Abstract
Background and aims
Late-night-dinner eating is associated with increased risk for type-2-diabetes. The underlying mechanism is unclear. One explanatory hypothesis is that the concurrence of elevated circulating melatonin and high glucose concentrations (characterizing late-eating) leads to impaired glucose-tolerance. However, to date no study has tested the influence of physiological melatonin concentrations on glucose-tolerance. The discovery of melatonin receptor MTNR1B as a diabetes risk gene provides evidence for a role of physiological levels of melatonin in glucose control. The aim of our study was to test the hypothesis that endogenous melatonin worsens glucose control when eating-late. Registered under ClinicalTrials.gov Identifier no. NCT03003936
Methods
We performed a randomized, cross-over trial to compare glucose-tolerance in the presence (late-dinner) or absence (early-dinner) of physiological melatonin and we compared the results between homozygous carriers and non-carriers of the MTNR1B risk allele.
Results
The concurrence of meal timing with elevated endogenous melatonin concentrations resulted in impaired glucose-tolerance. This effect was stronger in MTNR1B risk-carriers than in non-carriers. Furthermore, eating-late significantly impaired glucose tolerance only in risk-carriers and not in the non-risk carriers.
Conclusions
The interaction of dinner timing with MTNR1B supports a causal role of endogenous melatonin in the impairment of glucose-tolerance. These results suggest that moving the dinner to an earlier time may result in better glucose-tolerance specially in MTNR1B carriers.
Keywords: Glucose tolerance, MTNR1B, meal timing, diabetes, melatonin
Introduction
Nocturnal eating has recently become more common due to the modern 24/7 economy and lifestyle (1). A large-scale study in over 60 thousand people has demonstrated that late-night-dinner eating is robustly associated with hyperglycemia independent of relevant confounders including BMI (2). However, the cause of the decreased glucose tolerance with night eating is still unknown. Although many factors may be involved (3, 4) previous studies support the hypothesis that food intake coincident with high melatonin levels may lead to impaired glucose tolerance. Indeed, we and others have shown that exogenous melatonin administration acutely decreased glucose tolerance in both older (5) and younger women (6). Melatonin is a hormone known for its central role in the circadian system as the signal of the biological night; and for its sleep-promoting effects, with therapeutic potential for insomnia and jet lag (7). Blood melatonin levels are high at night and near-undetectable during the day, and it may contribute to the nocturnal decrease in glucose tolerance (8). Nevertheless, to date no study has tested the influence of physiological melatonin concentrations on glucose tolerance.
Interestingly, the gene which encodes the melatonin receptor 1B (MTNR1B) has been identified as a Type 2 Diabetes (T2D) risk gene (9, 10). MTNR1B has a common SNP at rs10830963 (MAF: 30%) that has been associated with one of the strongest effects on oral disposition index (the product of both insulin secretion and insulin sensitivity) out of over 90 common variants identified for T2D to date (11–13). While this finding generated much excitement regarding the link between sleep, circadian rhythms and glucose metabolism, and the mechanism underlying the association is beginning to be defined, the path to clinical translation is unclear (13–15). Nevertheless, the functional effect of this MTNR1B variant on metabolic physiology is not very well characterized, since dynamic measures of glucose control have so far been performed in the daytime, with very low levels of melatonin and therefore without the ligand of the receptor required to induce its effect.
In order to increase the understanding of this genetic association with T2D, we previously completed a placebo-controlled study to investigate whether MTNR1B rs10830963 influences the effect of exogenous melatonin (5mg) administration on glucose tolerance. Our results showed that in carriers of the MTNR1B risk variant, melatonin administration (5 mg) significantly impaired glucose tolerance, with no effect in non-carriers (16). These results have been recently replicated, and are consistent with our findings even after chronic melatonin administration (13). However, such a melatonin dose results in super-physiological melatonin concentrations. Indeed, so far, no studies have examined the acute effect of the concurrence of physiological melatonin concentrations and food intake on glucose tolerance.
The aim of the current study was to test the hypothesis that the concurrence of meal timing with elevated endogenous melatonin concentrations results in impaired glucose control and that this effect is stronger in homozygous MTNR1B risk carriers than in non-carriers. To do so we tested glucose tolerance using identical mixed meals under two dinner conditions: a) delayed dinner or Late Eating (LE): starting 1 hour before their usual bed time, b) advanced dinner or Early Eating (EE): starting 4 hours before habitual bed time (Figure 1). The study was performed in 40 overweight or obese women including 20 homozygous risk allele carriers for MTNR1B rs10830963 (GG) and 20 matched homozygous non-carriers (CC), using a randomized, within-participant, cross-over design.
