Abstract
Background
Having 90% of patients on antiretroviral therapy (ART) and achieving an undetectable viral load (VL) is one of the 90:90:90 by 2020 targets. In this global analysis, we investigated the proportions of adult and paediatric patients with VL suppression in the first three years after ART initiation.
Methods
Patients from the IeDEA cohorts who initiated ART between 2010 and 2014 were included. Proportions with VL suppression (<1000 copies/mL) were estimated using: (i) strict intention-to-treat (ITT) – loss to follow-up (LTFU) and dead patients counted as having detectable VL; and (ii) modified ITT – LTFU and dead patients were excluded. Logistic regression was used to identify predictors of viral suppression at one year after ART initiation using modified ITT.
Results
A total of 35561 adults from 38 sites/16 countries and 2601 children from 18 sites/6 countries were included. When comparing strict with modified ITT methods, the proportion achieving VL suppression at three years from ART initiation changed from 45.1% to 90.2% in adults, and 60.6% to 80.4% in children. In adults, older age, higher CD4 count pre-ART, and homosexual/bisexual HIV exposure were associated VL suppression. In children, older age and higher CD4 percentage pre-ART showed significant associations with VL suppression.
Conclusions
Large increases in the proportion of VL suppression in adults were observed when we excluded those who were LTFU or had died. The increases were less pronounced in children. Greater emphasis should be made to minimise LTFU and maximise patient retention in HIV-infected patients of all age groups.
Keywords: HIV, suppression, paediatrics, adults, IeDEA
Introduction
Durable virologic suppression is a primary goal of antiretroviral therapy (ART). Having 90% of patients on ART with undetectable HIV viral load (VL) is the third “90” for global programs as part of the 90:90:90 targets 1. Increasingly, VL testing is offered as part of ART monitoring to confirm early treatment failure and to indicate second-line treatment switch in order to reduce the accumulation of HIV drug resistance mutations. The World Health Organization (WHO) now recommends routine VL testing 2 as the preferred method to detect ART failure rather than immunological and clinical monitoring.
The International Epidemiology Databases to Evaluate AIDS (IeDEA) global consortium was established by the U.S. National Institute of Allergy and Infectious Diseases in 2005. There are seven regional data centres within IeDEA in North America (The North American AIDS Cohort Collaboration on Research and Design, NA-ACCORD), the Caribbean, Central and South America (CCASAnet), the Asia-Pacific (AP), and Africa (East Africa, EA; Central Africa, CA; West Africa, WA; Southern Africa, SnA) 3,4. Currently, IeDEA includes data on more than one million people living with HIV/AIDS. According to individual country assessments 5 on HIV indicators for sites within NA-ACCORD and CCASAnet, the percentage of patients on ART in the United States was 67%, while the highest was reported for Mexico at 90%. In the African population, in particular EA and SnA, ART coverage increased from 24% in 2010 to 54% in 2015, while CA and WA had a lower percentage coverage at 28%. ART usage in AP doubled from 19% in 2010 to 41% in 2015 6. The proportion of patients with VL suppression across different IeDEA regions in recent years, however, remains unclear. The primary objective of this study was to estimate the proportions of adult and paediatric patients enrolled in IeDEA, who achieved undetectable VL in the first three years after initiating ART. The secondary objective was to determine factors associated with VL suppression at one year after ART.
Methods
Study population and inclusion criteria
Adult and paediatric patients enrolled in IeDEA were included if they had initiated ART between 2010-2014. Paediatric patients were defined as children and adolescents aged <18 years when starting ART; adults were those aged ≥18 years at ART start. ART was defined as three or more antiretroviral drugs in a single regimen; those who started treatment with mono- or dual-drug regimens were excluded. Sites within each respective participating region were included if they were confirmed to perform routine annual VL testing. If no specific information was provided regarding VL testing frequency, we performed a calculation by obtaining the average number of VL tests for each patient from the regional cohort enrolment date to the last follow-up date. If the median number of VL tests per patient per site was above 0.8, that site was included in the initial data capture. However, only patients with at least one VL test after ART initiation were included in the analyses.
Definitions
VL suppression was defined as VL <1000 copies/mL at one, two and three years from ART initiation to be consistent with the WHO definition for classifying virological failure 2. Moreover, due to the use of different virological assays across the regions with varying lower limits of detection, the use of this threshold of VL <1000 copies/mL allowed the inclusion of sites with higher undetectable cut-offs. This threshold also removed concern of unnecessarily excluding patients experiencing transient virological “blips” and then returning to virologic suppression” 7. The annual time points reflect the WHO recommendations for VL testing to monitor for treatment failure 8. We have chosen to include data up to three years after ART initiation to minimise LTFU as patient retention has been shown to decrease to 65% at three years 9. As different sites have different definitions of LTFU, patients in this study were considered to be LTFU according to the LTFU indicator provided in each regional database. If no LTFU information was available, patients who were not seen within six months 10 prior to the database closing date were considered lost at their final visit date defined as the latest of CD4, VL or clinic visit date.
Statistical analyses
Simple proportions were calculated by percentages. Two methods were used to estimate proportions of patients with undetectable VL.
Strict Intention-to-Treat
Patients who were LTFU or died were counted as having detectable VL after their last visit/ death date up until three years after ART initiation. Patients who were transferred out were removed from the analyses after their transfer date. The denominator for each 1-, 2-, and 3- year time point included patients who had VL testing at that time point and patients who were LTFU or died prior to that time point (counted as having detectable VL). Patients who did not have VL testing or transferred out prior to each time point were not included in the denominator (Supplementary Figure 1 and 3).
Modified Intention-to-Treat
The denominator at each time point included patients who had VL testing at that time point. Patients who did not have VL testing, or those who were LTFU, died or transferred out prior to each time point were not included in the denominator (Supplementary Figure 2 and 4).
Factors associated with VL suppression at one year, as defined by the modified intention-to-treat (ITT) method, were analysed using logistic regression methods. We chose to analyse VL suppression at one year in order to minimise LTFU cases. Additionally, as we included the VL measurement closest to the annual time point, our analyses would not be biased by how often VL was assessed. Covariates included were age at ART initiation, sex, prior AIDS diagnosis, pre-ART CD4 count or percent, HIV mode of exposure, and region. ART combinations were not included in the analyses due to potential collinearity with different regions. For example we would expect to see the majority of patients from resource-limited regions, such as in Asia and Africa, initiating on a nucleoside reverse transcriptase inhibitors (NRTI) and a non-NRTI (NNRTI) combination, while protease inhibitor (PI) and integrase inhibitor (IN) based regimens would be most commonly used in developed countries such as those in NA-ACCORD. All variables were entered in the multivariable model; no model selection was attempted. P-values <0.05 were considered statistically significant. Sensitivity analyses were performed using the strict ITT definition, as well as utilising VL failure as the outcome of interest, defined as VL ≥1000 copies/mL.
