Abstract
Differential experience leads infants to have perceptual processing advantages for own- over other-race faces, but whether this experience has down-stream consequences is unknown. Three experiments examined whether 7-month-olds (Range = 5.9-8.5 months, N = 96) use gaze from own- versus other-race adults to anticipate events. When gaze predicted an event’s occurrence with 100% reliability, 7-month-olds followed both adults equally; with 25% (chance) reliability, neither was followed. However, with 50% (uncertain) reliability, infants followed own- over other-race gaze. Differential face race experience may thus affect how infants use social cues from own- versus other-race adults for learning. Such findings suggest that infants integrate online statistical reliability information with prior knowledge of own- versus other-race to guide social interaction and learning.
In the first year of life, many infants are predominantly exposed to people from their own race and rarely encounter other-race individuals (Rennels & Davis, 2008; Sugden, Mohamed-Ali, & Moulson, 2014). It is now well established that this asymmetrical exposure has significant perceptual consequences (Bar-Haim, Ziv, Lamy, & Hodes, 2006; Kelly et al., 2007a; Kelly et al., 2005; Kelly et al., 2009; Kelly et al., 2007b; Liu et al., 2015). However, little is known about whether the asymmetrical exposure to own- versus other-race faces experienced by infants has down-stream social consequences. The present study addresses this significant gap in the literature by assessing whether infants are biased to rely more on eye gaze cues provided by own- versus other-race adults.
Face perception becomes specialized to the characteristics of own-race faces as opposed to those of other-race faces in the 1st year of life. For example, infants at 3 months with predominant own-race exposure from birth prefer to look more at own- over other-race faces (Bar-Haim et al., 2006; Kelly et al., 2005). Moreover, although 3-month-olds recognize own- and other-race faces equivalently, at 9 months, own-race faces are better recognized than other-race faces (Kelly et al., 2009; Kelly, Quinn et al., 2007). In addition, studies utilizing training procedures have revealed that the perceptual processing advantages for own-race faces are caused by the asymmetrical exposure of infants to own- versus other-race faces (Anzures et al., 2012; Bar-Haim et al., 2006; Heron-Delaney et al., 2011).
Would such asymmetrical experience own- versus other-race faces lead to broader social consequences in terms of how infants respond to own- versus other-race individuals? Quinn, Lee, Pascalis, and Tanaka (2016) found that Caucasian infants at 6 months formed separate categories for Caucasian, Asian, and African faces according to perceptual differences arising from facial physical characteristics. By contrast, at 9 months, Caucasian infants responded to the same set of faces based on whether a face was own race or other race. That is, they grouped Caucasian faces into one category and Asian and African faces into another despite the fact that the Asian and African faces are physically quite distinct. The outcomes suggest that infants between 6 and 9 months begin to dichotomize faces according to whether they belong to the familiar “own-race” group or the unfamiliar “other- race” group. Quinn et al. speculated that such dichotomous representation of faces by race might be a precursor to the race-based in-group versus out-group partitioning of faces in children and adults (e.g., Baron & Banaji, 2006; Dunham, Newheiser, Hoosain, Merrill, & Olson, 2014; Xiao et al., 2015).
To provide an account of how the initial beginnings of racial bias could actually arise in infants, Lee, Quinn, and colleagues have proposed a Perceptual-Social Linkage hypothesis (Lee, Quinn, & Heyman, in press-a; Lee, Quinn, & Pascalis, in press-b). This hypothesis posits that early asymmetrical exposure to own- versus other-race face experience has not only perceptual consequences regarding face recognition and categorization, but also social consequences in terms of racial bias. Due to asymmetrical exposure to own- versus other-race individuals, infants begin to perceive other-race members to be more homogenous than those within one’s own-race group (out-group homogeneity, Linville, Fischer, & Salovey, 1989). The out-group homogeneity leads to categorical rather than individual responding to other-race faces (Quinn et al., 2016). At the same time, due to such asymmetrical exposure, infants come to differentially associate positive or negative valence with own- versus other-race faces. This is because infants are typically exposed to own-race individuals who interact with them positively (Kim & Johnson, 2014; Malatesta & Haviland, 1982; Trainor, Austin, & Desjardins, 2000). Thus, infants may generalize and respond more positively to own-race individuals relative to other-race individuals. Lack of exposure to other-race individuals, and perceptual differences between the faces of own and other-race individuals, combined with a tendency to be wary of novel stimuli in general and strangers in particular (Bigelow, MacLean, Wood, & Smith, 1990; Bronson, 1972; Campos, Emde, Gaensbauer, & Henderson, 1975) may lead infants to respond more negatively to other-race individuals relative to own-race individuals. By these means, the tendency to categorize faces by race and the tendency to associate positive or negative valence with familiar versus unfamiliar stimulus categories may work in conjunction to form the basis from which racial bias emerges and develops.
The findings of a recent study (Xiao et al., in press) are consistent with the perceptual-social linkage hypothesis. In accord with the hypothesis, between 6 and 9 months of age, infants associate own-race faces with happy music and other-race faces with sad music. This finding suggests that older infants may already have begun to associate own- and other-race faces with differential valence. However, it is unclear whether such bias would also be observed in social interaction and learning. The current study specifically tested this possibility. We examined whether infants are more inclined to learn from an own-race adult than an other-race adult.
A common social activity in early development is learning from others. In this regard, many previous studies have shown that infants are sensitive to social cues (e.g., eye gaze, pointing) displayed by adults. From these studies, it is known that infants: (1) follow adult eye gaze as newborns (Farroni, Csibra, Simion, & Johnson, 2002), (2) follow adult eye gaze to locations to the left or right of the face around 3 to 6 months (D’Entremont, 2000; D’Entremont, Hains, & Muir, 1997; Hood, Willen, & Driver, 1998; Senju & Csibra, 2008), (3) use adult eye gaze to predict future events by 8 months (Tummeltshammer, Wu, Sobel, & Kirkham, 2014; Wu & Kirkham, 2010), and (4) use pointing cues to locate objects hidden behind occluded areas by 12 months (Brooks & Meltzoff, 2002; Gliga & Csibra, 2009). Most relevant to the present study, by 7 to 9 months, infants begin to use the historical behavior of adult informants to determine whether to rely on them to predict future events. For example, infants in this age range prefer to learn from more familiar, accurate, knowledgeable, consistent, and confident adults (Birch, Akmal, & Frampton, 2010; Buttelmann, Zmyj, Daum, & Carpenter, 2013; Chow, Poulin-Dubois, & Lewis, 2008; Poulin-Dubois, Brooker, & Polonia, 2011; Poulin-Dubois & Chow, 2009; Stenberg, 2012, 2013; Tummeltshammer et al., 2014).
However, all of the above-mentioned studies used social cues provided by own-race adults. It is therefore unclear whether infants would learn to follow and use social cues provided by own- versus other-race informants differently. To address this issue, we tested infants from 5.9 to 8.5 months of age (average age = 7 months) using a well-established gaze-cuing paradigm (Tummeltshammer et al., 2014; Wu, Gopnik, Richardson, & Kirkham, 2011; Wu & Kirkham, 2010; Wu, Tummeltshammer, Gliga, & Kirkham, 2014). This paradigm is comprised of a learning phase, a test phase, and a generalization phase. In the learning phase, infants watch a series of videos depicting an adult gazing at one of the four corners of a computer display. After the adult gazes at a particular location, an animal appears at the location. This pairing between the adult’s gaze and the appearance of the animal repeats several times. After the learning phase, the test and generalization phases involve the same adult gazing at a corner of the screen without the appearance of the animal. If infants learn that the adult’s gaze predicts the location of the upcoming animal during the learning phase, then they should use the adult’s gaze to anticipate the appearance of the animal during the test and generalization phases. In the standard paradigm, the adult is from the infant’s own race.
