Abstract
Background
Placebo-controlled HIV-1 prevention trials of pre-exposure prophylaxis (PrEP) have not generally used concurrent measurement of adherence due to the potential risk of unblinding. However, several PrEP trials for HIV-1 prevention among women failed to show effectiveness due to low product adherence. Evaluation of product adherence objectively during a study provides the opportunity for strengthening adherence activities at sites having low adherence.
Methods
During MTN-020/ASPIRE, a phase III, placebo-controlled trial of the dapivirine intravaginal ring, we implemented an adherence monitoring system. Monitoring began in Quarter 1 (Q1) 2013 and continued through the conclusion of the trial. Blood plasma was collected quarterly and tested for dapivirine concentrations while maintaining blinding among study team members involved in participant management. Dapivirine concentrations > 95 pg/mL, reflecting > 8 hours of continuous use, were assessed as signaling product use. Study leadership monitored results on a monthly basis and provided feedback to site investigators. Experiences were shared across sites to motivate staff and counsel participants to strive toward higher adherence levels.
Results
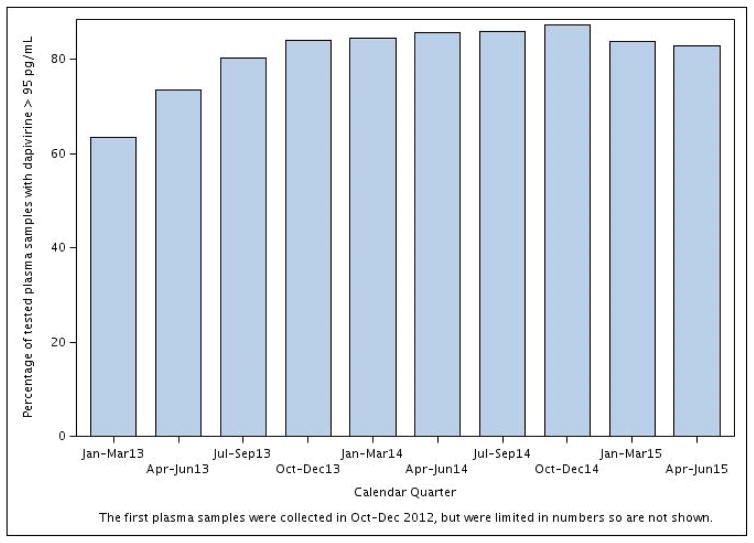
An upward trend in adherence was observed (p < 0.0001); the proportion of samples from subjects in the active arm with dapivirine > 95 pg/mL increased from 63% in Q1 2013 to 84% by Q1 2015.
Conclusions
Ongoing drug level testing as a marker of adherence in MTN-020/ASPIRE demonstrates the feasibility of real-time adherence monitoring while maintaining study blinding at the level of participants, sites, and study leadership. This approach is novel for large-scale effectiveness studies for HIV-1 prevention.
