Abstract
American trypanosomiasis, or Chagas disease, is a growing public health problem in Panama, and further forest degradation due to human population growth is expected to worsen the situation. Most people infected with the parasite Trypanosoma cruzi are silently ill, and their life expectancy is severely compromised, which contributes to further deterioration of living conditions in endemic regions. Here, we review the outcomes of nearly 100 years of ecological and epidemiological investigation about Chagas disease in Panama, in an attempt to highlight progress, identify needs, and re-orient future efforts. Rhodnius pallescens and Triatoma dimidiata are both primary vectors of T. cruzi in Panama, but R. pallescens seems more efficient in human-altered forest ecosystems due to a greater degree of association with Attalea butyracea. In contrast, T. dimidiata transmits T. cruzi efficiently under more sylvatic conditions (e.g. settlements inside old-growth or secondary forest patches), where its populations reach considerable numbers irrespective of the absence of A. butyracea. A trend of increasing forest degradation, suburbanization, and development of tourism in Panama favoring the establishment of A. butyracea and other palm tree species (Acrocomia sp.) suggests that a colonist species like R. pallescens will continue to play a more prominent role in the transmission of T. cruzi than a forest specialist like T. dimidiata. However, studies about the taxonomic status and ecology of these vectors are still needed in Panama to address their transmission potential fully. The implementation of an active surveillance system and education programs could greatly minimize the risk of Chagas disease transmission in Panama, preventing fatal infections in children from endemic areas.
Electronic supplementary material
The online version of this article (10.1186/s13071-017-2380-5) contains supplementary material, which is available to authorized users.
Keywords: Trypanosoma cruzi, Triatominae vectors, Animal reservoirs, Transmission cycles, Community ecology, Control
Background
American trypanosomiasis is a human parasitic infection caused by the protozoan Trypanosoma cruzi (Eucarya, Kinetoplastea, Trypanosomatidae). The infection, known as Chagas disease in honor of its discoverer, Carlos R. J. Chagas [1], is a zoonosis affecting a wide range of wildlife vertebrates, which spread to humans primarily by kissing-bug invertebrates [2–5]. In the Americas, 21 countries are considered endemic, including Panama, and between six and 12 million people are infected, mostly in Latin America [6–8]. In Panama, Trypanosoma rangeli is also known to infect humans, but its public health significance is negligible compared to that of T. cruzi. Other species of trypanosomes existing in the country that do not infect humans are Trypanosoma forattini, Trypanosoma hippicum, Trypanosoma vivax, Trypanosoma theileri and T. cruzi cruzi (s.l.) (Table 1) [9–15].
Table 1.
List of vertebrate hosts and reservoirs for trypanosome species in Panama
| Host order | Host family | Host species | Common names (English/Spanish) | Trypanosome species | Reference | Geographical origin | Diagnostic test |
|---|---|---|---|---|---|---|---|
| Chiroptera | Phyllostomidae | Desmodus rotundus (Geoffroy, 1810) | vampire bat/vampiro común | T. hippicum | [9] | Former Panama Canal Zone (Panama) | UND |
| Artiodactyla | Bovidae |
Bos taurus
Linnaeus, 1758 |
cattle/ganado vacuno | T. theileri | [10] | UND | UND |
| Carnivora | Canidae | Canis lupus familiaris Linnaeus, 1758 | dog/perro doméstico | T. cruzi | [17] | Former Panama Canal Zone (Panama) | Examination of blood by the thick film method; inoculation of guinea pigs, mice and rats with blood taken from infected dogs |
| Cingulata | Dasypodidae | Dasypus novemcinctus Peters, 1864 | nine-banded armadillo/armadillo | T. cruzi | [17] | Arraiján (Panama), Patuga and Parita (Herrera) | Examination of blood by the thick film method |
| Didelphimorfia | Didelphidae | Didelphis marsupialis Linnaeus, 1758 | opossum or black-eared opossum/zarigüeya común | T. cruzi, T. rangeli | [17, 24] | Chilibrillo Caves (Panama), Alhajuela (Panama), Parita and Patuga (Herrera) | Examination of blood by the thick film method |
| Rodentia | Sciuridae | Sciurus gerardi morulus Bangs, 1898 | squirrel/ardilla | T. cruzi | [17] | Former Panama Canal Zone (Panama) | Examination of blood by the thick film method |
| Chiroptera | Phyllostomidae | Hemiderma perspicillatum aztecum (Saussure, 1869) | short tail bat/murciélago de cola corta | T. cruzi | [17] | Chilibrillo Caves and Bella Vista (Panama) | Examination of blood by the thick film method; inoculation of laboratory animals |
| Chiroptera | Phyllostomidae |
Phyllostomus hastatus panamensis
Allen, 1904 |
spear-nosed bat/murciélago de nariz lanceolada | T. cruzi | [17] | Chilibrillo Caves (Panama) | |
| Chiroptera | Phyllostomidae | Uroderma bilobatum Peters, 1866 | tent-making bat/murciélago de orejas | T. cruzi | [17] | Summit (Panama) | |
| Chiroptera | Phyllostomidae | Glossophaga soricina leachi Gray, 1844 | pallas’s long-tongued bat/murciélago siricotero | T. cruzi | [17] | Bella Vista near Miraflores Locks (Panama) | |
| Chiroptera | Phyllostomidae |
Atribeus jamaicensis jamaicensis
Leach, 1821 |
fruit bat/murciélago zapotero | T. cruzi | [17] | Summit Zoo (Panama) | |
| Artiodactyla | Bovidae |
Bos taurus
Linnaeus, 1758 |
cattle/ganado vacuno | T. vivax | [11] | Aguadulce (Coclé) | Examination of thick blood smears |
| Didelphimorfia | Didelphidae | Caluromys derbianus (Waterhouse, 1841) | derby’s woolly opossum/comadreja | T. cruzi | [24] | UND | Examination of thick blood smears |
| Didelphimorfia | Didelphidae | Philander opossum (Linnaeus, 1758) | gray four-eyed opossum/zorra cuatro ojos | T. cruzi | [24] | UND | |
| Chiroptera | Phyllostomidae | Desmodus rotundus (Geoffroy, 1810) | vampire bat/vampiro común | T. cruzi | [24] | UND | |
| Chiroptera | Phyllostomidae | Carollia perspicillata (Linnaeus, 1758) | seba’s short-tailed bat/murciélago carolia | T. cruzi | [24] | UND | |
| Primates | Cebidae | Cebus capucinus (Linnaeus, 1758) | white-fronted capuchin/mono cariblanco | T. cruzi, T. rangeli, T. minasense | [24] b | Alanje and Barú (Chiriquí), Darién and Panama | Examination of thick and thin blood smears stained with Giemsa; inoculation into hemoculture tubes; direct microscopical examination |
| Primates | Atelidae |
Ateles fusciceps
Gray, 1866 |
black-headed spider monkey/yerre | T. cruzi | [24] b | Chepo, Panama and Darién | |
| Primates | Callitrichidae | Saguinus geoffroyi (Pucheran, 1845) | the Panamanian, red-crested or rufous-naped tamarin/mono tití | T. cruzi, T. rangeli, T. minasense | [24, 90] b | Panama and Colón | |
| Primates | Cebidae | Saimiri sciureus (Reinhardt, 1872) | common squirrel monkey/mono ardilla | T. cruzi | [24] b | Chiriquí | |
| Primates | Cebidae |
Alouatta villosa
(Gray, 1845) |
red howler monkey/mono aullador | T. mycetae | b | La Chorrera, Chepo, Panama, Darien and Los Santos | |
| Primates | Aotidae | Aotus trivirgatus (Humboldt, 1812) | three-striped night monkey/mono nocturno | T. sp. | b | La Chorrera, Capira, Arraiján, Panama, Colón and Darién | |
| Pilosa | Myrmecophagidae | Tamandua tetradactyla (Linnaeus, 1758) | collared anteater or lesser anteater/oso hormiguero | T. cruzi, T. rangeli, T. legeri | [24, 84] | Panama | Examination of fresh blood films; inoculation of culture media |
| Pilosa | Megalonychidae | Choleopus hoffmanni Peters, 1858 | two-toed sloth/perezoso de dos dedos | T. cruzi | [24] | UND | Examination of thick blood smears |
| Pilosa | Bradypodidae |
Bradypus variegatus infuscatus
Wagler, 1831 |
three-toed sloth/perezoso de tres dedos | T. cruzi | [24] | UND | |
| Rodentia | Sciuridae | Sciurus granatensis Humboldt, 1811 | red-tailed squirrel/ardilla colorada | T. cruzi | [24] | UND | |
| Rodentia | Echimyidae |
Proechimys semispinosus
Tomes, 1860 |
tome’s spiny rat/mocangué | T. cruzi | [24] | UND | |
| Rodentia | Dasyproctidae | Dasyprocta punctata Gray, 1842 | agouti or common agouti/neque | T. cruzi | [24] | UND | |
| Rodentia | Muridae |
Rattus rattus
(Linnaeus, 1758) |
black rat/rata negra de los tejados | T. cruzi | [24] b | UND | |
| Rodentia | Cricetidae | Tylomys panamesis (Gray, 1873) | panamanian climbing rat/rata trepadora | T. cruzi | [24] | UND | |
| Rodentia | Echimyidae | Diplomys labilis (Bangs, 1901) | rufous tree rat/rata espinosa | T. cruzi | [24] | UND | |
| Carnivora | Procyonidae | Nasua narica (Linnaeus, 1766) | white-nosed coati/gato solo | T. cruzi | [24] | UND | |
| Carnivora | Procyonidae |
Potos flavus
(Schreber, 1774) |
kinkajou/cusumbi o mico de noche | T. cruzi | [24] | UND | |
| Carnivora | Procyonidae | Bassaricyon gabbii Allen, 1876 | bushy-tailed olingo/olingo | T. cruzi | [24] | UND | |
| Rodentia | Muridae | Mus musculus Linnaeus, 1758a | mouse/ratón | T. rangeli | [24] | UND | |
| Rodentia | Muridae | Ratus norvegicus (Berkenhout, 1769)a | rat/rata | T. rangeli | [24] | UND | |
| Rodentia | Cricetidae | Oryzomys capito (Olfers, 1818) | large-headed rice rat/rata arrocera | T. forattinii | [12] | Trinidad forest (Panama) | Examination of heart blood smears |
| Squamata | Phyllodactylidae |
Tetradactylus rapicauda
(Houttuyn, 1782) |
turnip-tailed gecko/gecko | T. thecadactyli | b | UND | Examination of toe blood smears or brachial artery stained using Giemsa technique |
Research into epidemiological aspects of Chagas disease (herein CHD), including ecological factors, began in 1930 with the first case study published by Miller [16]. Initially, researchers did not have accurate diagnostic tools to identify the trypanosome species responsible for causing infection in humans. Therefore, scientific articles at the time referred to a parasite similar in morphology to T. cruzi [17–20]. In 1937, Johnson & Kelser [21] published the first epidemiological study about CHD in Panama; this effort looked at the incidence of human trypanosomes in endemic regions using an immunological test. However, during the following years, there were no related publications. Scientific efforts on CHD increased in Panama toward the end of the 1950s, with the seminal work of Dr. Octavio Sousa on the biology and ecology of triatomine bugs. Dr. Sousa reported three species of triatomines naturally infected with T. cruzi, worked on the development of diagnostic methods for T. cruzi, and investigated the distribution of T. cruzi and T. rangeli in endemic areas of Panama [22, 23]. In addition, Sousa identified a preliminary list of vertebrate hosts and reservoirs for these two trypanosome species (Table 1, Fig. 1) [13, 24–27]. As a result, efforts by Dr. Sousa contributed to a better understanding of CHD in southern Central America. Other researchers added important contributions between 1970 and 1990, which were largely about parasite biology, biochemistry, pathogenesis and treatment of CHD itself [28–34]. The focus of these investigations was on the transmission cycle, the taxonomy of insect vectors, and the identification of animal reservoirs of T. cruzi [35–40]. More recently, important scientific advances were made in the detection and identification of T. cruzi and T. rangeli using serological and molecular techniques [41–43], plus additional CHD foci were discovered in rural areas of Panama [44, 45].
