Abstract
To expand upon our previous observation that HIV-1 Gag-specific IgG antibodies were highest in HIV controllers not carrying HLA-B*57:01, we analysed these antibodies in a larger cohort of viremic controllers (VCs) or elite controllers (ECs) considering carriage of ‘protective’ HLA-B alleles. HIV-1 p24-specific IgG1 and IgG2 antibodies were higher only in HLA-B*57:01− VCs but there were no differences in ECs. Associations of HIV-1 gp140-specific IgG antibodies with HLA-B*57:01 carriage were inconsistent amongst VCs and ECs.
Keywords: HIV-1 p24-specific IgG antibodies, HIV-1 gp140-specific IgG antibodies, protective HLA-B alleles, viremic controllers, elite controllers
Defining immune responses that naturally control HIV-1 infection will enlighten therapeutic HIV-1 vaccine design. It is well-established that CD8+ T cell responses against peptides of HIV-1 capsid proteins encoded by gag (Gag proteins) and carriage of ‘protective’ alleles of genes for human leucocyte antigen (HLA)-B molecules, which present peptides of HIV-1 Gag proteins to CD8+ T cells, correlate with immune control of HIV-1 infection [1–3]. In individuals of European descent, the most ‘protective’ allele of HLA-B is HLA-B*57:01 [3], which encodes HLA molecules that bind fewer self-peptides and exhibit greater cross-reactive binding of viral peptides than other HLA-B alleles, contributing to exceptional long-term control of HIV-1 replication in carriers [4]. However, approximately 30–40% of individuals who naturally control HIV-1 infection (HIV controllers) do not possess a ‘protective’ HLA-B allele [1, 2], suggesting that immune responses other than those mediated by CD8+ T cells contribute to immune control of HIV-1 infection. Recently, Koofhethile et al. [5] demonstrated that viremic controllers (VCs) not carrying ‘protective’ alleles of HLA-B genes control HIV-1 infection more robustly than carriers, and that CD8+ T cells do not mediate this effect. In addition, Freund et al. [6] demonstrated that HIV-1 Env-specific antibodies with broad neutralising activity might have contributed to long-term control of HIV-1 infection in an elite controller (EC) who carried the ‘protective’ HLA-B alleles HLA-B*57:01 and HLA-B*27:05. However, the findings of previous studies on the role of antibodies in the natural control of HIV-1 infection have been inconclusive [2, 7–11].
We have previously provided evidence that HIV-1 Gag-specific IgG antibodies might contribute to control of HIV-1 infection [12–15], including in HIV controllers not carrying HLA-B*57:01 [13], and proposed that these antibodies mediate HIV-1 Gag-specific pDC-reactive opsonophagocytic antibody (PROAb) responses [14]. Importantly, in analysing the relationship between HIV-1 Gag-specific IgG antibodies and ‘protective’ HLA-B alleles in our previous study [13], we did not differentiate between VCs and ECs. We have therefore undertaken this analysis for the patients reported in Tjiam et al. [14]. As both IgG1 and IgG2 antibodies contribute to opsonophagocytic antibody responses that activate dendritic cells via FcγRIIa [16], we examined HIV-1 p24-specific IgG1 and IgG2 antibody levels and HIV-1 p24-specific PROAb responses in the previously reported VCs (n=29) and ECs (n=30) [14] stratifying them into subgroups based on carriage, or not, of HLA-B*57:01 or other ‘protective’ HLA-B alleles (HLA-B*14:02, 27:05, 52:01, 58:01 and 81:01) defined in European and African patients [3, 5]. Non-controllers (NCs; n=30) were included for comparison.