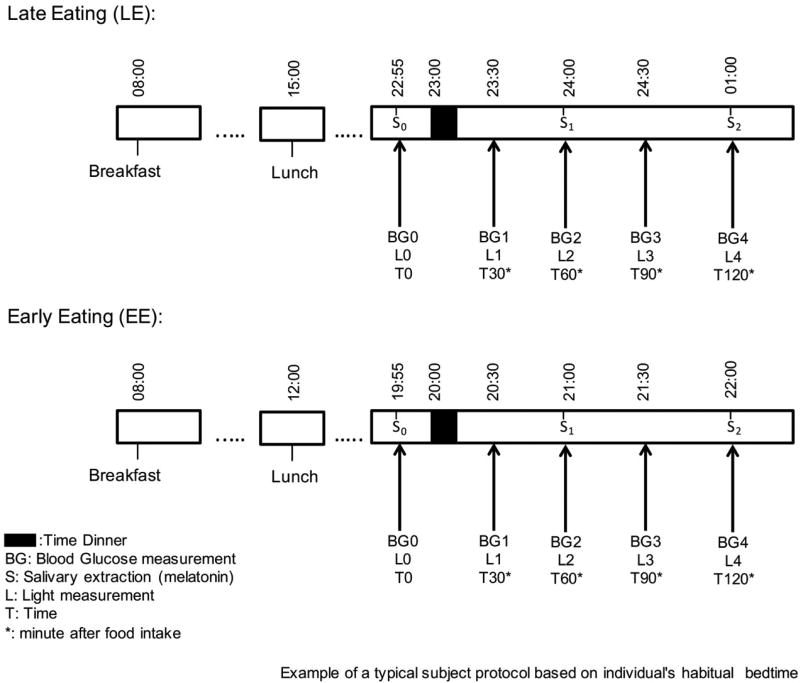
Figure 1. Experimental protocol.
Example of a typical protocol based on each individual´s habitual bedtime for EE and LE conditions is represented in this figure. In both conditions the subject ate the same food for breakfast, lunch and dinner, only the time of the food intake was modified between the two conditions. Just before the dinner, measurements of glucose, light intensity and saliva were collected. Light intensity and glucose were also measurements at 30, 60, 90 and 120 minutes after the start time for dinner, and saliva samples were also collected at 60 and 120 minutes after dinner. From at least 30 minutes before until 2 hours after the start of the test dinner, subjects were under moderately bright light conditions for the early eating condition, under low light conditions for the late eating condition.
Methods
Subjects
We recruited 40 overweight/obese women (BMI>25 kg/m2) of European ancestry who were habitual late eaters (those who self-reported dinner within 2.5 h of their habitual bedtime), including 20 homozygous GG risk allele carriers and 20 homozygous CC carriers for MTNR1B rs10830963 (Table 1). The 20 GG carriers were selected from a previously established database (n=94), then the CC carriers (n=20) were selected as controls to match for age, obesity and metabolic syndrome parameters (Table 1). As the previously selected database was mainly composed of females (»80%), we limited our investigation to female only. The exclusion criteria were: following a special diet, currently on weight loss medication, using sleeping medications or melatonin, or diagnosed with diabetes mellitus, chronic renal failure, hepatic diseases, or cancer (17). A written informed consent was obtained in accordance with the Helsinki Declaration of Human Studies and approved by the Ethical Committee of the University of Murcia (UMU, Murcia, Spain).
Table 1.
General characteristics of the study participants.
| Total Population (GG+CC) (n=40) |
Risk Allele (GG) (n=20) |
Non-Risk Allele (CC) (n=20) |
p values |
|
|---|---|---|---|---|
| Age (y) | 42±10 | 42±9 | 42±11 | 0.999 |
| Weight (kg) | 75.56±11.30 | 75.26±9.74 | 75.86±12.93 | 0.870 |
| BMI (kg/m) | 28.42±4.04 | 29.34±3.71 | 27.50±4.23 | 0.151 |
| Body fat (%) | 33.87±5.93 | 34.72±5.33 | 33.02±6.50 | 0.373 |
| Waist (cm) | 97.21±9.75 | 98.10±7.18 | 96.32±11.91 | 0.571 |
| Hip (cm) | 110.36±8.19 | 110.80±7.65 | 109.92±8.87 | 0.740 |
| WHR | 0.86±0.07 | 0.86±0.08 | 0.86±0.06 | 0.984 |
| Triglycerides (mmo l−1) | 1.08±0.47 | 1.11±0.48 | 1.04±0.46 | 0.637 |
| Cholesterol (mmo l−1) | 5.03±1.10 | 5.32±1.37 | 4.73±0.63 | 0.122 |
| HDL Cholesterol (mmo l−1) | 1.48±0.30 | 1.54±0.30 | 1.43±0.29 | 0.286 |
| Insulin (µUI/ml) | 17.21±22.77 | 14.96±23.92 | 19.61±22.00 | 0.564 |
| Glucose (mmo l−1) | 4.84±0.73 | 4.77±0.71 | 4.91±0.78 | 0.597 |
| HOMA | 1.59±1.16 | 1.05±0.65 | 2.17±2.97 | 0.161 |
| Systolic Blood pressure (mm Hg) | 113.90±19.74 | 111.80±25.73 | 116.00±11.37 | 0.510 |
| Diastolic Blood pressure (mm Hg) | 74.80±14.21 | 70.90±16.11 | 78.70±11.09 | 0.084 |
| MetS Score | 1.35±0.95 | 1.54±0.66 | 1.71±0.91 | 0.574 |
| MEQ Score | 54.40±9.65 | 56.90±9.04 | 51.90±9.81 | 0.102 |
Data are represented as means ± SD. Abbreviations: BMI. Body Mass Index; WHR. Waist hip ratio; HOMA. Homoeostasis model assessment; MetS. Metabolic Syndrome, MEQ: Morning-Evening Questionnaire. P values indicate comparison between carriers and non-carriers as tested by two-sample Student’s t-test.