Fig. 1.
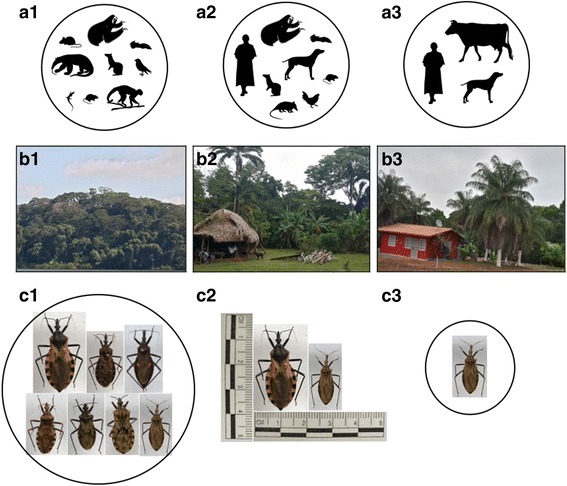
Eco-epidemiological transmission patterns of Trypanosoma cruzi in Panama: a1-a3 Animal hosts and/or reservoirs of T. cruzi across a gradient of forest habitat degradation. Main reservoirs Didelphis marsupialis (opossum), Choloepus hoffmanni (two-toed sloth), and Bradypus infuscatus (three-toed sloth); secondary reservoirs Proechymis semispinosus (prickly rat), Dasypus novemcinctus (armadillo), Tamandua tetradactyla (Anteater), Artibeus jamaicensis, Rattus rattus (common rat), Dasyprocta punctata (agouti) and Canis familiaris (dog). b1 Endemic transmission of T. cruzi at sylvatic enzootic foci (accidental transmission in humans). b2 Epidemic transmission in forest-altered habitats (indigenous communities). b3 Sporadic transmission in highly altered habitats (farmers “Campesinos”). c1-c3 Triatomine bugs vectors of T. cruzi across a gradient of natural forest habitat degradation. c1 top row, left to right, Triatoma dimidiata, Triatoma dispar, Eratyrus cuspidatus: bottom row Pastrongylus geniculatus, Pastrongylus rufotuberculatus, Pastrongylus humeralis, Rhodnius pallescens. c2 Triatoma dimidiata, and Rhodnius pallescens. c3 Rhodnius pallescens
The scope of eco-epidemiologic research about CHD in Panama has evolved through time. For most of the twentieth-century, scientific efforts adopted a pattern of discovery and data reporting type of research, which greatly helped to unravel the natural history of this complex zoonotic disease. At present, however, the focus is centered on studies trying to understand the community ecology of CHD [46–48]. Chagas disease, as with many other multilayered zoonotic diseases, requires a community-scale approach to complement traditional epidemiological approaches to untangle disease transmission. The proposed synthesis of “disease community ecology” offers a theoretical framework and the analytical tools to move beyond clinical outcomes of disease in humans, and considers the full suite of species that influence infection dynamics [48]. Moreover, in this more holistic conceptual framework, not only is the classic epidemiological triad considered (i.e. parasite-vector-host), which was widely studied between 1960 and 1990, but also the influence of habitat alteration on disease prevalence [2, 46–48]. Recent efforts in Panama investigated the impact of anthropogenic habitat alterations on the community structure of hosts and vectors and assessed mechanisms by which these changes may increase transmission risk. Gottdenker and colleagues [49, 50] applied community ecology as the framework, and hypothesis testing as the method, to understand how habitat fragmentation affects the interactions between parasites, vectors, and reservoirs comprising the enzootic cycle of CHD.
Lately, there has been a growing regional interest to review information about vector-borne infectious diseases affecting humans. These efforts are key to effectively manage neglected tropical zoonotic infections such as Plasmodium vivax malaria, leishmaniasis, and CHD, among others [3–5, 7, 51]. The information conveyed in these documents could be used to design efficacious prevention and mitigation strategies targeting the pathogens that cause these infections. The information can also help to appreciate the array of studies completed locally about these zoonoses, thus helping to avoid redundant research pursuits and to steer the scientific agenda further. The rationale for this review article is to describe the historical evolvement of scientific research about the ecology and epidemiology of CHD in Panama. In so doing, we aim at highlighting the work of prominent researchers and their key findings in studies conducted since 1930 (Additional file 1: Table S1). We put special emphasis on the transmission dynamics of T. cruzi, the bionomic of some species of Triatominae vectors, and the role of habitat degradation into transmission risk. Our considerations may potentially help to identify research needs and to re-orient future efforts about what is currently considered to be a growing public health concern in Panama.
Symptomatology, diagnosis and treatment
CHD is a two-phase clinical infection; the acute phase progresses in individuals of all ages, but children are typically most affected [24, 33, 34]. During the acute phase, a unilateral palpebral edema and conjunctivitis with ipsilateral regional lymphadenopathy, known as Romaña sign, develop around the eyebolt. However, many Panamanian patients do not have this symptom [24, 52]. Fatal cases during the acute phase of CHD in Panama are characterized by severe dyspnea and progressive myocarditis with arrhythmia, cardiomegaly, vomiting, and anuria. After the acute phase, infected people enter an indeterminate phase without symptomatology (i.e. chronic phase), but are still considered ill with positive serology [24, 50, 53, 54]. The most common clinical manifestations in confirmed cases of CHD in Panama are cardiac arrhythmia, cardiomegaly, dysfunction of nerve conduction, fever, and cervical and submaxillary adenitis. However, patients in both phases may have no apparent clinical symptoms [16, 19, 34].
In the Southern Cone region (southern South America), CHD is frequently associated with megaviscera (i.e. megaesophagus and megacolon), in Panama, several studies of esophageal transit failed to detect these manifestations in local patients. Parasitemia is short, and the chronic phase is benign in Panama compared to the one documented in South America [24, 28, 55, 56]. Some studies suggest that different clinical manifestations between Panama and South American countries could be the result of genetic divergence among discrete geographic strains of T. cruzi, or due to differences in human immune responses. Furthermore, similar clinical manifestations to those found in Panamanian patients were also detected in Rattus rattus (common rat) and Canis familiaris (domestic dog), which were either infected with T. cruzi naturally or experimentally [20, 30, 57]. These findings may indicate the existence of a distinct strain of T. cruzi in Panama, which may harbor specific phenotypic features regarding pathogenicity and virulence. This theory would explain the distinct clinical profile found locally. Scientific studies in Panama have confirmed the existence of a discrete strain of T. cruzi, which is thought to have co-evolved intimately with Didelphis marsupialis (black-eared or common opossum) and Rhodnius pallescens [58–61]. This strain is known as T. cruzi I, and it is genetically different from South American strains [58, 59, 62]. Currently, T. cruzi is subdivided into six discrete typing units (DTU) (i.e. TcI, TcII, TcIII, TcIV, TcV and TcVI), of which TcI is the most widely distributed in the Americas. Furthermore, there is empirical evidence suggesting that TcI transits between sylvatic and domestic cycles and that it is associated with cardiac complications in humans [63–65].