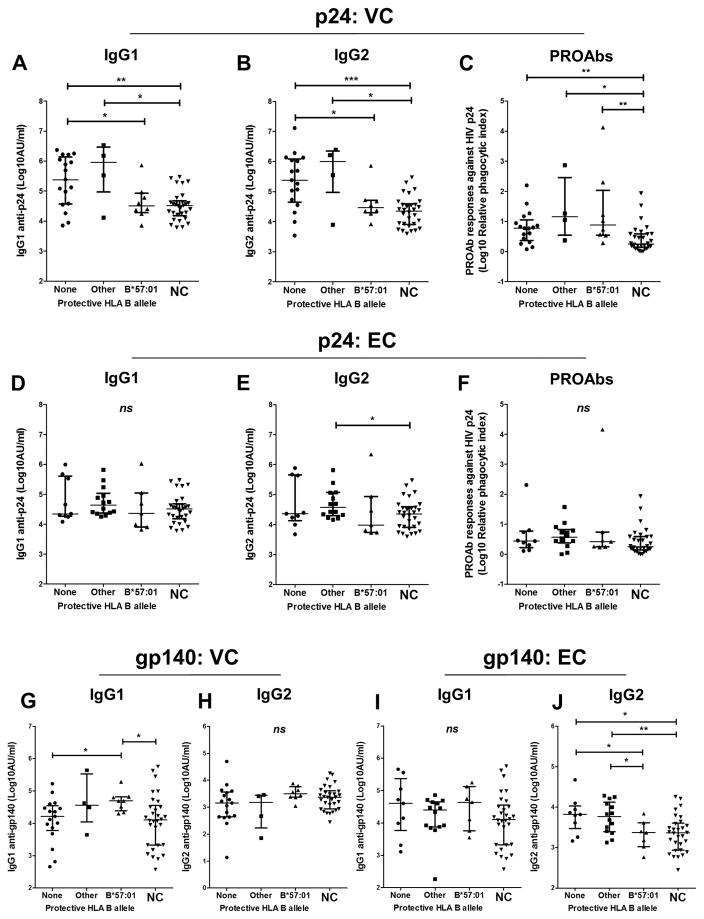
As shown in Figure 1, HIV-1 p24-specific IgG1 and IgG2 antibodies were higher in VCs than NCs, except for VCs carrying HLA-B*57:01 (Figures 1A, B). HIV-1 p24-specific PROAb responses were also higher in VCs than NCs but a relationship with ‘protective’ HLA-B alleles was not apparent (Figure 1C). In contrast, ECs exhibited essentially no differences in HIV-1 p24-specific IgG antibodies when compared with NCs (Figures 1D-F). We also examined the relationship of HIV-1 gp140-specific IgG1 and IgG2 antibody levels, demonstrated in these patients [14], with ‘protective’ HLA-B alleles (Figures 1G-J). HIV-1 gp140-specific IgG2 antibodies exhibited a relationship to HLA-B*57:01 that was similar to HIV-1 p24-specific IgG2 antibodies but in ECs rather than VCs (Figure 1J). In VCs, HIV-1 gp140-specific IgG1 antibodies were higher in patients carrying HLA-B*57:01 (Figure 1G).
Figure 1.
Based on plasma HIV-1 RNA levels, antiretroviral-naïve HIV-1-infected individuals were classified as either elite controllers (<75 copies/ml), viremic controllers (75–2000 copies/ml) or non-controllers (>10,000 copies/ml). Viremic and elite controllers were subgrouped based on possession of either HLA-B*57:01, other ‘protective’ HLA-B alleles (HLA-B*14:02, B*27:05, B*52:01, B*58:01, B*81:01) or no ‘protective’ HLA-B alleles. HIV non-controllers (NC) were included for comparison. Within viremic controllers, differences in IgG antibody responses against HIV-1 p24 (A–C) and HIV-1 gp140 (G, H) between subgroups are shown. Within elite controllers, differences in IgG antibody responses against HIV-1 p24 (D–F) and HIV-1 gp140 (I, J) between subgroups are shown. Differences between subgroups were compared using Mann-Whitney tests (* = p ≤ 0.05, ** = p < 0.01, *** = p <0.001, ns = p > 0.05).
