Abstract
Background
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are important disease activity biomarkers in rheumatoid arthritis (RA). This study aimed to determine to what extent obesity biases these biomarkers.
Methods
Body mass index (BMI) associations with CRP and ESR were assessed in two RA cohorts – 1) the cross-sectional Body Composition (BC) cohort (N = 451) including whole-body DXA measures of fat mass index, and 2) the longitudinal Veterans Affairs Rheumatoid Arthritis (VARA) registry (N = 1652), using multivariable models stratified by sex. For comparison, associations were evaluated in the general population using the National Health and Nutrition Examination Survey.
Results
Among women with RA and in the general population, greater BMI was associated with greater CRP, especially among women with severe obesity (all p < 0.001 for BMI ≥ 35 vs. 20–25 kg/m2). This association remained after adjustment for joint counts and patient global (p < 0.001 in BC and < 0.01 in VARA), but was attenuated after fat mass index adjustment (p = 0.17). Positive associations between BMI and ESR in women were more modest. In men with RA, lower BMI was associated with greater CRP and ESR, contrasting positive associations among men in the general population.
Conclusion
Obesity is associated with greater CRP and ESR in women with RA. This association is related to fat mass and not RA disease activity. Low BMI is associated with greater CRP in men with RA – this unexpected finding remains incompletely explained but likely is not a direct effect of adiposity.
Introduction
Laboratory measures of systemic inflammation, particularly C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are routinely used in the diagnosis and assessment of disease activity in patients with rheumatoid arthritis (RA) and may substantially impact physician decision-making. These biomarkers are also part of disease activity measures that serve as primary outcome measures in clinical trials (1).
In the general population, higher levels of CRP have been shown to be associated with greater body mass index (BMI) and adiposity, especially among women (2). Small studies with healthy subjects have also described similar associations with fibrinogen (3) and ESR (4,5). This association in the general population raises the question of how inflammatory markers may perform as a biomarker of disease activity among obese patients with RA. Indeed, Giles et al. found that truncal fat was associated with CRP and IL-6 in women, but not men, with RA (6). Inflammatory biomarkers that are elevated because of adiposity may lead to inaccurate assessment of clinical RA disease activity. There is surprisingly little recognition of this issue among practicing clinicians and little published data to illustrate the magnitude of effect among the severely obese.
The objectives of this study were to a) evaluate associations between BMI and inflammatory markers in patients with RA, b) determine whether these associations are similar to those in patients without RA, and c) examine the potential impact of obesity on disease activity measures. This study tested the hypothesis that inflammatory markers are higher among obese individuals with RA and correlate with excess adiposity, independent of other measures of RA disease activity.
Methods
Study Samples
Two RA cohorts were evaluated – a cross-sectional “Body Composition” (BC) cohort including whole-body DXA measures of adiposity, and the longitudinal Veterans Affairs Rheumatoid Arthritis Registry (VARA) cohort with a larger sample size and more men. Associations between BMI and inflammatory markers were also evaluated in the general population using data from the National Health and Nutrition Examination Survey (NHANES). Details of these cohorts are described below.
The “Body Composition” (BC) Cohort (N=451)
The BC cohort is pooled from three independent studies of patients with RA (see below) that included examination, laboratory analysis, as well as whole-body DXA measures of fat and lean mass.
University of California San Francisco (UCSF) Cohort (N = 140) (7): Details regarding this study cohort have been previously published (7,8). After telephone interviews in the study years 2007–2009, UCSF RA Panel participants were recruited for in-person assessments, including measurement of body composition. Exclusion criteria were non–English speaking, age <18 years, current daily oral prednisone dose >50 mg, current pregnancy, uncorrected vision problems that interfered with reading, and joint replacement within 1 year.
University of Pennsylvania (Penn) Cohort (N = 115) (9): The Penn cohort was initiated in 2012 to evaluate alterations in body composition and bone structure in patients with RA. Patients were recruited from the University of Pennsylvania Rheumatology practices and Philadelphia Veterans Affairs Medical Center and consisted of individuals with RA meeting 2010 American College of Rheumatology criteria aged 18–70 years. Patients with juvenile idiopathic arthritis (or another inflammatory arthritis), active cancer, chronic diseases known to affect bone health (e.g. chronic kidney disease, liver disease, malabsorption syndromes), or pregnancy were excluded. Patients weighing >300 pounds were excluded due to weight limitations of imaging equipment.
Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in RA Study (ESCAPE RA) Cohort (N = 197) (10): Patients were men and women in the ESCAPE RA cohort study between October 2004 and May 2006 (10). Patients with RA followed at the Johns Hopkins Arthritis Center or referred from local rheumatologists were enrolled, all of whom met American College of Rheumatology 1987 criteria, were 45–84 years old, weighed ≤ 300 pounds, and did not report any prior pre-specified cardiovascular events or procedures.
