Abstract
Comparative studies of primordial germ cell (PGC) development across organisms in many phyla reveal surprising diversity in the route of migration, timing and underlying molecular mechanisms, suggesting that the process of migration itself is conserved. However, beyond the perfunctory transport of cellular precursors to their later arising home of the gonads, does PGC migration serve a function? Here we propose that the process of migration plays an addition role in quality control, by eliminating PGCs incapable of completing migration as well as through mechanisms that favor PGCs capable of responding appropriately to migration cues. Focusing on PGCs in mice, we explore evidence for a selective capacity of migration, considering the tandem regulation of proliferation and migration, cell-intrinsic and extrinsic control, the potential for tumors derived from failed PGC migrants, the potential mechanisms by which migratory PGCs vary in their cellular behaviors and corresponding effects on development. We discuss the implications of a selective role of PGC migration for in vitro gametogenesis.
Keywords: primordial germ cell, migration, cell competition, germ cell tumor, germ cell selection
Graphical abstract
Throughout human history, journeys to sacred places have been undertaken in search of clarity, health, or successful reproduction. Pilgrims to Wutai Shan Mountain in Mongolia sought rebirth in a womb-like cave (Charleaux 2011); on Isla Mujeres, the sanctuary of the fertility goddess Ixchel first drew the ancient Mayans (McKillop 2004); travelers on the Camino de Santiago de Compostela in Spain carried a scallop shell, a symbol of fertility, as their badge (Wikipedia, 2016). In a striking parallel, fertility itself hinges upon a journey of cells across the developing embryo in many diverse organisms. Primordial germ cells (PGC) are among the first lineages established in development, and the successful passage of these dedicated precursors from their birthplace to the gonad primordia ensures an adequate supply of gametes for reproduction in the adult (McLaren 2003; Kunwar et al., 2006; Wong et al., 2013).
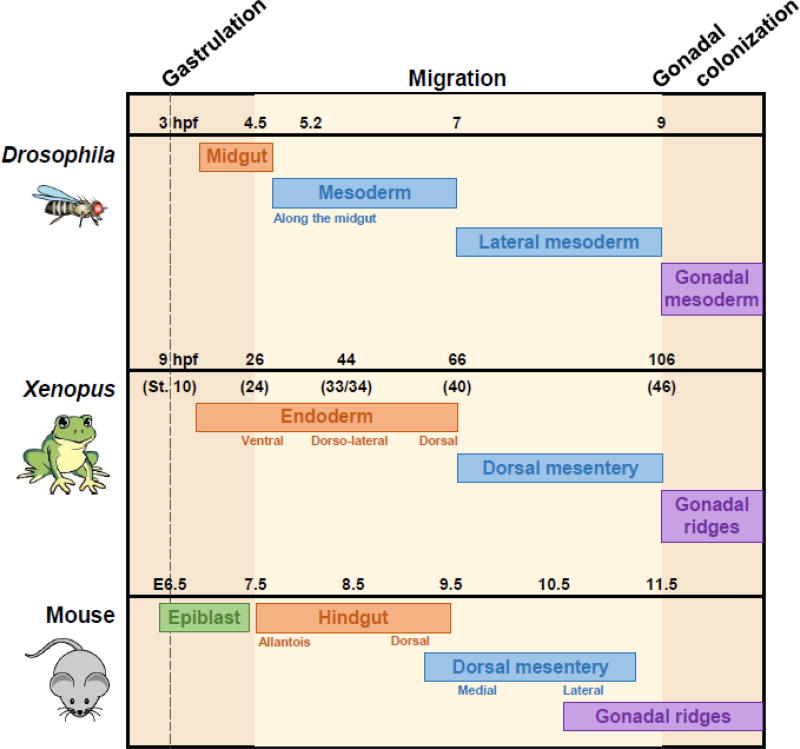
The study of PGC development in flies, fish, birds, amphibians and mammals reveals surprising diversity in the migratory circuits as well as the underlying molecular mechanisms. Migration initiates from the embryo posterior in most organisms; however, avian PGCs begin in the anterior germinal crescent (Nakamura et al., 2007). Transit through epithelial sheets of the endoderm is common to rodents, Xenopus, and Drosophila, and interstitial movement through mesoderm occurs in zebrafish, mammals, and Drosophila (Figure 1; Kamimura et al., 1976; Kunwar et al., 2006; Raz, 2004). Whereas fish PGCs move in clusters during gastrulation, this is the exception, as PGCs in most organisms move as single cells, with an extreme example as chick PGCs homing through the vasculature similar to lymphocytes (Nakamura et al., 2007). Common expression of the PIWI family of genes and RNA helicases such as VASA in PGCs of most organisms suggests that the cell lineages are homologous, in spite of differing modes of specification (Hay et al., 1990; Yoon et al., 1997; Megosh et al., 2006; Juliano et al., 2010); however, there is no such ancient molecular guidance system specific to PGCs. Rather, mechanisms of chemoattraction and repulsion appear to have been borrowed by PGCs from blood cells, neurons, and mesoderm (Richardson and Lehmann, 2010). Together these observations suggest that it is PGC migration itself that has been conserved during evolution rather than specific mechanisms (Figure 1).