Each regional data centre was responsible for ethics approval, development of data collection systems, extracting data from their regional database or requesting relevant data variables from designated programmes within their region, and verifying data quality. The datasets were then centrally aggregated and analysed at The Kirby Institute, UNSW Sydney (the University of New South Wales), Australia, the regional data centre of the IeDEA AP region. All data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) or Stata software version 14 (Stata Corp., College Station, TX, USA).
Results
Adults
There were a total of 38 sites from 16 countries: 12 sites/8 countries from AP, 6 sites/5 countries from CCASAnet, 14 sites/2 countries from NA-ACCORD, and six sites from South Africa (SA), a country within the IeDEA SnA regional cohort that met eligibility criteria for the adult analyses. Median VL testing frequency for each site ranged from 0.9 to 4.2 per patient per year. A total of 35561 patients were included in the analyses: 2121 (6.0%) from AP; 3404 (9.6%) from CCASAnet; 14579 (41.0%) from NA-ACCORD; and 15457 (43.5%) from SA (Table 1 and Supplementary Table 5). Sixty-one percent were male. At ART initiation the median age was 37 years (interquartile range (IQR 30-46 years) and the median CD4 cell count was 218 cells/μL (IQR: 105-344 cells/μL).
Table 1. Patient characteristics.
| Total adults: 35561 | Total children: 2601 | |
|---|---|---|
|
| ||
| Number* (%) | Number* (%) | |
|
| ||
| Median age at ART initiation, years | 37, (IQR 30-46) | 4.65, (IQR 1.02-9.75) |
|
| ||
| Sex | ||
| Male | 21623 (60.8) | 1299 (49.9) |
| Female | 13935 (39.2) | 1302 (50.1) |
| Unknown | 3 (0.0) | 0 (0.0) |
|
| ||
| Prior AIDS-defining illness | ||
| No | 15508 (43.6) | 164 (6.3) |
| Yes | 2695 (7.6) | 52 (2.0) |
| Unknown | 17358 (48.8) | 2385 (91.7) |
|
| ||
| Median Pre-ART CD4 count | 218 cells/μL, (IQR 105-344) | 15.89 percent, (IQR 8.70-23.24) |
|
| ||
| HIV mode of exposure | ||
| Homosexual/bisexual | 8537 (24.0) | 0 (0.0) |
| Heterosexual | 14216 (40.0) | 0 (0.0) |
| IDU | 1639 (4.6) | 0 (0.0) |
| Perinatal | 0 (0.0) | 2119 (81.5) |
| Other** | 645 (1.8) | 190 (7.3) |
| Unknown | 10524 (29.6) | 292 (11.2) |
|
| ||
| Region | ||
| Asia-Pacific | 2121 (6.0) | 291 (11.2) |
| Caribbean, Central and South America | 3404 (9.6) | 75 (2.9) |
| North America | 14579 (41.0) | 0 (0.0) |
| South Africa | 15457 (43.5) | 2235 (85.9) |
Unless otherwise specified.
For children, “Other” includes sexual behaviour (184), sexual abuse (2), blood transfusion (2) and breastfeeding (2).
Abbreviations: IQR – interquartile range; ART- antiretroviral therapy; IDU – injecting drug users
Using the strict ITT method, the overall proportion of adults with VL suppression at one year from ART initiation was 83.0%; 70.0% at two years; and 45.1% at three years. Figure 1a shows the proportions of adults with VL suppression decreasing after two years for NA-ACCORD and SA, with AP maintaining the highest VL suppression over the full three years. Using the modified ITT method where patients who were LTFU or died were excluded, of the 35561 adults patients, 26153 (73.5%) had VL testing at one year; 13602 (38.2%) at two years; and 4629 (13.0%) at three years. Overall VL suppression increased to 88.5%, 89.5% and 90.2% for years one to three, with all regions showing high proportions above 85% for all years (Figure 1b).
Figure 1. Proportion of adults with viral load (VL) suppression using (a) strict intention-to-treat; and (b) modified intention-to-treat methods.
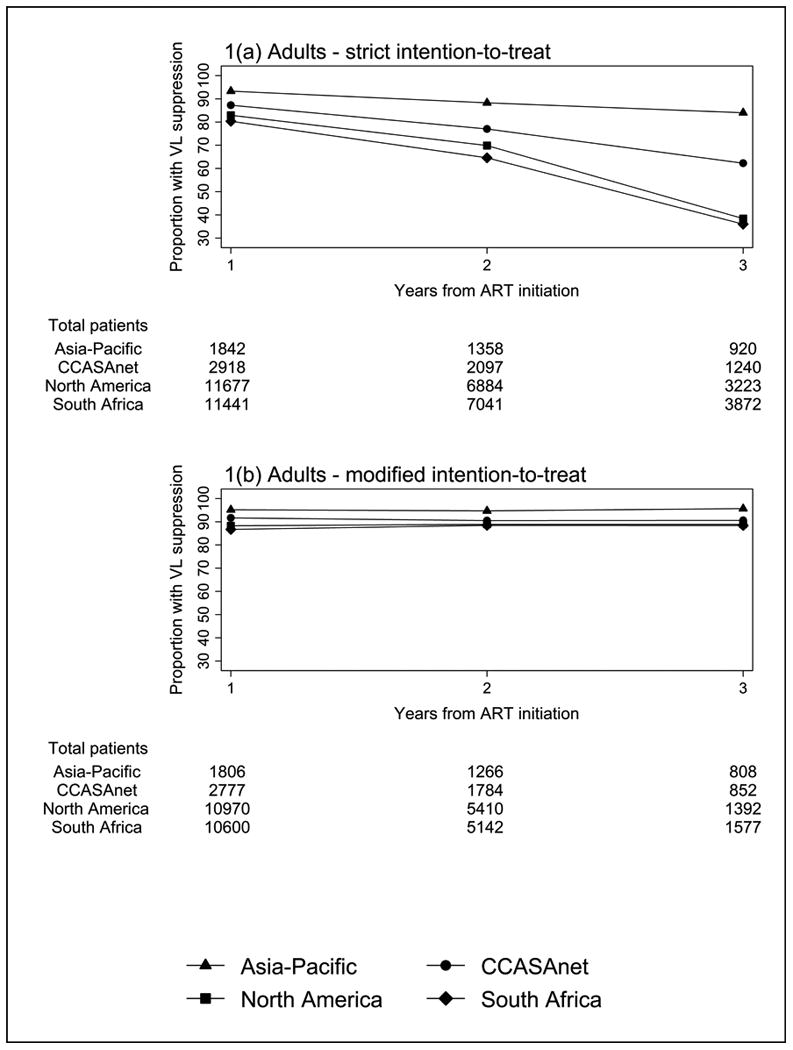
Abbreviations: CCASAnet - Caribbean, Central and South America; VL – viral load