VA Rheumatoid Arthritis (VARA) Registry Cohort (N=1652)
The VARA national repository and multicenter longitudinal, RA disease registry has operated for more than 10 years. At the time of this study, 12 VA sites participated (Salt Lake City, UT; Washington DC; Jackson, MI; Philadelphia, PA; Brooklyn, NY; Omaha, NE; Dallas, TX; Iowa City, IA; Denver, CO; Little Rock, AR; Portland, OR; Birmingham, AL). Veterans with RA are identified by the treating rheumatologist at individual sites. Veterans who fulfill the 1987 ACR criteria for RA and are over 18 years of age are eligible for enrollment. Physician-investigators at each site record clinical data at enrollment and at follow-up visits including the Multidimensional Health Assessment Questionnaire (MD-HAQ), swollen and tender joint counts (0 to 28), inflammatory markers, pain scores, and patient and physician disease assessment scores. Each individual site has Institutional Review Board (IRB) approval and all study patients provided informed written consent at enrollment.
NHANES Study (Controls)
NHANES is an ongoing program by the Centers for Disease Control and Prevention and National Center for Health Statistics, which interviews and examines a representative sample of the U.S. population. NHANES uses a complex, multistage, probability-sampling design with oversampling of certain populations. Survey methods and data collection for the 2007–2010 survey and 1971–1974 survey have previously been described (11,12). CRP was evaluated in 2007–2010 (11) and ESR in 1971–1974 (12) among non-pregnant adults ≥18 years old.
Body composition assessment
Weight in kilograms was converted to BMI by dividing by the square of height in meters. BMI categories were defined as underweight (BMI<20 kg/m2), normal-weight (BMI 20–<25 kg/m2), overweight (BMI 25–<30 kg/m2), obese (BMI 30–<35 kg/m2), and severely obese (BMI ≥ 35 kg/m2) (class II obesity by World Health Organization definitions) (13). In VARA, BMI values were determined for each clinical visit based on medical record data as previously described (14). The UCSF and ESCAPE studies used a Lunar Prodigy DXA system to assess fat and lean mass (UCSF: software version 9.3, ESCAPE: software version 05.60.003 GE/Lunar Radiation, Madison, WI), adjusted based on the method by Shepherd et al. to facilitate comparison to NHANES data that were generated using Hologic equipment (15). In vivo coefficients of variation for the Lunar Prodigy have been estimated at 1% or less (16). Patients from the Penn cohort underwent whole-body DXA assessment using a Hologic densitometer (Delphi Systems, Hologic, Inc., Bedford, MA) and did not require adjustment. The in vitro coefficient of variation for Hologic measurements was less than 0.6% and the in vivo coefficient of variation in adults was less than 1% (17). Whole-body DXA was performed in the BC cohort to estimate appendicular lean mass and total fat mass. Fat mass index (FMI) and appendicular lean mass index (ALMI) were determined by dividing fat or lean mass (in kg) by height-squared. Standard sex- and race/ethnicity-specific Z-Scores were generated relative to age using LMS (Lambda, Mu, Sigma) curves previously published by Hologic Inc to account for the non-linearity, heteroskedasticity, and skew noted in body composition outcomes (18).
Laboratory measures
CRP (mg/dL) was measured using standard clinical assays at each VA institution within the VARA registry. CRP was measured by nephelometry in the ESCAPE RA study cohort, UCSF cohort, and as previously described in NHANES (19). At Penn, CRP was measured by a Fixed Point Immuno Rate Assay. ESR (mm/hr) was measured using the Westergren method within each study.
Statistical analysis
Associations of BMI as a continuous variable or BMI category (compared to normal BMI) with CRP or ESR were evaluated using linear regression models adjusting for age, race, and smoking status (previously shown to be associated with CRP or ESR) (2,20,21). In the BC cohort, analyses were also adjusted for study site. In VARA, generalized estimating equations incorporated repeated measures, using population-averaged models, an exchangeable correlation matrix, and robust standard error estimates to account for within-patient correlations. All clinical visits without missing data for the primary variables were included. In all regression analyses, CRP and ESR were log-transformed to approximate a normal distribution when used as the outcome. Normal weight (BMI 20-25 kg/m2) was the reference category. Analyses were stratified by sex based on previous literature suggesting sex-specific relationships in healthy control populations (2) and in RA (6), and because of significant sex interactions in the current study. The predicted value for ESR and CRP was determined for each patient based on linear regression models using the means for all other covariates in the model.
Analyses of ESR and CRP in the general population using NHANES accounted for sampling design using Mobile Examination Center weights, strata variables, and Taylor Series Linearization methods for variance estimation. Current smoking was included in CRP analysis using NHANES 2007–2010 but was not available for ESR analysis using NHANES I.
Linear regression models evaluated associations between log-transformed CRP and BMI as a continuous variable (adjusted for age, race, and smoking status) among patients with available swollen joint count, tender joint count, and patient global scores. Sequential models assessed the potential for confounding by component RA disease activity measures; individual components of the DAS28 (without ESR or CRP) were adjusted for rather than DAS28 itself given strong correlations between ESR and CRP. Similar adjustment for FMI-Z scores (a measure of adiposity) in the BC cohort assessed whether associations between BMI and inflammatory markers were explained by greater adiposity. Additionally, associations between BMI and swollen joint count, tender joint count, and patient global scores were evaluated using linear regression adjusting for age, race, and smoking status.