In the present study, we modified the standard paradigm by having an own- or other-race adult gaze at the various locations. The critical measure was whether infants would use the own- or other-race gaze cues to predict the appearance of an animal during the test and generalization phases. The test and generalization phases involve the same adult gazing at a corner of the screen without the appearance of the animal; test trials depicted gaze to the same locations depicted in learning trials, while generalization trials depicted gaze to new locations. Based on the perceptual-social linkage hypothesis, we hypothesized that infants would display an own-race bias when using social cues.
An important aspect of the paradigm is that it allows for including adult informants in a range of reliability from being highly reliable to being highly unreliable. To test our hypothesis, we conducted three experiments using own- and other-race cues, when the cues were 50% reliable (Experiment 1), 100% reliable (Experiment 2), or 25% reliable (Experiment 3), respectively. In Experiment 1, infants learned that either an own- or other-race adult was reliable only 50% of the time. In other words, in the learning phase, when the adult gazed at a particular location, the animal appeared at the gazed-at location on 50% of the trials, and on the other 50% of the trials, the animal appeared at a non-gazed-at location. We reasoned that if infants had any own-race bias in social cue following and learning, the bias would likely emerge in this situation of uncertainty. This is because, with uncertainty, infants may find it difficult to determine whether to follow gaze based solely on its reliability. Thereby, the response of gaze following has to be based on additional cues. According to the perceptual-social linkage hypothesis, the greater familiarity infants have with own- than other-race faces may exert significant influence on their learning from social cues. Specifically, infants would be more likely to follow the gaze of own-race but not other-race faces to predict future events under uncertainty.
Two additional experiments assessed whether any bias to use own-race cues over other-race cues by infants is a general phenomenon across all situations or specific to situations of uncertainty. In Experiment 2, both the own- and other-race adults were 100% reliable. One possible outcome is that infants might be biased generally to rely on social cues provided by own-race adults. In this case, the perceptual-social linkage hypothesis would predict an own-race advantage even in a situation where both the own- and other-race adults are completely reliable. Alternatively, infants may not show any bias in a deterministic context (the logical approach) and would follow the gaze of both highly informative own- and other-race adults. In Experiment 3, both the own- and other-race adults were highly unreliable (i.e., at chance level, 25% reliability). In Tummeltshammer et al. (2014), infants did not follow the gaze of an own-race informant that displayed 25% reliability. Based on this finding, we expected that infants in the current study would respond randomly in the own-race condition as well as in the other-race condition. Thus, with the three experiments, we were able to test infant use of social cues provided by own- and other-race adults with high, medium, and low reliability.
Experiment 1
Method
Participants
We recruited 32 full-term Chinese infants to participate in this experiment and randomly assigned them to either the own-race condition (n = 16, 9 females and 7 males, M = 218.00 days, SD = 26.94 days, Range = 183 to 251 days) or other-race condition (n = 16, 8 females and 8 males, M = 204.68 days, SD = 27.69 days, Range = 177 to 254 days). Eleven additional infants were recruited, but excluded from the data analysis because of failure to complete the procedure due to fussiness (n = 10) or eye tracker calibration failure (n = 1). The number of participants for the current study was determined by referring to an existing study with a comparable experimental design (Tummeltshammer et al., 2014).
For all three experiments, all of the participants were recruited through a local public hospital, which was accessible to all families living in the community. Participation was entirely voluntary, and parents did not receive monetary compensation. Based on reporting from each infant’s primary caregiver, none of the participants had any direct contact with other-race individuals. In the current experiment, all of the participants were from middle class Mandarin speaking families. Eighteen of the 32 families had at least one parent with a post-secondary degree of bachelor’s or above; for the other 14, the highest education level was a high school diploma. Based on the most recent physical check-up records, all participants were full-term healthy infants. Experiments were approved by the Research Ethics Board of the University of Toronto and by the Institutional Ethics Board of Zhejiang Sci-Tech University. Informed consent was obtained from the caregivers of the infants. The data for all three experiments were collected from September 2014 to June 2015.
Materials and procedure
A pilot study with a within-subject design (N = 17) led to a high attrition rate (47%) and poor eye tracking quality in the second half of the session. Thus, a between-subjects design was utilized.
Each infant participant sat on the lap of his/her primary caregiver at a distance of 60 cm from the 17-inch computer monitor (35 × 26 cm2) used to present the stimuli, which was controlled by E-Prime software (2.0). A Tobii 1750 eye tracker (50 Hz) recorded infant eye movements, and was calibrated at the beginning of the experiment with an infant friendly calibration procedure (e.g., Tummeltshammer et al., 2014; Wu et al., 2011; Wu & Kirkham, 2010). During the calibration, infants were required to look at a cartoon animal continuously for at least 100 ms. This cartoon animal would appear at 5 locations on the monitor: the 4 corners and the center. The eyes of the caregivers were covered to avoid unintentional interference with infant responding.
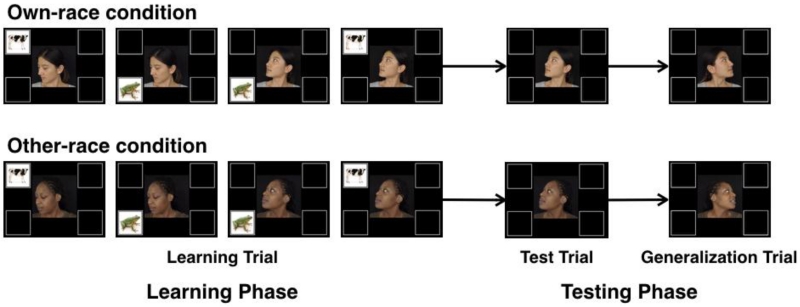
In the full experiment, infants saw a series of video clips. In each clip, a female actor first appeared in frontal view with a smile and provided direct eye gaze. There were four white boxes presented at each corner of the screen (8.54 × 8.54 cm2, 7.77° × 7.98° visual angle, Figure 1). After 0.50 seconds on the screen, the actor started to speak to the infant in infant-directed speech, saying “Hey baby, look at this” in Mandarin, which lasted for 1.30 s. The actor then turned her head towards one of the four boxes after she finished speaking, which lasted for 0.70 s.
Figure 1.
An example of the stimulus display including four boxes and an informant. The letters (A, B, C, & D) are used here and in the text to refer to the four boxes, but the letters themselves did not appear in any of the experiments.
There were 8 valid trials and 8 invalid trials presented in a random order. On the 8 valid trials, an animal appeared in the box cued by the actor’s head turn (e.g., the actor cued Box A, and an animal appeared in Box A, see Figure 2). In this instance, the actor’s head turn was a reliable cue for the appearance of the animal at the cued location. Following Tummeltshammer et al. (2014), the animals depicted in each of 4 boxes were a cow, a frog, a dog, and a bird. The animal was shown with its special sound and animated movement (i.e., a cow that moos and moves up and down, a bird that chirps and moves left and right, a dog that barks and rotates, and a frog that croaks and changes size). The animal event was presented for 3 s during which the actor remained in the head-turned posture. The 8 invalid trials were the same as the valid trials except that the animal appeared in a box that was not cued by the actor’s head turn (e.g., the actor cued Box A, but the animal appeared in Box B).
Figure 2.
Examples of the learning, test, and generalization trials. On the 16 learning trials, 4 different trials were presented in each block: 2 valid trials and 2 invalid trials, randomly mixed. On valid trials, the actor’s gaze predicted the location of an upcoming animal in the gazed-at location. On invalid trials, the actor’s gaze did not predict the animal’s occurrence in the gazed-at location. In the test phase, for the 2 test trials, the actor gazed at one of the two locations that she had looked at during the learning trials. For the 2 generalization trials, the actor gazed at one of the two locations that she had not looked at during the learning trials. No animal appeared in the test or generalization trials.