Procedures
First, the subjects were contacted via a phone call in which they were informed about the study, and then a personal appointment was arranged in a nutrition clinic. During the personal meeting in the nutrition clinic, a more detailed explanation of the experimental study was given and written informed consent was obtained, after which the intervention dates were established and blood extraction, anthropometric evaluation and blood pressure determinations were performed. On the two days of the intervention, a study investigator performed the 2-h glucose tolerance tests the saliva sampling, and light readings at the houses of the study participants.
Experimental Design
A randomized, cross-over study design was used. Participants were tested under two dinner conditions: a) Delayed dinner or Late Eating (LE): 1 hour before their usual bed time, with dinner starting at approximately 23 h; b) Advanced dinner or Early Eating (EE): 4 hours before habitual bed time, with dinner starting at approximately 20 h (Figure 1). Randomization was performed by the UMU staff with a block size of 2 using a balanced design using a computer-executed software (http://www.randomization.com). Blood glucose levels were determined by glucometer (A. Menarini Diagnostics S.r.l., Firenze, Italy) immediately prior to dinner and at 30, 60, 90, and 120 minutes after the start of the meal. We also collected saliva samples every hour (immediately prior to dinner, and at 60 and 120 minutes after the start of the meal) for the assessment of salivary melatonin concentrations. Light exposure was also measured using a HOBO Pendant Temperature/Light Data Logger UA-002–64 (Onset Computer, Bourne, MA, USA) on a neck chain as close as possible to the eyes as previously described by Martinez-Nicolas A et al.(17). Participants were studied in their natural environment (their homes) in the two dinner conditions with a 6-day washout period between visits, i.e., thus the same day of the week on two consecutive weeks. As expected, on the two days of the experiment (Table 2), melatonin levels were higher and light intensity was lower in the LE condition than in the EE. Moreover, during the week prior to each experiment no significant differences in the food intake and sleep schedules were found between the early and late eating condition. Similarly, on the day of the experiment, sleep and food intake timing was the same in the two conditions (EE and LE), except for the start of lunch and dinner that was fasting (8h) before dinner in both conditions.
Table 2.
Comparison of melatonin, light, and sleep and food intake timing the day of the experiment between EE and LE eating conditions within each genotype group.
| Risk Allele (GG) (n=20) |
Non-Risk Allele (CC) (n=20) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| EE | LE | P value | EE | LE | P value | |
| Melatonin (pg/mL)* | ||||||
| T0’ | 5.67±1.72 | 15.45±9.68 | <0.0001 | 5.19±2.61 | 12.87±5.97 | <0.0001 |
| T60’ | 6.21±1.81 | 14.13±5.56 | <0.0001 | 5.21±3.84 | 14.37±4.61 | <0.0001 |
| T120’ | 8.03±4.63 | 16.83±7.30 | <0.0001 | 6.13±5.69 | 15.99±5.61 | <0.0001 |
| Light (luxes)* | ||||||
| T0’ | 282±248 | 5±5 | <0.0001 | 317±262 | 5±5 | <0.0001 |
| T60’ | 290±253 | 5±5 | <0.0001 | 312±222 | 4±2 | <0.0001 |
| T120’ | 296±252 | 6±7 | <0.0001 | 318±262 | 4±2 | <0.0001 |
| Timing of sleep | ||||||
| Sleep onset (hh:mm)° | 23:56±0:52 | 24:26±0:59 | 0.057 | 23:50±0:52 | 23:50±0:34 | 0.966 |
| Sleep offset (hh:mm)° | 7:29±0:12 | 7:34±0:13 | 0.524 | 7:36±0:14 | 7:41±0:14 | 0.719 |
| Sleep duration (hh:mm)° | 7:33±0:11 | 7:09±0:14 | 0.154 | 7:39±0:11 | 7:45±0:12 | 0.675 |
| Timing of food intake | ||||||
| Start lunch (hh:mm) | 12:08±0:32 | 15:03±0:28 | <0.0001 | 11:46±0:30 | 14:48±0:27 | <0.0001 |
| Start dinner (hh:mm) | 20:04±0:26 | 23:02±0:27 | <0.0001 | 19:51±0:28 | 22:48±0:27 | <0.0001 |
Before (T0’) and after (T60’ and T120’) dinner;
Night before the experiment, morning of the experiment and the duration; EE (Early eating condition), LE (Late eating condition). Habitual P values indicate comparison between EE and LE as tested by paired Student’s t-test for each genotype group.
General measurements
Obesity and metabolic syndrome parameters (MetS)
Using the protocol described earlier by our group (18), body weight was determined in barefooted participants wearing light clothes using a digital scale (Tanita Corporation of America, Arlington Heights, IL) accurate to the nearest 0.1 Kg. Height was measured using a stadiometer (rank, 0.14–2.10). The participants were positioned upright, relaxed and with the head in the Frankfort plane. These data were used to calculate the body mass index according to the formula: weight (kg)/height2 (m2). Blood pressure was measured with participants seated with their arm resting on a table.