Independent associations between DXA measured body composition measures (ALMI, FMI) and inflammatory markers were directly assessed in multivariable models.
Disease activity score in 28 joints with CRP (DAS28-CRP) was calculated according to standard formulas with the contribution of CRP calculated as 0.36*ln(CRP in mg/L +1). To estimate the potential impact that the high CRP values observed in obese women may have on the DAS28, a corrected CRP was calculated among women with severe obesity by taking the natural log of CRP, subtracting the expected difference compared to normal weight patients (beta coefficient) from linear regression analysis in BC or VARA, and exponentiating the result (1). Adjusted DAS28 scores were determined among severely obese individuals using corrected CRP. DAS28 categories (remission, low, moderate, high) using corrected CRP were compared to original DAS28 categories to determine the frequency of disease activity category change with CRP correction.
Analyses were conducted using Stata 13.1 (StataCorp, 2015).
Results
Patient Characteristics
Characteristics of RA patients in BC and VARA cohorts and control patients in the NHANES cohorts are shown in Table 1. BMI distribution was similar in BC and VARA. Patients in VARA were more frequently male, older, had higher rates of smoking, higher CRP and ESR, and were less frequently using biologic DMARDs.
Table 1.
Baseline Cohort characteristics
| Rheumatoid Arthritis Cohorts | General Population Cohorts | |||
|---|---|---|---|---|
|
| ||||
| Veterans Affairs Rheumatoid Arthritis Registry (VARA) | Body Composition (BC) | NHANES 2007–2010 | NHANES I 1971–1974 | |
|
|
||||
| N | 1652 | 451 | 10813 | 12227 |
| Age | 63.5 ± 11.1 | 58.3 ± 10.5 | 50.1 ± 17.7 | 45.1 ± 18.0 |
| Female, N (%) | 150 (9%) | 263 (58%) | 5510 (51%) | 7449 (61%) |
| White, N (%) | 1207 (73%) | 285 (69%) | 5231 (48%) | 9976 (82%) |
| Black, N (%) | 269 (16%) | 63 (14%) | 1958 (18%) | 2099 (17%) |
| Body mass index (kg/m2) | 28.5 ± 5.4 | 28.2 ± 6.2 | 29.0 ± 6.7 | 25.3 ± 5.2 |
| Body mass index, N (%) | ||||
| < 20 kg/m2 | 48 (3%) | 27 (6%) | 469 (4%) | 1488 (12%) |
| 20–25 kg/m2 | 412 (25%) | 133 (29%) | 2615 (24%) | 5164 (42%) |
| 25–30 kg/m2 | 629 (38%) | 141 (31%) | 3708 (34%) | 3704 (30%) |
| 30–35 kg/m2 | 381 (23%) | 90 (20%) | 2292 (21%) | 1317 (11%) |
| ≥35 kg/m2 | 182 (11%) | 60 (13%) | 1729 (16%) | 554 (5%) |
| Fat mass index (FMI) Za | - | −0.18 (1.18) (n = 442) | - | - |
| CRP (mg/dL) | 0.70 [0.37,1.80] | 0.44 [0.13,0.86] | 0.19 [0.08,0.45] | - |
| ESR (mm/hr)b | 22 [10,38] (n = 1482) | 14 [5,30] (n = 251) | - | 13 [7,21] |
| Current smoking, N (%) | 439 (26%) | 57 (13%) | 2396 (22%) | - |
| Swollen joint count 28c | 3 [0,8] | 5 [2,7] | ||
| Tender joint count 28c | 3 [0,9] | 3 [1,8] | ||
| Patient Global (0–100)c | 44 [20,60] | 62 [35,84] | ||
| CCP positive, N (%)b | 1194/1520 (79%) | 179/253 (71%) | ||
| Glucocorticoid use, N (%) | 634 (38%) | 168/447 (38%) | ||
| Non-biologic DMARDc | 1500 (91%) | 265/311 (85%) | ||
| Biologic DMARDc | 346 (21%) | 148/311 (48%) | ||
Characteristics summarized by frequency (%), mean ± SD, or median [IQR].
Sex- and race/ethnicity-specific Z-scores of DXA derived fat mass index (mean of 0 and standard deviation of 1 in a reference population),
measured in UCSF and PENN studies among the BC cohort,
measured in ESCAPE RA and PENN studies among the BC cohort.