Very few CHD cases were diagnosed in Panama at the time of its discovery [16, 19]. In fact, detection of T. cruzi is rare in the country thus far, even with better diagnostic methods, and people often visit hospitals for other reasons without knowing they are infected. Johnson & Kelser [21] attributed this difficulty to the irregular occurrence of parasites in peripheral blood and to the lack of accuracy in microscopic examination intended to identify T. cruzi. Also, inoculation and culture of T. cruzi in susceptible animals or xenodiagnostic techniques using insect vectors are limited approaches to detect low volumes of parasites in the blood. Johnson & Kelser [21, 66] demonstrated the presence of trypanosomes throughout most of Panama. These authors inspected 10,570 human samples for the presence of trypanosomatids using the fixation complement test and estimated an overall infection rate of 1.86%. However, most positive samples came from the former Panama Canal Zone. The fixation complement test, based on cultures of T. cruzi, was considered an adequate diagnostic tool at the time because it did not display cross-reaction among different species of trypanosomes. Later on, other studies provided serological and biochemical markers for the detection of T. cruzi, and these have been widely used since the 1970s [22, 24, 67]. More recently, significant advances were made in parasite diagnosis and identification through molecular techniques such isoenzyme genotyping, multiplex PCR, and automated Sanger DNA sequencing for nuclear loci [43, 60, 68]. Because of reagent- and equipment-related costs, these modern molecular techniques are expensive, whereas rapid serological tests are cost-effective and useful tools to diagnose T. cruzi infections in humans. However, there is still a concern about false-positive results due to cross-reactivity with T. rangeli [2].
There are very few reports about treating or curing CHD in Panama [54]. The first clinical cases reported in the country were managed without medication [16]. In 1976, Blandón et al. [33] administered doses of metronidazole, primaquine, levofuraltadone, and isopentaquina to 47 patients, including 44 in the acute phase. Metronidazole was the most effective drug because it successfully cleared the infection without patient intolerance or death. Currently, the most used drugs to treat T. cruzi infections in Panama are nifurtimox [5-nitrofuran (3-methyl-4-(5′-nitrofurfurylideneamine)tetrahydro-4H-1,4-tiazine-1,1-dioxide] and benznidazole [2-nitroimidazole (N-benzyl-2-nitroimidazole acetamide]. These drugs were developed four decades ago, have limited efficacy in patients in the chronic phase, and can produce harmful side effects [2, 54, 69–72]. Recent advances in drug discovery in Panama, specifically bioactive components extracted from the bacterium Bacillus pumilus, which was isolated from the black coral Antipathes sp., have shown the ability to constrain the growth of T. cruzi [73]. Other compounds derived from Panamanian isolates of the mangrove Pelliciera rhizophorae have also demonstrated selective anti-parasitic activity against T. cruzi [74]. It is worth mentioning that drug trials in Panama using bioactive components extracted from bacteria, corals, and other trypanostatics have all been in vitro. Thus, there are still many steps before they can be considered in human trials.
Eco-epidemiology and transmission
Conventionally, T. cruzi infection in Panama has been typified as a forest zoonosis, with humans being casually infected when entering the enzootic cycle, which is disseminated to a great variety of animal vertebrates, by six species of blood-sucking triatomine bugs (Fig. 1). Notwithstanding, transmission can proceed along a gradient of forest degradation, not in a spillover fashion like in the case of arthropod-borne viral pathogens (i.e. arboviruses) and mosquitoes, but rather as a well-adapted system in which deforestation fosters biodiversity losses while boosting the ecologic links between primary triatomine vectors and major animal reservoirs of T. cruzi [49, 50]. Moreover, deforestation, urbanization, and other human activities can bring people into closer contact with triatomine disease-carrying vectors, thus creating opportunities for the colonization and establishment of these insects in human settlements [4]. However, not every kind of landscape change seems to increase CHD epidemiologic risk in Panama. Rather, transmission of T. cruzi in human-altered settings appears to be worsened by artisanal practices that use certain palm trees and their foliage and fruits for food, to build thatch roofs of houses or to make fermented wine-like beverages [75, 76]. Because of this type of exploitation, these palms proliferate abundantly throughout rural topographies of the country, and serve as a disease epicenter, favoring the aggregation of key vectors and reservoirs of T. cruzi near human habitations (Fig. 1). Research in Brazil suggested that the reproductive biology of some species of Attalea benefit the most from environmental changes such as deforestation and soil degradation [77].
Trypanosoma cruzi and T. rangeli are often found co-infecting animals or humans; both species are endemic to the neotropics and co-occur within Panama [22, 24]. Transmission of T. cruzi occurs during blood consumption when triatomine bugs deposit trypomastigote forms along with their feces near the bite site. Later, affected individuals scratch this area, dragging parasites into the wound or eyes, thus facilitating the invasion of internal tissues [2]. Trypanosoma rangeli, on the contrary, is transmitted via saliva when bugs are ingesting blood [78, 79]. The infection caused by T. rangeli, reported for the first time in Panama in 1957, is symptomless due to low pathogenicity compared to the infection with T. cruzi [22]. Trypanosoma rangeli is usually found infecting the digestive tract, hemolymph, and salivary glands of R. pallescens, but in Panama, it does not seem to be capable of infecting other bugs, including Triatoma dimidiata [24]. This implies that a high degree of specificity exists between the Panamanian strain of T. rangeli and R. pallescens [78, 79]. Other ways of transmission of T. cruzi and T. rangeli to humans, such as organ transplants or blood transfusion from infected donors, although possible, have not been reported in Panama.
CHD, mainly detected in the provinces of Panama, Coclé and Colon, seems largely localized to central Panama, where human infection rate normally ranges from 0.5 to 8.8% [22]. However, since adequate ecologic conditions for the transmission of T. cruzi have been reported from the entire country, this is likely due to the concentration of studies in this area, plus considerable case is underreporting nationwide [22, 24, 49, 80]. Sousa & Johnson [22] reported T. rangeli to be six times more prevalent in rural Provinces of Panama, Coclé and Colón than T. cruzi, based on microscopic examination. Sousa [24] speculated that this was due to a steady contact between people and R. pallescens since this vector is the only one capable of transmitting T. rangeli in Panama. However, R. pallescens is found infected with T. cruzi more frequently than with T. rangeli, which suggests differences in the vectorial competence of R. pallescens for these two parasite species [22, 42, 81]. The greater prevalence of T. rangeli in humans could be due to a more efficient way of transmission via saliva as compared to that of T. cruzi via contaminated feces [3]. Some studies also suggest that this outcome is likely due to the fact that an initial exposure to T. rangeli might confer immune protection against a subsequent infection with T. cruzi [22, 42, 81, 82]. Recently, new CHD foci were detected in the district of Santa Fe, located north of Veraguas’s Province, and also in Chepo and Chiman, in eastern Panama. Authors in these studies reported equivalent infection rates of T. cruzi and T. rangeli in humans as well as in R. pallescens [44, 45].
Animal reservoirs
CHD is in principle a neotropical zoonosis that involves a large variety of vertebrate and invertebrate species as hosts, reservoirs, and vectors. However, due to increased international travel in recent decades, this infection has greatly expanded from its original geographical range [2–4]. In 1972, Octavio Sousa [24] published a list of 26 species of mammals in Panama that were found infected with T. cruzi based on microscopy. This record included six species of rodents, five bats, four primates, three marsupials, edentates, and carnivores, in that order (Table 1; Fig. 1). Among the rodents and marsupials, R. rattus and D. marsupialis are considered major reservoirs of T. cruzi in peridomestic settings, whereas Proechimys semispinosus (prickly rat) is an important reservoir under sylvatic conditions [30, 83]. Other species of wild animals that serve as a reservoir of T. cruzi in Panama are Dasypus novemcinctus (armadillo), Tamandua tetradactyla (anteater), Bradypus infuscatus (three-toed sloth), and the bat Artibeus jamaicensis [17, 84, 85].
Birds in general, including chickens, are considered refractory to infection with T. cruzi, which might suggest that they could be good candidates for zoo-prophylactic control strategies [86]. Besides, chickens frequently eat triatomines in peridomiciliary and domiciliary areas and could diminish their populations to some degree. For example, Cecere et al. [87] proposed that the exclusion of chickens from peridomiciliary areas could increase T. cruzi infection rates in humans. Moreover, recent studies conducted in rural localities of central Panama reported dogs commonly infected with T. cruzi, reaching prevalence rates of up to 11.1%, which could also suggest a role as a domestic reservoir [88, 89].
Trypanosoma rangeli has been identified from 15 species of wild mammals including D. marsupialis, which is frequently found co-infected with T. cruzi (Table 1, Fig. 1) [17]. Sousa & Dawson [90] proposed Saguinus geoffroyi (titi monkey) as another natural reservoir of T. cruzi and T. rangeli in Panama and anticipated a high risk of infection to people adopting these animals as pets. These monkeys can migrate from nearby forested areas into houses, possibly searching for food. A high prevalence of T. rangeli in S. geoffroyi implies a close relationship with R. pallescens, but this could also be due to horizontal transmission during the rainy season when these monkeys feed massively on triatomine bugs [13]. Because of the pleomorphic nature of epimastigotes of T. cruzi, surveillance studies based on microscopy are insufficient to describe pathogen-host relationships. Follow-up studies, based on molecular approaches to identification (i.e. DNA barcoding), are needed to confirm the specificity of pathogen-host species associations.
Vectors of T. cruzi and T. rangeli
Triatominae bugs vectoring T. cruzi in Panama were identified during the 1930s; these insects belong to various genera within the subfamily Triatominae (order Hemiptera), and are commonly known in Panama as “chinches mamones” or “chinches de monte” [35, 38, 83, 91]. Triatominae species found naturally infected and capable of transmitting T. cruzi in Panama are Triatoma geniculata (named as Panstrongylus geniculatus later on) [17, 18], R. pallescens [92], Eratyrus cuspidatus [93] and T. dimidiata [94] (Table 2). Clark & Dunn [17] incriminated R. prolixus as one of the main vectors of T. cruzi in Panama, but the occurrence of this species was never confirmed in the country [24, 95]. Furthermore, mistakenly identified as Triatoma venosa by Champion [96] and Usinger [97], Triatoma dispar is another potential vector of T. cruzi in Panama, and was found naturally infected in the forest canopy of eastern Panama [23]. In addition, Panstrongylus humeralis and Panstrongylus rufotuberculatus were incriminated as vectors of T. cruzi in Panama, but these are primarily sylvatic species associated with animal caves, burrow nests, and tree holes in pristine forest environments [25]. Therefore, they are not usually found near human settlements [24]. Cavernicola pilosa, a cave-dwelling species parasitizing bats, was also found infected with T. cruzi in Panama [24, 91].