Total body fat was determined by bioelectrical impedance, using a TANITA TBF 300 (Tanita Corporation of America, Arlington Heights, IL). Body fat distribution was determined by the measurement of waist circumference at the umbilicus level and hip circumference (19) in order to calculate the waist-hip ratio (WHR).
Triglycerides (TG), cholesterol and glucose levels were assessed by automated chemical analysis (IL ILAB 600 Chemistry Analyzer of Instrumentation Laboratory). HDL-C was measured after precipitation of apoB-containing lipoproteins with dextran sulfate and magnesium (27). The homeostasis model assessment (HOMA) index of insulin resistance was calculated with the accepted formula: HOMA= fasting glucose (mmol/l) × fasting insulin (mIU/l)/22.5.
To determine the presence or absence of MetS, we followed the definition proposed by the International Diabetes Federation (20, 21). A MetS score was calculated for each participant by summating one unit for each of the MetS components (waist circumference, fasting glucose, triglycerides, HDL-c, and systolic or diastolic blood pressure) with a maximum value of 5 points, described by Corbalan-Tutau, Dolores et al. (22).
2h glucose profiles were obtained following identical mixed meals. Glucose was determined, in both days, right before dinner (time 0’) and every 30 minutes after the start of the meal, for 2 hours (time 30’, 60’, 90 and 120’) (Figure 2). Area under the curve with respect to ground (AUC) for glucose, for the first 120 min after dinner was calculated with the trapezoid method (23). Whole-blood glucose concentration measurements were determined with a GlucoMen®LX Plus+ (Menarini Diagnósticos S.A, Barcelona, Spain), which uses reactive strips to measure glucose.
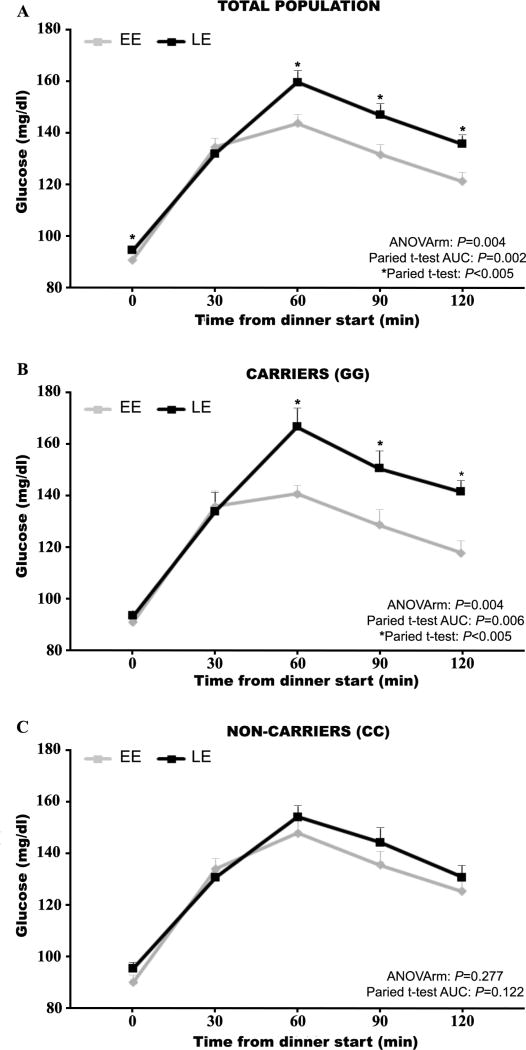
Figure 2. Glucose tolerance curve for total population and risk carriers and non-carriers of the MTNR1B gene.
Each curve represents the glucose concentrations just before dinner (T0) and 30, 60, 90 and 120 minutes after dinner. The black line is for the late diner condition (LE) and the gray line is for the early dinner condition (EE). Glucose profiles for the total population are represented in the panel 1A, while glucose profiles for the two MTNR1B genotypes, risk carriers (GG) and non-carriers (CC), are represented in the panels 2B and 2C, respectively.
Salivary melatonin
As previously described by Corbalan-Tutau, Dolores et al. (22), saliva was collected during both experimental days using the Salivette system (Sarstedt, Barcelona, Spain) immediately prior to dinner (time 0) and every hour after the start of dinner, for 2 hours (time 60 and 120 minutes) (Figure 2). Saliva samples were centrifuged and then frozen and stored at −80°C until analysis. Salivary melatonin concentrations were measured by radioimmunoassay (RIA) (IBL, Germany). The intra-and inter-assay precision was 6.7% and 10.4% respectively.
Light recording
The light exposure was measured every 30 s starting half an hour before dinner and until 2 hours after the start of the dinner with a HOBO Pendant Temperature/Light Data Logger UA-002–64 (Onset Computer, Bourne, MA, USA) on a neck chain as previously described by Martinez-Nicolas A et al.(17). Specific light measurements were also recorded every 30 minutes together with the glucose measurement. In the late dinner condition, participants were instructed avoid exposure to bright light (light intensities above 25 lux), while in the EE condition participants were exposed to their habitual light at home (300–500 lux) (Figure 2).