Keywords: HIV-1, adherence, dapivirine, women, pre-exposure prophylaxis, microbicide, intravaginal ring
Introduction
In Africa and worldwide, women account for more than half of all people living with HIV-1, and young women in Africa can often face strikingly high HIV-1 risk.1 Development of effective prevention options for women is thus a global health priority. Over the past decade, a number of studies have evaluated novel antiretroviral-based prevention strategies, including topical vaginal microbicides and oral pre-exposure prophylaxis (PrEP), for HIV-1 prevention in young African women. In three placebo-controlled, pivotal studies of oral tablet and vaginal gel formulations of the antiretroviral agent tenofovir among young African women, adherence to the investigational products was extremely low and HIV-1 protection was not seen,2–4 in contrast, these products demonstrated efficacy for HIV-1 protection in other studies where adherence was higher.5–10 Although all of these PrEP studies used multiple methods to evaluate product adherence while the trials were ongoing, including self-reported use and counts of unused study product returns, those approaches were subsequently found to have substantially over-estimated adherence, when compared with measurement of the active investigational medication in plasma or cervicovaginal fluids.2–4 The surprising blood and cervicovaginal testing results were not consistent with pre-study expectations that the target population had a high willingness to adhere to product use.11
The discovery of very low adherence on biologic samples tested after completion of tenofovir-based prevention trials among women was a surprise to the HIV prevention field,12 as an implicit assumption of randomized trials has been that study participants who regularly attend monthly study visits, have pregnancy and HIV testing, and receive risk reduction and product adherence counseling will also use investigational study products. Moreover, no difference was seen in women’s tendency to accurately report non-adherence at the study termination visit and at routine follow-up visits underscoring that women in resource limited settings often join studies for the benefits of study participation and fear that by revealing product non-use their study participation could be jeopardized.13 Strategies to evaluate adherence objectively during the study provides an opportunity for study investigators to strengthen adherence counseling, adjust study enrollment at some sites, or even terminate a study if low adherence suggests futility. However, blinded, placebo-controlled HIV-1 prevention clinical trials have not generally implemented real-time monitoring of adherence using measures of the active investigational medication due to the costs and the potential risk of unblinding of study leadership, clinical staff, and participants to randomization arm assignments.
An intravaginal ring containing the antiretroviral agent dapivirine was developed as a novel topical microbicide and tested in phase I-III trials,14–17 including two recently-completed phase III trials, MTN-020/ASPIRE18 and IPM 027/The Ring Study.19 Within the MTN-020/ASPIRE trial, we implemented an adherence monitoring system, concurrent with the conduct of the trial, using measurement of dapivirine concentrations in plasma as an objective adherence marker. Here we describe and assess the effectiveness of this adherence monitoring system.
Methods
Population and study procedures
The MTN-020/ASPIRE study was a randomized, double-blind, placebo-controlled phase III safety and effectiveness trial of a vaginal matrix ring containing dapivirine for the prevention of HIV-1 infection in African women (ClinicalTrails.gov number NCT01617096). Study design, methods and results are described elsewhere.18,20 In brief, study enrollment began in August 2012 and was completed in June 2014 at 15 sites in four countries (Malawi, Uganda, South Africa, and Zimbabwe); follow-up was completed in June 2015. At enrollment, participants were randomized in a 1:1 fashion to blinded active dapivirine vaginal ring or placebo. Thereafter, they attended monthly follow-up visits at which HIV-1 serologic testing, product resupply, and questionnaire-based assessments of sexual behavior and adherence were completed. Plasma samples to be used for measurement of dapivirine concentrations were collected and archived on a quarterly basis.
At the enrollment visit, participants were taught how to insert and remove the vaginal ring, and clinic staff confirmed via a digital examination that the ring was properly in place prior to the participant leaving the clinic. Clinic staff counseled participants to leave the ring in place throughout the four week period between scheduled monthly follow-up visits and to return to the clinic with the ring in place at their next monthly visit. Participants were counseled to reinsert a clean ring any time it fell out or was taken out and to contact the clinic to receive a new ring if needed. Other than male or female condoms and tampons during menses, the participant was told to avoid use of all other vaginal products, since they could impact drug levels. Regular, ongoing ring use was stressed and additional rings were provided if a participant anticipated not being able to attend a regularly scheduled monthly visit. All sites received institutional review board approval and participants provided written informed consent.
