Abstract
Objective
Seizures in temporal lobe epilepsy (TLE) disturb brain networks and lead to connectivity disturbances. We previously hypothesised that recurrent seizures in TLE may lead to abnormal connections involving subcortical activating structures including the ascending reticular activating system (ARAS), contributing to neocortical dysfunction and neurocognitive impairments. However, no studies of ARAS connectivity have been previously reported in patients with epilepsy.
Methods
We used resting-state functional MRI recordings in 27 patients with TLE (67% right sided) and 27 matched controls to examine functional connectivity (partial correlation) between eight brainstem ARAS structures and 105 cortical/subcortical regions. ARAS nuclei included: cuneiform/subcuneiform, dorsal raphe, locus coeruleus, median raphe, parabrachial complex, pontine oralis, pedunculopontine and ventral tegmental area. Connectivity patterns were related to disease and neuropsychological parameters.
Results
In control subjects, regions showing highest connectivity to ARAS structures included limbic structures, thalamus and certain neocortical areas, which is consistent with prior studies of ARAS projections. Overall, ARAS connectivity was significantly lower in patients with TLE than controls (p<0.05, paired t-test), particularly to neocortical regions including insular, lateral frontal, posterior temporal and opercular cortex. Diminished ARAS connectivity to these regions was related to increased frequency of consciousness-impairing seizures (p<0.01, Pearson’s correlation) and was associated with impairments in verbal IQ, attention, executive function, language and visuospatial memory on neuropsychological evaluation (p<0.05, Spearman’s rho or Kendell’s tau-b).
Conclusions
Recurrent seizures in TLE are associated with disturbances in ARAS connectivity, which are part of the widespread network dysfunction that may be related to neurocognitive problems in this devastating disorder.
INTRODUCTION
Temporal lobe epilepsy (TLE) is the most common epilepsy syndrome in which seizures typically arise from limbic temporal lobe structures such as the hippocampus and amygdala.1 Patients with TLE often experience impaired consciousness during seizures (the ictal period), even when seizure activity does not propagate to distal brain regions, and they frequently develop neurocognitive and psychosocial problems that persist even in the absence of seizure activity (the interictal period).2,3 A perplexing question in TLE is why do seizures originating in a focal brain region lead to more global ictal and interictal problems, such as loss of consciousness and neurocognitive impairment, which suggest disturbance of widespread brain networks?
In recent studies using magnetoencephalography (MEG) and functional MRI (fMRI) in patients with TLE, we observed reduced resting-state functional connectivity in widespread neocortical and subcortical areas distal from the epileptogenic zone (EZ), suggesting large-scale network disruption.4,5 Also, our group and others have noted associations between altered connectivity and neuropsychological deficits in this disorder, suggesting that network disturbances may lead to neurocognitive problems.6,7 While the mechanistic underpinnings of diffuse network impairment in TLE remain unknown, we recently postulated that recurrent seizures may lead to abnormal connectivity patterns involving subcortical structures important for cortical activation, leading in turn to aberrant neocortical connectivity and function.8
Our hypothesis regarding interictal neocortical dysfunction in human TLE results in part from ictal studies in rodents showing that the spread of limbic seizure activity to subcortical activating structures leads to neocortical inhibition.9,10 For instance, these animal studies have demonstrated neocortical deactivation with seizure spread to regions within the ascending reticular activating system (ARAS)—a network of brainstem structures critical for arousal and vigilance through its direct and indirect activation of cortical and subcortical regions.9,11,12 However, to our knowledge, no prior study has examined ARAS connectivity patterns in patients with epilepsy versus normal controls.
In the present study, we used resting-state fMRI to examine functional connectivity of ARAS structures in TLE. These ARAS structures included eight pontomesencephalic brainstem regions: the cuneiform/subcuneiform nuclei, dorsal raphe nucleus (DR), locus coeruleus (LC), median raphe nucleus (MR), parabrachial complex (PBC), pontine nucleus oralis (PO), pedunculopontine (tegmental) nucleus (PPN) and ventral tegmental area (VTA). We used an MRI-based atlas of ARAS structures recently developed by others using high angular resolution diffusion imaging together with postmortem microscopic histopathological examination of adult human brains.13 We compared ARAS connectivity patterns in control subjects versus patients with TLE, and related connectivity to disease-related parameters and neuropsychological performance in TLE.
METHODS
Subjects
Study subjects included patients with TLE undergoing evaluation for epilepsy surgery at Vanderbilt University Medical Center between June 2012 and June 2016. Inclusion criterion required a diagnosis of unilateral TLE based on structural imaging with MRI, ictal and interictal electroencephalogram, analysis of seizure semiology and functional imaging with positron emission tomography. Exclusion criteria included structural abnormalities other than hippocampal sclerosis and previous intracranial surgery. Overall, 27 patients and 27 control subjects were included in the study. Healthy control subjects were recruited by email from a database of control subjects maintained by the institution’s imaging centre. Controls had no history of neurological disorders and were individually matched to each patient by age, gender and handedness (table 1). All procedures were approved by the Vanderbilt University Institutional Review Board.
Table 1.