NHANES: National Health and Nutrition Examination Survey, DMARD: disease modifying anti-rheumatic drugs
Associations between BMI and CRP in women
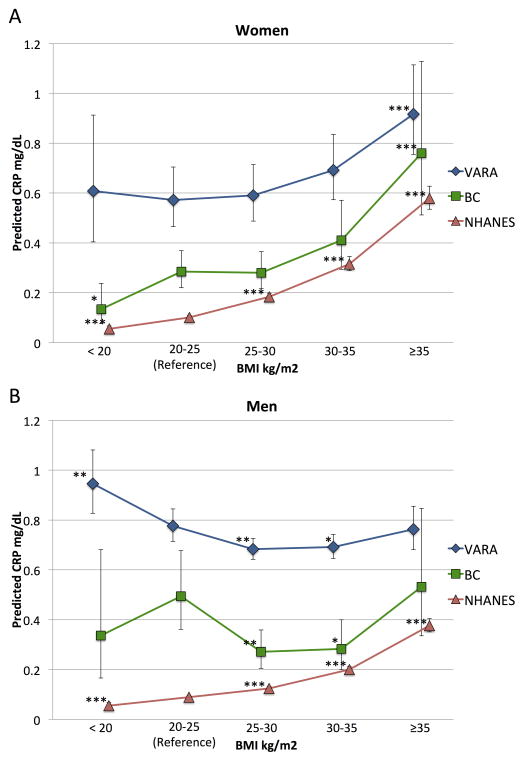
Among women, BMI as a continuous variable was positively associated with CRP in both RA cohorts and in the general population after adjustment for age, race, and smoking status in BC (β [95% CI] 0.057 [0.035 to 0.080]), in VARA (0.030 [0.014 to 0.045]), and in NHANES (0.097 [0.092 to 0.101]) (all p < 0.001). Women with RA in higher BMI categories had higher predicted CRP (expected values from multivariable linear regression models) in both VARA and BC, mirroring the pattern seen among non-RA female controls from the NHANES cohort (Figure 1A). In particular, women who were severely obese (BMI ≥35 kg/m2) had a significantly higher CRP than women with normal BMI (20–25 kg/m2) in both RA cohorts and in the general population (all p < 0.001) (Table 2, Figure 1A). Further adjustment for glucocorticoid and DMARD use did not substantially change results (not shown).
Figure 1. Predicted CRP in men and women by BMI category in patients with rheumatoid arthritis (VARA and BC) and the general population (NHANES 2007–2010).
CRP predicted from linear regression models or GEE models (in VARA) of CRP at the means of age, race, smoking status. BMI: body mass index; VARA: Veteran’s Affairs Rheumatoid Arthritis Registry; BC: 3 pooled body composition studies of rheumatoid arthritis patients. NHANES: National Health and Nutrition Examination Survey 2007–2010. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. reference range BMI 20–25 kg/m2 category within each cohort.
Table 2.
Linear regression analyses assessing the association between ln(CRP) and BMI categories in the BC and VARA rheumatoid arthritis cohorts.
| Women
|
||
|---|---|---|
| BC n = 263 β (95% CI) |
VARA n = 149, obs = 1532 β (95% CI) |
|
|
| ||
| BMI <20 | −0.75 (−1.37, −0.13)* | 0.06 (−0.35, 0.48) |
| 20–25 | Ref | Ref |
| 25–30 | −0.02 (−0.38, 0.35) | 0.03 (−0.23, 0.29) |
| 30–35 | 0.36 (−0.06, 0.79) | 0.19 (−0.08, 0.46) |
| ≥35 | 0.98 (0.50, 1.46)*** | 0.47 (0.19, 0.75)*** |
|
| ||
| Men | ||
|
| ||
| BC n = 188 β (95% CI) |
VARA n = 1503, obs = 15013 β (95% CI) |
|
|
| ||
| BMI <20 | −0.39 (−1.16, 0.39) | 0.20 (0.07,0.32)** |
| 20–25 | Ref | Ref |
| 25–30 | −0.60 (−1.03, −0.18)** | −0.13 (−0.22, −0.04)** |
| 30–35 | −0.56 (−1.03, −0.09)* | −0.11 (−0.22, −0.01) |
| ≥35 | 0.07 (−0.48, 0.63) | −0.02 (−0.16, 0.13) |
p < 0.05,
p < 0.01,
p < 0.001.
Adjusted for age, race, smoking. BC also adjusted for study site. Coefficients represent mean difference in ln(CRP) compared to the reference group. BMI: body mass index; BC: 3 pooled body composition studies of patients with rheumatoid arthritis; VARA: Veterans Affairs Rheumatoid Arthritis Registry
Associations between BMI and CRP in men
BMI as a continuous variable was also positively associated with CRP among men in the general population after adjustment (β [95% CI] 0.085 [0.078 to 0.091] p < 0.001), although the strength of the association was weaker than among women (p for interaction < 0.01). Among men with RA, however, BMI was not associated with CRP in BC (β [95% CI] 0.004 [−0.025 to 0.034] p = 0.78) or in VARA (β [95% CI] −0.008 [−0.017 to 0.001] p = 0.09). In contrast to what was seen in the general population, men with RA in higher BMI categories did not have significantly higher CRP levels compared to normal weight men in either VARA or BC (Table 2, Figure 1B). In VARA, underweight men had significantly higher CRP than normal weight men (p < 0.01) (Table 2, Figure 1B). In both BC and VARA, normal-weight men had significantly higher predicted CRP than overweight or obese men (Table 2, Figure 1B).