Rationale to initiate ongoing monitoring of adherence
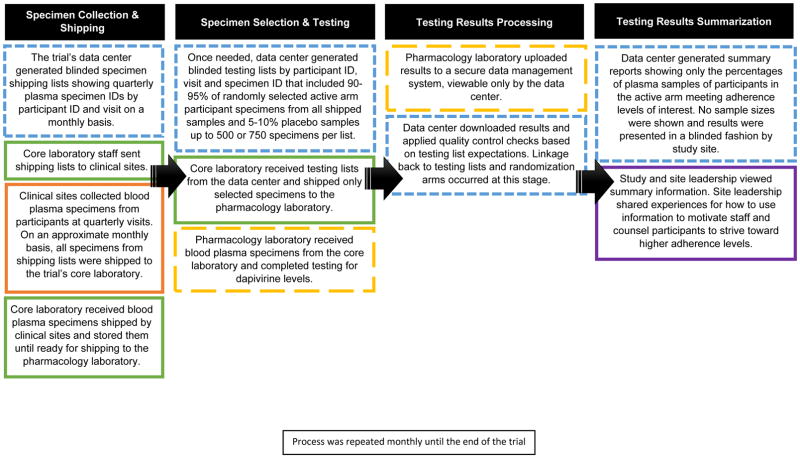
Prior to initiation of MTN-020/ASPIRE, the study’s leadership team determined that an adherence monitoring program concurrent to study implementation could prove beneficial to the overall conduct of the trial. Such a program would have to preserve blinding to arm assignments for investigators and participants, and otherwise maintain the overall integrity of the study data. Prior to implementation, a standardized approach to monitor adherence during the study by testing plasma samples for dapivirine was designed and presented to the trial’s independent Data and Safety Monitoring Board. During the first three months after the study initiated, the adherence monitoring system was commenced. Importantly, seven months after initiation of the MTN-020/ASPIRE study (March 2013), the results of the MTN-003/VOICE trial were reported publicly, revealing very low adherence to tenofovir-based PrEP as measured by objective levels of drug in blood plasma and reinforcing the need for an adherence monitoring strategy for MTN-020/ASPIRE.2 Figure 1 displays a flow diagram of the steps involved and entities responsible for implementation of the real-time adherence monitoring system.
Figure 1.
Flow diagram of MTN-020/ASPIRE real time adherence monitoring system1,2
1 Orange represents the clinical sites, blue represents the data center, green represents the core laboratory, yellow represents the pharmacology testing laboratory, and purple represents the MTN-020/ASPIRE study leadership team.
2 Solid lines indicate that study blinding, defined as the identification of the linkage of participant identifiers (IDs) to the randomization arm, is upheld by staff for the organizations indicated by the color of the box. Small dashed lines indicate that randomization assignments are linked by participant ID so therefore staff are unblinded to the randomization assignment by participant ID. Large dashed lines indicate that the randomization arm may be deduced based on levels of blood plasma found through dapivirine test results so therefore staff were considered partially blinded to the randomization assignment.
Specimen collection and shipping
The trial’s data center staff created lists of aliquots of blood plasma specimens, referred to as specimen shipping lists. The shipping lists included both the active dapivirine and placebo arm participants, and were based on specimens known in the trial’s database to have been collected and archived from quarterly participant visits. The data center staff posted the shipping lists to a secure website on a monthly basis where core laboratory staff retrieved and sent them to the study sites. Site staff subsequently prepared shipments of plasma samples and sent them to the core laboratory.
Specimen selection and testing
Prior to January 2014, all available plasma aliquots from both the active and placebo arms were shipped from the core laboratory and tested at the pharmacology laboratory on a monthly basis. Due to cost considerations, in February 2014, the data center created testing lists containing randomly selected specimens by participant ID, visit and specimen ID, with approximately 90–95% of available specimens from active dapivirine arm participants and the remaining 5–10% from randomly-selected specimens from placebo arm participants. The number of specimens was capped at 500 initially and later at 750, the maximum affordable for testing in a month at the pharmacology laboratory. Core laboratory staff sent selected specimens to the pharmacology laboratory where dapivirine testing was completed. The relative proportions of active and placebo arm specimens were known only to the data center staff.