Figure 1. Conservation of PGC migration between multiple species.
Following gastrulation (dashed line), PGCs in Drosophila, Xenopus, and mouse undergo lengthy migrations through endodermal sheets (orange) and mesodermal tissues (blue) to reach the developing gonads (purple). Time scales of the migratory period are noted for each species; hpf = hours post-fertilization, E = embryonic day. Light beige background denotes the migratory period; darker beige background represents pre- and post-migratory periods. Annotations underneath each bar represent specific locations and timing of PGC movement within the more general tissue type.
Why does PGC development across so many phyla involve a pilgrimage within the embryo? Whether germline fate is acquired by inheritance of cytoplasmic determinants or inductive signals delivered to pluripotent cells (Extavour and Akam, 2003), the early specification of PGCs mandates a strategy for awaiting organogenesis and transiting to their eventual home of the gonad. Thus, migration fulfills this perfunctory requirement, but does it serve a function beyond transport? Here we propose that the process of migration plays an additional role in germline quality control. We suggest that negative selection occurs via elimination of PGCs incapable of completing migration as well as through mechanisms that favor PGCs capable of responding appropriately to migration cues. In this review, we will explore evidence for a selective capacity of migration, focusing primarily on PGCs in mice.
1 The yin and yang of mouse PGC migration
Mouse PGCs are specified from epiblast at E7.5, travel within the growing hindgut epithelium, then egress through the mesentery before colonizing the emerging gonads by E11.5 (Figure 1; Saitou, 2012). Only after this point does sex-specific differentiation proceed as PGCs, now termed gonocytes, enter meiosis in females and mitotic arrest in males at ~E13 (Chiquoine, 1954; McLaren, 2003). While undergoing migration, these PGCs are also coordinating other cell processes important for their development, including epigenetic reprogramming and expansion. Distinct from other model organisms in which proliferation follows migration (Su et al., 1998; Richardson and Lehmann, 2010; de Melo Bernardo et al., 2012), mammalian PGCs are actively proliferating during their migration, increasing in population size from approximately 45 cells at E7.5 to ~200 at E9.5 (Saitou et al., 2002; McLaren, 2003; Seki et al., 2007), ~2500 at E11.5 (Laird et al., 2011), and peaking around 25,000 at E13.5 (Tam and Snow, 1981).
1.1 Regulation of PGC migration by intrinsic versus extrinsic signaling mechanisms
PGCs are a unique model for parsing the effects of intrinsic and extrinsic signaling owing to their known interaction with a diversity of cell types as they move from their point of specification in the epiblast to their ultimate residence in the gonads. The mammalian germline is particularly interesting due to the multitude of cellular processes that take place concurrently with PGC migration – proliferation, survival, and epigenetic reprogramming (reviewed in Ewan and Koopman 2010). This complexity of development across several, distinct microenvironments has generated many questions regarding the role of the soma in regulating PGC development. Previous work identified a requirement for KitL as well as Sdf1 (also known as Cxcl12) from somatic cells in regulating PGC survival and proliferation while simultaneously guiding their movement in mice (Gu et al., 2009; Gu et al., 2011; Runyan et al., 2006; Ara et al., 2003; Molyneaux et al., 2003). Both factors provide chemotactic and survival signals; thus, loss of KitL, Sdf1, or their respective receptors, cKit and Cxcr4, leads to inefficient colonization of the gonads and diminished numbers of PGCs.