Table 2 shows factors associated with VL suppression at one year using the modified ITT method. The multivariate results show that after adjustment for all variables, sex was the only factor showing no association with VL suppression (p=0.358). The odds for VL suppression increased with age 25-49 years (OR=1.42, 95% CI 1.24-1.63), and ≥50 years (OR=2.20, 95% CI 1.86-2.60), all p<0.001, compared to age ≤24 years. Pre-ART CD4 count also showed an increasing trend: 200-349 cells/μL (OR=1.60, 95% CI 1.44-1.78), 350-499 cells/μL (OR=1.73, 95% CI 1.48-2.02), and ≥500 cells/μL (OR=1.91, 95% CI 1.62-2.26), all p<0.001, compared to CD4 <200 cells/μL. Patients with homosexual/bisexual mode of HIV exposure were more likely to have VL suppression (OR=1.66, 95% CI 1.46-1.89, p<0.001), while injecting drug users (IDU) had reduced odds compared heterosexual mode of exposure (OR=0.69, 95% CI 0.58-0.83, p<0.001). Having a prior AIDS-defining illness also negatively affected VL response (OR=0.82, 95% CI 0.71-0.95, p=0.008). Comparison of different regions showed that when compared to NA-ACCORD, AP (OR=2.78, 95%CI 2.2-3.52, p<0.001) and CCASAnet (OR=1.70, 95%CI 1.45-2.00, p<0.001) had higher proportions of VL suppression. When AP was the reference group, patients in CCASAnet (OR=0.61, 95% CI 0.47-0.79, p<0.001); NA-ACCORD (OR=0.36, 95% CI 0.28-0.45, p<0.001); and SA (OR=0.37, 95% CI 0.29-0.48, p<0.001), all had smaller proportions of patients with VL suppression. Additional tests for multicollinearity showed there was no collinearity amongst the included variables.
Table 2. Factors associated with viral load suppression at one year from ART initiation, adult analysis using modified intention-to-treat method, N=26153.
| Total patients | VL <1000 copies/mL | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |||
|
| ||||||||
| Age at ART initiation (years) | <0.001 | <0.001 | ||||||
| 18-24 | 2065 | 1773 | Ref | Ref | ||||
| 25-49 | 19275 | 16974 | 1.21 | (1.07, 1.39) | 0.004 | 1.42 | (1.24, 1.63) | <0.001 |
| ≥50 | 4813 | 4393 | 1.72 | (1.47, 2.02) | <0.001 | 2.20 | (1.86, 2.60) | <0.001 |
|
| ||||||||
| Sex | ||||||||
| Male | 16323 | 14565 | Ref | Ref | ||||
| Female | 9828 | 8573 | 0.82 | (0.76, 0.89) | <0.001 | 1.04 | (0.95, 1.14) | 0.358 |
| Unknown | 2 | 2 | ||||||
|
| ||||||||
| Prior AIDS-defining illness | 0.027 | 0.008 | ||||||
| No | 11027 | 9705 | Ref | Ref | ||||
| Yes | 2000 | 1725 | 0.85 | (0.74, 0.98) | 0.027 | 0.82 | (0.71, 0.95) | 0.008 |
| Unknown | 13126 | 11710 | 1.13 | (1.04, 1.22) | 0.003 | 0.83 | (0.69, 0.99) | 0.036 |
|
| ||||||||
| Pre-ART CD4 count (cells/μL) | <0.001 | <0.001 | ||||||
| <200 | 10000 | 8590 | Ref | Ref | ||||
| 200-349 | 6241 | 5661 | 1.60 | (1.45, 1.77) | <0.001 | 1.60 | (1.44, 1.78) | <0.001 |
| 350-499 | 2631 | 2393 | 1.65 | (1.43, 1.91) | <0.001 | 1.73 | (1.48, 2.02) | <0.001 |
| ≥500 | 2363 | 2160 | 1.75 | (1.50, 2.04) | <0.001 | 1.91 | (1.62, 2.26) | <0.001 |
| Missing | 4918 | 4336 | 1.22 | (1.10, 1.36) | <0.001 | 1.29 | (1.16, 1.44) | <0.001 |
|
| ||||||||
| HIV mode of exposure | <0.001 | <0.001 | ||||||
| Homosexual/bisexual | 6676 | 6139 | 1.73 | (1.56, 1.92) | <0.001 | 1.66 | (1.46, 1.89) | <0.001 |
| Heterosexual | 10403 | 9036 | Ref | Ref | ||||
| IDU | 1229 | 1017 | 0.73 | (0.62, 0.85) | <0.001 | 0.69 | (0.58, 0.83) | <0.001 |
| Other | 504 | 449 | 1.24 | (0.93, 1.64) | 0.148 | 1.10 | (0.82, 1.48) | 0.534 |
| Unknown | 7341 | 6499 | 1.17 | (1.07, 1.28) | 0.001 | 1.23 | (1.12, 1.36) | <0.001 |
|
| ||||||||
| Region | <0.001 | <0.001 | ||||||
| Asia-Pacific | 1806 | 1719 | 0.38 | (0.31, 0.48) | <0.001 | 2.78 | (2.20, 3.52) | <0.001 |
| Caribbean, Central and South America | 2777 | 2546 | 0.68 | (0.59, 0.79) | <0.001 | 1.70 | (1.45, 2.00) | <0.001 |
| North America | 10970 | 9685 | Ref | Ref | ||||
| South Africa | 10600 | 9190 | 1.16 | (1.07, 1.25) | <0.001 | 1.03 | (0.85, 1.25) | 0.767 |
Note: Values in bold represent significant covariates in the adjusted model.
Abbreviations: OR – odds ratio, 95%CI – 95% confidence interval, IDU – injecting drug use, ART –antiretroviral therapy.