Laboratory testing methods
Plasma specimens were tested using a validated ultra-high pressure liquid chromatographic-tandem mass spectrometric (UHPLC-MS/MS) method.21 Lower (LLOQ) and upper (ULOQ) limits of quantification for the assay were 20 pg/mL and 10,000 pg/mL, respectively. Values below the LLOQ were reported as below the limit of quantification of the assay; no results were above the assay ULOQ. The assay was validated in accordance with the FDA, Guidance for Industry Bioanalytical Method Validation recommendations.22
Testing results processing
Pharmacology laboratory staff uploaded plasma test results to a secure data management system where data center staff downloaded, conducted data quality checks, and linked results to randomization arm assignments. Other members of the study team, including leadership and all site staff, did not have access to the raw or linked data. Instead, the data center staff summarized results showing only the percentages and no sample sizes from plasma samples among participants in the active arm where dapivirine was > 20 pg/mL and > 95 pg/mL for each study site, and overall. The > 95 pg/mL level was chosen as an indicator for use of the vaginal ring for at least eight hours, and to exclude those who inserted the ring just prior to the study visit. Prior studies showed that plasma dapivirine levels reached > 95 pg/mL with use of 8 hours or more.15
Testing results summarization
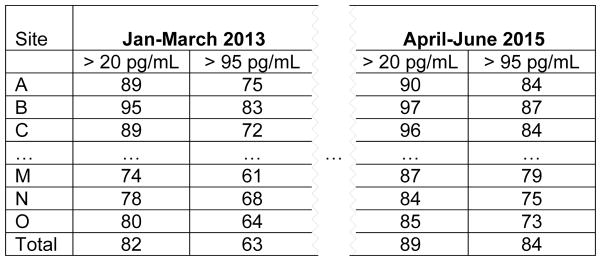
On a monthly basis, data center staff prepared summary reports of the plasma dapivirine results and communicated them to study leadership who, in turn, distributed the results to the study site leadership. The data were presented across calendar quarters overall, and by study site since interest was in changes in adherence behavior over calendar time. The first results were shared with study sites in March 2013 and continued on a monthly basis for the duration of the study. Figure 2 shows an example of how the tabular results were summarized. Study site leadership were given only their site’s letter code and could see the relative ranking of their performance over time in a blinded fashion. Only this summary information was provided – no information on adherence by individual participants was given, either to study site teams or to the study leadership. At the study sites, adherence data were used to modify counseling messages, motivate staff, and create opportunities for participant engagement in the research process. As reported previously,18 adherence data were also used to close enrollment at two sites where adherence and quality of study conduct was suboptimal.
Figure 2.
Example of summary table presented to study sites – percentage of plasma samples among participants in the active arm with dapivirine > 20 pg/mL and > 95 pg/mL
Preserving the study blind
At each step, preserving study blind was considered essential and only the unblinded statistical staff within the data center had knowledge of the adherence results and randomization arm assignment of individual subjects. Initially when all samples were shipped, approximately equal numbers of specimens from active and placebo arm participants were tested making identification of arm assignment impossible. Later in the study, specimens in the placebo arm were randomly distributed throughout specimen testing lists using the parameters discussed previously, again making it impossible to identify a participant’s arm assignment. The pharmacology laboratory staff who conducted the drug level testing had sample identifying information based on test results only but did not have contact with the study sites and only shared results directly with the data center. Thus, study participants, clinical staff, site leadership, and study leadership remained blinded throughout the entire adherence monitoring process.
Communications within the study team
Regular, on-going communications between and within organizations was required. A weekly conference call was conducted between the core laboratory and the data center to work through any challenges in sample identification, testing, and data processing. Communications occurred regularly between the core, site and pharmacology laboratories, and data quality checks were on-going between the data center and laboratories. In addition, the protocol coordination center organized a monthly call between study and site leadership to discuss results.