Patient and control subject characteristics
| Patients (n=27) | Controls (n=27) | p-Value | |
|---|---|---|---|
| Age (mean±SD) | 38.2±12.8 | 39.6±13.0 | 0.68 (t-test) |
| Gender (% female) | 51.9 | 51.9 | 1.0 (χ2) |
| Handedness (% right) | 88.9 | 88.9 | 1.0 (χ2) |
| Epilepsy duration, years (mean±SD) | 22.0±2.8 | ||
| Seizure frequency, monthly | |||
| SPS (mean±SD) | 2.7±2.2 | ||
| CPS (mean±SD) | 6.5±1.5 | ||
| GTCS (mean±SD) | 0.4±0.2 | ||
| Side (% right) | 66.7 | ||
| MTS (% yes) | 74.1 |
CPS, complex-partial seizure; GTCS, generalised tonic–clonic seizure; MTS, mesial temporal sclerosis; SPS, simple-partial seizure.
Imaging
Imaging was performed with a Philips Achieva 3T MRI scanner (Philips Healthcare, Best, Netherlands) using a 32-channel head coil. The acquisition included: (1) three-dimensional, T1-weighted whole-brain series for normalisation and tissue segmentation (gradient echo, repetition time (TR)=9.1 ms, echo time (TE)=4.6 ms, 192 shots, flip angle=8°, matrix=256×256, 1×1×1 mm3); (2) two-dimensional, T1-weighted axial image series in the same slice locations as the fMRI scans for functional to structural data coregistration (1×1×4 mm3); (3) T2*-weighted fMRI blood oxygenation level-dependent image series at rest with eyes closed (80×80, field of view=240 mm, 30 axial slices, TE=35 ms, TR=2 s, slice thickness=3.5 mm/0.5 mm gap), with 300 volumes during a 10 min scan. Physiological monitoring of cardiac and respiratory fluctuations was monitored at 500 Hz using the integrated pulse oximeter and the respiratory belt.
Image preprocessing and functional connectivity measurements
fMRI images were preprocessed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and MATLAB v 2016a (The MathWorks, Natick, Massachusetts, USA). Preprocessing steps included slice timing correction, motion correction (three translational and three rotational axes), physiological noise correction using a RETROICOR protocol14 and spatial normalisation to the Montreal Neurological Institute (MNI) template. Spatial smoothing using a 6×6×6 mm3 full width, half maximum Gaussian kernel was also performed for cortical and subcortical regions but not for small brainstem ARAS regions to avoid signal blur. Then, the normalised fMRI time series were temporally low-pass filtered at 0.1 Hz.15 The normalised 3D T1-weighted image was segmented into grey matter, white matter and cerebrospinal fluid components. The average time series over all voxels in an eroded white matter mask was used as a confound for connectivity analyses. Voxel-wise temporal signal-to-noise ratio (tSNR) maps (mean signal/SD, over time) were also created for each fMRI time series.
Regions for functional connectivity analysis included: (1) eight brainstem ARAS structures from the Harvard Ascending Arousal Network Atlas, provided by the Martinos Center for Biomedical Imaging, Charleston, Massachusetts, USA (https://www.martinos.org/resources/aan-atlas); (2) 105 cortical and subcortical regions—including left-sided, right-sided and midline regions—from the Harvard-Oxford atlas, provided by the Harvard Center of Morphometric Analysis, Cambridge, Massachusetts, USA (http://www.fmrib.ox.ac.uk/fsl/), excluding cerebellar regions. The MNI coordinates of structures in the ARAS atlas were provided by previous investigators13 as depicted in online Supplementary figure 1. Functional connectivity was then computed between each ARAS structure and each cortical/subcortical region (8×105) as the partial Pearson’s correlation between the mean preprocessed fMRI time series in each region, with the mean white matter time series and six motion time series as confounds. A Fisher Z-score matrix was then calculated for each subject.
Clinical and neuropsychological data
Patient gender, age, handedness, side of the EZ and the results of neuroimaging or electrographic diagnostic studies were recorded, including the presence or absence of mesial temporal sclerosis on MRI. Details regarding patients’ epilepsy history and seizure semiology, including epilepsy duration, seizure type and frequency, were obtained from comprehensive preoperative clinical assessments by epileptologists. Seizure types investigated included consciousness-impairing seizures, including complex-partial seizures (CPS) and secondarily generalised tonic–clonic seizures (GTCS), as well as consciousness-sparing simple partial seizures (SPS). Patient characteristics are summarised in table 1.