These findings expand upon our previous findings [13] by demonstrating that the association between HIV-1 p24-specific IgG antibodies and natural control of HIV-1 infection in individuals not carrying HLA-B*57:01 is only observed in VCs. This observation was made for HIV-1 p24-specific IgG1 and IgG2 antibodies but not for PROAb responses, possibly because they are detected by a functional antibody assay that may be affected by factors other than binding of antibodies to antigens in an enzyme immunoassay. Furthermore, our findings provide more evidence that IgG2 antibodies to HIV-1 Env antigens are associated with control of HIV-1 infection [10, 17], but only in ECs not carrying HLA-B*57:01. We suggest that HIV-1 p24-specific IgG1 and IgG2 antibodies, possibly including PROAb responses, might contribute to non-CD8+ T cell-mediated control of HIV-1 infection in patients with low-level HIV-1 replication [5] and that this requires further investigation. We also suggest that these investigations should consider the possibility that HIV-1 Gag-specific IgG antibodies mediate an antibody response against HIV-1 capsids extracellularly via PROAb responses mediated through FcγRIIa [14] and/or intracellularly via the neonatal Fc receptor and the cytosolic Fc receptor TRIM21/Ro52 [18, 19]. The former type of antibody response has been associated with immune control of non-enveloped RNA viruses [20–22], where antibody-mediated opsonisation of virions leads to their phagocytosis by pDCs and interferon-α production through viral RNA binding to TLR7, and also with control of enveloped influenza viruses by a similar mechanism in mouse models of influenza virus disease [23]. We also demonstrated that carriage of HLA-B*57:01 was associated with HIV-1 gp140-specific antibody levels in HIV controllers but in contrast to HIV-1 p24-specific antibodies, the patterns of association were inconsistent. Thus, HIV-1 gp140-specific IgG1 antibody levels were higher in VCs carrying HLA-B*57:01 while IgG2 antibody levels were higher in ECs not carrying HLA-B*57:01. While it was notable that lack of HLA-B*57:01 carriage was associated with higher HIV-1 gp140-specific IgG2 antibodies in ECs as well as higher HIV-1 p24-specific IgG1 and IgG2 antibodies in VCs, it was not possible to determine if gp140-specific IgG2 antibodies in ECs correlated negatively with HIV viral load, as was shown for HIV-1 p24 IgG antibodies in viremic patients [14].
In conclusion, our findings highlight the importance of examining patients with active HIV-1 replication, and considering the exceptional effects of HLA-B*57:01 on control of HIV-1 infection, when investigating the role of HIV-1 Gag-specific IgG antibodies in controlling HIV-1 infection.
Acknowledgments
Funding Information
This work was funded by the National Health and Medical Research Council of Australia (grant 510448) and the Medical Research Foundation of Royal Perth Hospital (grant 2011/027). S.F. was supported by a fellowship from the Royal Perth Hospital Medical Research Foundation. The University of California San Francisco SCOPE cohort was supported by Delaney AIDS Research Enterprise (grant AI096109), University of California San Francisco/Gladstone Institute of Virology and Immunology Center for AIDS Research (grant P30 AI027763), and by Center for AIDS Research Network of Integrated Systems (grant R24 AI067039).
Footnotes
Contributions
The manuscript was written by M.A.F., M.C.T and S.F. Laboratory work was performed by M.C.T., M.A.M., L.S., S.L., S.F. and D.B.A.T. Data analyses were performed by M.C.T. Patient plasma samples were provided by S.G.D and J.N.M. All authors reviewed and provided comment on the manuscript.