Table 2.
Triatominae species found naturally infected and capable of transmitting T. cruzi and T. rangeli in Panama
| Triatominae taxa | Trypanosome species [ref] | Habitat | Host-feeding range [ref] | Geographical location [Province] | Sampling site | Ecotype | Methodology |
|---|---|---|---|---|---|---|---|
| Panstrongylus geniculatus |
T. cruzi
[17] |
Sylvatic species specialized on subterranean host habitats: caves, nests and tree holes. It has been associated with the armadillo (Dasypus novemcinctus) | UND | Chilibrillo caves [Panama] | Bat cave | Sylvatic | Examination of bug feces; inoculation of guinea pigs with macerated bugs; feeding bugs on guinea pigs (xenodiagnostic technique) |
| Rhodnius pallescens s.l. |
T. cruzi
[92]; T. rangeli [79] |
Anthropic colonist species, invades both indoor and outdoor environments depending on ecological, enviromental and soci-economic conditions. It is strongly associated with species of palm trees, e.g. Attalea butyracea a and also Acrocomia spp. | Bradypodidae, Cracidae, Didelphidae, Echimyidae, Myrmecophagidae, Sauria (Lizards), Sciuridae, [100] |
Aguas Buenas [Panama] | Inside house | Domestic | Feeding bugs on guinea pigs (xenodiagnostic technique) |
| Amphibia, Canidae, Cebidae, Columbidae, Cracidae, Cricetidae, Dasyproctidae, Didelphidae, Echimyidae, Felidae, Hominidae, Muridae, Leporidae, Phasianidae, Psittacidae, Reptilia, Rallidae, Suidae, Strigidae (Sauria), [37] | |||||||
| Birds, dogs, humans, opossum, rats [104] | |||||||
| Artiodactyla, Carnivora, Caudata, Chiroptera, Ciconiiformes, Falconiformes, Galliformes, Marsupialia, Passeriformes, Primata, Rodentia, Squamata, Xenarthra, [50] | |||||||
| Eratyrus cuspidatus |
T. cruzi
[93] |
Sylvatic species rarely found near human habitations in rural areas | UND | Retiro Matias Hernandez [Panama] | Inside building | Domestic | Feeding bugs on guinea pigs (xenodiagnostic technique) |
| Triatoma dimidiata |
T. cruzi
[94] |
Sylvatic or semi-anthropic species found occasionally indoor, but mainly encountered around houses in domestic animal shelters | Accipitridae, Amphibia, Ardeidae, Bovidae, Canidae, Columbidae, Cricetidae, Felidae, Hominidae, Leporidae, Muridae, Passeriformes, Phasianidae, Psittacidae, [39] |
Chorrera [Panama] | Inside house | Domestic | Feeding bugs on guinea pigs (xenodiagnostic technique) |
| Cavernicola pilosa |
T. cruzi [91] |
Sylvatic species associated with the caves inhabited by various species of bats | UND | Panama City [Panama] | Bat cave | Sylvatic | – |
| Triatoma dispar |
T. cruzi
[23] |
Sylvatic species found in the canopy of mature old-growth type of forest | UND | Cerro Quia [Darien] | Forest canopy | Sylvatic | Examination of bug feces; inoculation of white mice with suspension of fecal material from bugs |
| Panstrongylus rufotuberculatus |
T. cruzi
[24] |
Sylvatic species, specializes on subterranean host habitats: caves, nests and tree holes. It is often found in pristine seasonal tropical rainforest forest | UND | Cerro Quia [Darien] | Forest canopy | Sylvatic | Examination of bug feces; inoculation of white mice with suspension of fecal material from bugs |
| Panstrongylus humeralis |
T. cruzi
[25] |
Sylvatic species that specializes on subterranean host habitats: caves, nests and tree holes. It has been found sporadically around houses in rural areas | UND | Bayano Lake [Panama] | Forest understory | Sylvatic | Inoculation of white mice with suspension of fecal material from bugs |
a Formerly known as Scheela zonensis
Reference: ref
Undetermined: UND
Rhodnius pallescens and T. dimidiata are considered to be the primary vectors of T. cruzi in Panama [22, 24, 35, 38, 76, 97]. The former appears to predominate in the central part of the country, whereas T. dimidiata is most commonly known from the western region [22, 24]. This apparent inter-species spatial segregation seems more associated with discrete environmental circumstances in these areas matching the ecologic requirements of each species. For instance, R. pallescens thrives in central Panama, where in the last 60 years human population growth has prompted the transformation of forest into land for agriculture and livestock production. These landscape changes favor the proliferation of certain palm tree species [76], which in turn, seem to facilitate the demographic expansion of R. pallescens [98]. In contrast, T. dimidiata appears to be more associated with less-altered forest habitats in western Panama, where it is able to maintain large and stable population sizes without palm trees [24, 99].
Vector bionomics of R. pallescens and T. dimidiata
Pipkin [85] posited that ecological niche, the degree of domiciliation, host feeding behavior, and rate of infection with T. cruzi are the most important factors shaping the local transmission role of different species of triatomines. In Panama, R. pallescens is closely associated with Attalea butyracea (e.g. Royal, Wine, or Corozo palm), a species of palm tree formerly known as Scheelea zonensis [75, 99, 100]. Attalea butyracea is prevalent across the country, is found in both primary and secondary forest habitats, but most commonly in savanna ecosystems, prairies, and realms for agriculture and livestock development, often in close proximity to human habitations [75, 76, 100]. This palm offers proper conditions of humidity and temperature as well as food (i.e. D. marsupialis) for the development of R. pallescens [98, 100]. Moreover, recent studies suggested that R. pallescens, which under laboratory conditions can fly up to 5 km before tiring, could invade houses attracted by light from nearby palm trees [101, 102]. Therefore, the presence of A. butyracea near human communities is considered an important risk factor for CHD transmission.
Pipkin [85] deduced that the abundance of R. pallescens in houses of CHD endemic communities from central Panama exceeded that of other triatomines. He found nymphs and adults of R. pallescens inside houses, showing for the first time that this triatomine species could enter households and nourish on humans. However, in other studies, R. pallescens was collected most commonly outdoors than indoors [101, 103]. Differences in the degree of domiciliation of R. pallescens across Panama could be an artifact of ecological, demographic, and socioeconomic disparities among different study sites. For example, particular housing conditions are essential for the colonization and adaptation of R. pallescens; houses built with mud, clay, and palm leafs may be more vulnerable to invasion than those built with brick, cement, and metal roofs. Moreover, houses surrounded by palm trees (e.g. Attalea spp. and Acrocomia spp.) and animal shelters might promote a faster invasion of R. pallescens as opposed to others that lack these conditions [2, 3, 24, 100].
Initial studies about host-feeding ranges of Panamanian triatomines indicated that R. pallescens feeds mainly on D. marsupialis and humans, but it can also take blood from rodents, canines, felines, monkeys, reptiles, and wild/domestic birds (Table 2) [37, 100, 104]. Gottdenker et al. [49] proposed that host species spectra serving as food sources for R. pallescens vary as a function of habitat fragmentation, with Choleopus hoffmanni (i.e. two-toed sloth) being the primary host in areas of old-growth and secondary forests, and D. marsupialis being the primary host in forest-altered settings close to human settlements (Fig. 1). Some researchers consider R. pallescens to be a forest specialist in Panama, but its opportunistic feeding behavior and a remarkable capacity to invade and adapt to different environmental conditions (including human-related niches) [105] confer it an advantage over other triatomines. This capacity allows it to flourish in both sylvatic and peridomestic areas, where wildlife and humans are the main sources of blood, correspondingly [50]. Contrary to R. pallescens, T. dimidiata has not been commonly associated with palm trees, nor has it frequently been found indoors in Panama [45]. Christensen et al. [39] hypothesized that western populations of T. dimidiata feed mainly on humans, chickens, and dogs, but they do not seem to feed on D. marsupialis. However, some authors consider this finding to be erroneous and attribute it to low specificity by the precipitin test employed in previous studies. Although studies conducted in Panama provided a general view of the host-feeding ranges of R. pallescens and T. dimidiata, it is discernible that both species are catholic feeders that take blood from a large variety of vertebrates, probably depending on their availability and biomass, which in the peridomestic setting may be mostly rodents and humans [37, 104, 106, 107].
Sousa & Johnson [70] estimated T. cruzi infection rates of triatomines from central Panama to be between 3.1–21.5%. Vásquez et al. [103] used microscopy and reported 85.4 and 14.6% infection rates with T. cruzi and T. rangeli in R. pallescens, respectively. Calzada et al. [101] used molecular techniques and estimated the infection rates of R. pallescens with T. cruzi and T. rangeli at 72.7 and 40.0%, respectively. As expected, results obtained with molecular methods were superior to those obtained with microscopy, where only 27.3% of specimens tested positive for trypanosomes. In contrast, the infection rate of T. dimidiata with T. cruzi in Panama was significantly lower than that of R. pallescens, ranging between 13.5–17.7% [22, 45]. Gottdenker et al. [49, 50] suggested that the infection rate of R. pallescens with T. cruzi is influenced by the degree of habitat fragmentation, which in turn determines host species composition and availability. They reported a higher infection rate of R. pallescens with T. cruzi in deforested and fragmented forest sites compared with more contiguous and less altered forest habitats. Future studies aiming to investigate T. cruzi infection rates and host-feeding ranges in Panamanian triatomines must control the degree of habitat alteration and for the availability of hosts as potential biases when assessing pathogen-vector-host interactions.