Paediatrics
The paediatric analysis included 18 clinical centres from three IeDEA regions with 2601 children overall: 291 (11.2%) from 10 AP sites/3 countries, 75 (2.9%) from four CCASAnet sites/2 countries and 2235 (85.9%) from four sites in SA (Table 1 and Supplementary Table 6). Median VL testing frequency for each site ranged from 1.5 to 2.7 per patient per year. At ART initiation, the median age was 4.7 years (IQR 1.0-9.8). For 1677 children with available data the median CD4 percentage was 15.9 (IQR 8.70-23.24) with 477 (18.3%) of children having CD4 percentage <10%. Small proportion (0.2) had experienced WHO clinical stage 4 events before ART. These baseline patient characteristics differed between regions. Median CD4 percentage and age were 12% and eight years in AP, 23% and 11 years in CCASAnet, and 16% and four years in SA.
Using strict ITT methods as shown in Figure 2a, a decrease in the proportion of children with VL <1000 copies/mL over time was seen for CCASAnet (80.3% at year one, 68.3% at year two and 50.0% at year three) and SA regions (73.3% to 67.5% and 56.7%). Using the modified ITT approach, CCASAnet still showed a decline in the proportion of children with viral suppression from 81.7% to 73.7% and 69.2% for years one to three. Overall, during the follow-up period, 69.2-83.0% of children maintained VL <1000 copies/mL (Figure 2b).
Figure 2. Proportion of paediatric patients with viral load (VL) suppression using (a) strict intention-to-treat; and (b) modified intention-to-treat methods.
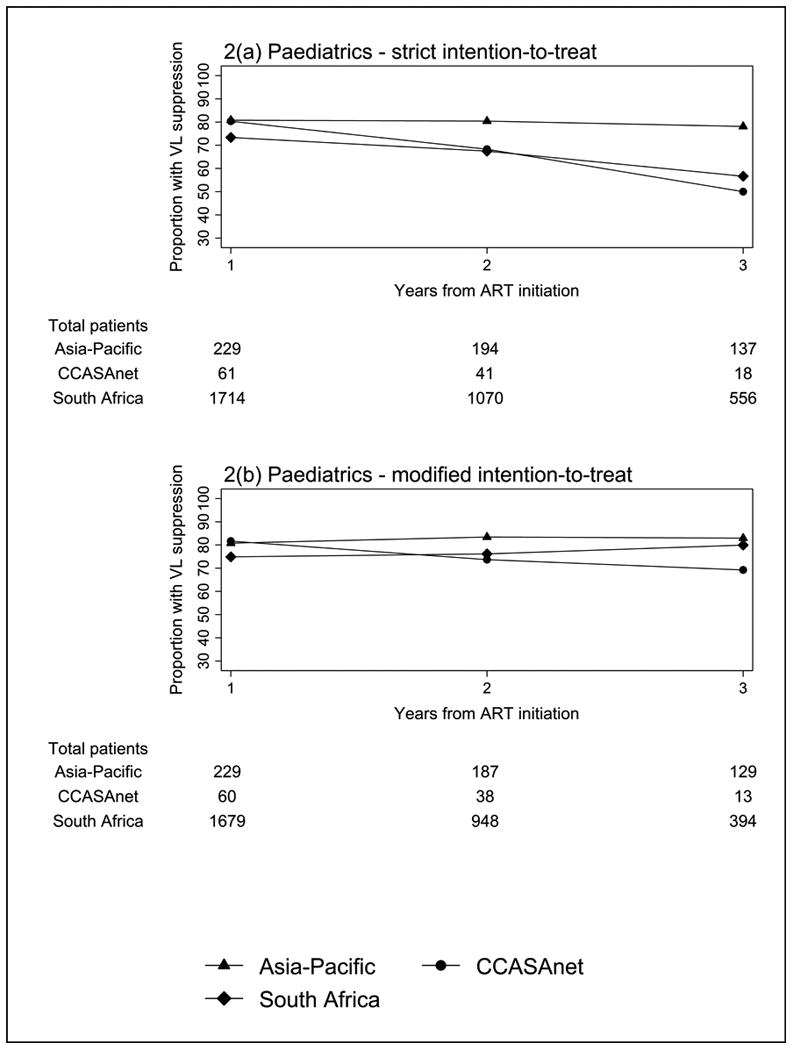
Abbreviations: CCASAnet - Caribbean, Central and South America; VL – viral load.
The adjusted statistical analysis (Table 3) identified the following baseline characteristics to be associated with VL suppression at one year after ART initiation: age 1.5-4 years (OR=2.33, 95% CI 1.73-3.14, p<0.001); 5-9 years (OR=2.79, 95% CI 2.06-3.78, p<0.001), 10-14 years (OR=2.32, 95% CI 1.70-3.16, p<0.001), and 15-17 years (OR=2.34, 95% CI 1.28-4.27, p=0.006) compared to children <1.5 years (the reference group); and pre-ART CD4 percentage 15-24% (OR=2.38, 95% CI 1.67-3.41, p<0.001) and ≥25% (OR =1.81, 95% CI 1.24-2.64, p=0.002) versus CD4 <10%. Other factors, including sex, WHO clinical stage 4, mode of exposure and region were not significantly associated with VL suppression. No collinearity was detected among the variables.
Table 3. Factors associated with viral suppression at one year from ART initiation, paediatric analysis using modified intention to treat method, N=1968.