DNA purification and MNTR1B genotyping
Applying the same approach described above by Bandín C et al. (24), DNA was isolated from blood samples using standard procedures (Qiagen, Valencia, CA, USA). Genotyping of the MTNRIB SNP was performed using a TaqMan assay with allele-specific probes on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to standardized laboratory protocols (25).
Dietary conditions
During the week prior to each experiment a “Seven-day dietary record” was completed reporting the amount and type of food that they ate and the onset time of every meal and snack (Table 3).
During the both experimental days (early dinner and late dinner) a high-glycemic index dinner was provided to each participant by the Department of Physiology (UMU). This dinner consisted of ravioli with tomato sauce and 1 piece of peach (Hero, S.A. Murcia, Spain) with a nutritional composition of 58–60% carbohydrate, 15–17% protein, and 25–27% fat. The energy content of this test meal represented 30 to 35 % of total energy intake of the day for each participant. In early and late dinner conditions, we gave the subjects a fixed menu to follow so they ate the same food for breakfast, lunch and dinner in both experimental days. The meal prior to dinner (lunch; which acted as a pre-meal) consisted of a high protein/low carbohydrate meal with a total energy content of 650 Kcal and started 8 hours before the dinner, both for the early and late dinner conditions, to ensure the same fasting duration before the start of the test dinner. Furthermore, breakfast was consumed always at the same time in both conditions.
Table 3.
Comparison of sleep, food intake timing, dietary intake and energy distribution the weeks before the day of the experiment (habitual characteristics) between EE and LE eating conditions within each genotype group.
| Risk Allele (GG) (n=20) |
Non-Risk Allele (CC) (n=20) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| EE | LE | P value | EE | LE | P value | |
| Sleep characteristics | ||||||
| Sleep onset (hh:mm) | 24:30±0:46 | 24:29±0:38 | 0.897 | 24:10±0:48 | 24:04±0:48 | 0.278 |
| Sleep offset (hh:mm) | 7:58±1:01 | 7:47±1:00 | 0.302 | 7:34±0:40 | 7:43±0:37 | 0.227 |
| Sleep duration (hh:mm) | 7:25±0:41 | 7:17±0:50 | 0.415 | 7:24±0:35 | 7:39±0:35 | 0.115 |
| Timing of food intake | ||||||
| Start breakfast (hh:mm) | 8:43±0:53 | 8:48±0:47 | 0.330 | 8:41±0:56 | 8:42±0:54 | 0.783 |
| Start lunch (hh:mm) | 14:40±0:20 | 14:46±0:24 | 0.346 | 14:31±0:51 | 14:45±0:23 | 0.284 |
| Start dinner (hh:mm) | 21:31±0:33 | 21:35±0:33 | 0.566 | 21:33±0:24 | 21:27±0:26 | 0.169 |
| Dietary Intake | ||||||
| Total Energy Intake (Kcal/day) | 1391±606 | 1332±260 | 0.703 | 1337±234 | 1466±74 | 0.090 |
| Distribution of energy (day)† | ||||||
| Breakfast (%) | 19±7 | 19±5 | 0.870 | 17±7 | 17±6 | 0.987 |
| Lunch (%) | 39±10 | 37±7 | 0.561 | 42±11 | 41±11 | 0.753 |
| Dinner (%) | 26±9 | 31±9 | 0.218 | 29±11 | 29±9 | 0.597 |
Distribution of the energy with respect of the total energy intake; the difference to 100% corresponds to inter-meal snacks consumption; EE (Early eating condition), LE (Late eating condition). Habitual P values indicate comparison between EE and LE as tested by paired Student’s t-test for each genotype group.
Questionnaires
Dietary intake and sleep recording
Habitual energy and nutrient intake was determined by a Seven-day dietary record where the participants recorded their intake (food amount and type) and the onset time of every meal and snack during the week before the day of the EE condition, and during the week before the LE condition (Table 3). The participants were educated in the proper collection of these data, and were asked to write in detail every foodstuff that they ate or drank, the grams of each nutrient that they consumed, the time when they started and finished the food, and the duration of consumption and the place where they consumed the items.
They were also instructed to record their sleep characteristics in a diary designed by the Murcia University Chronobiology Laboratory (26) which included information about the time they went to bed, wake-up time and the duration of sleep (Table 3). These questions were applicable for night-time sleep as well as for sleep during the afternoon or siesta.
Morningness/eveningness questionnaire (MEQ)
Participants also completed the MEQ, a 19-item scale developed by Horne and Ostberg (1976). Participants with scores below 41 were defined as evening types and with scores above 59 as morning types. Participants within the range of 42–58 were classified as neither type (27).