Statistical analysis
To assess the feasibility of this adherence monitoring system, percentages of samples with dapivirine detected are summarized overall by quarterly calendar time. Plasma results from participants who were on a product hold or off product due to pregnancy are included. Changes in adherence to ring use are assessed over calendar time using generalized estimating equations with a logit link and exchangeable working correlation structure from all available plasma dapivirine test results. Analyses were conducted in SAS, version 9.4 (SAS Institute).
Results
A total of 2,629 women were enrolled in MTN-020/ASPIRE. Their median age was 26 years (IQR 22–31), and median follow-up was 1.6 years (IQR 1.1–2.3). From October 2012 through January 2014 all quarterly plasma samples were shipped and tested; 4,618 total with 2,322 in the active arm and 2,296 in the placebo arm. From February 2014 until the end of the study in June 2015 only selected samples were tested; 8,548 total with 7,036 in active arm and 1,512 in the placebo arm.
Adherence as defined by dapivirine levels of > 95 pg/mL of plasma steadily increased across the 15 study sites (Figure 3). An upward linear trend was observed over calendar time, from 63% during Quarter 1 2013 to 84% during Quarter 1 2015 (p < 0.0001).
Figure 3.
Percentage of tested plasma samples among participants in the active arm across all 15 study sites with dapivirine > 95 pg/mL by calendar quarter beginning January 2013
Discussion
In this phase III, blinded, placebo-controlled study evaluating a vaginal ring containing 25 mg of dapivirine, a novel real-time adherence monitoring system was shown to be feasible and the feedback and corrective actions taken as a result of this feedback were temporally associated with improved adherence to vaginal ring use. Maintaining blinding to study product assignments for study participants, site staff, and study leadership was considered an essential aspect of the feasibility of this novel system, and was shown to be possible. The success of the system also involved regular, on-going communication between the trial’s data center, laboratories, study site staff, and study site leadership. To our knowledge, this is the first example to report on the process for and outcomes of real-time adherence monitoring in a blinded clinical trial of an HIV-1 prevention agent.
Other HIV-1 prophylaxis studies in African women have observed a strong relationship between adherence and HIV-1 protection, with two prominent studies finding no protection and very low adherence, which were only discovered after the trials were completed.2,3 Those studies did not conduct real-time adherence monitoring, thus did not have the opportunity to modify their approaches to actively promote adherence, as was the case in this study. The VOICE study, found that clinic-based product counts, face-to-face interviews and audio computer-assisted self-interviewing widely overestimated adherence (86%, 90%, 88% respectively) compared to post-hoc testing of tenofovir levels in plasma (<30%).2 Qualitative examination in the VOICE study and other studies found several factors that influenced adherence to PrEP such as uncertainty of whether the investigational drug was efficacious, ambivalence about randomization that included a placebo, differences in participants’ perceived HIV risk, uncertainty about use of an antiretroviral drug for HIV prevention, concerns about side effects, stigma, and partner support and/or acceptance of their participation in the study.23–26 Moreover, motivation to take part in a phase III trial may have been based on interest in access to health services, and fees for participation rather than actual use of the investigational products, although participants were reluctant to disclose non-use due to fears of termination of current or disqualification from future research.12 Additional qualitative studies showed that participants indicated that individual, social and structural factors were barriers to high adherence to PrEP,25 and the use of a pictorial tool that supported discussion about drug levels between clinic staff and participants helped elucidate patterns of drug use and adherence behaviors.12 Several of these qualitative studies concluded that the use of objective measures for adherence would result in a more accurate account of participants’ true levels of use, and that evaluating objective measures of adherence in real-time were warranted.2,11–13
A qualitative sub-study conducted at 6 of the 15 study sites evaluated the acceptability of and adherence to the dapivirine vaginal ring.27 Salient findings showed first that although participants initially feared use of the vaginal ring, they grew to like it and had a sense of ownership and ease of use over time. Second, support by staff and their peers aided in the uptake and sustained use of the ring. Participants developed a sense of contributing to a common good and felt part of a team at their study site, due to blinded adherence feedback. Over time, the women developed a sense of ownership to a shared cause of contributing to a large-scale study of a novel HIV prevention tool with the potential to show efficacy and eventual licensure for future use in their communities. Last, male partner relationship dynamics were the most prevalent determinant for participants’ acceptability and willingness to use the ring. If their partners were accepting of their participation, and participated in site activities promoting adherence, they were more likely to stay committed and adhere. These qualitative results support the notion that changes in adherence over study follow-up stemmed from the feedback provided by the real-time adherence monitoring system implemented in MTN020/ASPIRE.