In the own-race condition, the actor was an Asian female adult; in the other-race condition, the actor was an African female adult. The African female adult had been studying Mandarin in China for 2 years, and was able to speak fluent Mandarin. To ensure that the own- and other-race models both presented native speech, the original soundtracks of the own- and other-race actors were replaced with soundtracks of two other native Mandarin speakers. These two speakers were required to imitate the mouth movements of the two actors as closely as possible. After repeated practice imitating the mouth movements of the actors, the voices of the speakers were recorded. This procedure ensured that the soundtracks matched the mouth movements of the two actors. We used Adobe Premiere to closely match the mouth movements of the two actors with the replacement speech soundtracks to produce a seamless synchronized audio-video stream. Adobe Audition was used to equalize the perceived loudness of the voices in the own- and other-race videos. We adjusted the image sizes of the two faces to make them comparable. Their size was 8.96 × 12.03 cm2 with 8.34° × 11.39° visual angle.
Infants first participated in the learning phase, consisting of 16 trials split into four blocks of four trials each: two valid trials and two invalid trials. On the two valid trials, the actor gazed at two different locations respectively (e.g., Box A or Box B) and two different animals appeared subsequently. On the two invalid trials, the actor also gazed at these two locations but the animals appeared in a different, non-cued location. For example, on a trial when the actor gazed at Box A, the animal appeared at Box B; on a trial when the actor gazed at Box B, the animal appeared at Box A.
After the learning trials, two test and two generalization trials ensued. These trials were identical to the learning trials except that no animal appeared after the same actor gazed at a box. On the test trials, the actor gazed at one of the boxes that she had gazed at during the learning trials (e.g., Box A or Box B). On the generalization trials, the actor gazed at a box that she had not gazed at during the learning trials (e.g., Box C or Box D). Thus, the only difference between the test and generalization trials was that on test trials the actor gazed at a box that was previously cued during learning trials, whereas on generalization trials the actor gazed at a novel box that was never cued during learning trials. The purpose of including both test and generalization trials was to examine whether infants learned the reliability of the actor and therefore followed the actor’s gaze to a previously looked-at location even if no animal appeared (test trials), and whether this learning could be generalized to a novel location in which the actor had not yet looked previously (generalization trials).
The test and generalization trials were interleaved, with each test trial presented first followed by each generalization trial (i.e., Test Trial 1 – Generalization Trial 1 – Test Trial 2 – Generalization Trial 2). The selection of the cued boxes in the learning, test, and generalization trials was counterbalanced across infants. Thus, each infant saw 16 learning trials, 2 test trials, and 2 generalization trials.
To ensure the accuracy and precision of eye tracking across the trials, in addition to the initial calibration that occurred before the learning trials, we performed four more calibrations during the trials: the first between the 4th and 5th learning trials, the second between the 8th and 9th learning trials, the third between the 12th and 13th learning trials, and the fourth between the 16th learning trial and 1st test trial. For each calibration, infants had to fixate on the center of the screen for 1 s to trigger the calibration procedure. The overall experimental session lasted about 130 s ([16 learning trials + 2 test trials + 2 generalization trials] 9 6.50) plus calibration time (Total M = 206.33 s).
Results and Discussion
We calculated the proportional looking time of the infants to each of the four boxes at the four corners of the screen separately for the learning, test, and generalization trials after the adult had shifted her gaze.
Gaze following on learning trials
For the valid learning trials on which the adult’s gaze correctly predicted the location where an animal appeared, infants mostly looked at the cued box (M = 95.77%, SD = 6.04%). An independent-samples t-test showed that the looking time for the cued box in the own-race condition (M = 97.61%, SD = 3.82%) was not significantly different from that in the other-race condition (M = 93.93%, SD = 7.32%, t[30] = 1.78, p = .085). For the invalid learning trials on which the adult’s gaze incorrectly predicted the location where the animal appeared, we also found no significant race effect in looking time to the cued boxes (Mown = 5.22%, SDown = 7.91%, Mother = 5.93%, SDother = 9.24%, independent sample t-test: t[30] = 0.23, p = .816). Thus, during the learning phase, the looking pattern of the infants was not affected by the race of the informants.
Gaze following on test and generalization trials
To assess whether infants followed the gaze of the own-race adult more than the other-race adult on test and generalization trials, we used a mixed analysis of variance (ANOVA). We examined whether face race (own vs. other) and trial type (test vs. generalization) affected the mean looking time of infants to the cued box on test and generalization trials. Trial type was a repeated measures variable, and face race was a between-subject variable. We found that infants followed the gaze of the own-race adult (M = 61.19%, SD = 35.95%) more than that of the other-race adult (M = 30.10%, SD = 38.91%, F[1, 30] = 14.30, p < .001, ηP2 = .32). Neither the effect of trial type (Mtest = 49.40%, SDtest = 41.49%, Mgeneralization = 41.89%, SDgeneralization = 39.43%, F[1, 30] = 0.51, p = .482, ηP2 = .02) nor the interaction between the two variables (F[1, 30] = 0.04, p = .849, ηP2 = .001) was significant. Thus, infants were more likely to follow the gaze of an own- than other-race adult when both were only 50% reliable.
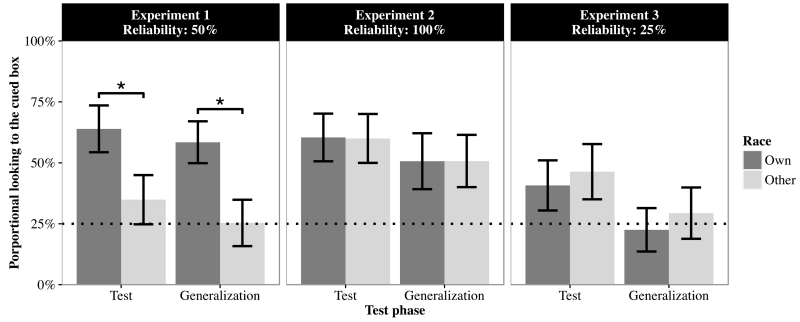
To examine whether infant gaze follow- ing was reliably above chance, we compared their proportional looking time at the cued box to the chance level of 25% (i.e., equal looking time at each of the four boxes). One-sample t tests revealed that in the own-race condition, infant looking time to the cued box was significantly above chance on test trials (M = 63.93%, SD = 38.40%), t(15) = 4.06, p < .001, and generalization trials (M = 58.44%, SD = 34.36%), t(15) = 3.89, p = .001. In the other- race condition, infant gaze following to the cued box did not differ from chance on test (M = 34.87%, SD = 40.40%), t(15) = 0.98, p = .344, or generalization trials (M = 25.33%, SD = 38.06%), t(15) = 0.03, p = .973. Moreover, we used independent sample t tests to examine whether infant gaze following in the own-race condition differed from that in the other-race condition on test and generalization trials. The results showed that infant gaze following to the cued box was significantly higher in the own-race condition than in the other-race condition for both test trials, t(30) = 2.09, p = .046, and generalization trials, t(30) = 2.58, p = .015 (see Figure 3, left panel).
Figure 3.
Mean proportional looking times for the cued boxes on test and generalization trials where the own- and other-race adults were 50% (Experiment 1: left panel), 100% (Experiment 2: central panel), and 25% reliable (Experiment 3: right panel). The dashed line indicates chance-level looking percentage. Asterisks indicate significant differences in infant gaze following between the own- and other-race conditions. Error bars indicate one standard error.