Rhodnius pallescens and T. dimidiata are both primary vectors of T. cruzi in Panama, but the former seems more important from an epidemiologic standpoint due to its greater degree of association with Attalea butyracea in rural communities of Panama [35, 40, 85, 98]. Rural workers, “campesinos,” in these settings use leafs (Pencas) of A. butyracea to assemble the roof of their shacks [76], which might expedite invasion and also contribute to the spread of eggs, nymphs, and/or adults of R. pallescens. As a result, its genetic diversity is elevated by favoring gene flow among distantly located geographical populations [72]. Triatoma dimidiata, in contrast, is the primary vector of T. cruzi under more sylvatic conditions, in woody areas of Panama, where human settlements are established inside old-growth or secondary forest patches, and its populations reach large numbers regardless of the absence of A. butyracea [22, 45]. A trend of increasing forest degradation, suburbanization, and development of tourism in Panama indicates that a colonist species (i.e. disturbance tolerant) such as R. pallescens will continue to play a more prominent role in the transmission of T. cruzi than a forest specialist (i.e. disturbance intolerant) like T. dimidiata (Fig. 1). Other biologic attributes of R. pallescens supporting this view are high physiological plasticity, notable flight range by T. cruzi-infected individuals, and relatively short developmental time [40, 87, 102, 105].
Taxonomic status
Triatoma dimidiata and R. pallescens distribute extensively across the neotropics, but the former has a greater geographical distribution. Triatoma dimidiata most likely originated in northern Central America (i.e. Mexico and Guatemala) and colonized southward through Mesoamerica and northern South America, whereas R. pallescens originated in South America (i.e. Colombia and Ecuador) and colonized northward across Mesoamerica [108–110]. Both species experienced episodes of vicariance and secondary admixture in the past, and face considerable environmental variability across their ranges at present. Therefore, they depict substantial phenotypic variance in color, size, behavior, and various levels of molecular divergence in mitochondrial and nuclear loci [105, 109, 111]. Several lines of evidence support the existence of at least three taxa within T. dimidiata (s.l.) (i.e. cryptic species complex), including T. dimidiata capitata, which is found in Panama and Colombia. Molecular divergence in T. dimidiata has been attributed to geographical range expansion following adaptation to local climatic conditions during its colonization of South America (e.g. Pleistocene climatic changes) or to more recent anthropogenic habitat degradation [108, 109, 112]. Likewise, R. pallescens depicts significant population structure across Panama, Colombia and Ecuador [110]. Two molecular lineages and a putative sympatric sibling species, Rhodnius colombiensis, were predicted to occur between northern South America and Panama. Rhodnius pallescens may be a complex of two isomorphic species with different chromosomal attributes. Values of molecular divergence between lineages I and II of R. pallescens were similar to those between R. colombiensis and these lineages, suggesting a very close phylogenetic relationship and perhaps similar ecologic niche among these three sister taxa (e.g. sylvatic habitat). Diversification of R. pallescens (lineages I and II) was attributed to the formation of the Isthmus of Panama, vicariance, and subsequent range re-colonization [110]. Studies about the population genetic structure and taxonomic status of R. pallescens and T. dimidiata capitata (i.e. the presence of additional cryptic evolutionary units) have not been conducted systematically across Panama, despite their potential to inform about the applicability of genetic vector control strategies.
Prevention and control
Despite the ongoing expansion of CHD throughout ecologically altered areas of Panama, the Panamanian Ministry of Health (MINSA) does not consider this infection a priority in terms of control, limiting mitigation efforts to treat severe cases detected mostly by passive surveillance. Although the inattention to CHD by MINSA is likely due to the enzootic epidemiologic characteristic and chronic nature of CHD [2], some researchers attribute it to a significant degree of underreporting, and to the non-domiciliary behavior of R. pallescens in Panama [101, 103]. For instance, Panamanian populations of R. pallescens are highly susceptible to deltamethrin and lambda-cyhalothrin, but it is impractical to implement pyrethroid residual spraying to kill a vector that does not reside indoors [113]. CHD prevention and control programs in Panama must focus on putting into action an active surveillance program for accurate case detection. This program must also increase epidemiologic surveillance into unexplored areas of the countryside to detect new transmission foci [44, 45]. Recent work directed at CHD in Panama has highlighted the need to implement interdisciplinary approaches to prevent transmission, taking into account local changes in disease patterns due to anthropogenic and/or climatic changes, but actively involving community members in mitigation actions [2, 107]. The implementation of educational programs targeting vulnerable communities can help to minimize risk by teaching people how to improve house quality using appropriate construction materials, thus helping them to reduce human-vector contact [107, 114].
Future research agenda
Once an active surveillance program is put into place and underreporting of T. cruzi is no longer an issue, forthcoming research about CHD in Panama must center on identifying epidemiologic risk factors in endemic areas, including ecologic (i.e. landscape uses affecting major vectors and reservoirs of CHD), demographic (i.e. gender and age range in human populations), environmental (i.e. temperature, precipitation, forest cover, and seasonality) and social variables (i.e. level of poverty and occupation) that may be related to a higher infection risk under certain conditions. Moreover, spatial and temporal clusters of CHD must be defined using hotspot analysis, geographical information system (GIS) mapping techniques, or landscape genetics as having been done recently for other vector-borne diseases in Panama [115]. Modeling the impact of climate change and/or forest alteration on the prevalence of T. cruzi can help to prevent future outbreaks. A recent study demonstrated the utility of the macro-ecological approach to better understand the spatial-temporal transmission dynamic of leishmaniasis in Panama [116], but similar work on CHD has not yet been conducted. In addition, there is a need for developing new and innovative strategies for vector control and more effective and less toxic drugs to treat infected people [72, 74]. More specifically, scientific studies about population genetics and niche dynamics of major epidemiologic components of CHD (i.e. parasite-vector-host) are lacking in Panama. The taxonomic status of T. dimidiata capitata and R. pallescens must be further evaluated, as well as possible differences in vector bionomic or insecticide resistance profiles among different subpopulations (e.g. lineages or sister taxa) of these vectors [117]. In addition, no systematic study of the distribution of vector and non-vector triatomines or about inter-specific competition among triatomine species has ever been conducted in the country. Tourism is growing in Panama, and certain real estate developments use A. butyracea and other species of palm trees (i.e. Acrocomia sp.) for esthetic purposes, which could potentially open new niches for some triatomine species [98]. It is also necessary to investigate the role of recurrent infections with T. rangeli on CHD transmission in Panama because this sympatric parasite greatly decreases the fitness of R. pallescens. These and other topics must be tackled to understand the evolutionary potential of CHD under an increasing scenario of climate change and urbanization.
Conclusions
Extensive ecological and epidemiological investigation about CHD has been undertaken in Panama since the beginning of last century, and several generations of Panamanian scientists have been involved in these efforts. Furthermore, lately there has been a growing interest in investigating CHD in Panama, and a new theoretical approach linking anthropogenic degradation of forest ecosystems with CHD emergence has been implemented. However, very few attempts have been made in Panama to integrate all this information into prevention and mitigation actions for CHD control [118–120]. The first logical step in planning effective strategies to prevent and manage CHD expansion across the country is to summarize existing information on scientific research. Here we make progress toward this end, reviewing knowledge about the ecology and epidemiology of CHD since the 1930s. Seemingly easy and yet challenging at once, this task is often neglected in countries with a rich history of research in tropical medicine, where past scientific information is no longer read by newer generations of scientists and might be scattered or lost [118, 120]. This might be the case in Panama, where no review article has ever been written in English about CHD, despite the rich history of scientific investigations on this neglected vector-borne infection. As a philosophical conclusion, we posit that scientific research about CHD must continue in Panama, and will prove to be the best weapon to lessen transmission risk in endemic areas. However, the government should make it a public health priority and establish an effective active surveillance program as the first step to mitigating this problem [107, 118, 120]. Finally, future research plans about CHD in Panama must continue using community ecology and hypothesis testing as the primary instruments to understand the overlooked complexity of this disease system better. For now, this is a more realistic approach than the pattern of discovery/data reporting type of research, and it will result in the generation of valid scientific information that could be used to design integrated and effective mitigation strategies. We hope that our review article will contribute to the very first step of this crucial goal, as it provides a summary of information on ecologic and epidemiologic research, which along with knowledge about the impact of anthropogenic habitat alterations and climate change into CHD transmission will help to diminish the burden of this neglected tropical infection in Panama.
Acknowledgements
We express gratitude to Julio Rodríguez Lascano for helping to generate the sketch on Fig. 1. We also want to thank Dr Matthew J. Miller from the University of Oklahoma for providing valuable remarks to our work. Dr Yves Basset from the CTFS Arthropod Initiative at STRI facilitated the specimens of P. humeralis and E. cuspidatus used in Fig. 1. Jose Alejandro Ramirez Silva, also from STRI, provided the rest of specimens used in Fig. 1 (e.g. T. dimidiata, T. dispar, P. rufotuberculatus, P. geniculatus and R. pallescens), from his personal collection. Finally, we want to acknowledge the encouraging comments from three anonymous reviewers, which improved the clarity, quality and impact of our manuscript.
Funding
Financial support was provided by the Instituto de Investigaciones Científicas y Servicios de Alta Tecnología (INDICASAT-AIP) and the Smithsonian Tropical Research Institute (STRI). The Secretariat for Science, Technology and Innovation of Panama (SENACYT), through the research grants ITE11–015 and COL11–044 and the National System of Investigation (SNI), supports research activities by JRL. Financial support for IGR comes from various student fellowships including SNI and the Fundación Benéfica Deveaux.
Availability of data and materials
Not applicable.