More recently, the non-canonical Wnt receptor Ror2 and its main ligand Wnt5a were implicated in the migration of PGCs by a forward genetic screen in mice (Laird et al., 2011; Chawengsaksophak et al., 2012). In contrast to the temporal and spatial restriction to PGCs of the receptors cKit and Cxcr4, Ror2 is expressed on both PGCs and their somatic cell neighbors, most highly in the hindgut epithelium, and at lower levels in the dorsal mesentery and gonadal ridges. In PGCs, Ror2 provides autonomous control of motility, as evidenced by an increase in the number of germ cells remaining outside the gonadal ridges at the conclusion of migration in both the Ror2Y324C full loss-of-function mutant and the PGC-specific Ror2cKO mutant (Laird et al., 2011; Cantú et al., 2016; Cantú and Laird, unpublished results). Another autonomous function for Ror2 is in the regulation of PGC proliferation, with aberrantly high rates of cycling PGCs found in the hindgut of both ubiquitous and PGC-specific mutants (Cantú et al., 2016). However, unlike the persistence of motility defects in Ror2 mutants for the duration of PGC migration, the specificity of proliferation phenotypes implies that the control of the PGC cell cycle by Ror2 signaling is limited to a single somatic compartment. This suggests that mitogenic signals from the microenvironment differ between somatic compartments, while the factors that enable PGC movement are more stable and consistent. The high level of WNT5a in the hindgut compared to the surrounding mesentery is the most likely basis for this location-specific phenotype, but does not explain the absence of proliferation defects in the gonadal ridges, where WNT5A is also high. Other secreted factors such as SFRP1 may regulate the availability of Wnt ligands or alter the balance of signaling in a compartment-specific manner. Future studies to profile mitogenic regulators specific to each somatic niche will be needed to test this hypothesis. Furthermore, given the high level of expression, the possible function of Ror2 in the PGC niche remains to be identified.
The work described above takes a PGC-centric view in assessing the interactions between the germline and the soma. However, evidence from other species suggests that contact between PGCs and their somatic neighbors can be mutually beneficial for the development of both tissues. In the human embryo, PGCs and nerve fibers colonize the gonadal ridges at the same time (Møllgård et al., 2010). Image analysis shows human PGCs in the dorsal mesentery to the gonadal ridges in close association with developing nerve fibers and Schwann cells, and intriguingly PGC chemoattractants such as KitL (Høyer et al., 2005), and Sdf1 (Belmadani et al. 2005) are expressed in the developing nervous system. While Møllgård and colleagues conclude that the nervous system guides the PGCs to the gonads, one might speculate that the interplay between the cell types is more complex. Migratory PGCs in Drosophila have been found to regulate and direct the movement of the caudal visceral mesoderm (CVM), progenitors of the midgut muscles, a neighboring cell type that shares a common migratory route with PGCs (Stepanik et al., 2016). Live imaging in PGC migration mutants found that CVM cells exhibit an affinity for PGCs and will invade inappropriate tissues to localize with mismigrated germ cells. When PGCs are absent, CVM migratory behavior is altered, resulting in muscle defects in the midgut. Although migratory PGCs in Drosophila influence the development of their microenvironment, a similar education of the somatic tissues by PGCs has not yet been identified in the mouse. Thus far, it seems that embryogenesis occurs normally in the absence of PGCs (Mintz and Russell 1957; Chen et al., 2013; Hayashi et al., 2011); however, the development and movement of the somatic cell types that comprise the migratory route during PGC migration have yet to be comprehensively characterized.
1.2 Cell migration versus proliferation
In many organisms, the period of PGC migration is distinct from their proliferation, with migration following initial specification and proliferation commencing upon arrival in the gonads (reviewed in Richardson and Lehmann 2010). The mammalian system, however, is an exception. In the mouse embryo, PGCs begin dividing as soon as they are specified and continue to expand in population size until meiotic entry in the female or mitotic arrest in the male (Tam and Snow 1981; Seki et al., 2007). This concurrence of migration and proliferation is surprising based on the differences in cell morphology and adhesion each process requires; elongation, formation of protrusions, and dynamic adhesions with the microenvironment during migration would appear to be at odds with the rounding up and loss of adhesion that occur during proliferation.