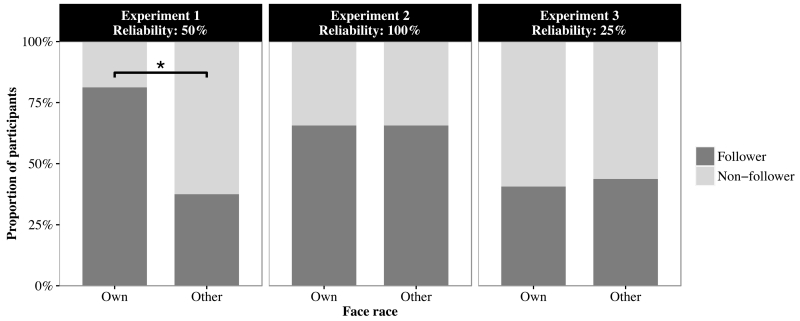
To consider the performance of individual infants, we used nonparametric analyses to compare the proportions of infants who followed the adult’s gaze to the cued boxes in the own- versus other-race conditions on test and generalization trials. The infants whose proportional looking time to the cued box were above chance (i.e., > 25%) were defined as Follower. By contrast, the infants whose proportional looking time to the cued box were not different from chance (i.e., 25%) were defined as Non-Follower. We used a generalized linear mixed model (GLMM) to examine whether face race (own vs. other), trial type (test vs. generalization), or their interaction affected the proportion of participants who followed to the cued boxes. Trial type was a repeated measures variable, and face race was a between-subjects variable. We found that more infants followed to the gazed-at boxes in the own-race condition than in the other-race condition (z = 2.11, p = .035, see Figure 4, left panel). Neither the effect of trial type (z = 0.00, p = 1.000) nor the interaction between the two variables (z = 0.45, p = .646) was significant.
Figure 4.
Proportion of participants whose gaze following to the cued boxes was above chance on test and generalization trials in which the own- and other-race adults were 50% (Experiment 1: left panel), 100% (Experiment 2: center panel), and 25% reliable (Experiment 3: right panel). The asterisk indicates a significant difference in the proportion of gaze followers between the own- and other-race conditions.
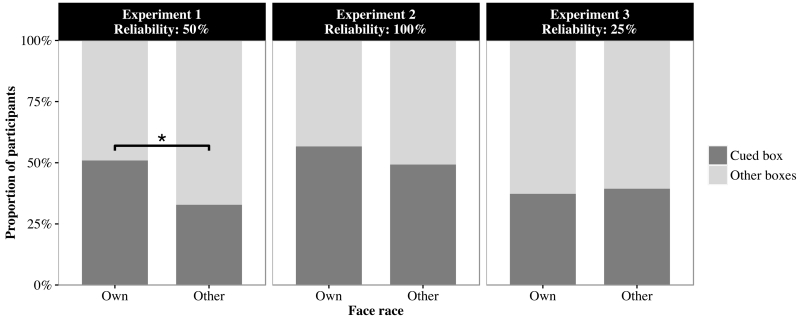
Next we examined the initial looking location of infants after the own- and other-race informants cued the box on test and generalization trials. Specifically, we analyzed first fixation location of the infants after the actor finished turning their head to the cued boxes. Infants then were classified into two groups based on their initial fixation location: those who looked at the cued box and those who looked at the other uncued boxes. We used a GLMM to examine whether face race (own vs. other), trial type (test vs. generalization) affected the proportion of participants who followed to the cued boxes on their first fixation. Trial type was a repeated measures variable, and face race was a between-subjects variable. We found that more infants followed to the gazed-at boxes in the own-race condition than in the other-race condition on their first fixation (z = 2.02, p = .043, see Figure 5, left panel). The effect of trial type was not significant (z = 0.87, p = .385).
Figure 5.
Proportion of participants whose first fixations followed the actor to the cued box on test and generalization trials in which the own- and other-race adults were 50% (Experiment 1: left panel), 100% (Experiment 2: center panel), and 25% reliable (Experiment 3: right panel). The asterisk indicates a significant difference between the own- and other-race conditions in the proportion of participants whose first fixations was to the cued box.
The analyses of proportional looking time, proportion of followers, and initial looking taken together suggest that after learning that the gaze cues of the own- and other-race adults were only 50% reliable, infants were more inclined to follow the gaze of the own-race adult to anticipate an upcoming event. This finding indicates that infants as young as approximately 7 months of age (range from 5.9 to 8.5 months) are biased to rely on the social cues provided by an own-race individual relative to an other-race individual in an uncertain context.
It is, however, unclear whether the bias seen here is a general phenomenon or specific to situations with some degree of uncertainty. To examine this question directly, we conducted Experiments 2 and 3. In Experiment 2, the own- and other-race adults were 100% reliable and thus their gaze signals predicted the location of every animal that appeared. In Experiment 3, the reliability of the own- and other-race cues was at chance level (i.e., 25% reliability) during the learning phase. If infants are generally biased to rely on the social cues of own-race adults over those of other-race adults, then they should be more inclined to follow the gaze of own-race adults than other-race adults in both Experiments 2 and 3. Alternatively, if infants are biased to rely on own-race cues only under uncertainty, then no bias should be observed in Experiments 2 and 3. Finally, if infants are biased to rely on own-race cues more than other-race cues when own-race cues are at least moderately reliable, then infants should follow own-race cues in Experiment 2, but not in Experiment 3.
Experiment 2
Method
Participants
We recruited 32 full-term Chinese infants and randomly assigned them to either the own- (n = 16, 8 females and 8 males, M = 214.13 days, SD = 28.53 days, Range = 182 to 246 days) or other-race conditions (n = 16, 6 females and 10 males, M = 216.13 days, SD = 27.25 days, Range = 183 to 252 days). We recruited 8 additional infants, who were excluded from data analyses because of failure to complete the procedure due to fussiness (n = 7) or eye tracker calibration failure (n = 1). Based on report from each infant’s primary caregiver, none of the participants had any direct contact with other-race individuals. All of the participants were from middle class Mandarin speaking families. Fifteen of the 32 families had at least one parent with a post-secondary degree of bachelor’s or above; for the other 17, the highest education level was a high school diploma. Based on the most recent physical check-up records, all participants were full-term healthy infants.
Materials and procedure
Stimuli and procedure were identical to those in Experiment 1 except that the adults in the learning phase displayed valid cuing on 100% of the trials.
Results and Discussion
Gaze following on learning trials
Participants mostly looked at the cued box (M = 96.41%, SD = 4.24%). An independent-sample t-test showed that infant looking time to the cued box in the own-race condition (M = 97.50%, SD = 3.05%) was not significantly different from that in the other-race condition (M = 95.31%, SD = 5.02%, t[30] = 1.49, p = .147). These results suggest that during the learning phase the looking pattern of the infants was not affected by face race.
Gaze following on test and generalization trials
An ANOVA similar to that performed on the Experiment 1 data revealed no significant effects of race (F[1, 30] < 0.01, p = .985, ηP2 < .01), trial type (F[1, 30] = 0.14, p = .656, ηP2 = .21), or the interaction between them (F[1, 30] < 0.01, p = .982, ηP2 < .01). One-sample t-tests showed that infant following of the 100% reliable adult gaze on test (M = 60.20%, SD = 39.13%, t[15] = 2.24, p = .003) and generalization trials (M = 50.70%, SD = 45.90%, t[15] = 3.49, p = .041) in the own-race condition was significantly above chance (Figure 3, center panel). It was also above chance in the other-race condition on test (M = 59.99%, SD = 40.16%, t[15] = 3.49, p = .003) and generalization trials (M = 50.57%, SD = 42.86%, t[15] = 2.40, p = .030).
The same GLMM that was performed for Experiment 1 revealed no significant effects of race in the proportion of infant following to the cued boxes (z = 0.00, p = 1.000, see Figure 4, center panel). Neither the effect of trial type (z = 0.37, p = .710) nor the interaction between the two variables (z = 0.00, p = 1.000) was significant. These results indicate that infants followed the gaze of own- and other-race adults equally when they were 100% reliable.
Next, we used the same GLMM that was performed for Experiment 1 to examine whether the initial fixation of the infants to the cued box was affected by face race. Neither the effect of face race (z = 0.86, p = .392, see Figure 5, center panel) nor the effect of trial type (z = 0.97, p = .330) was significant. These results indicate that the initial fixation to the cued box was not different between the own- and other-race conditions.