An important goal in randomized, placebo-controlled phase III trials is to obtain an unbiased estimate of the true effect of treatment. Intent-to-treat analyses, the required primary analysis for regulatory submission of an investigational product, is based on participants’ original randomization assignment, or equivalently, the policy of receiving one treatment versus another, regardless of adherence to assigned product, time off product, cross-over and loss to follow-up.28 Adherence to assigned product during a phase III trial is, thus, a very important aspect of study conduct that ensures an intent-to-treat effect estimate is closest to a causal estimate. For example, when adherence to daily use of oral PrEP tenofovir-based products was shown to be high in four out of six trials in serodiscordant couples, injection drug users in Thailand, men who have sex with men, and heterosexual men and women intent-to-treat analyses were found to be statistically significant and ranged from 44–75%.5,7,9,10 Adherence in these studies was > 50% and a linear relationship between effectiveness estimates and adherence was seen. Furthermore, additional analyses among active arm participants where tenofovir was present in plasma showed increased effectiveness estimates ranging from 85–100%.5,6,9 Two trials, VOICE and FEM-PrEP, with detection levels of tenofovir in plasma of < 30% were not able to show an effect in intent-to-treat analyses of similar products.2,3 These results underscore the importance of improving adherence in phase III trials in order to provide an unbiased estimate of effect.
Notably, a statistically significant estimate of HIV-1 protection was found in MTN-020/ASPIRE. The overall effectiveness of the dapivirine vaginal ring in providing protection against HIV-1 infection over the duration of the trial was 27%, 37% after excluding the data from two sites with the poorest adherence to product and study visits, and 56% in women over 21 years of age.18 In other HIV-1 studies of treatment and prevention it has been observed that use of product declines over time.8,29 However, in this study, we observed an increase in product use in calendar time from 63–84% based on plasma measures, suggesting that real-time adherence monitoring could promote and sustain participant behaviors that lead to product adherence. The difference between this degree of adherence (63–84%) and the levels of HIV-1 protection seen (27–56%) could indicate that plasma dapivirine levels are an imperfect reflection of adherence, potential white coat dosing30, or the maximum HIV-1 protection provided by the dapivirine vaginal ring, even with perfect use, may be less than 100%. Ongoing exploratory analyses are assessing the relationship between adherence and HIV-1 protection for this product.18
Whether it is feasible to monitor and counsel for adherence in the “real-world” roll out of PrEP is an important question to answer, and is currently under investigation in a phase IV, prospective study among young African HIV-uninfected woman.31 Standard behavioral adherence support will be compared to an enhanced version including counseling based on real-time drug levels. The MTN-025/HOPE study, a phase IIIB open-label follow-on study examining safety and adherence to the dapivirine vaginal ring is also underway.32 Study staff are providing drug levels to participants choosing to use the ring starting one month from enrollment and until the 12-month follow-up visit. Furthermore, tenofovir urine testing was found to be effective for assessing adherence and work is currently underway to develop point of care urine assays.33 These initiatives will help to answer the question of feasibility of monitoring and counseling for adherence in a “real-world” setting among women using PrEP.