References
- 1.Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. Journal of virology. 2008;82:5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. Journal of Infectious Diseases. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 3.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PIW, Walker BD. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science (New York, NY) 2010;330:1551. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Košmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465:350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koofhethile C, Ndhlovu Z, Thobakgale-Tshabalala C, Prado J, Ismail N, Mncube Z, et al. CD8+ T cell breadth and ex vivo virus inhibition capacity distinguish between viremic controllers with and without protective HLA class I alleles. Journal of virology. 2016;90:6818–6831. doi: 10.1128/JVI.00276-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freund NT, Wang H, Scharf L, Nogueira L, Horwitz JA, Bar-On Y, et al. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Science Translational Medicine. 2017;9:eaal2144. doi: 10.1126/scitranslmed.aal2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS (London, England) 2009;23:897. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee K, Klasse P, Sanders RW, Pereyra F, Michael E, Lu M, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS research and human retroviruses. 2010;26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PloS one. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai JI, Licht AF, Dugast A-S, Suscovich T, Choi I, Bailey-Kellogg C, et al. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. Journal of virology. 2014;88:2799–2809. doi: 10.1128/JVI.03130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French MA, Tanaskovic S, Law MG, Lim A, Fernandez S, Ward LD, et al. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high-affinity’ FcγRIIa genotype. Aids. 2010;24:1983–1990. doi: 10.1097/QAD.0b013e32833c1ce0. [DOI] [PubMed] [Google Scholar]
- 13.French MA, Center RJ, Wilson KM, Fleyfel I, Fernandez S, Schorcht A, et al. Isotype-switched immunoglobulin G antibodies to HIV Gag proteins may provide alternative or additional immune responses to ‘protective’human leukocyte antigen-B alleles in HIV controllers. Aids. 2013;27:519–528. doi: 10.1097/QAD.0b013e32835cb720. [DOI] [PubMed] [Google Scholar]
- 14.Tjiam MC, Taylor JP, Morshidi MA, Sariputra L, Burrows S, Martin JN, et al. Viremic HIV Controllers Exhibit High Plasmacytoid Dendritic Cell–Reactive Opsonophagocytic IgG Antibody Responses against HIV-1 p24 Associated with Greater Antibody Isotype Diversification. The Journal of Immunology. 2015;194:5320–5328. doi: 10.4049/jimmunol.1402918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjiam MC, Sariputra L, Armitage JD, Taylor JPA, Kelleher AD, Tan DBA, et al. Control of early HIV-1 infection associates with plasmacytoid dendritic cell-reactive opsonophagocytic IgG antibodies to HIV-1 p24. AIDS. 2016;30:2757–2765. doi: 10.1097/QAD.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 16.den Dunnen J, Vogelpoel LT, Wypych T, Muller FJ, de Boer L, Kuijpers TW, et al. IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood. 2012;120:112–121. doi: 10.1182/blood-2011-12-399931. [DOI] [PubMed] [Google Scholar]
- 17.Martinez V, Costagliola D, Bonduelle O, N’go N, Schnuriger A, Théodorou I, et al. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. Journal of Infectious Diseases. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 18.Watkinson RE, McEwan WA, James LC. Intracellular antibody immunity. Journal of clinical immunology. 2014;34:30–34. doi: 10.1007/s10875-014-0017-4. [DOI] [PubMed] [Google Scholar]
- 19.Stapleton NM, Einarsdóttir HK, Stemerding AM, Vidarsson G. The multiple facets of FcRn in immunity. Immunological reviews. 2015;268:253–268. doi: 10.1111/imr.12331. [DOI] [PubMed] [Google Scholar]
- 20.Palmer P, Charley B, Rombaut B, Daëron M, Lebon P. Antibody-dependent induction of type I interferons by poliovirus in human mononuclear blood cells requires the type II Fcγ receptor (CD32) Virology. 2000;278:86–94. doi: 10.1006/viro.2000.0627. [DOI] [PubMed] [Google Scholar]
- 21.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. Journal of Immunology. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 22.Lannes N, Python S, Summerfield A. Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Vet Res. 2012;43:64. doi: 10.1186/1297-9716-43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMere MW, Lam H-T, Moquin A, Haynes L, Lund FE, Randall TD, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. Journal of Immunology. 2011;186:4331–4339. doi: 10.4049/jimmunol.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]