Experiment 3
Method
Participants
We recruited 32 full-term Chinese infants and randomly assigned them to either the own- (n = 16, 11 females and 5 males, M = 213.75 days, SD = 29.81 days, Range = 181 to 248 days) or other-race conditions (n = 16, 9 females and 7 males, M = 199.56 days, SD = 25.42 days, Range = 181 to 256 days). We recruited 13 additional infants, who were excluded from the data analyses because of failure to complete the procedure due to fussiness (n = 10) or program error (n = 3). Based on report from each infant’s primary caregiver, none of the participants had any direct contact with other-race individuals. All of the participants were from middle class Mandarin speaking families. Seventeen of the 32 families had at least one parent with a post-secondary degree of bachelor’s or above; for the other 15, the highest education level was a high school diploma. Based on the most recent physical check-up records, all participants were full-term healthy infants.
Materials and procedure
Stimuli and procedure were identical to those in Experiment 1 except that the adults in the learning phase showed valid cuing on 25% of the trials.
Results and Discussion
Gaze following on learning trials
For the valid learning trials on which the adult’s gaze correctly predicted the location where an animal appeared, infants mostly looked at the cued box (M = 95.59%, SD = 7.30%). An independent-samples t-test showed that the looking time for the cued box in the own-race condition (M = 97.45%, SD = 6.24%) was not significantly different from that in the other-race condition (M = 93.74%, SD = 8.00%, t[30] = 1.46, p = .155). For the invalid learning trials on which the adult’s gaze incorrectly predicted the location where the animal appeared, we also found no significant race effects in looking time to the cued boxes (Mown = 4.80%, SDown = 4.89%, Mother = 5.74%, SDother = 5.63%, independent sample t-test: t[30] = 0.51, p = .617). Thus, during the learning phase, the looking pattern of the infants was not affected by the race of the informants.
Gaze following on test and generalization trials
An ANOVA similar to that performed on the data from Experiments 1 and 2 revealed no significant effects of race (F[1, 30] = 0.48, p = .492, ηP2 = .02), trial type (F[1, 30] = 2.35, p = .135, ηP2 = .07), or their interaction (F[1, 30] < 0.01, p = .958, ηP2 < .01). One-sample t-tests revealed that infant gaze following to the cued box did not differ from chance on test (M = 40.73%, SD = 41.17%, t[15] = 1.53, p = .147) or generalization trials (M = 22.51%, SD = 35.57%, t[15] = −0.28, p = .784) in the own-race condition (Figure 3, right panel). Non-significant differences from chance were also observed on test (M = 46.35%, SD = 45.33%, t[15] = 1.88, p = .079) and generalization trials (M = 29.37%, SD = 42.08%, t[15] = 0.42, p = .684) in the other-race condition.
The same GLMM that was performed for Experiments 1 and 2 revealed no significant effects of race in the proportion of infants who followed to the cued boxes (z = 0.35, p = .723, see Figure 4, right panel). Neither the effect of trial type (z = 1.07, p = .284) nor the interaction between the two variables (z = 0.24, p = .809) was significant. These findings indicate that infants did not follow the gaze of either the own- or other-race adult when they were 25% reliable.
Next, we used the same GLMM that was performed for Experiments 1 and 2 to examine whether the initial fixation of infants to the cued box was affected by face race. Neither the effect of face race (z = 0.24, p = .809, see Figure 5, right panel) nor the effect of trial type (z = 1.52, p = .129) was significant. These results indicate that the initial fixation to the cued box was not different between the own- and other-race conditions.
Comparisons of gaze following on test and generalization trials across the three experiments
To probe further how reliability of the informants affected gaze following of the infants in the test phase, we performed an omnibus ANOVA to examine the mean gaze following percentages on the test and generalization trials across the three levels of reliability in the own- and other-race conditions. Face race, reliability, and trial type were the independent variables. The mean gaze following percentage was the dependent variable. The results showed a significant interaction between face race and reliability, F(2, 90) = 5.15, p = .008, ηP2 = .10. This interaction indicated that infant responses to adults with various reliabilities were affected by the race of the adults. The ANOVA also showed a significant main effect of reliability, F(2, 90) = 5.55, p = .005, ηP2 = .11. Infant gaze following increased with the reliability.
We did not observe a significant difference in infant gaze following between the test and generalization phases across the three experiments, F(1, 90) = 3.15, p = .079, ηP2 = .03. This result suggests that infant gaze following in the test phase was not purely based on memory related factors. The non-significant result is consistent with the argument that infant gaze following reflected their learning of the reliability of the adult. The ANOVA also did not show any other significant results (ps > .104).
To further probe the significant interaction between face race and reliability, we conducted two separate one-way ANOVAs to compare the mean gaze following percentages on test and generalization trials across the three levels of reliability in the own- and other-race conditions. In the own-race conditions, gaze following percentages of the infants were significantly different in the three reliability conditions, F(2, 45) = 7.69, p = .001, ηP2 = .25. Considering our expectation that 50% reliability would be the critical condition to reveal the differential responses of infants to own- versus other-race adults, we set it as the a priori reference level and contrasted it with the 25% and 100% reliability conditions, respectively. These a priori contrasts revealed that the mean gaze following percentage in the 50% condition (M = 61.19%, SD = 35.95%) was significantly higher than that in the 25% condition (M = 31.62%, SD = 38.96%, p < .001), but was not different from that in the 100% condition (M = 55.53%, SD = 42.25%, p = .194). In other words, the infants responded to the own-race adult informant in the 50% condition differently from the own-race adult informant that was 25% reliable, but not differently from the own-race adult informant that was 100% reliable.
In the other-race conditions, the reliability of the other-race adult also significantly affected their gaze following, F(2, 45) = 3.69, p = .033, ηP2 = .14. A priori contrasts with the 50% condition as the reference revealed that the mean gaze following percentage in the 50% condition (M = 30.10%, SD = 38.92%) did not differ significantly from that in the 25% condition (M = 37.86%, SD = 43.88%, p = .420), but was significantly lower than that in the 100% condition (M = 55.37%, SD = 41.12%, p = .013). Thus, the infants responded to the other-race adult informant in the 50% condition differently from the other-race adult informant that was 100% reliable, but not differently from the other-race adult informant that was 25% reliable.
Together, the results from these cross-experiment comparisons revealed that infants treated the gaze cues of the own- and other-race informants under uncertainty differently. They responded to the 50% reliable own-race informant’s gaze cues as if she was 100% reliable and responded to the 50% reliable other-race informant’s gaze cues as if she was at the chance level of 25% reliability.
General Discussion
The present study examined whether infants in the first year of life differentially use social cues from own- versus other-race individuals to anticipate events. We found that 7-month-old infants (ranging from 5.9 to 8.5 months of age) were biased to use the social cues provided by own- over other-race adults. This bias in gaze following and learning was, however, limited to a situation of uncertainty. Specifically, when an adult informant’s gaze signals were only 50% reliable, infants followed own-race gaze cues more than other-race cues. By contrast, when the adults were 100% reliable, infants followed own- and other-race gaze equally, and when both adults were entirely unreliable (i.e., chance level, 25% reliability), infants did not follow either to predict a future event. Instead, they searched randomly in all possible locations.
Infant Use of Social Cues in a Context of Uncertain Reliability
Why did infants behave differentially in the three experiments? According to Tummeltshammer et al. (2014), infants from an early age are sensitive to the statistical information provided by gaze cues from adult informants and their learning is largely dependent on such information. Thus, infants in the present study used an informant’s gaze cues when the informant was 100% reliable, and did not use them when the informant’s reliability was at chance (i.e., 25%). These results indicate that cue reliability supersedes own-race face bias in either highly reliable or highly unreliable situations. However, when the informant was 50% reliable, although such reliability was above the chance level, infants might find it to be inadequate for determining whether to follow the informant’s gaze, and consequently use additional information about the informant as a basis for following their gaze. In accord with the perceptual-social linkage hypothesis, because face race becomes increasingly salient to infants from 3 to 9 months (e.g., Kelly et al., 2007b; Quinn et al., 2016), they might readily use this information in an uncertain context.