In general, the ability of actively migrating cells to divide is not unusual; neural crest cells and lateral line primordium in zebrafish proliferate during their embryonic migrations (Huszar et al., 1991; Laguerre et al., 2005; Ciruna et al., 2006). However, these cell types utilize collective cell migration rather than migrating singly like PGCs, and it is likely that passive movement of cells within the group is more compatible with dividing, effectively eliminating interruptions in their migration. PGC motility and the cell cycle are much more likely to be conflicting processes, requiring that the cell choose one at the expense of the other. We see this antagonism most clearly at the period of active PGC migration – invasion through the basement membrane and egress from the hindgut. In this specific microenvironment, PGC proliferation is most strongly suppressed (Cantú et al., 2016), allowing cells to be more highly motile. We further showed that these dueling cell functions are regulated by antagonism between two arms of the Wnt pathway, where noncanonical Wnt5a-Ror2 signaling promotes cell motility while inhibiting β-catenin-mediated proliferation. Additionally, Ror2-mutant PGCs are more rounded in shape, likely as a result of increased cell division, akin to what we observe in the highly proliferative gonadal ridge (Laird et al., 2011). Thus, we propose that the antagonism is mutual – in mutants with higher rates of proliferation, such as Ror2Y324C and βcatGOF, there is a migratory delay (Laird et al., 2011; Cantú et al., 2016). In support of these findings in PGCs, suppression of canonical Wnt signaling by the noncanonical Wnt pathway has been found in neuronal migration during C. elegans development as well as several cell lines (Forrester et al., 2004; Mikels and Nusse 2006; Mikels et al., 2009), although downstream effectors remain unknown.
The conflict between migration and proliferation of PGC was also described in the developing reproductive tract of C. elegans, where the anchor cells are only capable of invading into the vulval epithelium during G1/G0 cell cycle arrest (Matus et al., 2015). Induction of proliferation in these normally quiescent cells blocks their invasive function. While this model is unique in that the anchor cells are not migratory – they breach the basement membrane with protrusions but do not translocate the cell body – these findings have implications for other highly invasive cell types such as metastatic cancers. Using experimental data, in silico modeling of the interplay between migration and proliferation of cancer cells predicts that highly proliferative cells have impaired movement when confronted with limited metabolic resources and physical barriers than their less proliferative counterparts (Hecht et al., 2015). Traditional treatment of cancer in patients has targeted highly proliferative cells, potentially leaving behind quiescent and invasive cell populations. One such drug, Palbociclib (PD-0332991), directly targets the cell cycle by inhibiting the G1/S transition via CDK4/6 (Baughn et al., 2006) and is currently being tested in clinical trials for many different cancers. While Palbociclib has shown great promise in blocking the cell cycle and suppressing tumor growth, it has also induced EMT and invasion in pancreatic cancer cell lines (Liu and Korc 2012). This raises the possibility that inhibition of proliferation in cancer may induce metastatic behavior. Thus, the antagonism between cell migration and proliferation could have severe consequences on the progression of human disease and patient outcomes.
1.3 Wnt5a-Ror2 versus KitL-cKit signaling in PGCs
Because mammalian PGC migration remains largely understudied, most work thus far has focused on singular and specific signaling pathways, without much clear overlap. Our previous work on Ror2 in the germline opened the door for examining the interactions between key pathways with the observation that Ror2-mutant PGCs are less responsive to KitL in terms of migratory morphology (Laird et al., 2011). Based on the further characterization of PGC phenotypes in multiple models of Ror2 loss (Ror2Y324C, Ror2−/− and Ror2cKO), we have uncovered distinct areas of separation and overlap between the Wnt5a-Ror2 pathway and KitL-cKit signaling. In order to promote PGC motility, these pathways work together to induce cell elongation and polarity, general requirements for effective migration (Laird et al., 2011). However, they diverge in the regulation of PGC proliferation and survival. Wnt5a-Ror2 signaling can suppress proliferation in regions of high KitL expression, such as the E9.5 hindgut, which we demonstrated by the elevation of proliferation of Ror2-mutants in this microenvironment. We furthermore observed that germ cells manage to survive despite autonomous loss of Ror2, leading to an overall doubling of the PGC population in Ror2cKO mutants compared to controls (Cantú and Laird, unpublished results). Without the current knowledge of Ror2 phenotypes and potential interplay with KitL-cKit, initial studies of PGCs using combinations of genetic mutants in these pathways proved difficult to interpret. Return to this line of inquiry will be feasible with conditional alleles of each receptor, candidate downstream readouts, and emerging testable hypotheses about the function of each pathway in the motility, proliferation, and survival of PGCs.