Associations between BMI and ESR in men and women
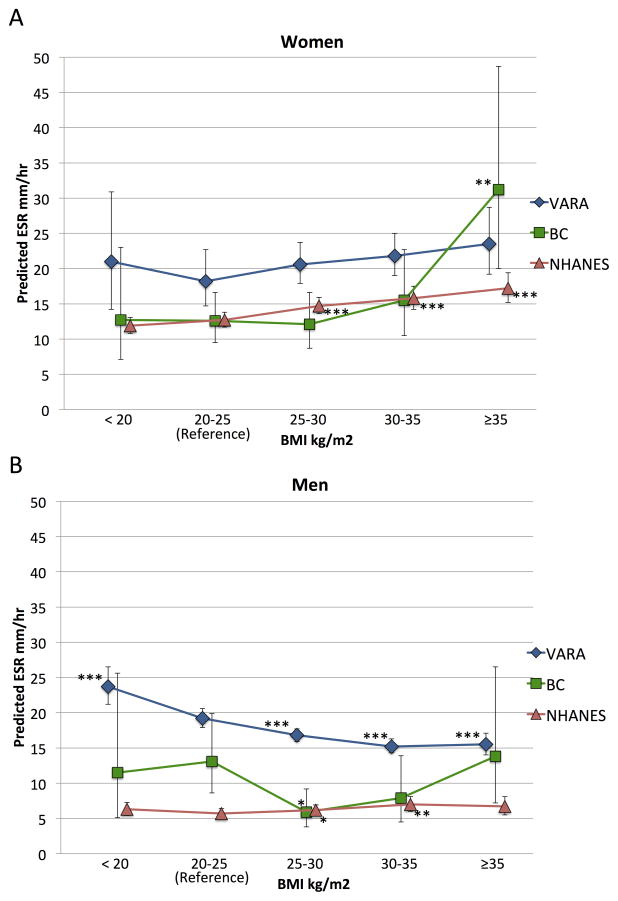
ESR was associated with BMI among women in the general population in NHANES (β [95% CI] 0.020 [0.016 to 0.024] p <0.001) (Figure 2A). Associations were similar among women with RA in BC (β [95% CI] 0.033 [0.010 to 0.057] p = 0.006) and in VARA (β [95% CI] 0.016 [−0.001 to 0.032] p = 0.051). A similar pattern of higher ESR in higher BMI categories was observed among women in the two RA cohorts, however only severely obese women in the BC cohort (BMI ≥35 kg/m2) had significantly higher ESR compared to normal-weight women (Figure 2A, Supplemental Table 1).
Figure 2. Predicted ESR in men and women by BMI category in patients with rheumatoid arthritis (VARA and BC) and the general population (NHANES I).
ESR predicted from linear regression models or GEE models (in VARA) of ln(ESR) at the means of age, race. VARA and BC also adjusted for smoking. BMI: body mass index; VARA: Veteran’s Affairs Rheumatoid Arthritis Registry; BC: 3 pooled body composition studies of rheumatoid arthritis patients. NHANES I: National Health and Nutrition Examination Survey 1971–1974. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. reference range BMI 20–25 kg/m2 category within each cohort.
ESR was modestly associated with BMI among men in the general population (β [95% CI] 0.014, [0.005 to 0.022] p = 0.002) although severely obese men in the general population did not have significantly higher ESR than normal weight men (Figure 2B). ESR was not associated with BMI among men with RA in BC (β [95% CI] −0.005 [−0.045 to 0.036], p = 0.81) and was negatively associated with BMI among men in VARA (β [95% CI] −0.026 [−0.034 to −0.018], p < 0.001). Underweight and normal-weight men in VARA had significantly higher ESR than men in higher BMI categories (all p < 0.001) (Figure 2B, Supplemental Table 1).
Factors implicated in the associations between BMI and CRP
Greater BMI was not associated with greater swollen joint count, tender joint count, or patient global score in women with RA in similar analyses (not shown), and the positive association between BMI and CRP among women with RA was not attenuated with adjustment for swollen joint count, tender joint count, and patient global scores in either cohort (Table 3). In contrast, adjustment for fat mass (FMI Z-score) in the BC cohort fully attenuated the association between BMI and CRP (Table 3). CRP and ESR were positively associated with fat mass but not lean mass (ALMI Z-Score) in women after adjusting for age, race, and smoking (Table 4).
Table 3.
