being related to changes in either SP or AP amplitudes. At least in mice, the loss of the apical OHCs results in a significantly increased SP amplitude, 53 which might be interpreted as suggesting the potential for OHC loss in the high-risk college student participants in the study by Liberman et al (because high-risk participants had increased SP amplitudes). 26 Although DPOAEs were not significantly different when the high-risk and low-risk groups were compared, the DPOAE tests provided limited data concerning the status of the apical OHCs. The larger SP amplitude seems unlikely to be the consequence of a selective neural pathology, given that loss of the IHC population has the opposite effect, resulting in a significantly decreased SP amplitude in chinchillas. 54
Conclusions
There are a variety of data that support a relationship between suprathreshold function and exposure to noise (for recent review, see Le Prell and Clavier 20 ). Although the present study did not find significant associations between 1-year noise exposure history (L Aeq8760 ) and wave I amplitude of the ABR, or between wave I amplitude and functional measures, it remains possible that deficits may be present in those with louder, longer, or more frequent noise exposure. We suggest that future studies seeking evidence of hidden hearing loss should prioritize prospective documentation of TTS as in the recent report by Grinn et al. 55 If possible, the inclusion of participants with exposure to blast and/or weapon fire would present an opportunity to clarify the relationship between wave I amplitude and extreme noise, particularly as related to speech-in-noise abilities. 56 Although data suggest that exposure to blast may result in lingering difficulty with speech-in-noise communication, tinnitus, and reduced quality of life, 57 other data have indicated no significant differences when wave I amplitude was compared for blast-exposed subjects and nonblast-exposed controls. 58 The potential for suprathreshold functional deficits associated with lasting neural trauma, despite threshold recovery, would have significant implications not only for workers who are potentially at risk for suprathreshold deficits, but also for military service members exposed to hazardous noise conditions. If currently hypothesized relationships between noise exposure and suprathreshold functional performance are ultimately confirmed, then it may become reasonable to work toward the development of a protocol that could be used in worker monitoring programs, community screenings, and routine diagnostic tests.
Acknowledgments
This study was completed by Angela Fulbright in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Florida. 59 Funding was provided by The Hearing Research Center at the University of Florida. The authors thank Rich Douglas for contributions to the statistical analysis, Shinichi Someya and Steven Anton for helpful comments and suggestions, and Hannah Medina and Frank Ross for technical assistance.
References
- 1.NIOSH.Criteria for a Recommended Standard, Occupational Noise Exposure, DHHS (NIOSH) Publication No98–126;1998. Cincinnati, OH
- 2.OSHA. 29 CFR 1910.95. Occupational Noise Exposure; Hearing Conservation Amendment; Final Rule, effective 8 March1983;1983. Available at:https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9735. Accessed September 13, 2017
- 3.Figueiredo R R, Azevedo A A, Oliveira P M, Amorim S P, Rios A G, Baptista V. Incidence of tinnitus in mp3 player users. Rev Bras Otorrinolaringol (Engl Ed) 2011;77(03):293–298. doi: 10.1590/S1808-86942011000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim M G, Hong S M, Shim H J, Kim Y D, Cha C I, Yeo S G. Hearing threshold of Korean adolescents associated with the use of personal music players. Yonsei Med J. 2009;50(06):771–776. doi: 10.3349/ymj.2009.50.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Prell C G, Spankovich C, Lobariñas E, Griffiths S K. Extended high-frequency thresholds in college students: effects of music player use and other recreational noise. J Am Acad Audiol. 2013;24(08):725–739. doi: 10.3766/jaaa.24.8.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng J H, Tao Z Z, Huang Z W. Risk of damage to hearing from personal listening devices in young adults. J Otolaryngol. 2007;36(03):181–185. [PubMed] [Google Scholar]
- 7.Derebery M J, Vermiglio A, Berliner K I, Potthoff M, Holguin K. Facing the music: pre- and postconcert assessment of hearing in teenagers. Otol Neurotol. 2012;33(07):1136–1141. doi: 10.1097/MAO.0b013e31825f2328. [DOI] [PubMed] [Google Scholar]
- 8.Opperman D A, Reifman W, Schlauch R, Levine S. Incidence of spontaneous hearing threshold shifts during modern concert performances. Otolaryngol Head Neck Surg. 2006;134(04):667–673. doi: 10.1016/j.otohns.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Ramakers G GJ, Kraaijenga V JC, Cattani G, van Zanten G A, Grolman W. Effectiveness of earplugs in preventing recreational noise-induced hearing loss: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2016;142(06):551–558. doi: 10.1001/jamaoto.2016.0225. [DOI] [PubMed] [Google Scholar]