Our findings are consistent with the existing evidence regarding how infants use social cues under uncertainty. For example, Tamis-LeMonda and colleagues (2008) investigated whether use of parental encouragement as a social cue would affect the attempts of 18-month-olds to walk down slopes of varying risk levels: safe, risky, or borderline where the safety was uncertain. Infants made significantly more walking attempts with parental encouragement, but only in the borderline situation where the perceptual cue alone was insufficient to indicate the safety of the slope. By contrast, infants disregarded the parental encouragement when the slope was safe or risky. The findings suggest that infants selectively rely on social cues to guide action when perceptual cues are not adequately informative. Consistent with this suggestion, infants in the present study used face race as a source of information to guide their gaze following when the reliability of the informant was uncertain.
Potential Mechanisms
The current data indicate that face race affects the learning of infants in a situation of uncertainty where they rely on social cues provided by own- rather than other-race adults. This finnding suggests that during uncertain social interactions, infants do not use an online probability matching mechanism to determine whether to rely on an adult informant’s gaze cues. Had the infants used probability matching, no difference should have been observed between the own- and other-race conditions. Furthermore, infants should have followed the gaze of the 100% reliable adult the most, followed by the 50% reliable adult, and the 25% reliable adult the least. However, our cross-experiment analyses failed to support this prediction based on probability matching. We found that in the own-race condition, infants followed the gaze of the 50% reliable own-race adult as if she was 100% reliable, whereas in the other-race condition, they followed the gaze of the 50% reliable other-race adult as if she were only 25% reliable.
Why would infants follow own-race, but not other-race, informants under uncertainty? A Bayesian mechanism (Aslin, 2011; Gopnik & Tenenbaum, 2007; Spiegelhalter & Rice, 2009) may parsimoniously explain the current findings. This account would suggest that infants use their prior statistical knowledge of adult reliability and current statistical knowledge of the informant’s reliability together to determine how to respond under uncertainty. It is well known that infants interact mostly with own-race individuals (Rennels & Davis, 2008; Sugden et al., 2014), such as caregivers, relatives, and neighbors, who usually offer highly reliable information (Gergely, Egyed, & Király, 2007; Goldstein & Schwade, 2008). Based on this finding and as suggested by the perceptual-social linkage hypothesis, infants may generalize and associate own-race individuals with high reliability. In Experiment 1, the own-race adults displayed uncertain reliability during the learning trials. This level of reliability was likely lower than what infants brought to the laboratory about the reliability of own-race adults. A Bayesian mechanism based on prior and current statistical knowledge should therefore bias infants to follow the gaze cues of own-race adults at a level greater than their current reliability. Indeed, consistent with this account, we found that infants followed the gaze of the own-race adult in the 50% reliability condition at a level comparable to the gaze following of the own-race adult in the 100% reliability condition.
In contrast, for the other-race informant, it is well known that infants begin to develop negative emotional affect towards unfamiliar individuals after 5 months, i.e., stranger anxiety (Bigelow et al., 1990; Bronson, 1972; Campos et al., 1975). According to the perceptual-social linkage hypothesis, given that other-race faces are perceptually different from faces of own-race strangers in terms of salient facial information (Anzures, Quinn, Pascalis, Slater, & Lee, 2010), infants may show even stronger “stranger anxiety” towards other-race faces. Although our infants did not have any prior statistical knowledge about the reliability of the other-race adults, heightened negative affect towards such strangers along with the current statistical knowledge about the other-race informant’s 50% reliability might lead to responding to the informant as more unreliable than she actually was. Consistent with this suggestion, we found that infants followed the gaze of the other-race adult in the 50% reliability condition at a level comparable to the gaze following of the other-race adult in the 25% reliability condition.
Advantages and Disadvantages of a Bias in Social Cue Use Under Uncertainty
The early bias in social cue use under uncertainty may be an adaptive strategy. This is because, as mentioned above, infants tend to encounter mostly own-race adults in their environment who typically provide social cues in a highly reliable manner. In an uncertain situations, it is useful for infants to recruit their prior knowledge about the type of adults with whom they are familiar as a disambiguating cue. In this situation, they can use their existing knowledge that own-race individuals are generally reliable and transfer that knowledge to determine whether to learn from a new own-race adult.
Like any strategy of generalization in learning, there are potential pitfalls. For example, race-based generalization may lead infants to rely on own-race adults whose social cues are indeed unreliable and should not be followed (e.g., an adult may be ignorant or have malicious intentions). In the same vein, infants may also miss opportunities for interacting with and learning from other-race individuals whose social cues could be informative or benevolent. Moreover, although race-based generalization may provide a useful shortcut in early social interaction and learning, overuse of such a strategy may lead to the racial biases against other-race individuals seen in the preschool years and beyond (Baron & Banaji, 2006; Qian et al., 2016; Xiao et al., 2015). Interestingly, the current study revealed that infants did not seem to have difficulty learning to use other-race cues when they were highly reliable, suggesting that infants can learn from adults from unfamiliar race categories under optimal conditions.
Perceptual-Social Linkage
The present findings offer support for the perceptual-social linkage hypothesis (Lee et al., in press-a, in press-b). As outlined in the introduction, this hypothesis suggests that greater exposure to own-race faces accompanied with mostly positive experiences with such faces lead infants to favor own-race individuals over other-race individuals. Existing infant evidence supporting the perceptual-social linkage hypothesis has included the finding that infants between 6 and 9 months of age respond to different other-race face categories equivalently, but differently from one’s own-race face category (Quinn et al., 2016), and develop associations between face race and music emotional valence (Xiao et al., in press). The current findings extend these results by showing that infants in the first year of life are biased in social learning. The findings taken together suggest that perceptual-social linkage begins to emerge in infancy. A possibly analogous perceptual-social linkage may exist in the auditory domain where infants exhibit a social preference for individuals who present the auditory properties that dominate in their everyday experiences, such as a native accent, native speech tones, and culturally-specific musical rhythms (Begus, Gliga, & Southgate, 2016; Kinzler & Spelke, 2011; Kinzler et al., 2007; Paquette-Smith & Johnson, 2016; Soley & Sebastián-Gallés, 2015; Soley & Spelke, 2016).
Additional evidence regarding perceptual-social linkage comes from studies with children. For example, research using an implicit racial bias measure has revealed that children’s perceptual categorization of faces by race significantly predicts their implicit racial bias (Dunham, Chen, & Banaji, 2013). This work suggests that children’s tendency to categorize faces by race may trigger the development of intergroup bias, and reducing this tendency may lead to reduction in intergroup bias. Consistent with this suggestion, Xiao et al. (2015) measured Asian preschooler’s implicit racial bias against Africans. The children were then trained to recognize individual other-race African faces or own-race Asian faces. Training significantly reduced implicit racial bias against Africans only for those children who were trained to recognize African faces. This finding suggests that young children’s perceptual recognition of other-race faces may be causally linked to their implicit racial bias against them. Future infant training studies are needed to extend the preschool findings to infancy to establish the causal relations driving the perceptual-social linkage throughout early development, and whether it is possible to forestall the emergence of racial bias in infancy. In addition, longitudinal work with both infants and children could be undertaken to determine whether perceptual-social linkage may arise from a single causal direction or whether the relation may be bi-directional and emerge through multiple developmental pathways.
A Pre-Existing Bias?