| Total patients | VL <1000 copies/mL | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |||
|
| ||||||||
| Age at ART initiation (years) | <0.001 | <0.001 | ||||||
| <1.5 years | 569 | 364 | Ref | Ref | ||||
| 1.5-4 | 441 | 355 | 2.32 | (1.74, 3.11) | <0.001 | 2.33 | (1.73, 3.14) | <0.001 |
| 5-9 | 492 | 405 | 2.62 | (1.97, 3.50) | <0.001 | 2.79 | (2.06, 3.78) | <0.001 |
| 10-14 | 395 | 311 | 2.09 | (1.55, 2.80) | <0.001 | 2.32 | (1.70, 3.16) | <0.001 |
| 15-17 | 71 | 56 | 2.10 | (1.16, 3.81) | 0.014 | 2.34 | (1.28, 4.27) | 0.006 |
|
| ||||||||
| Sex | ||||||||
| Male | 977 | 731 | Ref | Ref | ||||
| Female | 991 | 760 | 1.11 | (0.90, 1.36) | 0.333 | 1.11 | (0.89, 1.37) | 0.350 |
|
| ||||||||
| Prior AIDS-defining illness | 0.199 | 0.613 | ||||||
| No | 127 | 111 | Ref | Ref | ||||
| Yes | 38 | 30 | 0.54 | (0.21, 1.38) | 0.199 | 0.78 | (0.30, 2.03) | 0.613 |
| Unknown | 1803 | 1350 | 0.43 | (0.25, 0.73) | 0.002 | 0.53 | (0.31, 0.92) | 0.024 |
|
| ||||||||
| Pre-ART CD4 % | 0.021 | <0.001 | ||||||
| <10% | 396 | 291 | Ref | Ref | ||||
| 10-14% | 228 | 175 | 1.19 | (0.81, 1.74) | 0.366 | 1.43 | (0.97, 2.11) | 0.074 |
| 15-24% | 419 | 356 | 2.04 | (1.44, 2.89) | <0.001 | 2.38 | (1.67, 3.41) | <0.001 |
| ≥25% | 293 | 226 | 1.22 | (0.86, 1.73) | 0.274 | 1.81 | (1.24, 2.64) | 0.002 |
| Missing | 632 | 443 | 0.85 | (0.64, 1.12) | 0.242 | 1.11 | (0.83, 1.49) | 0.491 |
|
| ||||||||
| HIV mode of exposure | 0.238 | 0.164 | ||||||
| Perinatal | 1592 | 1197 | 0.76 | (0.49, 1.19) | 0.238 | 0.72 | (0.46, 1.14) | 0.164 |
| Sexual behaviour | 129 | 103 | Ref | Ref | ||||
| Other/Unknown | 247 | 191 | 0.86 | (0.51, 1.45) | 0.575 | 0.86 | (0.50, 1.48) | 0.591 |
|
| ||||||||
| Region | 0.083 | 0.384 | ||||||
| Asia-Pacific | 229 | 185 | Ref | Ref | ||||
| Caribbean, Central and South America | 60 | 49 | 1.06 | (0.51, 2.20) | 0.877 | 1.02 | (0.47, 2.21) | 0.959 |
| South Africa | 1679 | 1257 | 0.71 | (0.50, 1.00) | 0.051 | 0.80 | (0.56, 1.14) | 0.217 |
Note: Values in bold represent significant covariates in the adjusted model.
Abbreviations: OR – odds ratio, 95%CI – 95% confidence interval, ART – antiretroviral therap
The strict ITT sensitivity analyses (Supplementary Tables 1 and 2) showed similar results to the main analyses. In VL failure analyses (Supplementary Tables 3 and 4), the ORs of the covariates were simply the reciprocal of the ORs reported in the main analyses, with the same p-values. This indicates that the use of logistic regression was appropriate for both VL suppression and VL failure outcomes in adults and children.
Discussion
Our study included data from four IeDEA regions covering 35561 patients from 38 adult sites and 2601 patients from 24 paediatric sites who initiated ART between 2010-2014. By using the modified ITT approach that excludes LTFU and those who died, the proportions of patients with VL suppression was 90% for adults and 80% for children at three years. However, when the strict ITT approach was used including LTFU and deceased patients and categorising them as having detectable VL, these estimates decreased to 45% in adults and 61% in children. In adults, older age, higher pre-ART CD4 count, homosexual/bisexual and other modes of HIV exposure were associated with a better chance of achieving VL suppression at one year from ART initiation. In children, age >1.5 years and CD4 ≥15% were associated with higher chance of achieving VL suppression. Adults from the AP region performed significantly better than other regions. In children, VL suppression at one year did not differ significantly between regions.
Patients included in this study were those from sites that offered routine annual VL testing. Many resource-limited countries throughout the world currently do not offer routine VL tests for the detection of HIV treatment failure. For example, all sites within the WA, CA and EA IeDEA regions and countries within the SnA IeDEA region outside of SA did not have annual VL testing for the 2010-2014 time period. Some countries in the Asia-Pacific region, including Cambodia and Vietnam, also did not perform routine VL testing. The WHO 2 guidelines have recently recommended that VL testing be the preferred method of detecting treatment failure, and many countries have adopted this recommendation and are scaling up their VL monitoring capacity 11,12. Studies have shown that the WHO's immunologic and clinical failure criteria have performed poorly in predicting virological treatment failure leading to unnecessary switch to second-line ART during periods of VL suppression, or delayed switch due to the misclassification of treatment failure 13-15. Using CD4 monitoring in the presence of HIV drug resistance mutations during periods of viraemia may also lead to delayed ART switches compared to VL monitoring alone 16. Delayed second-line ART switch can lead to the accumulation of drug resistance mutations 17,18, which can compromise treatment options for second-line therapy, particularly in resource-limited countries. In addition, low positive predictive value of current immunological criteria may result in increased costs because of unnecessary switches to second-line therapy in people with adequate VL suppression 14. Unfortunately, some countries that do not yet offer VL monitoring continue to refer to CD4 measurements and clinical monitoring in the assessment of HIV treatment outcomes.
The overall high proportions of VL suppression under the modified ITT analyses indicate that patients who are followed-up and retained in care have good response to treatment. This is in contrast to the decrease in the proportion of adults and children achieving VL suppression when LTFU and dead patients were included as being detectable under strict ITT methods. The decrease in the proportion of patients with VL suppression was less pronounced in children. When compared with adults, children had higher rates of suppression when we considered LTFU and death as detectable. This might be explained by a lower rate of LTFU in children (6%) compared with adults (12%) in this study. The decrease in VL suppression when patients who were LTFU or dead were assumed to have detectable viraemia has also been reported in another study 19, which suggests the importance of retention in HIV care. Mortality rates were often found to be higher in children and adults who were LTFU or transferred out compared to patients who were retained in care 20,21. An Australian study, however, showed no association between LTFU and mortality, possibly due to unreported re-engagement into care 22.
The multivariate analyses in this study indicate that the adult AP cohort has performed significantly better than NA-ACCORD as well as other cohorts, although proportions of VL suppression were above 85% for all regions. These results most likely reflect the patient recruitment process within AP. Sites in AP are urban referral centres and patients were recruited based on the likelihood of remaining in care 23. These results therefore do not represent the general HIV-infected population in Asia, and should be interpreted with caution. In contrast, for children the chance of VL suppression did not differ across regions which may indicate less between-region heterogeneity and less variations in both patient-level and site-specific factors. High clinical resources and access to paediatric antiretroviral formulations were reported in a survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa 24.