Statistical Methods
Student’s t-test was used to analyze possible differences in the general characteristics and biochemical data of the population between GG and CC carriers (Table 1). Moreover, a paired t-test was performed to study statistical differences between the two LE and EE conditions within each group of women (GG or CC carriers) for a) glucose tolerance (AUC with respect to ground), b) melatonin, c) light conditions, d) habitual sleep characteristics and e) habitual timing of food intake. Moreover, an ANOVA of repeated measurements (ANOVArm) was used to test for differences in glucose across the different times (T0, T30, T60, T90, T120) between EE and LE and also to test whether there was an interaction between meal timing (EE vs. LE) and genotype (GG vs. CC) for AUC and glucose values across the different times (T0, T30, T60, T90, T120) (JMP Pro 12, SAS Institute). Data was adjusted to menstrual cycle phase by adding the menstrual phase as a covariate and also to MEQ score for meal timing *genotype for AUC.
Results
Figure 2 shows glucose profiles following identical mixed meals for the total population and for the MTNR1B risk carriers (GG) and non-carriers (CC) separately. As hypothesized, in the total population, LE significantly impaired glucose tolerance relative to EE (mean±SD; AUC=284.74±32.67 mmol/L×h and AUC=269.61±34.8 mmol/L×h, respectively, P=0.004). Furthermore, repeated measures ANOVA also showed a significant difference between both dinner conditions in glucose concentration (P=0.004).
More importantly, when the population was divided according to the MTNR1B genotype (GG and CC) (Figure 2B and 2C), our results show that among the risk carriers GG, late dinner timing impaired glucose tolerance compared to early dinner timing as assessed by glucose AUC (LE: AUC=292.2±33.8 mmol/L×h and EE: AUC= 270.9±30.4 mmol/L×h, P=0.006). Glucose values showed similar results as assessed by repeated measures ANOVA (P=0.004). Nevertheless, the dinner timing condition (early or late) did not differentially affect postprandial glucose tolerance as assessed by AUC (LE: 277.3±30.5 mmol/L × h and EE: 268.2±38.2 mmol/L×h, P=0.122) or repeated measures ANOVA (P=0.277) among non-risk carriers (CC).
Further analyses demonstrated a significant interaction between meal timing (EE vs. LE) and genotype (GG vs. CC) for AUC (P=0.045) and for the repeated measures for glucose (P=0.012).
Discussion
The current results support our hypothesis that late evening high melatonin levels in conjunction with food intake cause impairment of glucose tolerance. The fact that this effect was magnified in homozygous risk carriers of MTNR1B rs10830963 SNP suggests causality of melatonin in the impairment of glucose tolerance. A potential explanation of these results is that under normal conditions, melatonin released by the pineal gland at night binds to MTNR1B receptor (also named MT2) in pancreatic-islet beta cells. As a consequence it reduces ATP conversion to cAMP; it also reduces the activation of the stimulatory protein kinase A and as a result melatonin inhibits glucose-stimulated insulin secretion. In carriers of the MTNR1B risk variant (G), MT2 receptors are up regulated. For that reason, G carriers are likely to have a greater inhibition of cAMP and an even larger inhibition of glucose-stimulated insulin secretion in pancreatic-islet beta cells than non-carriers. This reduction in insulin release may further be exacerbated by increases in circulating melatonin, particularly in G carriers in whom greater numbers of MT2 receptors are expressed than in non-carriers. Other studies have shown that MTNR1B risk SNP, melatonin and their interaction on insulin sensitivity may also explain the decrease in glucose tolerance (11, 16). These mechanisms may explain why MTNR1B genetic variant G is associated with impaired glucose tolerance and a higher risk of type 2 diabetes (28).
Results of the current study have clinical and public health relevance given that: a) the risk G allele is highly prevalent, with ∼51% of individuals of European ancestry being carriers of the G allele (MAF ∼30%); b) risk carriers have enhanced expression of the melatonin receptor 1b in pancreatic islets (9, 13) implicating MTNR1B gain-of-function in diabetes risk (15); and c) the MTNR1B risk allele has one of the strongest adverse effects on insulin secretion, insulin sensitivity and glucose tolerance of all known T2D associations reported so far (11, 12). For these reasons, MTNR1B signaling has already been proposed as a therapeutic target in the prevention of T2D (29).
Previous placebo-controlled human clinical studies have demonstrated that exogenous (pharmacological) melatonin administration worsens morning and evening glucose tolerance, both in older (5) and younger women (6). Furthermore, we found that the MTNR1B rs10830963 risk variant dramatically worsens the effect of exogenous melatonin on glucose tolerance (16). These results as a whole suggest vulnerability in carriers of the risk allele at MTNR1B rs10830963 who consume food when melatonin levels are elevated, such as risk allele carriers who are also late eaters or shift workers. However, up to now no investigations of the impact of MTNR1B variants on glucose metabolism has been performed in the context of elevated endogenous (physiological) melatonin concentrations with concurrent food intake (as seen in late night eaters). The impact of endogenous melatonin on glucose control is an important and broadly relevant question. While an estimated 5–12 million people in the USA use exogenous melatonin to treat sleeping problem (30), an even larger proportion of the population typically eats late at night when endogenous melatonin levels are high, including not only in the ∼10–20 million night shift workers (31), but also the general population in which late night eating appears to be even more common than previously recognized (32).