Association between BMI and ln(CRP) before and after adjusting for disease activity or fat mass
| Women
|
|||
|---|---|---|---|
| Baseline Model β (95% CI) |
Baseline + Joint count/patient global β (95% CI) |
Baseline + FMI-Z β (95% CI) |
|
|
| |||
| BC Cohort (n = 169) | 0.061 (0.035, 0.088)*** | 0.062 (0.036, 0.088)*** | −0.049 (−0.118, 0.021) |
| BMI (per 1 kg/m2) | |||
| VARA Cohort (n = 135, obs = 773) | 0.029 (0.010, 0.046)** | 0.026 (0.007, 0.044)** | |
| BMI (per 1 kg/m2) | |||
|
| |||
| Men | |||
|
| |||
| Baseline Model β (95% CI) |
Baseline + Joint Counts/Patient Global β (95% CI) |
Baseline + FMI-Z β (95% CI) |
|
|
| |||
| BC Cohort (n = 131) | −0.015 (−0.052, 0.022) | −0.013 (−0.050, 0.025) | −0.039 (−0.128, 0.049) |
| BMI (per 1 kg/m2) | |||
| VARA Cohort (n = 1424, obs = 8148) | −0.005 (−0.014, 0.005) | −0.003 (−0.012, 0.006) | |
| BMI (per 1 kg/m2) | |||
p <0.01,
p < 0.001.
Baseline model adjusted for age, race, smoking status, and study site (in BC). Disease activity adjusted by including swollen joint count, tender joint count, and patient global scores in multivariable models. BMI: body mass index; BC: 3 pooled body composition studies of patients with rheumatoid arthritis; VARA: Veterans Affairs Rheumatoid Arthritis Registry. FMI-Z: DXA derived fat mass index z-score (mean of 0 and standard deviation of 1 in a reference population)
Table 4.
Associations between fat mass index and adjusted lean mass index Z-scores and inflammatory markers
| Women
|
||
|---|---|---|
| ln(CRP) n = 253 β (95% CI) |
ln(ESR) n = 141 β (95% CI) |
|
|
| ||
| FMI-z | 0.52 (0.35, 0.69)*** | 0.20 (0.01, 0.40)* |
| ALMI-z | −0.18 (−0.36, −0.01)* | −0.01 (−0.24, 0.23) |
|
| ||
| Men | ||
|
| ||
|
ln(CRP) n = 187 β (95% CI) |
ln(ESR) n = 106 β (95% CI) |
|
|
| ||
| FMI-z | 0.002 (−0.14, 0.15) | −0.10 (−0.31, 0.11) |
| ALMI-z | −0.01 (−0.18, 0.16) | 0.01 (−0.21, 0.24) |
p <0.05,
p < 0.001.
Multivariable models included both FMI-z and ALMI-z and were also adjusted for age, race, smoking, and study site. FMI-z: fat mass index z-score. ALMI-z: appendicular lean mass index z-score (mean of 0 and standard deviation of 1 in a reference population)
Among men, the overall association between BMI and CRP was not significant in either RA cohort before or after adjustment for swollen joint count, tender joint count, and patient global scores (Table 3). Additionally, ESR and CRP were not associated with DXA measured fat mass or lean mass after adjusting for age, race, and smoking (Table 4). BMI was not associated with joint counts or patient global in men except for a modest negative association between BMI and log transformed swollen joint count in VARA (β [95% CI] −0.01 [−0.02, −0.003] p = 0.002). The association between underweight (BMI < 20 kg/m2) and higher CRP in VARA remained similar in magnitude although was no longer statistically significant after adjustment for joint counts and patient global scores (β [95% CI] 0.21 [−0.001 to 0.42] p = 0.051).
Estimated Impact of BMI Effects on the DAS28-CRP in severely obese women
Based on the contribution of severe obesity to CRP predicted from linear regression models, women with severe obesity on average would be expected to have a DAS28-CRP score 0.29 points higher in BC and 0.15 points higher in VARA. If CRP were corrected to what would be expected for normal weight individuals, 3/31 (9.8%) DAS28 measurements in BC and 18/158 (11.4%) measurements in VARA among severely obese women would be re-categorized to a lower disease activity category (Supplemental Figure 1).
Discussion
In this study, obesity was associated with greater CRP in women with RA, independent of other components of disease activity. A similar association was observed in a non-RA sample, suggesting that elevated CRP values among obese women with RA are not reflective of greater RA disease activity but rather are an expected phenomenon related to adiposity. In contrast to associations observed in women, low BMI and not obesity was associated with greater CRP in men with RA.
Smaller studies, most notably a study by Giles et al. using the ESCAPE-RA cohort (one of three studies included in the BC cohort), have demonstrated associations between fat mass and CRP in women with RA (6,20,22). The current study is the largest to address this issue and illustrates the effect of inflammatory markers in specific BMI categories; BMI is an imperfect measure of adiposity, especially in RA, but is widely available clinically and, as shown in this study, is also highly associated with inflammatory markers in women with RA. The large number of male patients in the VARA cohort improves characterization of the different associations among men. The inclusion of a non-RA control population demonstrates the normal relationship between BMI and inflammatory markers as a reference.