Abbreviations
- CHD
Chagas disease
- CTFS
Center for Tropical Forest Science
- DNA
Deoxyribonucleic acid
- DTU
Discrete typing units
- GIS
Geographical information system
- INDICASAT-AIP
Instituto de Investigaciones Científicas y Servicios de Alta Tecnología
- MINSA
Panamanian Ministry of Health
- PCR
Polymerase chain reaction
- SNI
National System of Investigation
- STRI
Smithsonian Tropical Research Institute
Additional file
References concerning the ecology and epidemiology of American trypanosomiasis or Chagas Disease, in Panama. (XLSX 497 kb)
Authors’ contributions
Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-017-2380-5) contains supplementary material, which is available to authorized users.
References
- 1.Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–18.
- 2.Telleria J, Tibayreng M. American trypanosomiasis Chagas disease: one hundred years of research. 1. Amsterdam: Elsevier; 2010. [Google Scholar]
- 3.Gourbière S, Dorn P, Tripet F, Dumonteil E. Genetics and evolution of triatomines: from phylogeny to vector control. Heredity. 2012;108:190–202. doi: 10.1038/hdy.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steverding D. The history of Chagas disease. Parasit Vectors. 2014;7:317. doi: 10.1186/1756-3305-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bern C. Chagas Disease N Engl J Med. 2015;373:5. doi: 10.1056/NEJMp1506446. [DOI] [PubMed] [Google Scholar]
- 6.Chaniotis B. Outline of principal diseases transmitted by insects in Panama. Rev Med Panamá. 1987;12:0379–1623. [PubMed] [Google Scholar]
- 7.Hotez P, Woc-Colburn L, Bottazzi M. Neglected tropical diseases in Central America and Panama: review of their prevalence, populations at risk and impact on regional development. Int J Parasitol. 2014;44:597–03. [DOI] [PubMed]
- 8.World Health Organization. Chagas disease (American trypanosomiasis), World Health Organ Fact Sheet. 2015;340. http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 14 Oct 2014.
- 9.Darling S. Equine trypanosomiasis in the canal zone. Bull Soc Pathol Exot. 1910;3:381–384. [Google Scholar]
- 10.Teague O, Clark H. A trypanosome of Panamanian cattle and a method for concentrating trypanosomes in peripheral blood. J Infect Dis. 1918;22:154–158. doi: 10.1093/infdis/22.2.154. [DOI] [Google Scholar]
- 11.Johnson C. Bovine trypanosomiasis in Panama. Am J Trop Med Hyg. 1941;21:289–297. doi: 10.4269/ajtmh.1941.s1-21.289. [DOI] [Google Scholar]
- 12.Everard C, Sousa O. Recovery of Trypanosoma forattinii Coutinho and Trypanosoma pattoli from a Trinidadian rodent. Rev Saúde Públ. 1972;6:283–285. doi: 10.1590/S0034-89101972000300007. [DOI] [PubMed] [Google Scholar]
- 13.Sousa O, Rossan R, Berg D. The prevalence of trypanosomes and microfilariae in Panamanian monkeys. Am J Trop Med Hyg. 1974;23:862–868. doi: 10.4269/ajtmh.1974.23.862. [DOI] [PubMed] [Google Scholar]
- 14.Miguel-Pinto C, Kalko E, Cottontail I, Wellinghausen N, Cottontail V. TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infect Genet Evol. 2012;12:1328–1332. doi: 10.1016/j.meegid.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Cottontail V, Kalko E, Cottontail I, Wellinghausen N, Tschapka M, Perkins S, et al. High local diversity of Trypanosoma in a common bat species, and implications for the biogeography and taxonomy of the T. cruzi clade. PLoS One. 2014;9:e108603. doi: 10.1371/journal.pone.0108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J. Chagas disease in Panama: report of three cases. South Med J. 1931;24:645–647. doi: 10.1097/00007611-193107000-00014. [DOI] [Google Scholar]
- 17.Clark H, Dunn L. Experimental studies on Chagas disease in Panama. Am J Trop Med Hyg. 1932;12:49–77. doi: 10.4269/ajtmh.1932.s1-12.49. [DOI] [Google Scholar]
- 18.De Coursey E. The first fatal case of Chagas disease observed on the isthmus of Panama. Am J Trop Med Hyg. 1935;15:33–39. doi: 10.4269/ajtmh.1935.s1-15.33. [DOI] [Google Scholar]
- 19.Johnson C, De Rivas G. Six new cases of Chagas disease in Panama with review of previous cases. Am J Trop Med Hyg. 1935;16:47–57. [Google Scholar]
- 20.Johnson C. Cardiac changes in dogs experimentally infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1938;18:197–206. doi: 10.4269/ajtmh.1938.s1-18.197. [DOI] [Google Scholar]
- 21.Johnson C, Kelser R. The incidence of Chagas disease in Panama as determined by the complement fixation test. Am J Trop Med Hyg. 1937;17:385–392. doi: 10.4269/ajtmh.1937.s1-17.385. [DOI] [Google Scholar]
- 22.Sousa O, Johnson C. Frequency and distribution of Trypanosoma cruzi and Trypanosoma rangeli in the Republic of Panama. Am J Trop Med Hyg. 1971;20:405–410. doi: 10.4269/ajtmh.1971.20.405. [DOI] [PubMed] [Google Scholar]
- 23.Sousa O, Galindo P. Natural infections of Triatoma dispar (lent 1950) with Trypanosoma cruzi in Panama. Am J Trop Med Hyg. 1972;21:293–295. doi: 10.4269/ajtmh.1972.21.293. [DOI] [PubMed] [Google Scholar]
- 24.Sousa O. Anotaciones sobre la enfermedad de Chagas en Panamá. Frecuencia y distribución de Trypanosoma cruzi y Trypanosoma rangeli. Rev Biol Trop. 1972;20:167–179. [PubMed] [Google Scholar]
- 25.Sousa O, Adames A. Geographical extension in a new ecological association of Panstrongylus humeralis (Hemiptera: Reduviidae), natural host of Trypanosoma cruzi in Panamá. J Med Entomol. 1977;13:748–749. doi: 10.1093/jmedent/13.6.748. [DOI] [PubMed] [Google Scholar]
- 26.Sousa O, Herman C. Blood parasites of birds from Chiriquí and Panama provinces in the Republic of Panama. J Wildl Dis. 1982;18:205–221. doi: 10.7589/0090-3558-18.2.205. [DOI] [PubMed] [Google Scholar]
- 27.Sousa O. Isolation and amplification techniques. Rev Soc Bras Med Trop. 1985;18:23–27. doi: 10.1590/S0037-86821985000100006. [DOI] [Google Scholar]
- 28.Blandón R, Guevara J, Johnson C. Tránsito esofágico en pacientes con enfermedad de Chagas en Panamá. Arch Gastroenterología. 1969;6:189–196. [Google Scholar]
- 29.Johnson C, Edgcomb J, Kinney R. Chagasic myocardiopathy. N Engl J Med. 1971;285:1262. doi: 10.1056/NEJM197111252852214. [DOI] [PubMed] [Google Scholar]
- 30.Edgcomb J, Walker D, Johnson C. Pathological features of Trypanosoma cruzi infections of Rattus rattus. Arch Pathol Lab Med. 1973;96:36–39. [PubMed] [Google Scholar]
- 31.Wood D. Trypanosoma cruzi: fatty acid metabolism in vitro. Exp Parasitol. 1975;37:60–66. doi: 10.1016/0014-4894(75)90052-1. [DOI] [PubMed] [Google Scholar]
- 32.Wood D, Sousa O. Trypanosoma cruzi: effects of Rhodnius prolixus extracts on in vitro development. Rev Inst Med Trop São Paulo. 1976;18:93–96. [PubMed] [Google Scholar]
- 33.Blandón R, Guevara J, Johnson C. Enfermedad de Chagas aguda en niños. Sintomatología y tratamiento Rev Med Panamá. 1976;1
- 34.Blandón R, Johnson C, Leandro I, Acuna E. Chagas disease in children. Pediatr Cardiol. 1981;4:614–618. [Google Scholar]
- 35.Méndez E, Sousa O. Identificación y distribución de los triatominos de Panamá (Hemiptera: Reduviidae: Triatominae) Rev Med Panamá. 1979;4:258–280. [Google Scholar]
- 36.Dujardin JP, Schofield CJ, Panzera F. Los vectores de la Enfermedad de Chagas. Academie Royale des Sciences D’Outre-Mer: Bruxelles; 2002. [Google Scholar]
- 37.Christensen H, De Vásquez A. Host feeding profiles of Rhodnius pallescens (Hemiptera: Reduviidae) in rural villages of central Panama. Am J Trop Med Hyg. 1981;30:278–283. doi: 10.4269/ajtmh.1981.30.278. [DOI] [PubMed] [Google Scholar]
- 38.Sousa O, Wolda H, Batista F. Triatominos encontrados en el ambiente silvestre de la Isla Barro Colorado. Rev Med Panamá. 1983;8:50–55. [PubMed] [Google Scholar]
- 39.Christensen H, Sousa O, De Vásquez A. Host feeding profiles of Triatoma dimidiata in peridomestic habitats of western Panama. Am J Trop Med Hyg. 1988;38:477–479. doi: 10.4269/ajtmh.1988.38.477. [DOI] [PubMed] [Google Scholar]
- 40.Méndez E, Sousa O, Turner A. Caracterización biológica y ecológica de los triatominos panameños (Hemiptera: Reduviidae) Scientia. 1997;12:7–66. [Google Scholar]
- 41.Saldaña A, Sousa O, Örn A. Virulence clones from Panama: humoral immune responses and antigenic cross-reactions with Trypanosoma rangeli in experimentally infected mice. Scand J Immunol. 1995;42:644–650. doi: 10.1111/j.1365-3083.1995.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 42.Vásquez J, Krusnell J, Sousa O, Harris R. Serological diagnosis of Trypanosoma rangeli infected patients. A comparison of different methods and its implications for the diagnosis of Chagas disease. J Immunol. 1997;45:322–330. doi: 10.1046/j.1365-3083.1997.d01-405.x. [DOI] [PubMed] [Google Scholar]
- 43.Brandao A, Samudio F, Fernández O, Calzada J, Sousa O. Genotyping of Panamanian Trypanosoma cruzi stocks using the calmodulin 3’UTR polymorphisms. Parasitol Res. 2007;102:523–526. doi: 10.1007/s00436-007-0775-5. [DOI] [PubMed] [Google Scholar]
- 44.Calzada J, Pineda V, Garisto J, Samudio F, Santamaría A, Saldaña A. Short report: human trypanosomiasis in the eastern region of the Panama province: new endemic areas for Chagas disease. Am J Trop Med Hyg. 2010;82:580–582. doi: 10.4269/ajtmh.2010.09-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saldaña A, Pineda V, Martínez I, Santamaría A, Miranda A, Calzada J. A new endemic focus of Chagas disease in the northern region of Veraguas Province, Western Half Panama, Central America. Plos One. 2012;7:e34657. [DOI] [PMC free article] [PubMed]
- 46.Bradford HA. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bain L, Awah P. Eco-epidemiology: challenges and opportunities for tomorrow’s epidemiologists. Pan Afr Med J. 2014;17:317. doi: 10.11604/pamj.2014.17.317.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson PTJ, De Roode JC, Andy F. Why infectious disease research needs community ecology. Science. 2015;4:349(6252). doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottdenker N, Calzada J, Saldaña A, Carrol R. Association of anthropogenic land use change and increased abundance of the Chagas disease vector Rhodnius pallescens in a rural landscape of Panama. Am J Trop Med Hyg. 2011;84:70–77. doi: 10.4269/ajtmh.2011.10-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottdenker N, Chaves L, Calzada J, Saldaña A, Carrol R. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. PLoS Negl Trop Dis. 2012;6:1–11. doi: 10.1371/journal.pntd.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutari L, Loaiza J. American cutaneous leishmaniasis in Panama: a historical review of entomological studies on anthropophilic Lutzomyia sand fly species. Parasit Vectors. 2014;7:218. doi: 10.1186/1756-3305-7-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.La conjuntivitis schizotripanosómica unilateral. Mision de Estudios de Patologia Regional Argentina (MEPRA). 1935;22:16–25.