During the presurgical evaluation, patients received a comprehensive neuropsychological examination by a licensed neuropsychologist. The Full Scale IQ Score, Verbal Comprehensions Index (or verbal IQ) and Perceptual Reasoning Index (or performance IQ) were measured using the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV). In addition, the following tests were used, although examinations were customised to each patient at the discretion of the neuropsychologist. Tests evaluating attention and concentration included the Trail Making Test Part A, Digit Span Forward, Digit Span Backward and Digit Span Sequencing. Cognitive processing was assessed using the Working Memory Index Score and Processing Speed Index Score, and evaluation of the Full Scale IQ on the WAIS-IV. Executive function was evaluated using the Wisconsin Card Sorting Test, F-A-SWords, Animal Naming, and Trail Making Test Part B. Assessment of language abilities included the WAIS-IV Vocabulary Scaled Score and the Boston Naming Test or Neuropsychological Assessment Battery Naming Test. Verbal memory was evaluated using the California Verbal Learning Test, part II, and the Wechsler Memory Scale (WMS), Third and Fourth Edition. Finally, visuospatial memory testing included the Continuous Visual Memory Test or WMS-III Family Pictures and the Rey-Osterrieth Complex Figure Text. To summarise results from each of these six batteries and relate performance to functional connectivity patterns, patient performance was estimated as above average (60th–100th percentile), average (40th–60th percentile), low/below average (20th–40th percentile) or severely below average (0–20th percentile) compared with a standard normative population. One patient without available neuropsychological data was excluded.
Statistical analyses
To compare mean connectivity patterns between various ARAS structures within the control group, one-way analysis of variance (ANOVA) was used with Duncan’s multiple range test (MRT) post hoc analysis. To ensure adequate tSNR in the brainstem versus other subcortical regions, we compared mean tSNR in ARAS structures with tSNR of the thalamus using a two-tailed t-test. Mean ARAS connectivity between patients and controls (across all regions, by side and by ARAS structure) was compared using paired two-tailed t-tests with subjects individually matched for age, gender and handedness. Mean connectivity values were calculated within each individual subject prior to statistical comparison of patients versus controls. To relate overall connectivity in each patient with TLE to other factors of interest, ‘global’ ARAS connectivity was estimated as the mean connectivity between all 105 cortical/subcortical regions and all eight ARAS structures. Meanwhile, ‘regional’ ARAS connectivity was estimated as the mean connectivity among the 20 cortical/subcortical regions with the largest differences in connectivity in patients versus controls, across all eight ARAS structures. To examine the relationship between regional or global connectivity and other factors in patients, Pearson’s correlation was used for parametric comparisons (seizure frequency and epilepsy duration), Spearman’s rho for non-parametric testing (IQ scores) and Kendell’s tau-b for ordinal variables (neuropsychological performance). Statistical analyses were performed using MATLAB 2016a and SPSS 22 with significance assessed at p<0.05. Statistical tests were corrected for multiple comparisons using the Bonferroni-Holm method.
RESULTS
ARAS connectivity patterns in non-epileptic control subjects
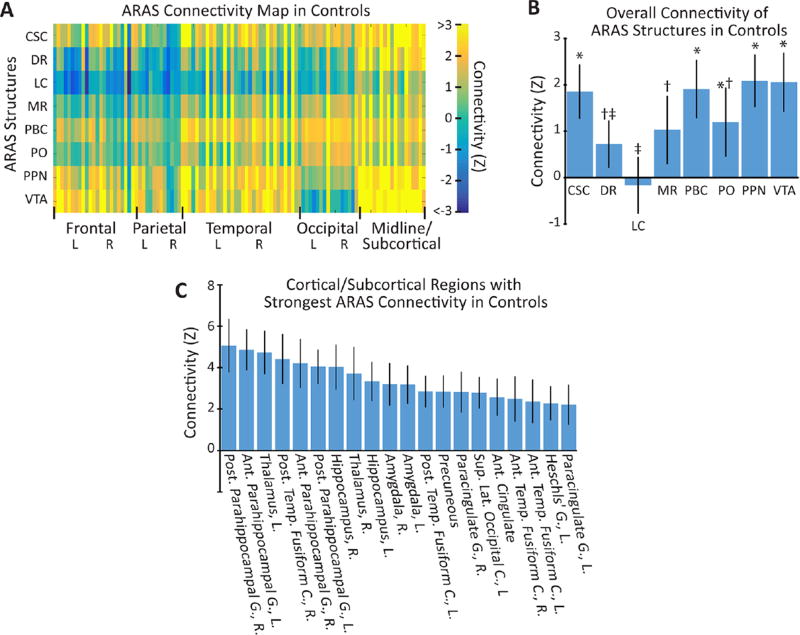
We first examined connectivity and tSNR maps in control subjects, and observed tSNR values in ARAS structures (159±17, mean±95% CI) that was comparable to the bilateral thalami (149±9, mean±95% CI), suggesting adequate signal for analysis (p>0.2, paired t-test). Within controls, we noted mostly positive but also negative network correlations between ARAS structures and cortical/subcortical regions (figure 1A). Across all cortical/subcortical connections, we observed differences in mean connectivity among certain ARAS structures (p<0.001, one-way ANOVA), with the strongest positive connections to cuneiform/subcuneiform nucleus (CSC), PBC, PO, PPN and VTA and weakest connectivity to LC and DR (figure 1B). We then examined which cortical/subcortical regions exhibited the strongest mean positive or negative connectivity to ARAS structures overall. The 20 strongest connectivity relationships (figure 1C) included positive connections to limbic structures (hippocampus, amygdala and parahippocampal gyrus), thalamus and certain mesial neocortical regions including precuneus and anterior cingulate cortex. Other strong relationships (not shown) included positive connections to nucleus accumbens/basal forebrain, as well as negative connections to precentral and postcentral gyrus. These results demonstrate that connectivity patterns in controls are relatively consistent with expected ARAS projections.