2 Infidelities in germ cell migration
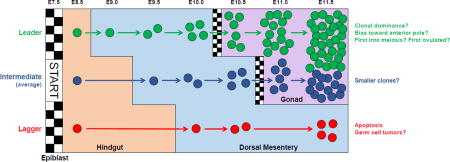
Molecular regulation of PGC migration in mice appears to be complex and redundant, which confers robustness, but comes with a downside: heterogeneity of cellular behavior. This complexity is evidenced by the changing cellular morphology and variety of different kinds of movements observed during the 5-day odyssey from PGC specification to colonization of the gonad. Redundancy in genetic control of PGC migration is suggested by the absence of mouse mutants in which migration of PGCs is completely disrupted. This includes spontaneous, targeted or chemically-induced mutants, and an ENU screen corresponding to ~10% of the genome (D. Laird, unpublished data). A consequence of multiple redundant processes operating simultaneously is fluctuation, and quantification of PGC location by embryonic age reveals a high degree of variability during the migratory period (Cantu et al., 2016). By the conclusion of migration at E11.5, about 5% of PGCs remain outside of the gonads (Laird et al., 2011), and these are eliminated by apoptosis (Runyan et al., 2006).
The trigger for programmed cell death of PGCs that wander off route is believed to be the withdrawal from support factors, such as KitL (Dolci et al., 1991; Matsui et al., 1991; Godin et al., 1991; Pesce et al., 1993). Apoptosis of PGCs during the migratory period occurs via the intrinsic pathway (Stallock et al., 2003; Rucker et al., 2000); however, elimination of wayward PGCs can fail, as evidenced by their capacity to give rise to disorganized tumors known as teratomas that contain derivatives of all three germ layers. The PGC origin of testicular teratomas was demonstrated by abolishing tumors in a susceptible mouse strain by crossing to a cKit mutant, which lacks PGCs (Stevens 1967, 1974, 1984). Mutations in the oncogenes Pten, CyclinD1, Dmrt1 and Dnd1 have been shown to cause testicular teratomas in mice (Kimura et al., 2003; Cook et al., 2009; Krentz et al., 2009; Heaney et al., 2012) and variants in some of these loci are associated with the same tumors in humans (Litchfield et al., 2015). The functions of these genes suggest that neoplasms from fetal gonocytes that fail to maintain proliferative arrest or resist differentiation (Peterson 2012). However this may be distinct from the origin of teratomas from PGCs that fail migration (Runyan et al., 2008).
Teratomas are also found in other locations, including the spine and coccyx, mediastinum and even intracranially (Grosfeld 1985). With an occurrence of ~1 of every 30,000–40,000 live births, Saccrococcygeal Teratoma (SCT) is the most common tumor in newborns and young children, whereas it is exceedingly rare in adults (Rescorla 1998). The most likely source of extragonadal germ cell tumors is a lesion in a migratory PGC (reviewed in Looijenga 2013) because their predominance in midline structures correlates with the migration of PGCs through the hindgut and medial mesentery before bifurcating to the gonads (reviewed in Bustamante-Marin 2013). The not infrequent observation of PGCs left in these locations in wild-type mice after migration is complete argues that the PGC origin of SCTs is more feasible than from derivatives of the embryonic signaling center known as the node (Moore 2003) or as a result of somatic cells reacquiring pluripotency (Economou 2016). Although the presence of a latent pluripotency program in PGCs (Matsui 1992, Resnik 1992) might render them more susceptible to oncogenic transformation, concrete evidence linking SCTs to failed PGC migrants is lacking. Molecular characterization of SCTs in humans as well as the development of a mouse model are much needed in this field.
3. Germ cell quality and selection
Germ cell development is a lengthy and complex process that starts with specification in the early embryo and proceeds through stages of migration, proliferation, epigenetic reprogramming, sex differentiation, and gametogenesis to ultimately produce mature oocytes and sperm (reviewed in Ewan and Koopman 2010). Here we posit that each step following specification can potentially function as a selective mechanism to ensure that that highest quality germ cells become the adult gametes. Even though their migration occurs early in gametogenesis, PGCs could harbor defects that negatively impact later development and future progeny. Migratory PGCs may acquire genetic mutations resulting from rapid proliferation in the blastocyst prior to germline specification (MacAuley et al., 1993). Recent measurement of mutation at one locus using the BigBlue mouse demonstrated a decrease in the mutational load in the germline as development proceeded, arguing for a process of selection against the most damaged germ cells; the overall decrease in germ cell mutational load as compared to somatic cell lineages could indicate a greater capacity for DNA repair in addition to negative selection in the germline (Murphey et al., 2015).