The association between older age and higher pre-ART CD4 count with VL suppression, and the increased risk of VL failure in patients with IDU mode of exposure and those who had a prior AIDS defining illness in adults are consistent with other published literature 19,25-27. Although homosexual mode of exposure is often associated with lower adherence levels leading to poorer treatment outcomes 28, the positive effect of this transmission group could possibly be explained by better ART adherence levels reported in some patients 29-31. We found that children who initiated ART when CD4 >10% and those started at age >1.5 years were more likely to achieve VL suppression. This may reflect the impact of early access to ART and higher baseline level of RNA in infants and young children. An early study conducted in America found that infants whose disease progressed rapidly have high numbers of HIV-1 RNA copies during the first 24 months of life 32. The association between high baseline viral load (>1 million copies/ml) and VL failure has been also reported by a more recent study conducted in children in SA 33. In addition, adherence issues related to taste and formulation, dosing and/or high pharmacokinetic variability of drugs might adversely affect virological response and contribute to poorer responses in younger children 34.
Our study has several limitations including the classification of LTFU and dead patients as having detectable VL. Classifying dead patients as virological failure is debatable in terms of 90:90:90 and treatment as prevention. However, we have used this definition to be consistent with that used in clinical trials where LTFU and dead patients would generally be classified as “failed”. It is also consistent with a strict ITT approach which includes all patients. Known transferred cases were excluded from the calculations, but there may be instances where patients have self-transferred without the knowledge of the treating physician. Patients in follow-up without VL testing were also not included in our analyses. This could be considered a potential bias as targeted VL testing to confirm treatment failure often occurs in resource-limited settings. However, as our study only included sites with annual VL testing, we assume that the bias caused by targeted VL testing would be minimised. Lastly, the lack of data completeness and heterogeneity of treatment approaches and settings are another concern when analysing large collaborative dataset. There may be discrepancies between the actual last follow-up date and the final visit date calculated using our definition which could lead to misclassifications of LTFU patients. Furthermore, 86% of children in this study are from SA, therefore the generalisability of our paediatric findings is limited. Data on ART adherence and factors related to ART adherence such as disclosure and orphan status in children were not available in our dataset and therefore not included in the multivariate analyses. As adherence level is a known predictor of virological outcomes 35 and disclosure in children is associated with ART adherence 36, our analysis results should be interpreted with this in mind.
Conclusions
This multiregional collaborative study showed that a high level of VL suppression can be achieved among children and adults receiving ART in resource-limited settings. Our findings highlight that even for those retained in care, achieving 90:90:90 for children may be more challenging. Sustainable approaches are needed to ensure optimal clinical outcomes and to minimise LTFU and increase patient retention.
Supplementary Material
Supplementary Table 1 - Factors associated with viral load suppression at one year from ART initiation, adult analysis using strict intention-to-treat method
Supplementary Table 2 - Factors associated with viral load suppression at one year from ART initiation, paediatric analysis using strict intention-to-treat method
Supplementary Table 3: Factors associated with viral load failure at one year from ART initiation, adult analysis using modified intention-to-treat method
Supplementary Table 4: Factors associated with viral load failure at one year from ART initiation, paediatric analysis using modified intention to treat method
Supplementary Table 5: Patient characteristics in adults by region
Supplementary Table 6: Patient characteristics in children by region
Supplementary Figure 1: Flow chart for adult strict ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 2: Flowchart for adult modified ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 3: Flow chart for paediatric strict ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 4: Flow chart for paediatric modified ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Acknowledgments
The TREAT Asia HIV Observational Database (TAHOD): PS Ly* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee* †, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
S Pujari*, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India;
TP Merati*, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
E Yunihastuti*, D Imran and A Widhani, Working Group on AIDS Faculty of Medicine, University of Indonesia/ Cipto Mangunkusumo Hospital, Jakarta, Indonesia;
S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
BLH Sim*, YM Gani, and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines;
WW Wong* ‡, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee and R Martinez-Vega, Tan Tock Seng Hospital, Singapore;
P Phanuphak*, K Ruxrungtham, A Avihingsanon and C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul*, S Sungkanuparph, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand;
P Kantipong* and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
KV Nguyen*, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
TT Pham*, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn*, JL Ross* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, A Jiamsakul* and DC Boettiger, The Kirby Institute, UNSW Sydney, Australia.
* TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair.
The TREAT Asia Pediatric HIV Network (TApHOD): PS Ly*, and V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia;
J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia;
N Kumarasamy*, S Saghayam, and E Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India;
DK Wati*, LPP Atmikasari, and IY Malino, Sanglah Hospital, Udayana University, Bali, Indonesia;
N Kurniati*, and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
SM Fong*†, M Lim, and F Daut, Hospital Likas, Kota Kinabalu, Malaysia;
NK Nik Yusoff*, and P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia;
KA Razali*, TJ Mohamed, and NADR Mohammed, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia;
R Nallusamy*, and KC Chan, Penang Hospital, Penang, Malaysia;
T Sudjaritruk*, V Sirisanthana, L Aurpibul, and P Oberdorfer, Department of Pediatrics, Faculty of Medicine, Chiang Mai University and Research Institute for Health Sciences, Chiang Mai, Thailand;
R Hansudewechakul*, S Denjanta, W Srisuk, and A Kongphonoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
P Lumbiganon*‡, P Kosalaraksa, P Tharnprisan, and T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand;
G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand;
T Bunupuradah*, T Puthanakit, W Prasitsuebsai, and C Phadungphon, HIV-NAT, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
K Chokephaibulkit*, K Lapphra, W Phongsamart, and S Sricharoenchai, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand;
KH Truong*, QT Du, and CH Nguyen, Children's Hospital 1, Ho Chi Minh City, Vietnam;
VC Do*, TM Ha, and VT An Children's Hospital 2, Ho Chi Minh City, Vietnam;
LV Nguyen*, DTK Khu, AN Pham, and LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam;
ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam;
AH Sohn*, JL Ross, and C Sethaputra, TREAT Asia/amfAR -- The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, UNSW Sydney, Australia;
*TApHOD Steering Committee member
† Current Steering Committee Chair; ‡ co-Chair
IeDEA Caribbean, Central, and South America (CCASAnet): Fundación Huésped and Hospital Fernández, Argentina: Pedro Cahn, Carina Cesar, Valeria Fink, Omar Sued, Emanuel Dell'Isola, Hector Perez, Jose Valiente, Cleyton Yamamoto
Instituto Nacional de Infectologia Evandro Chagas - Fiocruz, Brazil: Beatriz Grinsztejn, Valdilea Veloso, Paula Luz, Raquel de Boni, Sandra Cardoso Wagner, Ruth Friedman, Ronaldo Moreira.