Figure 1.
Brainstem ARAS functional connectivity in control subjects (n=27). (A) Colour map representing mean functional connectivity Z-score values between eight ARAS structures and 105 cortical/subcortical regions across all control subjects. (B) Summary of the connectivity (mean±95% CI) for each ARAS structure to all cortical/subcortical regions in controls. Significant variability is observed between the groups (p<0.001, one-way ANOVA), and post hoc analysis (Duncan MRT) revealed three homogeneous but overlapping data subsets in which connectivity strength was similar. These homogeneous but overlapping subsets are indicated by the symbols *, † and ‡. (C) The 20 cortical/subcortical regions with the strongest connectivity (largest positive or negative Z-score) to all ARAS regions (mean±95% CI). Strong positive connectivity is observed to limbic structures, thalamus and certain neocortical regions, with no negative connections observed among the 20 strongest relationships. ANOVA, analysis of variance; Ant, anterior; ARAS, ascending reticular activating system; C, cortex; CSC, cuneiform/subcuneiform nucleus; DR, dorsal raphe nucleus; G, gyrus; L, left; LC, locus coeruleus; lat, lateral; MR, median raphe nucleus; MRT, multiple range test; PBC, parabranchial complex; PO, pontine nucleus oralis; post, posterior; PPN, pedunculopontine nucleus; R, right; sup, superior; temp, temporal; VTA, ventral tegmental area.
ARAS connectivity is diminished in patients with TLE compared with controls
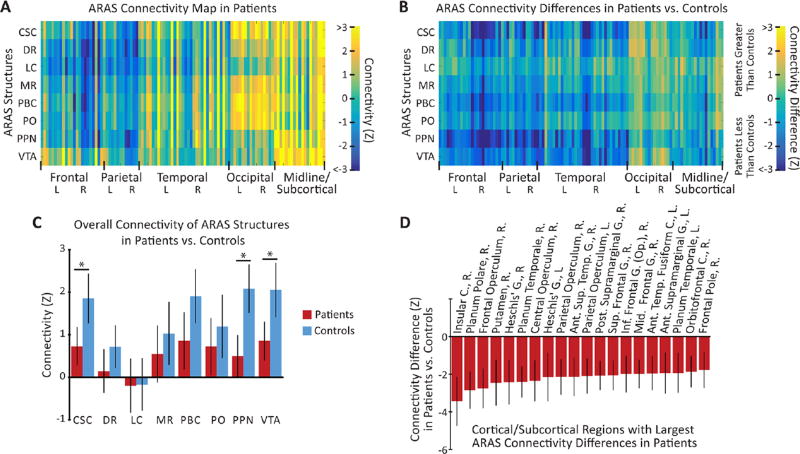
We then compared ARAS connectivity patterns in patients with TLE versus controls. As in control subjects, tSNR values in patients were comparable between ARAS structures (171±18, mean±95% CI) and bilateral thalami (159±13, mean±95% CI), and tSNR values did not significantly differ between patients and controls (p>0.2, paired t-tests). While both positive and negative connectivity relationships were found between ARAS structures and cortical/subcortical regions in patients (figure 2A), patients exhibited an overall decrease in mean ARAS connectivity (p<0.05, paired t-test) compared with controls (figure 2B). Across individual ARAS regions, we observed a significant decrease in mean connectivity to all cortical/subcortical areas from CSC, PPN and VTA in patients (figure 2C). Next, we evaluated which cortical/subcortical regions exhibited the largest overall differences in ARAS connectivity in patients, averaged across all ARAS regions together for summary purposes (figure 2D). Among 20 cortical/subcortical regions with the largest decreases in ARAS connectivity in patients, most areas were neocortical structures (95%) and the majority were lateralised to the right hemisphere (75%), including insular, lateral frontal, posterior temporal and opercular regions (figure 2D).
Figure 2.
ARAS connectivity is diminished in patients with epilepsy (n=27) versus controls (n=27). (A) Colour map representing mean functional connectivity Z-score values between eight ARAS structures and 105 cortical/subcortical regions in patients with epilepsy. (B) Colour map representing connectivity Z-score differences in patients versus controls across all regions, with more connectivity decreases than increases seen in patients. (C) Summary of connectivity (mean±95% CI) for each ARAS structure to all cortical/subcortical regions in patients versus controls, revealing overall diminished connectivity in patients, with significant differences in CSC, PPN and VTA. (D) The 20 cortical/subcortical regions with the largest differences in overall ARAS connectivity between patients and controls (mean±95% CI), averaged across all ARAS structures. Overall, 95% of these regions are neocortical, 75% are right sided and connectivity is reduced in patients among all regions with the largest differences. Ant, anterior; ARAS, ascending reticular activating system; C, cortex; CSC, cuneiform/subcuneiform nucleus; DR, dorsal raphe nucleus; G, gyrus; inf, inferior; L, left; LC, locus coeruleus; mid, middle; MR, median raphe nucleus; Op, opercularis; PBC, parabranchial complex; PO, pontine nucleus oralis; post, posterior; PPN, pedunculopontine nucleus; R, right; sup, superior; temp, temporal; VTA, ventral tegmental area. *p<0.05, paired t-test with Bonferroni-Holm correction for multiple comparisons.