It is unclear whether our infants had a pre-existing bias in responding towards the gaze shift of own- versus other-race individuals prior to their participation in the study. This is due to a limitation in the design of the present study: we did not assess whether the infant participants had an overall bias in favor of own-race adults before they started to learn about the reliability of the own- versus other-race informants. Moreover, although infants were not initially biased to follow the gaze of own- over other-race informants during learning trials, the null result may be at least partially attributable to infant attention being captured by the dynamic animal stimulus appearing in the box. A pre-existing bias would be consistent with the perceptual-social linkage hypothesis and with infant use of “priors” in a Bayesian account, but future specifically designed studies are needed to assess it. One potential approach would be to modify the current paradigm such that prior to the commencement of the learning phase, pre-test trials the same as the current generalization trials could be implemented. That is, after the own- versus other-race adults shift their gaze, the animal does not appear. Infant attention immediately following the gaze shift of the adult should reveal whether infants are already biased to follow the gaze of own- relative to other-race adults. Such future studies are important because they will help to determine whether existing extensive experience with own-race faces (“Bayesian priors”) will initially bias infants to rely on the gaze cues of own-race adults more than those of other-race adults before a specific informant’s reliability is known. The initial bias would presumably either be corrected after learning other-race informants to be highly reliably or continue to operate when the informant’s reliability is uncertain.
Conclusions
The present findings from the three experiments together reveal that infants in the first year of life integrate online statistical information with prior knowledge to guide learning from social cues in real time. Infants are biased to follow the social cues of own-race individuals over other-race adults under situations of uncertainty. When own- and other-race individuals are highly reliable, infants follow own- and other-race social cues equally. By contrast, when the social cues of own- and other-race adults are highly unreliable, infants follow neither. Although differential reliance on social cues of own-race versus other-race adults in uncertain contexts may be adaptive for early socialization and learning, it may also be a source for the emergence and development of racial biases in early childhood and beyond.
Acknowledgments
This research was supported by grants from the Natural Science and Engineering Research Council of Canada, the National Institutes of Health (R01 HD046526), the National Science Foundation of China (31070908, 31300860, 31470993, and 31671146), and the Zhejiang Provincial Natural Science Foundation of China (LY16C90006). We thank Lauren Emberson and three anonymous reviewers for their comments, Xiaoli Jiao and Jun Li for their assistance in data collection.
References
- Anzures G, Quinn PC, Pascalis O, Slater AM, Lee K. Categorization, categorical perception, and asymmetry in infants’ representation of face race. Developmental Science. 2010;13:553–564. doi: 10.1111/j.1467-7687.2009.00900.x. doi:10.1111/j.1467–7687.2009.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzures G, Wheeler A, Quinn PC, Pascalis O, Slater AM, Heron-Delaney M, et al. Brief daily exposures to Asian females reverses perceptual narrowing for Asian faces in Caucasian infants. Journal of Experimental Child Psychology. 2012;112:484–495. doi: 10.1016/j.jecp.2012.04.005. doi:10.1016/j.jecp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN. Perceptual organization of visual structure requires a flexible learning mechanism. Infancy. 2011;16:39–44. doi: 10.1111/j.1532-7078.2010.00053.x. doi:10.1111/j.1532-7078.2010.00053.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own-race face processing. Psychological Science. 2006;17:159–163. doi: 10.1111/j.1467-9280.2006.01679.x. doi:10.1111/j.1467–9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Baron AS, Banaji MR. The development of implicit attitudes: Evidence of race evaluations from ages 6 and 10 and adulthood. Psychological Science. 2006;17:53–58. doi: 10.1111/j.1467-9280.2005.01664.x. doi:10.1111/j.1467–9280.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- Begus K, Gliga T, Southgate V. Infants’ preferences for native speakers are associated with an expectation of information. Proceedings of the National Academy of Sciences. 2016;113:12397–12402. doi: 10.1073/pnas.1603261113. doi:10.1073/pnas.1603261113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow A, MacLean J, Wood C, Smith J. Infants’ responses to child and adult strangers: An investigation of height and facial configuration variables. Infant Behavior and Development. 1990;13:21–32. doi:10.1016/0163-6383(90)90003-Q. [Google Scholar]
- Birch SAJ, Akmal N, Frampton KL. Two-year-olds are vigilant of others’ non-verbal cues to credibility. Developmental Science. 2010;13:363–369. doi: 10.1111/j.1467-7687.2009.00906.x. doi:10.1111/j.1467-7687.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Bronson GW. Infants’ reactions to unfamiliar persons and novel objects. Monographs of the Society for Research in Child Development. 1972;37:1–46. doi:10.2307/1165685. [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The importance of eyes: How infants interpret adult looking behavior. Developmental Psychology. 2002;38:958–966. doi: 10.1037//0012-1649.38.6.958. doi:10.1037/0012-1649.38.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttelmann D, Zmyj N, Daum M, Carpenter M. Selective imitation of in-group over out-group members in 14-month-old infants. Child Development. 2013;84:422–428. doi: 10.1111/j.1467-8624.2012.01860.x. doi:10.1111/j.1467-8624.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Emde RN, Gaensbauer T, Henderson C. Cardiac and behavioral interrelationships in the reactions of infants to strangers. Developmental Psychology. 1975;11:589–601. doi:10.1037/0012-1649.11.5.589. [Google Scholar]
- Chow V, Poulin-Dubois D, Lewis J. To see or not to see: Infants prefer to follow the gaze of a reliable looker. Developmental Science. 2008;11:761–770. doi: 10.1111/j.1467-7687.2008.00726.x. doi:10.1111/j.1467-7687.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains SMJ, Muir DW. A demonstration of gaze following in 3- to 6-month-olds. Infant Behavior and Development. 1997;20:569–572. doi:10.1016/S0163-6383(97)90048-5. [Google Scholar]
- D’Entremont B. A perceptual–attentional explanation of gaze following in 3- and 6-month-olds. Developmental Science. 2000;3:302–311. doi:10.1111/1467-7687.00124. [Google Scholar]
- Dunham Y, Chen EE, Banaji MR. Two signatures of implicit intergroup attitudes: Developmental invariance and early enculturation. Psychological Science. 2013;24:860–868. doi: 10.1177/0956797612463081. doi:10.1177/0956797612463081. [DOI] [PubMed] [Google Scholar]
- Dunham Y, Newheiser A, Hoosain L, Merrill A, Olson KR. From a different vantage: Intergroup attitudes among children from low-and intermediate-status racial groups. Social Cognition. 2014;32:1–21. doi:10.1521/soco.2014.32.1.1. [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. doi:10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely G, Egyed K, Király I. On pedagogy. Developmental Science. 2007;10:139–146. doi: 10.1111/j.1467-7687.2007.00576.x. doi:10.1111/j.1467-7687.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Gliga T, Csibra G. One-year-old infants appreciate the referential nature of deictic gestures and words. Psychological Science. 2009;20:347–353. doi: 10.1111/j.1467-9280.2009.02295.x. doi:10.1111/j.1467-9280.2009.02295.x. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. Social feedback to infants’ babbling facilitates rapid phonological learning. Psychological Science. 2008;19:515–523. doi: 10.1111/j.1467-9280.2008.02117.x. doi:10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Gopnik A, Tenenbaum JB. Bayesian networks, Bayesian learning, and cognitive development. Developmental Science. 2007;10:281–287. doi: 10.1111/j.1467-7687.2007.00584.x. doi:10.1111/j.1467-7687.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Heron-Delaney M, Anzures G, Herbert JS, Quinn PC, Slater AM, Tanaka JW, et al. Perceptual training prevents the emergence of the other race effect during infancy. PLoS ONE. 2011;6:e19858. doi: 10.1371/journal.pone.0019858. doi:10.1371/journal.pone.0019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM, Willen JD, Driver J. Adult’s eyes trigger shifts of visual attention in human infants. Psychological Science. 1998;9:131–134. doi:10.1111/1467-9280.00024. [Google Scholar]