- 10.Keppler H, Dhooge I, Vinck B. Hearing in young adults. Part II: the effects of recreational noise exposure. Noise Health. 2015;17(78):245–252. doi: 10.4103/1463-1741.165026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberman M C. Hidden hearing loss. Sci Am. 2015;313(02):48–53. doi: 10.1038/scientificamerican0815-48. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J B, Lysaght A C, Liberman M C, Qvortrup K, Stankovic K M. Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS One. 2015;10(05):e0125160. doi: 10.1371/journal.pone.0125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman A C, Kujawa S G, Liberman M C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(03):577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujawa S G, Liberman M C. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H W, Furman A C, Kujawa S G, Liberman M C. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12(05):605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Ren C. Effects of repeated “benign” noise exposures in young CBA mice: shedding light on age-related hearing loss. J Assoc Res Otolaryngol. 2012;13(04):505–515. doi: 10.1007/s10162-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kujawa S G, Liberman M C.Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss Hear Res 2015330(Pt B):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Prell C G, Lobarinas E. New York, NY: Humana Press; 2015. Strategies for assessing antioxidant efficacy in clinical trials; pp. 163–192. [Google Scholar]
- 19.Le Prell C G, Brungart D S. Speech-in-noise tests and supra-threshold auditory evoked potentials as metrics for noise damage and clinical trial outcome measures. Otol Neurotol. 2016;37(08):e295–e302. doi: 10.1097/MAO.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 20.Le Prell C G, Clavier O H. Effects of noise on speech recognition: challenges for communication by service members. Hear Res. 2017;349:76–89. doi: 10.1016/j.heares.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Hickox A E, Liberman M C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111(03):552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez K A, Jeffers P WC, Lall K, Liberman M C, Kujawa S G. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannström P, Kirkegaard M, Ulfendahl M. Repeated moderate noise exposure in the rat–an early adulthood noise exposure model. J Assoc Res Otolaryngol. 2015;16(06):763–772. doi: 10.1007/s10162-015-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobie R A, Humes L E. Commentary on the regulatory implications of noise-induced cochlear neuropathy. Int J Audiol. 2017;56 01:74–78. doi: 10.1080/14992027.2016.1255359. [DOI] [PubMed] [Google Scholar]
- 25.Bramhall N, Ong B, Ko J, Parker M. Speech perception ability in noise is correlated with auditory brainstem response wave I amplitude. J Am Acad Audiol. 2015;26(05):509–517. doi: 10.3766/jaaa.14100. [DOI] [PubMed] [Google Scholar]
- 26.Liberman M C, Epstein M J, Cleveland S S, Wang H, Maison S F. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One. 2016;11(09):e0162726. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamper G C, Johnson T A. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015;36(02):172–184. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamper G C, Johnson T A. Letter to the editor: examination of potential sex influences in auditory function in normal-hearing, noise-exposed human ears, Ear Hear, 36, 172–184. Ear Hear. 2015;36:738–740. doi: 10.1097/AUD.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast G, Guest H, Munro K J et al. Effects of noise exposure on young adults with normal audiograms I: electrophysiology. Hear Res. 2017;344:68–81. doi: 10.1016/j.heares.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spankovich C, Le Prell C G, Lobarinas E, Hood L J. Noise history and auditory function in young adults with and without Type-1 diabetes. Int J Audiol. 2017;56:716–722. doi: 10.1097/AUD.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 31.Bramhall N F, Konrad-Martin D, McMillan G P, Griest S E. Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear Hear. 2017;38(01):e1–e12. doi: 10.1097/AUD.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickox A E, Larsen E, Heinz M G, Shinobu L, Whitton J P. Translational issues in cochlear synaptopathy. Hear Res. 2017;349:164–171. doi: 10.1016/j.heares.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar U A, Ameenudin S, Sangamanatha A V. Temporal and speech processing skills in normal hearing individuals exposed to occupational noise. Noise Health. 2012;14(58):100–105. doi: 10.4103/1463-1741.97252. [DOI] [PubMed] [Google Scholar]
- 34.Stone M A, Moore B C, Greenish H. Discrimination of envelope statistics reveals evidence of sub-clinical hearing damage in a noise-exposed population with ‘normal’ hearing thresholds. Int J Audiol. 2008;47(12):737–750. doi: 10.1080/14992020802290543. [DOI] [PubMed] [Google Scholar]
- 35.Stone M A, Moore B C. Amplitude-modulation detection by recreational-noise-exposed humans with near-normal hearing thresholds and its medium-term progression. Hear Res. 2014;317:50–62. doi: 10.1016/j.heares.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ANSI.Specifications for Audiometers (ANSI S3.6 1996) New York, NY: American National Standards Institute; 1996 [Google Scholar]