Noting that most regions with large connectivity reductions in patients were right sided, we examined overall ARAS connectivity laterality. Compared with controls, patients had significantly reduced ARAS connectivity to cortical/subcortical regions in the right hemisphere (p<0.01, paired t-test with Bonferroni-Holm correction for multiple comparisons), but left-sided connectivity was not significantly different from controls (midline structures excluded). Given that the majority (67%) of patients harboured a right-sided seizure focus, we repeated this analysis with respect to EZ laterality instead of anatomic hemisphere. Overall, ARAS connectivity in patients was significantly decreased in the hemisphereipsilateral to the EZ (p<0.05, paired t-test with Bonferroni-Holm correction for multiple comparisons), but connectivity contralateral to the EZ was not significantly different from controls. These findings suggest that ARAS connectivity is reduced in patients with TLE, particularly to neocortical regions, with larger decreases observed ipsilateral to the EZ.
ARAS connectivity disturbances are related to consciousness-impairing seizure frequency
To summarise connectivity disturbances in patients and relate these changes to other variables, we defined ‘global’ ARAS connectivity as mean connectivity to all subcortical/cortical regions and ‘regional’ ARAS connectivity as mean connectivity to the 20 subcortical/cortical regions showing the largest differences in patients versus controls (see figure 2D). We then examined potential relationships between connectivity alterations in patients and severity or duration of illness. We noted that frequency of consciousness-impairing seizures (CPS, GTCS) was negatively correlated with both regional and global connectivity (p<0.05 for each, Pearson’s correlation with Bonferroni-Holm correction for multiple comparisons), whereas no relationship was noted between frequency of consciousness-sparing seizures (SPS) and regional or global connectivity. Also, no relationship was seen between epilepsy duration and either connectivity measure (p>0.25 for each, Pearson’s correlation with Bonferroni-Holm correction for multiple comparisons). These results suggest that ARAS connectivity disturbances in TLE may be related to severity of illness, with respect to consciousness-impairing seizure frequency.
ARAS connectivity patterns are related to neuropsychological impairments in TLE
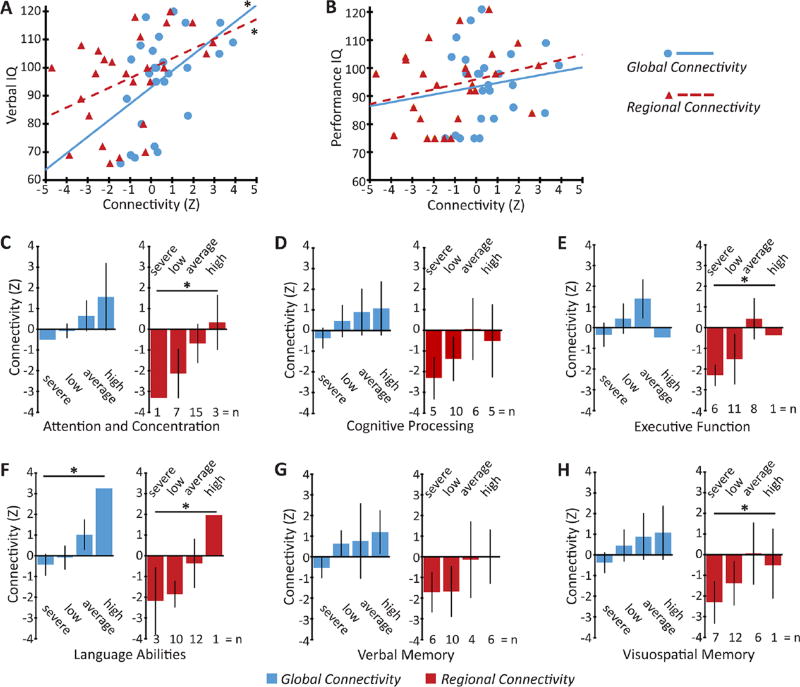
Finally, we evaluated potential relationships between ARAS connectivity patterns and performance on neuropsychological assessments in patients with TLE (figure 3). We noted significant positive relationships between verbal IQ and both regional and global connectivity in patients with TLE (p<0.05 for each, Spearman’s rho with Bonferroni-Holm correction for multiple comparisons), whereas we did not see a significant relationship between connectivity and performance IQ (figure 3A, B). On examining scores on subsets of the neuropsychological evaluation (figure 3C–H), we observed that diminished regional connectivity was significantly associated with worse performance in attention and concentration, executive function, language abilities and visuospatial memory, whereas decreased-global connectivity was related to poorer performance in language abilities (p<0.05 for each, Kendell’s tau-b with Bonferroni-Holm correction for multiple comparisons), although no significant relationships were observed in other instances. This suggests that decreased ARAS connectivity may be related to deficits in various neuropsychological parameters.
Figure 3.