While epidemiological studies have shown that late dinner timing associates with worse glucose control, such study designs cannot determine causality. In the current experimental study, using a within-subject design, we demonstrate that moving the dinner to a later time impairs glucose tolerance. More importantly, we show that differences in glucose tolerance between early (low melatonin) and late (high melatonin) dinner were confined to the risk carriers for MTNR1B. Indeed, among non-risk carriers the dinner timing condition did not significantly influence glucose tolerance. Supported by the reported increase in expression of melatonin receptor 1b in G carriers (9), we have provided the first evidence that melatonin is implicated in the glucose impairments that accompany late night eating.
There may be other factors that contribute to differences in glucose tolerance between late and early dinner timing besides differences in the presence or absence of melatonin, such as differences in insulin sensitivity, and gastric, intestinal and pancreatic function, which show daily changes (3, 4). Regardless, the fact that late dinner timing only impaired glucose tolerance in risk carriers of MTNR1B and not in non-risk carriers provides evidence for a important role of melatonin in this outcome.
Our novel findings lead us to recommend consuming dinner no later than 2–4 hours prior to the habitual sleep timing. This will allow recovery of postprandial glycaemia to fasting values prior to the rise of endogenous melatonin levels. The elevation of glucose levels after a meal can take 1–2 hours in normoglycemic individuals and longer in pre-diabetic and diabetic individuals, while the melatonin onset typically starts on average approximately 2 h before habitual bedtime. This recommendation will limit the concurrence of elevated glucose and melatonin concentrations.
These advice may be particularly beneficial for many populations such as: a) Western societies having the largest energy load in the evening, such as Canadians (33) or Americans (34) who consume ∼40% of total energy intake at dinner (35); b) for some countries including some Mediterranean countries in which dinner is taken at late hours (within 1 hour before going to bed)(36); c) those suffering from Night-Eating-Syndrome which affects at 0.5 % to 5.7 % of the general population (1); and d) for shift workers who account for the 15–20% of the working population and need to eat frequently at an atypical time, i.e., late in the evening and or at night (37). Within these populations, these recommendations should be mostly directed to G carriers which account to the ∼51% of the population with a European ancestry.
The strength of the study includes the randomized, cross-over design performed in a population of natural late eaters, the selection of matched homozygous risk carriers and non-carriers, the identical fasting duration and pre-meal conditions preceding the differentially timed dinner test meal. Our primary aim was to answer a practical question which refers to “at what time can we best eat dinner according to our genetics” and specifically to determine the effect of dinner timing and its interaction with melatonin on glucose tolerance. This is first experimental investigation of these important questions using a randomized cross-over design. Due to various limitations in conducting at-home glucose tolerance tests, for the purpose of mimicking real life conditions at homes, placing an intravenous line for venous blood draw was not feasible in that setting. Instead, we opted for capillary glucose assessments. Nevertheless, previous studies conclude that there exists a strong correlation between capillary and intravenous blood glucose samples ranging from r=0.933 to 0.973 (P<0.01). Moreover, no significant time lag during glucose excursions was observed among the ISF, and capillary and venous glucose levels (38, 39). In addition, if capillary glucose measures are less robust than venous blood glucose measures and do indeed introduce greater variance in our data, this increased noise would have biased our finding towards the null hypothesis. The fact we observed highly statistical significant differences suggest that was a robust finding.
This present manuscript is a preliminary investigation in unraveling nighttime glucose intolerance and further studies are necessary to elucidate the underlying mechanism related to this meal timing question and the separate effects on insulin release, insulin sensitivity, glucagon release and other factors implicated in the regulation of glucose tolerance.
These findings could support a clinical application for the screening of this SNP and the possibility of implementing tailored and cost-effective behavioral interventions to prevent T2D in vulnerable populations.
Supplementary Material
Acknowledgments
We want to knowledge this study to the National Institute of Health (USA) R01-DK-105072-01A1 and R01DK10269 and to Spanish Ministry of Economy and Competitiveness including FEDER co-funding (Spain/Europe).
Statement of authorship
Lopez-Minguez J. performed recruitment, laboratory assays and conducted the study, Saxena R: designed the study, discussed data and wrote the paper; Bandín C performed recruitment, Scheer FA and Garaulet M: designed the study, wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Founding Sources
This study was supported by grants from Spanish Government of Economy and Competitiveness (SAF2014-52480-R), and European Regional Development Fund (ERDF) and R01 DK102696 and DK-105072-01A1 to Marta Garaulet, and NIH grants R01 DK102696, R01 DK099512 and R01 HL118601, DK-105072-01A1 to Frank AJL Scheer, and NIH grant R01 DK102696 and DK-105072-01A1 to Richa Saxena.