- 53.Calero C. Clinical forms of the chronic type of Chagas disease, observed in the isthmus of Panama. Arch Med Panameños. 1963;12:3–7. [PubMed] [Google Scholar]
- 54.Blandón R. The clinical experience in Panama with metronidazole in treating Chagas' disease in the acute and chronic phases. Rev Med Panamá. 1993;18:0379–1623. [PubMed] [Google Scholar]
- 55.Johnson C. The 32nd annual report of the Gorgas Memorial Laboratory. Washington D.C.: U.S. Gov. Printing Office. 1960:12.
- 56.Johnson C, Sabonge R, Gálvez A, Pinilla C. Tránsito esofágico en enfermos con tripanosomiasis americana. Arch Med Panameños. 1962;11:93. [Google Scholar]
- 57.Blandón R, Edgcomb J, Guevara F, Johnson C. Electrocardiographic changes in Panamanian Rattus rattus naturally infected by Trypanosoma cruzi. Am Heart J. 1974;88:758–764. doi: 10.1016/0002-8703(74)90286-5. [DOI] [PubMed] [Google Scholar]
- 58.Briones M, Souto R, Stolf B, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999;104:219–232. doi: 10.1016/S0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- 59.Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, Miles G, et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 60.Sousa O, Samudio F, Juncá C, Calzada J. Molecular characterization of human Trypanosoma cruzi isolates from endemic areas in Panamá. Mem Inst Oswaldo Cruz. 2006;101:455–457. doi: 10.1590/S0074-02762006000400018. [DOI] [PubMed] [Google Scholar]
- 61.Samudio F, Ortega E, Saldaña A, Calzada J. A Predominance of Trypanosoma cruzi I among Panamanian sylvatic isolates. Acta Trop. 2007;101:178–81. [DOI] [PubMed]
- 62.Ruíz R, De León M, Matta V, Reyes P, López R, Jay D, et al. Trypanosoma cruzi isolates from Mexican and Guatemalan acute and chronic chagasic cardiopathy patients belong to Trypanosoma cruzi I. Mem Inst Oswaldo Cruz. 2005;100:281–283. doi: 10.1590/S0074-02762005000300012. [DOI] [PubMed] [Google Scholar]
- 63.Zingales B, Andrade S, Briones M, Campbell D, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 64.Ramírez J, Guhl F, Rendón L, Rosas F, Marin-Neto J, Morillo C. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis. 2010;4(11):e899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrasco H, Segovia M, Llewellyn M, Morocoima A, Urdaneta-Morales S, Martínez C, et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis. 2012;6(6):e1707. doi: 10.1371/journal.pntd.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelser R. A complement-fixation test for Chagas disease employing an artificial culture antigen. Am J Trop Med Hyg. 1936;16:405–415. doi: 10.4269/ajtmh.1936.s1-16.405. [DOI] [Google Scholar]
- 67.Sousa O, Johnson C. Prevalence of Trypanosoma cruzi and Trypanosoma rangeli in triatomines (Hemiptera: Reduviidae) collected in the Republic of Panama. Am J Trop Med Hyg. 1973;22:18–23. doi: 10.4269/ajtmh.1973.22.18. [DOI] [PubMed] [Google Scholar]
- 68.Kreutzer R, Sousa O. Biochemical characterization of Trypanosoma spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1981;30:308–317. doi: 10.4269/ajtmh.1981.30.308. [DOI] [PubMed] [Google Scholar]
- 69.Coura J, De Castro S. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/S0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 70.Rocha M, Teixeira M, Ribeiro A. An update on the management of Chagas cardiomyopathy. Expert Rev Anti-Infect Ther. 2007;5:727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- 71.Soeiro M, Dailyri A, Silva C, Batista D, Souza E, Oliveira G, et al. Experimental chemotherapy for Chagas disease: 15 years of research contributions through in vivo and in vitro studies. Mem Inst Oswaldo Cruz. 2009;104:301–310. doi: 10.1590/S0074-02762009000900040. [DOI] [PubMed] [Google Scholar]
- 72.Romanha A, Lisboa S, Correia M, Lannes-Vieira B, Zani C, Spadafora C, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 73.Martínez-Luis S, Gómez J, Spadafora C, Guzmán H, Gutiérrez M. Antitrypanosomal allkaloids from the marine bacterium Bacillus pumilus. Molecules. 2012;17:11146–55. [DOI] [PMC free article] [PubMed]
- 74.López D, Cherigo L, Spadafora C, Loza-Mejía M, Martínez-Luis S. Phytochemical composition, antiparasitic and α-glucosidase inhibition activities from Pelliciera rhizophorae. Chem Cent J. 2015;9:53. doi: 10.1186/s13065-015-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitlaw J, Chaniotis B. Palm tree and Chagas disease in Panama. Am J Trop Med Hyg. 1978;27:873–881. doi: 10.4269/ajtmh.1978.27.873. [DOI] [PubMed] [Google Scholar]
- 76.Aguilar S, Condit R. Use of native tree species by a Hispanic community in Panama. Econ Bot. 2001;55:223–235. doi: 10.1007/BF02864560. [DOI] [Google Scholar]
- 77.Aguiar A, Tabarelli M. Edge effects and seedling bank depletion: the role-played by the early successional palm Attalea oleifera (Arecaceae) in the Atlantic Forest. Biotropica. 2009;42:158–166. doi: 10.1111/j.1744-7429.2009.00555.x. [DOI] [Google Scholar]
- 78.Pifano F, Mayer M. Hallazgo de formas evolutivas del Trypanosoma rangeli en el jugo de la trompa de Rhodnius prolixus de Venezuela. Arch Venez Med Trop Parasitol Med. 1949;2:153–158. [Google Scholar]
- 79.Zeledón R. Trypanosoma rangeli en glándulas salivales de Rhodnius pallescens de Panamá. Rev Biol Trop. 1965;13:157–159. [Google Scholar]
- 80.Jaffe L. Chagas' disease in Bocas del Toro, Panama: a pilot study. Am J Trop Med Hyg. 1960;63:0022–5304. [PubMed] [Google Scholar]
- 81.Zúñiga C, Palau T, Penin P, Gamallo C, De Diego J. Protective effect of Trypanosoma rangeli against infections with a highly virulent strain of Trypanosoma cruzi. Tropical Med Int Health. 1997;2:482–487. doi: 10.1111/j.1365-3156.1997.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 82.Palau M, Mejía A, Vergara U, Zúñiga C. Action of Trypanosoma rangeli in infections with virulent Trypanosoma cruzi populations. Mem Inst Oswaldo Cruz. 2003;98:543–548. doi: 10.1590/S0074-02762003000400022. [DOI] [PubMed] [Google Scholar]
- 83.The 37th annual report of the Gorgas Memorial Laboratory. Washington D.C.: U.S. Gov. Printing Office; 1966. p. 51.