ARAS connectivity disturbances are associated with neuropsychological impairments in patients with epilepsy. (A,B) Global and regional ARAS connectivity are significantly and positively correlated with verbal IQ (A) but not performance IQ (B) in patients. (C–H) Across all patients, global and regional connectivity values (mean±95% CI) are stratified by performance on various neuropsychological batteries. Significantly decreased regional connectivity is related to poorer performance in several batteries, including attention and concentration (C), executive function (E), language abilities (F) and visuospatial memory (H), whereas diminished global connectivity is associated with impaired language abilities (F). Data reflect the results of neuropsychological evaluations including Wechsler Adult Intelligence Scale (A,B) and various other tests listed in the Methods section (C–H). For C–H, performance is estimated by the treating neuropsychologist as severely impaired (0–20th percentile), low (20th–40th percentile), average (40th–60th percentile) or high/above average (60th–100th percentile), compared with a normative population. Global connectivity reflects mean connectivity between ARAS structures and all cortical/subcortical regions, whereas regional connectivity includes only the 20 cortical/subcortical regions with largest connectivity differences in patients versus controls (see figure 2D). n=26 of 27 patients in which full neuropsychological reports were available. *p<0.05, Spearman’s rho (A,B) or Kendell’s tau-b (C–H) with Bonferroni-Holm correction for multiple comparisons. ARAS, ascending reticular activating system.
DISCUSSION
The present study is the first to examine ARAS functional connectivity in patients with epilepsy, and relate connectivity reorganisation to disease characteristics and neuropsychological parameters. The brainstem ARAS contains several structures that are important for maintaining arousal and vigilance, in large part through monoaminergic projections to subcortical regions involved in cortical activation.11,12 Overall, ARAS connectivity patterns in non-epileptic control subjects in this study resembled those expected based on prior neuroimaging studies (figure 1). For instance, previous fMRI investigations examining ARAS resting-state connectivity have also reported strong positive connectivity to subcortical (hippocampus, amygdala) and cortical (anterior cingulate) limbic regions and the precuneus, as well as areas important for neocortical activation and reward, such as thalamus and basal forebrain/nucleus accumbens.16,17 Meanwhile, we observed negative correlations between ARAS structures and the precentral and postcentral gyri, which has been observed in previous studies examining connectivity of excitatory brainstem nuclei18,19 and has been hypothesised to reflect monoaminergic modulation of nociceptive pathways.18 We also noted that LC—a major source of noradrenergic projections—was the only region showing more negative than positive cortical/subcortical connections. Another fMRI study comparing LC and VTA functional connectivity found more widespread negative connections involving LC compared with VTA,20 with LC showing positive connectivity to thalamus and limbic areas and negative connectivity to rolandic and association neocortex, resembling our observations. Concordance between our findings in control subjects and prior studies may help validate the reliability of our results.
Overall, we found reduced connectivity between ARAS structures and cortical/subcortical regions in patients versus controls (figure 2). Among ARAS structures, we observed the largest decreases in connectivity involving CSC, PPN and VTA, which on neuroanatomical examination have been shown to possess more extensive ascending projections among ARAS structures.13 CSC and PPN regions are important for both cortical arousal and control of locomotion and muscle tone via glutamatergic and cholinergic connections, while VTA possesses dopaminergic projections involved in reward circuits.21,22 Among cortical/subcortical regions, ARAS connectivity reductions in patients were most pronounced in neocortical structures including lateral frontal, posterior temporal, insular, central opercular and paracingulate regions. Decreases in connectivity were greater in the right hemisphere, which may be influenced by laterality of the EZ, as most patients had a right-sided seizure focus. It is possible that connectivity impairments between the ARAS and the cortex in focal epilepsy may contribute to widespread reductions in neocortical connectivity reported in previous fMRI, structural MRI and MEG studies.4,5,23–26 We also found greater reductions in ARAS connectivity associated with a higher frequency of consciousness-impairing but not consciousness-sparing seizures. This suggests a relationship between ARAS connectivity patterns and severity of illness, although causation cannot be inferred. This is consistent with previous MEG-based connectivity analysis inpatients with focal epilepsy, relating frequency of consciousness-impairing but not consciousness-sparing seizures to cortical connectivity reductions.5 Notably, an association between epilepsy duration and connectivity was not observed here, although it has been reported in previous studies examining other networks.4,5,27
Next, a relationship between reduced ARAS connectivity and poor performance in certain neuropsychological functions was noted, including verbal IQ, attention and concentration, executive function, language abilities and visuospatial memory (figure 3). These deficits appeared more closely related to ‘regional’ connectivity between ARAS and areas showing large connectivity decreases in patients, suggesting an association between altered connectivity in these regions and neuropsychological impairment, although the directionality of this relationship cannot be determined. Indeed, patients demonstrated pronounced decreases in ARAS connectivity to lateral, frontal and paracingulate areas important for executive function and attention, as well as inferior frontal and posterior temporal neocortex involved in language.28–30 Other groups have also related functional connectivity decreases in frontal neocortex to various neurocognitive impairments.7,31,32
Based on our present results and prior investigations, we hypothesise that: (1) seizures in TLE result in aberrant ictal activity in ARAS structures;9,33 (2) frequent seizures are then associated with interictal connectivity disturbances between ARAS and other cortical/subcortical regions; (3) diminished connectivity between ARAS and these regions may be related to long-term reductions in neocortical metabolism, grey matter volume and connectivity seen in epilepsy34,35; (4) these network disturbances may ultimately be associated with neurocognitive and psychosocial problems suffered by patients with epilepsy. However, it is important to note that this is only a hypothesis, and while associations between ARAS connectivity and disease and neurocognitive parameters were observed in the present study, causation cannot be inferred. Progressive network dysfunction and neuropsychological impairments seen in focal epilepsy demonstrate the critical importance of achieving seizure freedom in this disorder through medical or surgical intervention.36 In future studies, it will be important to determine whether definitive epilepsy treatment, including successful epilepsy surgery, is associated with reversal of connectivity reorganisation. Finally, while subcortical activating structures have been discussed as potential neuromodulation targets to improve the level of consciousness in epilepsy,33,37 the potential effects of ARAS neurostimulation on neurocognitive profiles in this disorder may warrant consideration.