Universidade Federal de Minas Gerais, Brazil: Jorge Pinto, Flavia Ferreira, Marcelle Maia.
Universidade Federal de São Paulo, Brazil: Regina Célia de Menezes Succi, Daisy Maria Machado, Aida de Fátima Barbosa Gouvêa
Fundación Arriarán, Chile: Marcelo Wolff, Claudia Cortes, Maria Fernanda Rodriguez, Gladys Allendes
Les Centres GHESKIO, Haiti: Jean William Pape, Vanessa Rouzier, Adias Marcelin, Christian Perodin.
Hospital Escuela Universitario, Honduras: Marco Tulio Luque.
Instituto Hondureño de Seguridad Social, Honduras: Denis Padgett.
Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico: Juan Sierra Madero, Brenda Crabtree Ramirez, Paco Belaunzaran, Yanink Caro Vega.
Instituto de Medicina Tropical Alexander von Humboldt, Peru: Eduardo Gotuzzo, Fernando Mejia, Gabriela Carriquiry.
Vanderbilt University Data Center, USA: Catherine C McGowan, Bryan E Shepherd, Timothy Sterling, Karu Jayathilake, Anna K Person, Peter Rebeiro, Mark Giganti, Jessica Castilho, Stephany N Duda, Hilary Vansell, Fernanda Maruri.
NA-ACCORD Collaborating Cohorts and Representatives: Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch
AIDS Link to the IntraVenous Experience: Gregory D. Kirk
Fenway Health HIV Cohort: Stephen Boswell, Kenneth H. Mayer and Chris Grasso
HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio SG Montaner, Angela Cescon and Tareq Ahmed
HIV Outpatient Study: Kate Buchacz and John T. Brooks
HIV Research Network: Kelly A. Gebo and Richard D. Moore
Johns Hopkins HIV Clinical Cohort: Richard D. Moore
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg
Kaiser Permanente Northern California: Michael J. Silverberg
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne
Multicenter Hemophilia Cohort Study–II: James J. Goedert
Multicenter AIDS Cohort Study: Lisa P. Jacobson and Gypsyamber D'Souza
Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein
Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann N. Burchell and Anita R. Rachlis
Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor
Southern Alberta Clinic Cohort: M. John Gill
Study of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Pragna Patel and John T. Brooks
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero and James Willig
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik
University of Washington HIV Cohort: Mari M. Kitahata, Heidi M. Crane and Daniel R. Drozd
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, Sally Bebawy and Megan Turner
Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin
Women's Interagency HIV Study: Stephen J. Gange and Kathryn Anastos
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Keri N. Althoff, Rosemary G. McKaig, Amy C. Justice and Aimee M. Freeman
Administrative Core: Richard D. Moore, Aimee M. Freeman and Carol Lent
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Daniel R. Drozd, Liz Morton, Justin McReynolds and William B. Lober
Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Sharada Modur, Cherise Wong, Brenna Hogan, Weiqun Tong, Bin Liu and Bin You
IeDEA-Southern Africa Steering Committee: Matthias Egger (co-PI), University of Bern, Switzerland;
Mary-Ann Davies (co-PI), University of Cape Town, South Africa;
Frank Tanser, Africa Centre for Health and Population Studies, University of Kwazulu-Natal, South Africa;
Michael Vinikoor, Centre for Infectious Disease Research in Zambia;
Eusebio Macete, Centro de Investigação em Saúde de Manhiça, Mozambique;
Robin Wood, Desmond Tutu HIV Centre (Gugulethu and Masiphumelele clinics), South Africa;
Andrew Boulle, Khayelitsha ART Programme and Médecins Sans Frontières, South Africa; Geoffrey Fatti, Kheth'Impilo Programme, South Africa;
Sam Phiri, Lighthouse Trust Clinic, Malawi;
Cleophas Chimbetete, Newlands Clinic, Zimbabwe;
Kennedy Malisita, Queen Elizabeth Hospital, Malawi;
Brian Eley, Red Cross War Memorial Children's Hospital and Department of Paediatrics and Child Health, University of Cape Town, South Africa;
Jochen Ehmer, Solidarmed, Switzerland;
Christiane Fritz, SolidarMed SMART Programme, Lesotho;
Michael Hobbins, SolidarMed SMART Programme, Mozambique;
Kamelia Kamenova, SolidarMed SMART Programme, Zimbabwe;
Matthew Fox, Themba Lethu Clinic, South Africa;
Hans Prozesky, Tygerberg Academic Hospital, South Africa;
Karl Technau, Empilweni Clinic, Rahima Moosa Mother and Child Hospital, South Africa;
Shobna Sawry, Harriet Shezi Children's Clinic, Chris Hani Baragwanath Academic Hospital, South Africa.
Source of Funding: This study was supported by the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Cancer Institute under the following award numbers by region – Asia-Pacific: U01AI069907; Caribbean, Central and South America (CCASAnet): U01AI069923; Southern Africa: U01AI069924; and North America (NA-ACCORD): U01AI069918, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24DA000432, K24AI065298, KL2TR000421, M01RR000052, N02CP055504, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA004334, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided to NA-ACCORD by the Intramural Research Program of the National Cancer Institute. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney (The University of New South Wales). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above. We thank all patients and their families, and all staff at participating sites for preparation of data contributed to this collaborative work. We also thank the IeDEA-WHO Collaboration for their guidance and expertise.
Footnotes
Conflicts of interest: All authors stated that they have no conflicts of interest.
Presentation of data: Oral presentation: The Australasian HIV&AIDS Conference, November 17th 2016, Adelaide – Australia.