Beyond integrity of the genome, precise control of epigenetic reprogramming is necessary to maintain PGC identity and function (reviewed in De Felici 2011). The process of genome-wide DNA demethylation that occurs during PGC development is not indiscriminate, as genes critical to post-migratory PGC function, imprinted genes, and retrotransposons remain highly methylated until after gonadal colonization (Maatouk et al., 2006; Seisenberger et al., 2012; Hackett et al., 2012). Additionally, histone modifications that occur during migration, including erasure of H3K9me2 and addition of H3K27me3 and H4/H2AR3me2, appear to move the genome toward greater transcriptional plasticity while preventing inappropriate differentiation (Seki et al., 2005; Ancelin et al., 2006). It remains unclear whether this phase of epigenetic reprogramming is linked to PGC migration and movement through different somatic microenvironments or might be intrinsically regulated by developmental timing. Thus, selective mechanisms may be important for eliminating PGCs carrying aberrant epigenetic marks to preserve the integrity of the germline.
A mechanism relevant to selection during development is cell competition, which is an emergent behavior upon interaction between cells that express differing levels of certain proteins. Cell competition has been studied in the Drosophila embryo (wing imaginal disc) as well as the epiblast of the mouse embryo, both of which are epithelial tissues (Morata and Ripoll 1975; Clavería et al., 2013). In these cases, heterogeneity between adjacent cells enables some with a greater ability to expand, via proliferation, and kill their weaker neighbors (Morata and Ripoll 1975; Moreno et al., 2002; Clavería et al., 2013). Thus, the surviving cells are considered to be more fit than their counterparts and go on to contribute a greater share of progeny to the developing tissue or organism (reviewed in Amoyel and Bach 2014).
It is easiest to imagine how cell competition might act in the developing gonads, where PGCs cluster in tight colonies (Tam and Snow 1981) and are close enough to outcompete and kill their neighbors. During the migratory period, however, PGCs are dispersed throughout multiple tissues as they move as single cells (Cantú et al., 2016) and interaction between germ cells prior to gonadal colonization remains functionally unclear. Examination of PGCs in histological sections revealed contact between some germ cells via long and thin protrusions, potentially forming a network of distantly connected cells (Gomperts et al., 1994). Additionally, it is known that gap junctions are required for early PGC development; embryos deficient in Gja1 (known as Connexin43) exhibit a loss of germ cells at E11.5 as a result of migratory defects and increased apoptosis (Francis and Lo 2006). Cyst formation in PGCs via intercellular bridges begins at E10.5, prior to the conclusion of migration (Pepling and Spradling 1998; Lei and Spradling 2013; Greenbaum et al., 2009). However, live imaging of germ cells in embryonic slice culture suggests that migratory PGCs make more brief contacts with each other before gonadal colonization (Molyneaux et al., 2001). Also, PGC-PGC coupling was not directly observed in the Connexin43 study, and it remains unclear if cyst formation at E10.5 occurs in all germ cells or specifically those already in the gonad and thus post-migratory (Francis and Lo 2006; Pepling and Spradling 1998; Lei and Spradling 2013).Given the conflicting results described above, we propose that any competition occurring at this stage of development would rely primarily on the fundamental and relative fitness of each cell rather than direct interactions between PGCs. This raises questions about what parameters make one migratory cell more fit than another and whether fitness at this stage of germ cell development translates into the production of better gametes in adulthood.