- 84.Walton B, Sousa O. Trypanosomes of the lesser anteater, Tamandua tetradactyla from Panama. J Parasitol. 1967;53:956–961. doi: 10.2307/3276816. [DOI] [PubMed] [Google Scholar]
- 85.Pipkin A. Domiciliary reduviid bugs and the epidemiology of Chagas disease in Panama (Hemiptera: Reduviidae: Triatominae) J Med Entomol. 1968;5:107–124. doi: 10.1093/jmedent/5.1.107. [DOI] [PubMed] [Google Scholar]
- 86.Hollingsworth T, Emily R, Roy M, Katherine A, Sarah B, María-Gloria B, et al. Quantitative analyses and modeling to support achievement of the 2020 goals for nine neglected tropical diseases. Parasit Vectors. 2015;8:630. doi: 10.1186/s13071-015-1235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cecere M, Gurtler R, Chuit R, Cohen J. Effects of chickens on the prevalence of infestation and population density of Triatoma infestans in rural houses of north-west Argentina. Med Vet Entomol. 1997;11:383–388. doi: 10.1111/j.1365-2915.1997.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 88.Calzada J, Pineda V, Saldaña A, Monfante I, Santamaria A, Gottdenker N, et al. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama, Central America. Vet Parasitol. 2011;178:360–3. [DOI] [PubMed]
- 89.Saldaña A, Calzada J, Pineda V, Perea M, Rigg C, González K, et al. Risk factors associated with Trypanosoma cruzi exposure in domestic dogs from a rural community in Panama. Mem Inst Oswaldo Cruz. 2015;110:936–944. doi: 10.1590/0074-02760150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sousa O, Dawson G. Trypanosome infections in the marmoset (Saguinus geoffroyi) from the Panama Canal zone. Am J Trop Med Hyg. 1976;25:407–409. doi: 10.4269/ajtmh.1976.25.407. [DOI] [PubMed] [Google Scholar]
- 91.Méndez E. Insectos y otros artrópodos de importancia médica y veterinaria. Impresora Pacífico, S.A: Panamá, República de Panamá; 1999. [Google Scholar]
- 92.Dunn L. A natural infection of Trypanosoma cruzi Chagas found in Rhodnius pallescens Barber in Panama. Am J Trop Med Hyg. 1933;13:471–3.
- 93.Dunn L. Notes on the reduviid bug, Eratyrus cuspidatus Stal., naturally infected with Trypanosoma cruzi Chagas found in Panama. Am J Trop Med Hyg. 1934;14:291–2.
- 94.Rozeboom L. Triatoma dimidiata Latr. Found naturally infected with Trypanosoma cruzi Chagas in Panama. Am J Trop Med Hyg. 1936;16:481–484. doi: 10.4269/ajtmh.1936.s1-16.481. [DOI] [Google Scholar]
- 95.Hashimoto K, Schofield C. Elimination of Rhodnius prolixus in Central America. Parasit Vectors. 2012;5:45. [DOI] [PMC free article] [PubMed]
- 96.Champion G. Insecta Rhynchota. Hemiptera-Heteroptera Biologia Centrali Americana Rhynchota. 1899;2:229–243. [Google Scholar]
- 97.Usinger R. The Triatominae of North and Central America and the West Indies and their public health significance. Public Health Bull. 1944;288:81.
- 98.Abad-Franch F, Lima M, Sarquis O, Gurgel-Gonçalves R, Sánchez-Martín M, Calzada J, et al. On palms, bugs, and Chagas disease in the Americas. Acta Trop. 2015;151:126–141. doi: 10.1016/j.actatropica.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Jurberg J, Galvao C, Noireau F, Carcavallo R, Silva Rocha D, Lent H. Uma iconografia dos Triatomíneos: une iconografie des Triatomes (Hemiptera: Reduviidae) Rio de Janeiro: Co-edição IRD Editions e Editora Gama Filho; 2005. [Google Scholar]
- 100.Christensen H, Vásquez A, Chaniotis B, Whitlan J. Sylvatic hosts of Rhodnius pallescens (Hemiptera: Reduviidae) nymphs in the Panama Canal zone. J Med Entomol. 1980;17:182. doi: 10.1093/jmedent/17.2.182. [DOI] [PubMed] [Google Scholar]
- 101.Calzada J, Pineda V, Montalvo E, Alvarez D, Santamaria A, Samudio F, et al. Human trypanosome infection and the presence of intradomicile Rhodnius pallescens in the western border of the Panama Canal. Panama Am J Trop Med Hyg. 2006;74:762–765. [PubMed] [Google Scholar]
- 102.Castro L, Peterson J, Saldaña A, Perea M, Calzada J, Pineda V, et al. Flight behavior and performance of Rhodnius pallescens (Hemiptera: Reduviidae) on a tethered flight mill. J Med Entomol. 2014;51:1010–1018. doi: 10.1603/ME14014. [DOI] [PubMed] [Google Scholar]
- 103.Vásquez A, Samudio F, Saldaña A, Paz H, Calzada J. Eco-epidemiological aspects of Trypanosoma cruzi, Trypanosoma rangeli and their vector (Rhodnius pallescens) in Panamá. Rev Inst Med Trop. 2004;46:217–222. doi: 10.1590/S0036-46652004000400008. [DOI] [PubMed] [Google Scholar]
- 104.Pineda V, Montalvo E, Alvarez D, Santamaria A, Saldaña A, Calzada J. Feeding source and trypanosome infection index of Rhodnius pallescens in a Chagas disease endemic area of Amador County, Panama. Rev Inst Med Trop. 2008;50:113–6. [DOI] [PubMed]
- 105.Arboleda S, Gorla D, Porcasi X, Saldaña A, Calzada J, Jaramillo-O N. Development of a geographical distribution model of Rhodnius pallescens (Barber, 1932) using environmental data recorded by remote sensing. Infect Genet Evol. 2009;9:441–8. [DOI] [PubMed]
- 106.Navia-Gine W, Loaiza JR, Miller M. Mosquito-host interactions during and after an outbreak of equine viral encephalitis in eastern Panama. PLoS One. 2013;8:e1788. doi: 10.1371/journal.pone.0081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ricardo EG, Yadon ZE. Eco-bio-social research on community-based approaches for Chagas disease vector control in Latin America. Trans R Soc Trop Med Hyg. 2015;109:91–98. doi: 10.1093/trstmh/tru203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bargues M, Klisiowics D, Gonzalez-Candelas F, Ramsey J, Monroy C, Ponce C, et al. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl Trop Dis. 2008;2:e233. [DOI] [PMC free article] [PubMed]
- 109.Monteiro F, Peretolchina T, Lazoski C, Harris K, Dotson E, Abad-Franch F, et al. Phylogeographic pattern and extensive mitochondrial DNA divergence disclose a species complex within the Chagas disease vector Triatoma dimidiata. PLoS One. 2013;8:e70974. doi: 10.1371/journal.pone.0070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diaz S, Panzera F, Jaramillo-O N, Pérez R, Fernández R, Vallejo G, et al. Genetic, cytogenetic and morphological trends in the evolution of the Rhodnius (Triatominae: Rhodniini) trans-Andean group. PLoS One. 2014;9:e87493. doi: 10.1371/journal.pone.0087493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gomez-Palacio A, Triana O. Molecular evidence of demographic expansion of the Chagas disease vector Triatoma dimidiata (Hemiptera, Reduviidae, Triatominae) in Colombia. PLoS Negl Trop Dis. 2014;8:e2734. doi: 10.1371/journal.pntd.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panzera F, Ferrandis I, Ramsey J, Ordóñez R, Salazar-Schettino P, Cabrera M, Monroy M, et al. Chromosomal variation and genome size support existence of cryptic species of Triatoma dimidiata with different epidemiological importance as Chagas disease vectors. Tropical Med Int Health. 2006;2:1092–1103. doi: 10.1111/j.1365-3156.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 113.Cáceres L, Rovira J, Calzada J, Saldaña A. Evaluación de la actividad tóxica de los insecticidas piretroides deltametrina y lambdacihaltotrina en dos poblaciones de campo de Rhodnius pallescens (Hemiptera: Reduviidae) de Panamá. Rev Biomédica. 2011;31:1. doi: 10.1590/S0120-41572011000100002. [DOI] [PubMed] [Google Scholar]
- 114.Hurtado L, Calzada J, Pineda V, Gonzales K, Santamaria A, Caceres L, Wald C, Saldaña A. Conocimientos y factores de riesgo relacionados con la enfermedad de Chagas en dos comunidades panameñas donde Rhodnius pallescens es el vector principal. Rev Biomédica. 2014;34:260–270. doi: 10.1590/S0120-41572014000200012. [DOI] [PubMed] [Google Scholar]
- 115.Lainhart W, Dutari LC, Rovira JR, Sucupira IMC, Póvoa MM, Conn JE, et al. Epidemic and non-epidemic hot spots of malaria transmission occur in indigenous Comarcas of Panama. PLoS Negl Trop Dis. 2016;10(5):e0004718. doi: 10.1371/journal.pntd.0004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamada K, Valderrama A, Nicole G, Lizbeth C, Noboru M, Azael S, et al. Macroecological patterns of American cutaneous leishmaniasis transmission across the health areas of Panamá (1980–2012) Parasite Epidemiol Control. 2016;1(2):42–55. doi: 10.1016/j.parepi.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barber HG. A new species of Rhodnius from Panama. J Washington Acad Sci. 1932;22:514–517. [Google Scholar]
- 118.OPS/OMS. Iniciativa de los países de Centro América para la interrupción de la transmisión vectorial y transfusional de la Enfermedad de Chagas. Taller técnico de estudio sobre Rhodnius pallescens, su vigilancia y control, Panamá, Panamá. Ortega-Barría E y Romaña CA. 2002.
- 119.OPS/OMS. Iniciativa de los países de Centro América para la interrupción de la transmisión vectorial y transfusional de la Enfermedad de Chagas. Taller técnico de estudio sobre la palmera Attalea butyracea como un marcador eco-epidemiológico de riesgo para la enfermedad de Chagas en Panamá. Ortega-Barría E y Romaña CA. 2002.
- 120.Romaña C, Brunstein D, Collin-Delavaud A, Sousa O, Ortega-Barría E. Public policies of development in Latin America and Chagas disease. Lancet. 2003;362:579. doi: 10.1016/S0140-6736(03)14132-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