In both RA cohorts, women with severe obesity had a significantly greater CRP than women with a normal BMI. Several pieces of evidence suggest that this CRP elevation is due to adiposity and not increased RA disease activity. First, a strikingly similar association between greater BMI categories and CRP was observed in a control population without RA, demonstrating that this association is not an RA-specific phenomenon. Secondly, DXA measured fat mass (and not lean mass) was strongly associated with CRP, and adjustment for fat mass completely attenuated the association between BMI and CRP (suggesting that higher CRP among patients with elevated BMI is due to increased adiposity). Finally, elevations in CRP in patients with obesity were not explained by greater swollen joint count, tender joint count, or patient global scores – BMI was not associated with elevations in these disease activity measures, and adjustment for these measures did not attenuate the association between BMI and CRP. There is a physiologic basis for these findings, with evidence that production of IL-6 and other inflammatory cytokines by adipose tissue can lead to CRP elevation in obese individuals (23). The similar, although more modest, associations between ESR and BMI in women are not unexpected, as ESR levels are elevated by acute phase reactants produced by pathways involving IL-6 but are also influenced by additional non-inflammatory factors such as immunoglobulins and erythrocyte morphology (24).
Patients with RA who are obese have been shown in some studies to have higher disease activity scores and poorer response to therapy, but these findings may not be related to more severe RA-related inflammation (25–29). In fact, numerous reports demonstrate less radiographic damage in obese patients (30,31). In part, the perception that RA is more severe or refractory in obese patients may be due to the influence of obesity itself on subjective clinical disease activity measures; obesity is known to affect patient-reported outcomes among individuals with RA (32) as well as pain and function in non-RA patients (33). The association between obesity and CRP provides another potential contributor. If CRP elevations are partially related to obesity, improvements may be more modest in response to therapies aimed at reducing RA disease activity.
Interestingly, men with RA with higher BMI (or higher fat mass index) did not have higher CRP levels. In fact, underweight men with RA had significantly greater CRP and ESR. These results are similar to work by Giles et al showing no association between fat mass and CRP in men and an inverse association among a small subset of men with multiple swollen and tender joints (6). The evidence from the current study suggests that this inverse association between BMI and CRP in men is an RA-specific phenomenon and not a direct causal effect of adiposity – the positive association between BMI and CRP in the general population suggests that adiposity can contribute to greater CRP levels in men as well as women.
One hypothesis for these findings is that higher levels of systemic inflammation leading to weight loss (both lean and fat mass) could obscure the modest association between adiposity and CRP. Indeed, numerous studies have demonstrated that RA patients with low BMI have a greater risk of long-term complications of RA including radiographic progression, cachexia, and death (8,14,30,31,34–36). Inflammation may have different effects on body composition in men and women (7,8), potentially leading to the different associations seen among men and women with RA. Lower BMI was associated with a greater swollen joint count among men with RA in VARA, although differences in joint counts and patient global scores alone did not explain the higher CRP and ESR in men with lower BMI. It was beyond the scope of this study to fully evaluate the complex relationship between RA disease activity, disease severity, weight loss, frailty, comorbidities, advancing age, and other factors that might contribute to higher levels of systemic inflammation in low BMI men.
The effect of obesity on CRP may alter its performance as a biomarker of disease activity in clinical trials and in practice. While our conservative assessment suggests a modest effect of obesity on DAS28-CRP (0.15 to 0.30 units, on average), correction of CRP values did result in the reclassification of disease activity in approximately 10% of severely obese women. Furthermore, because obesity may be expected to impact the reliability of other component measures of disease activity, clinicians may tend to rely more on objective measures such as CRP for diagnosis or to make treatment decisions (25,32,37).
Although two large RA cohorts were evaluated in this study, there was limited power to evaluate effects in all BMI categories among specific subgroups. Disease activity and ESR were also not measured in all studies or at all study observations, reducing power in these analyses. DXA measures of fat mass were only available in the BC cohort and, given the smaller number of men in this cohort, more detailed analysis of fat mass associations among subgroups of men in particular were limited.
Strengths of the current study include the direct evaluation of associations between inflammatory markers and adiposity using DXA measured fat mass, evaluation of a large observational cohort from clinical practice with a large proportion of men, comprehensive assessment of the impact of potential confounders, and comparison to large general population cohorts.
In conclusion, obesity is associated with greater CRP in women with RA, similar to what is seen in the general population. This association is explained by greater fat mass and not greater RA disease activity, suggesting that CRP should be interpreted cautiously among women with obesity. Higher CRP levels are observed among low and normal weight men with RA and may reflect greater systemic inflammation related to RA or other comorbid conditions in this group.
Supplementary Material
Significance.
Severely obese women both with and without rheumatoid arthritis have high C-reactive protein and erythrocyte sedimentation rate levels.
Obesity-related elevations in inflammatory markers in women with RA are not related to greater RA disease activity and should be interpreted cautiously.
Low body mass index is associated with higher inflammatory markers among men with RA, a phenomenon not observed in the general population.