There were limitations to this adherence monitoring approach. First, only the active arm participants’ data was used in evaluating adherence. If a comparable placebo-arm adherence biomarker were available, estimates of overall ring use would have been possible. Second, although plasma dapivirine level is one objective measure of adherence, and the level of 95 pg/mL is a lower bound chosen based on previous phase 1 and 2 studies of the dapivirine ring14–16, this measure was likely not a perfect indicator of adherence across a full month, nor use around the time of HIV-1 exposure. Moreover, because women were expected to arrive for clinic visits with the ring in place, white coat dosing30 could have signaled adherence for participants who were otherwise non-adherent. With further pharmacokinetic studies, improvements could be made to prevent misclassification, as has been done in clinical trials of tenofovir-based strategies.29,34 Third, we measured plasma adherence levels only at quarterly visits, although ultimately our goal was to characterize participants’ adherence profile throughout the monthly visit window. One measure does not suffice to adequately capture patterns of ring use over the entire quarter.
Women in Africa remain at high risk for HIV-1 and the dapivirine ring has been shown to provide protection in a phase III trial with concurrent implementation of an adherence monitoring system. Future large-scale effectiveness studies for HIV-1 prevention, including those for microbicidal drugs delivered through a vaginal ring, should consider implementing a real-time adherence monitoring system to improve the validity of HIV-1 effectiveness estimates.
Acknowledgments
Sources of support: The MTN-020/ASPIRE study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were supplied by the International Partnership for Microbicides (IPM).
We wish to thank the MTN-020/ASPIRE study team, MTN leadership and MTN Laboratory Center staff, as well as the dedication of all study participants for making this research possible.
MTN-020/ASPIRE Study Team
Study Team Leadership: Jared Baeten, University of Washington (Protocol Chair); Thesla Palanee-Phillips, Wits Reproductive Health and HIV Institute (Protocol Co-chair); Elizabeth Brown, Fred Hutchinson Cancer Research Center (Protocol Statistician); Lydia Soto-Torres, US National Institute of Allergy and Infectious Diseases (Medical Officer); Katie Schwartz, FHI 360 (Clinical Research Manager)
Study sites and site Investigators of Record:
Malawi, Blantyre site (Johns Hopkins University, Queen Elizabeth Hospital): Bonus Makanani;
Malawi, Lilongwe site (University of North Carolina, Chapel Hill): Francis Martinson
South Africa, Cape Town site (University of Cape Town): Linda-Gail Bekker;
South Africa, Durban – Botha’s Hill, Chatsworth, Isipingo, Tongaat, Umkomaas, Verulam sites (South African Medical Research Council): Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, and Nitesha Jeenarain;
South Africa, Durban, eThekwini site (Center for the AIDS Programme for Research in South Africa): Gonasagrie Nair
South Africa, Johannesburg site (Wits RHI): Thesla Palanee-Phillips
Uganda, Kampala site (John Hopkins University, Makerere University): Flavia Matovu
Zimbabwe, Chitungwiza, Seke South and Zengeza sites (University of Zimbabwe, University of California San Francisco): Nyaradzo Mgodi
Zimbabwe, Harare, Spilhaus site (University of Zimbabwe, University of California San Francisco): Felix Mhlanga
Data management was provided by The Statistical Center for HIV/AIDS Research & Prevention (Fred Hutchinson Cancer Research Center, Seattle, WA) and site laboratory oversight was provided by the Microbicide Trials Network Laboratory Center (Pittsburgh, PA).
Footnotes
Meetings where data were presented: HIV Research for Prevention Conference, Chicago, Illinois, October, 2016.