It is useful to note that several previous investigations have demonstrated connectivity disturbances in widespread brain networks in TLE8; therefore, connectivity problems in this disorder are not specific to the ARAS alone. Interestingly, in the present study we did not observe significant connectivity differences between patients and controls related to certain other subcortical structures important for arousal, including thalamus and basal forebrain/nucleus accumbens (data not shown). While detailed characterisation of connectivity patterns in these other brain networks are beyond the scope of the present study, closer examination of other arousal systems will be worthwhile in future studies of TLE.
There are other limitations to the present study that meritdiscussion. First, ARAS brainstem nuclei studied here are quite small and not easily discerned on 3T MRI. Furthermore, fMRI signal from these regions may be susceptible to motion, physiological noise and partial volume artefacts, and accuracy may be limited by fMRI voxel size. Steps taken to help mitigate these issues include correction for movement and physiological parameters, the calculation of tSNR maps which revealed acceptable signal-to-noise values in the ARAS structures, and the removal of voxels with susceptibility artefact. Furthermore, the ARAS connectivity patterns we uncovered resemble known connections of these networks, helping validate our observations. Next, we observed both positive and negative correlations in this study, and connectivity decreases in patients versus controls incorporated negative connections. While some have argued that global signal regression in fMRI can artificially inflate negative connectivity findings,38 such signal correction was not performed here, and pathophysiological negative network correlations in epilepsy have been reported in previous neuroimaging studies.39,40 While state of arousal was not explicitly controlled for in this study and may influence ARAS activity, we looked only at resting-state connectivity and not activation patterns, and patients and controls were compared using identical scanning conditions. Antiepileptic medications may have significant effects on connectivity patterns, but nearly all connectivity studies in epilepsy patients share this limitation, which is challenging to overcome. Finally, our goal in the present study was to summarise overall ARAS connectivity trends in TLE and relate these patterns to other important factors, but the present results do not establish causation in these associations.
CONCLUSIONS
ARAS structures are critical for neocortical activation and arousal, but while patients with epilepsy exhibit problems in these domains, connectivity of ARAS networks in epilepsy has not previously been studied. We observed significantly decreased functional connectivity between ARAS structures and several cortical and subcortical regions in patients with TLE compared with controls, particularly involving neocortical areas. ARAS connectivity reductions were quantitatively related to increased frequency of consciousness-impairing seizures and impaired performance on several neuropsychological parameters. Recurrent seizures may be related to disturbances in ARAS connectivity in patients with epilepsy, which may then be associated with more widespread network dysfunction and neurocognitive problems suffered in this devastating disorder.
Supplementary Material
Acknowledgments
For brain atlases used in this study, we thank the Harvard Center of Morphometric Analysis (Harvard-Oxford Atlas) and the Martinos Center for Biomedical Imaging (Harvard Ascending Arousal Network Atlas). We also thank all members of the multidisciplinary epilepsy team at Vanderbilt University for their support of this work, and for continued excellence in patient care.
Funding This work was supported by the National Institutes of Health grants K99 NS097618 (DJE) and R01 NS075270 (VLM) and by an American Epilepsy Society/Epilepsy Foundation grant (DJE).
Footnotes
Contributors Data were primarily collected by VLM and analysed by DJE and VLM, with data analysis assistance provided by P-FDH. Neuropsychological data were primarily collected and interpreted by MLJ. JCG, PEK and BWA-K participated in the data interpretation. DJE produced the first draft of the manuscript. All authors read, provided input and approved the final manuscript.
Competing interests None declared.