Acknowledgments
Funding/Grant sources: Dr. George was supported by the National Institutes of Health (NIH 5T32AR007442-28) and the Rheumatology Research Foundation Scientist Development Award. Dr. Katz is funded by NIAMS grant P60 AR053308. Dr. Mikuls is supported though funding from a VA Merit Award (CX000896) and from the NIH/NIGMS. Dr. Caplan is supported by VA HSR&D IIR 14-048-3. Dr. Michaud receives research grant funding from the Rheumatology Research Foundation. Dr. Baker is funded by a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
Footnotes
Financial interests/commercial sources:
Dr. Michaud receives research grant funding from Pfizer. Dr. Ogdie receives consulting fees from Novartis and Pfizer and grant funding from Pfizer. Dr. Sauer receives grant funding from Amgen.
References
- 1.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA) Arthritis Care Res. 2011;63(Suppl 11):S14–36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 3.Piché M-E, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 4.Leff RD, Akre SP. Obesity and the erythrocyte sedimentation rate. Ann Intern Med. 1986;105:143. doi: 10.7326/0003-4819-105-1-143_2. [DOI] [PubMed] [Google Scholar]
- 5.Richter WO, Möhrle W, Schwandt P. Obesity and the erythrocyte sedimentation rate. Ann Intern Med. 1988;109:928–929. doi: 10.7326/0003-4819-109-11-928. [DOI] [PubMed] [Google Scholar]
- 6.Giles JT, Bartlett SJ, Andersen R, Thompson R, Fontaine KR, Bathon JM. Association of body fat with C-reactive protein in rheumatoid arthritis. Arthritis Rheum. 2008;58:2632–2641. doi: 10.1002/art.23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz PP, Yazdany J, Trupin L, Schmajuk G, Margaretten M, Barton J, et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res. 2013;65:62–70. doi: 10.1002/acr.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Are men at greater risk of lean mass deficits in rheumatoid arthritis? Arthritis Care Res. 2015;67:112–119. doi: 10.1002/acr.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JF, Von Feldt J, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res. 2014;66:1612–1618. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles JT, Malayeri AA, Fernandes V, Post W, Blumenthal RS, Bluemke D, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum. 2010;62:940–951. doi: 10.1002/art.27349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007–2010. http://wwwn.cdc.gov/nchs/nhanes/search/nhanes07_08.aspx. [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1971–1974. https://www.cdc.gov/Nchs/Nhanes/Nhanesi.htm. [Google Scholar]
- 13.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9(Suppl 4):228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 14.Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis Rheumatol Hoboken NJ. 2015;67:1711–1717. doi: 10.1002/art.39136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27:2208–2216. doi: 10.1002/jbmr.1654. [DOI] [PubMed] [Google Scholar]
- 16.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obes Silver Spring Md. 2012;20:30–39. doi: 10.1038/oby.2011.211. [DOI] [PubMed] [Google Scholar]
- 17.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–1052. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PloS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Laboratory Protocol. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2007–2010. https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/crp_e_met.pdf. [Google Scholar]
- 20.Dessein PH, Norton GR, Woodiwiss AJ, Joffe BI, Solomon A. Independent role of conventional cardiovascular risk factors as predictors of C-reactive protein concentrations in rheumatoid arthritis. J Rheumatol. 2007;34:681–688. [PubMed] [Google Scholar]
- 21.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 22.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol. 2009;28:439–444. doi: 10.1007/s10067-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 24.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res. 2012;64:1471–1479. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 26.Ajeganova S, Andersson ML, Hafström I BARFOT Study Group. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res. 2013;65:78–87. doi: 10.1002/acr.21710. [DOI] [PubMed] [Google Scholar]
- 27.Jawaheer D, Olsen J, Lahiff M, Forsberg S, Lähteenmäki J, da Silveira IG, et al. Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clin Exp Rheumatol. 2010;28:454–461. [PMC free article] [PubMed] [Google Scholar]
- 28.Heimans L, van den Broek M, le Cessie S, Siegerink B, Riyazi N, Han KH, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res. 2013;65:1235–1242. doi: 10.1002/acr.21978. [DOI] [PubMed] [Google Scholar]
- 29.Sandberg MEC, Bengtsson C, Källberg H, Wesley A, Klareskog L, Alfredsson L, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis. 2014;73:2029–2033. doi: 10.1136/annrheumdis-2013-205094. [DOI] [PubMed] [Google Scholar]
- 30.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–3582. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 31.Baker JF, Ostergaard M, George M, Shults J, Emery P, Baker DG, et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2 years. Ann Rheum Dis. 2014;73:1923–1928. doi: 10.1136/annrheumdis-2014-205544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Hazlewood GS, Kaplan GG, Eksteen B, Barnabe C. The Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016 doi: 10.1002/acr.22932. [DOI] [PubMed] [Google Scholar]
- 33.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain Off J Am Pain Soc. 2007;8:430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30:2350–2355. [PubMed] [Google Scholar]
- 35.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in US Veteran Men with Rheumatoid Arthritis. Arthritis Care Res. 2015 doi: 10.1002/acr.22642. [DOI] [PubMed] [Google Scholar]
- 36.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–1629. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 37.George MD, Baker JF. The Obesity Epidemic and Consequences for Rheumatoid Arthritis Care. Curr Rheumatol Rep. 2016;18:6. doi: 10.1007/s11926-015-0550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.