Abbreviations
- MTNR1B
Melatonin Receptor 1B
- T2D
Type 2 Diabetes
- BMI
Body Mass Index
- SNP
Single Nucleotide Polymorphism
- LE
Late Eating
- EE
Early Eating
- MetS
Metabolic Syndrome
- WHR
Waist Hip Ratio
- TG
Triglycerides
- HOMA
Homeostasis Model Assessment
- AUC
Area Under the Curve
- RIA
Radioimmunoassay
- MEQ
Morning-Evening Questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no competing financial interests
Clinical Trial Registration
References
- 1.Gallant A, Lundgren J, Drapeau V. Nutritional Aspects of Late Eating and Night Eating. Current obesity reports. 2014;3(1):101–7. doi: 10.1007/s13679-013-0081-8. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima K, Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. Journal of diabetes and metabolic disorders. 2015;14:16. doi: 10.1186/s40200-015-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoogerwerf WA. Biologic clocks and the gut. Current gastroenterology reports. 2006;8(5):353–9. doi: 10.1007/s11894-006-0019-3. [DOI] [PubMed] [Google Scholar]
- 4.Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, et al. Circadian regulation of islet genes involved in insulin production and secretion. Molecular and cellular endocrinology. 2004;226(1–2):59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 2001;54(3):339–46. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37(10):1715–9. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9(1):5–9. doi: 10.1016/j.smrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Qian J, Scheer FA. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends in endocrinology and metabolism: TEM. 2016;27(5):282–93. doi: 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson A, Ladenvall C, Ahluwalia TS, Kravic J, Krus U, Taneera J, et al. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on alpha- and beta-cell function and insulin action in humans. Diabetes. 2013;62(8):2978–83. doi: 10.2337/db12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Replication DIG, Meta-analysis C. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46(3):234–44. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuomi T, Nagorny Cecilia LF, Singh P, Bennet H, Yu Q, Alenkvist I, et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell metabolism. 2016 doi: 10.1016/j.cmet.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Karamitri A, Renault N, Clement N, Guillaume JL, Jockers R. Minireview: Toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Molecular endocrinology (Baltimore, Md) 2013;27(8):1217–33. doi: 10.1210/me.2013-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Magi R, Reschen ME, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nature genetics. 2015;47(12):1415–25. doi: 10.1038/ng.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garaulet M, Gomez-Abellan P, Rubio-Sastre P, Madrid JA, Saxena R, Scheer FA. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism: clinical and experimental. 2015;64(12):1650–7. doi: 10.1016/j.metabol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiology international. 2011;28(7):617–29. doi: 10.3109/07420528.2011.593278. [DOI] [PubMed] [Google Scholar]
- 18.Bandin C, Martinez-Nicolas A, Ordovas JM, Madrid JA, Garaulet M. Circadian rhythmicity as a predictor of weight-loss effectiveness. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.211. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario VF, Sforza C, Schmitz JH, Miani A, Jr, Taroni G. Fourier analysis of human soft tissue facial shape: sex differences in normal adults. Journal of anatomy. 1995;187(Pt 3):593–602. [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine : a journal of the British Diabetic Association. 2006;23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 22.Corbalan-Tutau D, Madrid JA, Nicolas F, Garaulet M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiology & behavior. 2014;123:231–5. doi: 10.1016/j.physbeh.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes care. 1995;18(2):245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 24.Bandin C, Martinez-Nicolas A, Ordovas JM, Ros Lucas JA, Castell P, Silvente T, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes (Lond) 2013;37(8):1044–50. doi: 10.1038/ijo.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ. Allelic discrimination using fluorogenic probes and the 5’ nuclease assay. Genetic analysis : biomolecular engineering. 1999;14(5–6):143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 26.Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiology & behavior. 2008;95(4):570–80. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Adan A, H A. Adaptation and standardization of a Spanish version of the morningness-eveningness questionnaire: Individual differences. Pers Indivd Differ. 1990;11:1223–30. [Google Scholar]
- 28.Persaud SJ, Jones PM. A Wake-up Call for Type 2 Diabetes? The New England journal of medicine. 2016;375(11):1090–2. doi: 10.1056/NEJMcibr1607950. [DOI] [PubMed] [Google Scholar]
- 29.Matuszek MA, Anton A, Thillainathan S, Armstrong NJ. Increased Insulin following an Oral Glucose Load, Genetic Variation near the Melatonin Receptor MTNR1B, but No Biochemical Evidence of Endothelial Dysfunction in Young Asian Men and Women. PloS one. 2015;10(7):e0133611. doi: 10.1371/journal.pone.0133611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. The Journal of allergy and clinical immunology. 2012;129(2):434–40. doi: 10.1016/j.jaci.2011.10.025. 40 e1–2. [DOI] [PubMed] [Google Scholar]
- 31.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2014;44(6):842–50. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 32.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–93. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garriguet D. Canadians’ eating habits. Health reports. 2007;18(2):17–32. [PubMed] [Google Scholar]
- 34.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27(Suppl 2):255–62. doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 35.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutrition research (New York, NY) 2014;34(11):930–5. doi: 10.1016/j.nutres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbalan-Tutau MD, Madrid JA, Garaulet M. Timing and duration of sleep and meals in obese and normal weight women. Association with increase blood pressure. Appetite. 2012;59(1):9–16. doi: 10.1016/j.appet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer causes & control : CCC. 2006;17(4):489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 38.Yaraghi A, Mood NE, Dolatabadi LK. Comparison of capillary and venous blood glucose levels using glucometer and laboratory blood glucose level in poisoned patients being in coma. Adv Biomed Res. 2015;4:247. doi: 10.4103/2277-9175.170242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol Ther. 2001;3(3):357–65. doi: 10.1089/15209150152607132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.