- Kelly DJ, Liu S, Ge L, Quinn PC, Slater AM, Lee K, Liu Q, Pascalis O. Cross-race preferences for same-race faces extend beyond the African versus Caucasian contrast in 3-month-old infants. Infancy. 2007a;11:87–95. doi: 10.1080/15250000709336871. doi:10.1080/15250000709336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, Ge L. Development of the other-race effect during infancy: Evidence toward universality? Journal of Experimental Child Psychology. 2009;104:105–114. doi: 10.1016/j.jecp.2009.01.006. doi:10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007b;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. doi:10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, et al. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. doi:10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Johnson SP. Detecting ‘infant-directedness’ in face and voice. Developmental Science. 2014;17:621–627. doi: 10.1111/desc.12146. doi:10.1111/desc.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KD, Spelke ES. Do infants show social preferences for people differing in race? Cognition. 2011;119:1–9. doi: 10.1016/j.cognition.2010.10.019. doi:10.1016/j.cognition.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KD, Dupoux E, Spelke ES. The native language of social cognition. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12577–12580. doi: 10.1073/pnas.0705345104. doi:10.1073/pnas.0705345104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Quinn PC, Heyman GD. Rethinking the emergence and development of implicit racial bias: A perceptual-social linkage hypothesis. In: Turiel E, Budwig N, Zelazo P, editors. New perspectives on human development. Cambridge University Press; Cambridge, UK: in press-a. [Google Scholar]
- Lee K, Quinn PC, Pascalis O. Face race processing and racial bias in early development: A perceptual-social linkage. Current Directions in Psychological Science. doi: 10.1177/0963721417690276. in press-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville PW, Fischer GW, Salovey P. Perceived distributions of the characteristics of ingroup and outgroup members: Empirical evidence and a computer simulation. Journal of Personality and Social Psychology. 1989;57:165–188. doi: 10.1037//0022-3514.57.2.165. doi:10.1037/0022-3514.57.2.165. [DOI] [PubMed] [Google Scholar]
- Liu S, Xiao WS, Xiao NG, Quinn PC, Zhang Y, Chen H, Ge L, Pascalis O, Lee K. Development of visual preference for own- versus other-race faces in infancy. Developmental Psychology. 2015;51:500–511. doi: 10.1037/a0038835. doi:10.1037/a0038835. [DOI] [PubMed] [Google Scholar]
- Malatesta CZ, Haviland JM. Learning display rules: The socialization of emotion expression in infancy. Child Development. 1982;53:991–1003. doi:10.2307/1129139. [PubMed] [Google Scholar]
- Paquette-Smith M, Johnson EK. I don’t like the tone of your voice: Infants use vocal affect to socially evaluate others. Infancy. 2016;21:104–121. doi:10.1111/infa.12098. [Google Scholar]
- Poulin-Dubois D, Chow V. The effect of a looker’s past reliability on infants’ reasoning about beliefs. Developmental Psychology. 2009;45:1576–1582. doi: 10.1037/a0016715. doi:10.1037/a0016715. [DOI] [PubMed] [Google Scholar]
- Poulin-Dubois D, Brooker I, Polonia A. Infants prefer to imitate a reliable person. Infant Behavior and Development. 2011;34:303–309. doi: 10.1016/j.infbeh.2011.01.006. doi:10.1016/j.infbeh.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Qian MK, Heyman GD, Quinn PC, Messi FA, Fu G, Lee K. Implicit racial biases in preschool children and adults from Asia and Africa. Child Development. 2016;87:285–296. doi: 10.1111/cdev.12442. doi:10.1111/cdev.12442. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Lee K, Pascalis O, Tanaka JW. Narrowing in categorical responding to other-race face classes by infants. Developmental Science. 2016;19:362–371. doi: 10.1111/desc.12301. doi:10.1111/desc.12301. [DOI] [PubMed] [Google Scholar]
- Rennels JL, Davis RE. Facial experience during the first year. Infant Behavior and Development. 2008;31:665–678. doi: 10.1016/j.infbeh.2008.04.009. doi:10.1016/j.infbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Csibra G. Gaze following in human infants depends on communicative signals. Current Biology. 2008;18:668–671. doi: 10.1016/j.cub.2008.03.059. doi:10.1016/j.cub.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Soley G, Sebastián-Gallés N. Infants prefer tunes previously introduced by speakers of their native language. Child Development. 2015;86:1685–1692. doi: 10.1111/cdev.12408. doi:10.1111/cdev.12408. [DOI] [PubMed] [Google Scholar]
- Soley G, Spelke ES. Shared cultural knowledge: Effects of music on young children’s social preferences. Cognition. 2016;148:106–116. doi: 10.1016/j.cognition.2015.09.017. doi:10.1016/j.cognition.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter D, Rice K. Bayesian statistics. Scholarpedia. 2009;4:5230. doi:10.4249/scholarpedia.5230. [Google Scholar]
- Stenberg G. Why do infants look at and use positive information from some informants rather than others in ambiguous situations? Infancy. 2012;17:642–671. doi: 10.1111/j.1532-7078.2011.00108.x. doi:10.1111/j.1532-7078.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- Stenberg G. Do 12-month-old infants trust a competent adult? Infancy. 2013;18:873–904. doi:10.1111/infa.12011. [Google Scholar]
- Sugden NA, Mohamed-Ali MI, Moulson MC. I spy with my little eye: Typical, daily exposure to faces documented from a first-person infant perspective. Developmental Psychobiology. 2014;56:249–261. doi: 10.1002/dev.21183. doi:10.1002/dev.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Adolph KE, Lobo SA, Karasik LB, Ishak S, Dimitropoulou KA. When infants take mothers’ advice: 18-month-olds integrate perceptual and social information to guide motor action. Developmental Psychology. 2008;44:734–746. doi: 10.1037/0012-1649.44.3.734. doi:10.1037/0012-1649.44.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor LJ, Austin CM, Desjardins RN. Is infant-directed speech prosody a result of the vocal expression of emotion? Psychological Science. 2000;11:188–195. doi: 10.1111/1467-9280.00240. doi:10.1111/1467-9280.00240. [DOI] [PubMed] [Google Scholar]
- Tummeltshammer KS, Wu R, Sobel DM, Kirkham NZ. Infants track the reliability of potential informants. Psychological Science. 2014;25:1730–1738. doi: 10.1177/0956797614540178. doi:10.1177/0956797614540178. [DOI] [PubMed] [Google Scholar]
- Wu R, Kirkham NZ. No two cues are alike: Depth of learning during infancy is dependent on what orients attention. Journal of Experimental Child Psychology. 2010;107:118–136. doi: 10.1016/j.jecp.2010.04.014. doi:10.1016/j.jecp.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Wu R, Gopnik A, Richardson DC, Kirkham NZ. Infants learn about objects from statistics and people. Developmental Psychology. 2011;47:1220–1229. doi: 10.1037/a0024023. doi:10.1037/a0024023. [DOI] [PubMed] [Google Scholar]
- Wu R, Tummeltshammer KS, Gliga T, Kirkham NZ. Ostensive signals support learning from novel attention cues during infancy. Frontiers in Psychology. 2014;5:251. doi: 10.3389/fpsyg.2014.00251. doi:10.3389/fpsyg.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao NG, Quinn PC, Liu S, Ge L, Pascalis O, Lee K. Older but not younger infants associate own-race faces with happy music and other-race faces with sad music. Developmental Science. doi: 10.1111/desc.12537. in press. doi:10.1111/desc.12537. [DOI] [PubMed] [Google Scholar]
- Xiao WS, Fu G, Quinn PC, Qin J, Tanaka JW, Pascalis O, Lee K. Individuation training with other-race faces reduces preschoolers’ implicit racial bias: A link between perceptual and social representation of faces in children. Developmental Science. 2015;18:655–663. doi: 10.1111/desc.12241. doi:10.1111/desc.12241. [DOI] [PubMed] [Google Scholar]