One proposed parameter of fitness is PGC speed or efficiency of migration. As we have shown, PGCs are found in multiple locations at a single time point, with each location differentially influencing the rate of PGC proliferation (Cantú et al., 2016). Thus, we predict that those leader PGCs first to exit the cell cycle-suppressive environment of the hindgut would gain a head start in proliferation over cells that exit later in development. Due to the dramatic morphological growth that occurs in tissues along the migratory route, early migrators, dubbed ‘pioneers’, are expected to more rapidly traverse the different somatic compartments to reach the gonads because these tissues are smaller and in closer proximity (Gomperts et al., 1994; Molyneaux et al., 2001). Thus, the proposed distance of travel for leading PGCs is predicted to be shorter than that of PGCs who initiate migration later in development. The consequence of this increased migratory efficiency is that a greater number of leading PGC progeny would be found in the gonadal ridges and likewise dominate the germ cell pool. However, the basis for differences in migratory capacity of PGCs remains unclear; do the lead migrants exhibit a greater capacity for movement, are they stochastically designated, or do they reflect the first PGCs specified? Migratory capacity may also function deterministically in the distribution of PGCs within the gonadal ridges and subsequently influence their progression through later stages of germ cell development, such as meiotic entry.
One hypothesis put forth nearly 50 years ago is that PGCs which enter meiosis first are the first to mature into follicles and be ovulated, also known as the “production line” hypothesis (Henderson and Edwards 1968). In the mouse, meiotic initiation occurs in a wave starting from the anterior end of the ovary and moving to the posterior (Menke et al., 2003), so location within the tissue dictates order of meiosis, which may be linked to timing of follicle formation and ovulation in adulthood (Zheng et al., 2014). How PGCs are distributed in the gonads during and following migration is unknown, although it is likely a mix of active and passive processes (Clark and Eddy 1975; Anderson et al., 2000). Thus far, live imaging has not revealed a clear bias for the first PGC migrators out of the hindgut to exit from a particular region along the anterior-posterior axis (Gomperts et al., 1994; Molyneaux et al., 2001), although this has not been rigorously examined. In systemic Ror2 loss-of-function mutants, we observed a semipenetrant defect in which PGCs are unable to colonize the tip of the gonad (Laird et al., 2011; Arora et al., 2016). This likely arises from a combination of defects in axis elongation, due to the function of Ror2 in mesoderm and somites (Takeuchi et al., 2000; Oishi et al., 2003), and reduced efficiency of migration due to the cell autonomous function of Ror2 in PGCs. Why PGCs in some of these animals fail to fill the gonadal ridge evenly remains a mystery; however, this defect does not produce a delay in meiotic entry when we controlled for changes in ovary size or position on the anteroposterior axis (Arora et al., 2016). Because Ror2-mutant embryos do not survive beyond birth (Takeuchi et al., 2000; DeChiara et al., 2000; Oishi et al., 2003), we have been unable to use these animals to test the link between migration, meiosis, and ovulation. Thus, we expect genetic lineage tracing approaches will provide the best means to track the fate of leader versus laggard PGCs.
Such approaches are not limited to testing the “production line” hypothesis and folliculogenesis, but could also be applied to the study of male germline development. The relationship between migratory fitness and survival during the apoptotic wave in later fetal development or clonal dominance in adult spermatogonial stem cells would be obvious questions to address (Coucouvanis et al., 1993; Goriely and Wilkie 2012).
5 Conclusions
There is much to be learned about the pilgrimage of PGCs to the temple of the gonad, with respect to the fundamental mechanisms as well as the potential function. Here we propose that the process of migration acts selectively to cull cells with insufficient ability to respond to chemotactic cues, metabolic deficiency, and conversely to favor the expansion and priority of meiotic initiation of those who migrate earlier, farther, or faster. The basis of such differences may lie in the number of genetic mutations or differences in epigenetic reprogramming. If our hypothesis is correct, then it will have implications for understanding of extragonadal germ cell tumors such as SCTs, which may result from failed quality control. Furthermore, it may be important to consider the role of PGC migration in the context of in vitro gametogenesis. The emerging possibility of growing eggs from pluripotent cells in a dish recapitulates much of development, but bypasses the process of migration (Hikabe et al., 2016). Would challenging in vitro derived PGCs to migrate increase the quality of the gametes obtained, as measured by the frequency of fertilizable eggs and embryo development?
Highlights.