- 37.ANSI.Manual Pure-tone Threshold Audiometry (ANSI S3.21–1978, R1992) New York, NY: American National Standards Institute; 1978 [Google Scholar]
- 38.Wilson R H, Burks C A. Use of 35 words for evaluation of hearing loss in signal-to-babble ratio: a clinic protocol. J Rehabil Res Dev. 2005;42(06):839–852. doi: 10.1682/jrrd.2005.01.0009. [DOI] [PubMed] [Google Scholar]
- 39.Lee G J, Lim M Y, Kuan A Y, Teo J H, Tan H G, Low W K. Relationship between leisure noise exposure and otoacoustic emissions in a young Asian population. Int J Audiol. 2014;53(07):462–468. doi: 10.3109/14992027.2014.893376. [DOI] [PubMed] [Google Scholar]
- 40.Oswald J A, Janssen T. Weighted DPOAE input/output-functions: a tool for automatic assessment of hearing loss in clinical application. Z Med Phys. 2003;13(02):93–98. doi: 10.1078/0939-3889-00148. [DOI] [PubMed] [Google Scholar]
- 41.Wagner W, Heppelmann G, Kuehn M, Tisch M, Vonthein R, Zenner H P. Olivocochlear activity and temporary threshold shift-susceptibility in humans. Laryngoscope. 2005;115(11):2021–2028. doi: 10.1097/01.MLG.0000181463.16591.A7. [DOI] [PubMed] [Google Scholar]
- 42.Yost W A. San Diego, CA: Academic Press; 2006. Fundamentals of Hearing: An Introduction, 5th ed. [Google Scholar]
- 43.Spankovich C. Nashville, TN: Vanderbilt University; 2010. Early Indices of Auditory Pathology in Young Adults with Type-1 Diabetes [dissertation] [Google Scholar]
- 44.Megerson S C. Lawrence, KS: University of Kansas; 2010. Development of a Screening Tool for Identifying Young People at Risk for Noise-induced Hearing Loss [dissertation] [Google Scholar]
- 45.Neitzel R, Seixas N, Goldman B, Daniell W. Contributions of non-occupational activities to total noise exposure of construction workers. Ann Occup Hyg. 2004;48(05):463–473. doi: 10.1093/annhyg/meh041. [DOI] [PubMed] [Google Scholar]
- 46.Le Prell C G, Dell S, Hensley B et al. Digital music exposure reliably induces temporary threshold shift in normal-hearing human subjects. Ear Hear. 2012;33(06):e44–e58. doi: 10.1097/AUD.0b013e31825f9d89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spankovich C, Griffiths S K, Lobariñas E et al. Temporary threshold shift after impulse-noise during video game play: laboratory data. Int J Audiol. 2014;53 02:S53–S65. doi: 10.3109/14992027.2013.865844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Prell C G, Fulbright A, Spankovich C et al. Dietary supplement comprised of β-carotene, vitamin C, vitamin E, and magnesium: failure to prevent music-induced temporary threshold shift. Audiol Neurotol Extra. 2016;6(02):20–39. doi: 10.1159/000446600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hope A J, Luxon L M, Bamiou D E. Effects of chronic noise exposure on speech-in-noise perception in the presence of normal audiometry. J Laryngol Otol. 2013;127(03):233–238. doi: 10.1017/S002221511200299X. [DOI] [PubMed] [Google Scholar]
- 50.Hall J W. Boston, MA: Pearson Education, Inc.; 2007. New Handbook of Auditory Evoked Responses. [Google Scholar]
- 51.Lobarinas E, Spankovich C, Le Prell C G. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res. 2017;349:155–163. doi: 10.1016/j.heares.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Gannouni N, Lenoir M, Ben Rhouma K et al. Cochlear neuropathy in the rat exposed for a long period to moderate-intensity noises. J Neurosci Res. 2015;93(06):848–858. doi: 10.1002/jnr.23567. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Xiong B, Xiong F, Chen G D, Hu B H, Sun W. Apical hair cell degeneration causes the increase in the amplitude of summating potential. Acta Otolaryngol. 2016;136(12):1255–1260. doi: 10.1080/00016489.2016.1203989. [DOI] [PubMed] [Google Scholar]
- 54.Durrant J D, Wang J, Ding D L, Salvi R J. Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am. 1998;104(01):370–377. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- 55.Grinn S K, Wiseman K B, Baker J A, Le Prell C G.Hidden hearing loss? No effects of recreational noise exposure on ABR wave-I amplitude in humans Front Neurosci 2017114651–24.. Doi: 10.3389/fnins.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon J S, Griest S E, Thielman E J et al. Audiologic characteristics in a sample of recently-separated military veterans: the noise outcomes in servicemembers epidemiology study (NOISE study) Hear Res. 2017;349:21–30. doi: 10.1016/j.heares.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Remenschneider A K, Lookabaugh S, Aliphas A et al. Otologic outcomes after blast injury: the Boston Marathon experience. Otol Neurotol. 2014;35(10):1825–1834. doi: 10.1097/MAO.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 58.Gallun F J, Lewis M S, Folmer R L et al. Implications of blast exposure for central auditory function: a review. J Rehabil Res Dev. 2012;49(07):1059–1074. doi: 10.1682/jrrd.2010.09.0166. [DOI] [PubMed] [Google Scholar]
- 59.Fulbright A NC. Gainesville, FL: University of Florida; 2016. Normal Hearing? What Functional Hearing Tests, Noise Exposure History and ABR Wave-I Amplitude Reveal [dissertation] [Google Scholar]