Conflicts of Interest and Source of Funding: Dr. Craig Hendrix is receiving funding through a grant or contract managed through Johns Hopkins University from the NIH, Gates Foundation, ViiV/GSK, and Gilead for conduct of clinical research. Dr. Sharon Hillier received honoraria from Merck, Cepheid and Symbiomix, LLC. For the remaining authors none were declared.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir based pre-exposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees H, Delany-Moretlwe SA, Lombard C, Baron D, Panchia R, Myer L. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women Conference on Retroviruses and Opportunitic Infections (CROI) [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberer JE, Baeten JM, Campbell J, et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. PLOS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 8.Karim AQ, Karim AS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant RM, Lama JR, Anderson PL, et al. Pre-exposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral pre-exposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 11.Mensch BS, van der Straten A, Katzen LL. Acceptability in microbicide and PrEP trials: current status and a reconceptualization. Curr Opin HIV AIDS. 2012;7:534–41. doi: 10.1097/COH.0b013e3283590632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence: results from a qualitative study. AIDS. 2015;29(16):2161–71. doi: 10.1097/QAD.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mensch BS, Brown ER, Liu K, et al. Reporting of Adherence in the VOICE Trial: Did Disclosure of Product Nonuse Increase at the Termination Visit? AIDS Behav. 2016;20:2654–2661. doi: 10.1007/s10461-016-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. Acquir Immune Defic Syndr. 2009;51(4):416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 15.Nel A, Haazen W, Nuttall J, et al. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS. 2014;28(10):1479–1487. doi: 10.1097/QAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 16.Nel AM, Haazen W, Nuttall JP, Romano JW, Mesquita PMM, et al. Pharmacokinetics and Safety Assessment of Anti-HIV Dapivirine Vaginal Microbicide Rings with Multiple Dosing. J AIDS Clin Res. 2014;5:355. [Google Scholar]
- 17.Chen B, Panther L, Marzinke M, et al. Phase I safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr. 2015;70(3):242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel A, van Niekerk N, Kapiga S, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med. 2016;375:2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 20.Palanee-Phillips T, Schwartz K, Brown ER, et al. Characteristics of Women Enrolled into a Randomized Clinical Trial of Dapivirine Vaginal Ring for HIV-1 Prevention. PLOS ONE. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seserko LA, Emory JF, Hendrix CW, et al. The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis. 2013;5(22):2771–2783. doi: 10.4155/bio.13.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, US FDA. Guidance for Industry: Bioanalytical Method Validation. US FDA; Rockville, MD, USA: Center for Drug Evaluation and Research, Center for Veterinary Medicine; 2001. [Google Scholar]
- 23.Ware NC, Wyatt MA, Haberer JE, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. JAIDS. 2012;59(5):463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amico KR, Mansoor LE, Corneli A, et al. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS and behavior. 2013;17(6):2143–2155. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Straten A, Stadler J, Luecke E, et al. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17(Suppl 2):19146. doi: 10.7448/IAS.17.3.19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery ET, van der Straten A, Chitukuta M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017 May 15;31(8):1159–67. doi: 10.1097/QAD.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsiatis A. Analysis and interpretation of trial results: Intent-to-treat analysis. JAIDS. 1990;3(Suppl2):S120–S123. [PubMed] [Google Scholar]
- 29.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. JAIDS. 2014;66:340–8. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podsadecki TJ, Vrijens BC, Tousset EP, et al. “White Coat Compliance” Limits the Reliability of Therapeutic Drug Monitoring in HIV-1—Infected Patients. HIV Clinical Trials. 2008;9(4):238–46. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 31.Hosek S, Celum C, Wilson CM, et al. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. Journal of the International AIDS Society. 2016;19(7Suppl 6) doi: 10.7448/IAS.19.7.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiweewa Flavia Matovu, Mugwanya Kenneth K, Kiweewa Francis. Anti-Retroviral–Based HIV Pre-Exposure Prophylaxis for Women: Recent Advances and Next Steps. In: Dumais Nancy., editor. HIV/AIDS - Contemporary Challenges. InTech; 2017. Available from: https://www.intechopen.com/books/hiv-aids-contemporary-challenges/anti-retroviral-based-hiv-pre-exposure-prophylaxis-for-women-recent-advances-and-next-steps. [DOI] [Google Scholar]
- 33.Koenig H, Mounzer K, Daughtridge G, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017 doi: 10.1111/hiv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]