Ethics approval This study was approved by the Vanderbilt University Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Engel J, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. Lippincott Williams & Wilkins; philadelphia, PA: 2007. pp. 2479–86. [Google Scholar]
- 2.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–77. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl 2):96–8. doi: 10.1111/j.1528-1167.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 4.Morgan VL, Conrad BN, Abou-Khalil B, et al. Increasing structural atrophy and functional isolation of the temporal lobe with duration of disease in temporal lobe epilepsy. Epilepsy Res. 2015;110:171–8. doi: 10.1016/j.eplepsyres.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englot DJ, Hinkley LB, Kort NS, et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015;138:2249–62. doi: 10.1093/brain/awv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes M, Folley BS, Sonmezturk HH, et al. Resting state functional connectivity of the hippocampus associated with neurocognitive function in left temporal lobe epilepsy. Hum Brain Mapp. 2014;35:735–44. doi: 10.1002/hbm.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet G, Osipowicz K, Sharan A, et al. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2013;34:2202–16. doi: 10.1002/hbm.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia. 2016;57:1546–57. doi: 10.1111/epi.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–72. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–81. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 12.Steriade M. Ascending control of thalamic and cortical responsiveness. Int Rev Neurobiol. 1970;12:87–144. doi: 10.1016/s0074-7742(08)60059-8. [DOI] [PubMed] [Google Scholar]
- 13.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–46. doi: 10.1097/NEN.0b013e3182588293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: retroicor. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Bär KJ, de la Cruz F, Schumann A, et al. Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage. 2016;134:53–63. doi: 10.1016/j.neuroimage.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Bianciardi M, Toschi N, Eichner C, et al. In vivo functional connectome of human brainstem nuclei of the ascending arousal, autonomic, and motor systems by high spatial resolution 7-Tesla fMRI. MAGMA. 2016;29:451–62. doi: 10.1007/s10334-016-0546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beliveau V, Svarer C, Frokjaer VG, et al. Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage. 2015;116:187–95. doi: 10.1016/j.neuroimage.2015.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Buckner RL, Liu H, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111:E4367–75. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Hu S, Chao HH, et al. Resting-State functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/Substantia nigra pars Compacta and the effects of age. Cereb Cortex. 2016;26:3413–27. doi: 10.1093/cercor/bhv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80:1148–55. doi: 10.1212/WNL.0b013e3182886a76. [DOI] [PubMed] [Google Scholar]
- 22.Ranaldi R, Dopamine RR. Dopamine and reward seeking: the role of ventral tegmental area. Rev Neurosci. 2014;25:621–30. doi: 10.1515/revneuro-2014-0019. [DOI] [PubMed] [Google Scholar]
- 23.Haneef Z, Lenartowicz A, Yeh HJ, et al. Network analysis of the default mode network using functional connectivity MRI in temporal lobe Epilepsy. J Vis Exp. 2014:e51442. doi: 10.3791/51442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucukboyaci NE, Kemmotsu N, Cheng CE, et al. Functional connectivity of the hippocampus in temporal lobe epilepsy: feasibility of a task-regressed seed-based approach. Brain Connect. 2013;3:464–74. doi: 10.1089/brain.2013.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittau F, Grova C, Moeller F, et al. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. 2012;53:1013–23. doi: 10.1111/j.1528-1167.2012.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widjaja E, Zamyadi M, Raybaud C, et al. Disrupted global and regional structural networks and subnetworks in children with localization-related epilepsy. AJNR Am J Neuroradiol. 2015;36:1362–8. doi: 10.3174/ajnr.A4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haneef Z, Chiang S, Yeh HJ, et al. Functional connectivity homogeneity correlates with duration of temporal lobe epilepsy. Epilepsy Behav. 2015;46:227–33. doi: 10.1016/j.yebeh.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson's disease. Neuropsychopharmacol. 2010;35:70–85. doi: 10.1038/npp.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 30.Dorsaint-Pierre R, Penhune VB, Watkins KE, et al. Asymmetries of the planum temporale and Heschl's gyrus: relationship to language lateralization. Brain. 2006;129:1164–76. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- 31.Braakman HM, Vaessen MJ, Jansen JF, et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia. 2013;54:446–54. doi: 10.1111/epi.12044. [DOI] [PubMed] [Google Scholar]
- 32.Doucet GE, Rider R, Taylor N, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56:517–26. doi: 10.1111/epi.12936. [DOI] [PubMed] [Google Scholar]
- 33.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring Conscious Arousal during Focal Limbic seizures with Deep brain stimulation. Cereb Cortex. 2016:35. doi: 10.1093/cercor/bhw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonilha L, Rorden C, Appenzeller S, et al. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage. 2006;32:1070–9. doi: 10.1016/j.neuroimage.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 35.Diehl B, LaPresto E, Najm I, et al. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia. 2003;44:559–64. doi: 10.1046/j.1528-1157.2003.36202.x. [DOI] [PubMed] [Google Scholar]
- 36.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37:389–405. doi: 10.1007/s10143-014-0527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gummadavelli A, Kundishora AJ, Willie JT, et al. Neurostimulation to improve level of consciousness in patients with epilepsy. Neurosurg Focus. 2015;38:E10. doi: 10.3171/2015.3.FOCUS1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He H, Liu TT. A geometric view of global signal confounds in resting-state functional MRI. Neuroimage. 2012;59:2339–48. doi: 10.1016/j.neuroimage.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campo P, Garrido MI, Moran RJ, et al. Network reconfiguration and working memory impairment in mesial temporal lobe epilepsy. Neuroimage. 2013;72:48–54. doi: 10.1016/j.neuroimage.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.