References
- 1.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. [Accessed 30 September, 2015];2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 2.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection: Recommendations for a public health approach. [Accessed January 8, 2014];2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 3.IeDEA. The International Epidemiologic Databases to Evaluate AIDS network. [Accessed 30 October, 2015];2015 http://www.iedea.org/
- 4.Duda SN, Farr AM, Lindegren ML, et al. Characteristics and comprehensiveness of adult HIV care and treatment programmes in Asia-Pacific, sub-Saharan Africa and the Americas: results of a site assessment conducted by the International epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. J Int AIDS Soc. 2014;17:19045. doi: 10.7448/IAS.17.1.19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Althoff KN, Rebeiro PF, Hanna DB, et al. A picture is worth a thousand words: maps of HIV indicators to inform research, programs, and policy from NA-ACCORD and CCASAnet clinical cohorts. J Int AIDS Soc. 2016;19(1):20707. doi: 10.7448/IAS.19.1.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. Global AIDS Update. 2016 [Google Scholar]
- 7.Department of Health and Human Services; [Accessed April 27, 2016]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. [Accessed January 18, 2017];2016 Jun; http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- 9.Mberi MN, Kuonza LR, Dube NM, Nattey C, Manda S, Summers R. Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004-2012: a cohort study. BMC health services research. 2015;15:259. doi: 10.1186/s12913-015-0912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86(7):559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis on HIV. [Accessed 22 January, 2016];2015 http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. [PubMed]
- 12.Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries. MMWR Morbidity and mortality weekly report. 2015;64(46):1287–1290. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23(6):697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawizza HE, Chaplin B, Meloni ST, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MA, Boulle A, Eley B, et al. Accuracy of immunological criteria for identifying virological failure in children on antiretroviral therapy - the IeDEA Southern Africa Collaboration. Tropical medicine & international health : TM & IH. 2011;16(11):1367–1371. doi: 10.1111/j.1365-3156.2011.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann CJ, Maritz J, van Zyl GU. CD4 count-based failure criteria combined with viral load monitoring may trigger worse switch decisions than viral load monitoring alone. Tropical medicine & international health : TM & IH. 2016;21(2):219–223. doi: 10.1111/tmi.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozzi-Lepri A, Paredes, Phillips AN, et al. The rate of accumulation of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance in patients kept on a virologically failing regimen containing an NNRTI*. HIV Med. 2012;13(1):62–72. doi: 10.1111/j.1468-1293.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Mu W, Harwell J, et al. Drug resistance profiles among HIV-1-infected children experiencing delayed switch and 12-month efficacy after using second-line antiretroviral therapy: an observational cohort study in rural China. J Acquir Immune Defic Syndr. 2011;58(1):47–53. doi: 10.1097/QAI.0b013e318229f2a2. [DOI] [PubMed] [Google Scholar]
- 19.Mekuria LA, Nieuwkerk PT, Yalew AW, Sprangers MA, Prins JM. High level of virological suppression among HIV-infected adults receiving combination antiretroviral therapy in Addis Ababa, Ethiopia. Antivir Ther. 2016 doi: 10.3851/IMP3020. [DOI] [PubMed] [Google Scholar]
- 20.Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. 2014;67(2):e67–75. doi: 10.1097/QAI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya”. J Acquir Immune Defic Syndr. 2011;57(3):e40–46. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McManus H, Petoumenos K, Brown K, et al. Loss to follow-up in the Australian HIV Observational Database. Antivir Ther. 2014 doi: 10.3851/IMP2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ie DEAPWG. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa--the International epidemiologic Databases to Evaluate AIDS (IeDEA) J Int AIDS Soc. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shet A, Neogi U, Kumarasamy N, DeCosta A, Shastri S, Rewari BB. Virological efficacy with first-line antiretroviral treatment in India: predictors of viral failure and evidence of viral resuppression. Tropical medicine & international health : TM & IH. 2015;20(11):1462–1472. doi: 10.1111/tmi.12563. [DOI] [PubMed] [Google Scholar]
- 26.Huang P, Tan J, Ma W, et al. Outcomes of antiretroviral treatment in HIV-infected adults: a dynamic and observational cohort study in Shenzhen, China, 2003-2014. BMJ open. 2015;5(5):e007508. doi: 10.1136/bmjopen-2014-007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collazos J, Asensi V, Carton JA, Grupo Espanol para el Estudio Multifactorial de la A. Association of HIV transmission categories with sociodemographic, viroimmunological and clinical parameters of HIV-infected patients. Epidemiol Infect. 2010;138(7):1016–1024. doi: 10.1017/S0950268809991282. [DOI] [PubMed] [Google Scholar]
- 28.Graham SM, Mugo P, Gichuru E, et al. Adherence to antiretroviral therapy and clinical outcomes among young adults reporting high-risk sexual behavior, including men who have sex with men, in coastal kenya. AIDS and behavior. 2013;17(4):1255–1265. doi: 10.1007/s10461-013-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiamsakul A, Kumarasamy N, Ditangco R, et al. Factors associated with suboptimal adherence to antiretroviral therapy in Asia. J Int AIDS Soc. 2014;17(1):18911. doi: 10.7448/IAS.17.1.18911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortego C, Huedo-Medina TB, Santos P, et al. Sex differences in adherence to highly active antiretroviral therapy: a meta-analysis. AIDS care. 2012;24(12):1519–1534. doi: 10.1080/09540121.2012.672722. [DOI] [PubMed] [Google Scholar]
- 31.Puskas CM, Kaida A, Miller CL, et al. The adherence gap: a longitudinal examination of men's and women's antiretroviral therapy adherence in British Columbia, 2000-2014. AIDS. 2017;31(6):827–833. doi: 10.1097/QAD.0000000000001408. [DOI] [PubMed] [Google Scholar]
- 32.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. The New England journal of medicine. 1997;336(19):1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 33.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–278. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker AS, Doerholt K, Sharland M, Gibb DM, Collaborative HIVPSSC Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18(14):1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 35.Glass TR, Sterne JA, Schneider MP, et al. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS. 2015;29(16):2195–2200. doi: 10.1097/QAD.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 36.Teasdale CA, Abrams EJ, Coovadia A, Strehlau R, Martens L, Kuhn L. Adherence and viral suppression among infants and young children initiating protease inhibitor-based antiretroviral therapy. The Pediatric infectious disease journal. 2013;32(5):489–494. doi: 10.1097/INF.0b013e31827e84ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 - Factors associated with viral load suppression at one year from ART initiation, adult analysis using strict intention-to-treat method
Supplementary Table 2 - Factors associated with viral load suppression at one year from ART initiation, paediatric analysis using strict intention-to-treat method
Supplementary Table 3: Factors associated with viral load failure at one year from ART initiation, adult analysis using modified intention-to-treat method
Supplementary Table 4: Factors associated with viral load failure at one year from ART initiation, paediatric analysis using modified intention to treat method
Supplementary Table 5: Patient characteristics in adults by region
Supplementary Table 6: Patient characteristics in children by region
Supplementary Figure 1: Flow chart for adult strict ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 2: Flowchart for adult modified ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 3: Flow chart for paediatric strict ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.
Supplementary Figure 4: Flow chart for paediatric modified ITT analyses
Abbreviations: LTFU – loss to follow-up ; VL – viral load.