Mechanisms of PGC migration vary by organism, though the process is highly conserved
Migratory PGCs may gain a proliferative edge by being first to reach pro-mitotic soma
Cell competition during migration may select PGCs based on relative fitness
Extragonadal germ cell tumors may arise from heterogeneous PGC migration and survival
Acknowledgments
Funding: This work was supported by a National Science Foundation predoctoral fellowship to A.V. Cantú, the University of California, San Francisco Program for Breakthrough Biomedical Research, and National Institutes of Health grants 1R21ES023297-01 and DP2OD007420 to D.J. Laird.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amoyel M, Bach EA. Cell competition: how to eliminate your neighbours. Development. 2014;141(5):988–1000. doi: 10.1242/dev.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8(6):623–630. doi: 10.1038/ncb1413. [DOI] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Schöler H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91(1–2):61–68. doi: 10.1016/s0925-4773(99)00271-3. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1) Proc Natl Acad Sci USA. 2003;100(9):5319–1523. doi: 10.1073/pnas.0730719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Abby E, Ross AD, Cantu AV, Kissner MD, Castro V, Ho HY, Livera G, Laird DJ. Meiotic onset is reliant on spatial distribution but independent of germ cell number in the mouse ovary. J Cell Sci. 2016;129(13):2493–2499. doi: 10.1242/jcs.189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R, Niesvizky R, Cho H, Ely S, Moore MA, Chen-Kiang S. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66(15):7661–7667. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25(16):3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marín X, Garness JA, Capel B. Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology. Int J Dev Biol. 2013;57(2–4):201–10. doi: 10.1387/ijdb.130136bc. [DOI] [PubMed] [Google Scholar]
- Cantú AV, Altshuler-Keylin S, Laird DJ. Discrete somatic niches coordinate proliferation and migration of primordial germ cells via Wnt signaling. J Cell Biol. 2016;214(2):215–229. doi: 10.1083/jcb.201511061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charleux Isabelle. Mongol Pilgrimages to Wutai Shan in the Late Qing Dynasty. Journal of the International Association of Tibetan Studies. 2011 Dec;(6):275–326. (2011). http://www.thlib.org?tid=T5712.
- Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954;118:135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Chen L, Faire M, Kissner MD, Laird DJ. Primordial germ cells and gastrointestinal stromal tumors respond distinctly to a cKit overactivating allele. Hum Mol Genet. 2013;22(2):313–327. doi: 10.1093/hmg/dds430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439(7073):220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Eddy EM. Fine structural observations on the origin and associations of primordial germ cells of the mouse. Dev Biol. 1975;47(1):136–155. doi: 10.1016/0012-1606(75)90269-9. [DOI] [PubMed] [Google Scholar]
- Clavería C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500(7460):39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- Cook MS, Coveney D, Batchvarov I, Nadeau JH, Capel B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev Biol. 2009;328(2):377–83. doi: 10.1016/j.ydbio.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MH, Kim SY, Ebbert K, Duncan FE, Ramalho-Santos J, Woodruff TK. Geography of Follicle Formation in the Embryonic Mouse Ovary Impacts Activation Pattern During the First Wave of Folliculogenesis. Biol of Reproduction. 2015;93(4):88–88. doi: 10.1095/biolreprod.115.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucouvanis EC, Sherwood SW, Carswell-Crumpton C, Spack EG, Jones PP. Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp Cell Res. 1993;209(2):238–247. doi: 10.1006/excr.1993.1307. [DOI] [PubMed] [Google Scholar]
- De Felici M. Nuclear reprogramming in mouse primordial germ cells: epigenetic contribution. Stem Cells Int. 2011;2011:425863. doi: 10.4061/2011/425863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24(3):271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- De Melo Bernardo A1, Sprenkels K, Rodrigues G, Noce T, Chuva De Sousa Lopes SM. Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol Open. 2012;1(11):1146–52. doi: 10.1242/bio.20122592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci S, Williams De, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, Boswell HS, Donovan PJ. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature. 1991;29:809–811. doi: 10.1038/352809a0. [DOI] [PubMed] [Google Scholar]
- Economou C, Tsakiridis A, Wymeersch FJ, Gordon-Keylock S, Dewhurst RE, Fisher D, et al. Intrinsic factors and the embryonic environment influence the formation of extragonadal teratomas during gestation. BMC Developmental Biology. 2016:1–15. doi: 10.1186/s12861-015-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323(1):76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130:5869–5884. doi: 10.1242/dev.00804. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 Inhibits EGL-20/Wnt Signaling in Cell Migration. Genetics. 2004;168(4):1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RJ, Lo CW. Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development. 2006;133(17):3451–3460. doi: 10.1242/dev.02506. [DOI] [PubMed] [Google Scholar]
- Godin I, Deed R, Cooke J, Zsebo K, Dexter M, Wylie CC. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991;29:807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- Gomperts M, García-Castro M, Wylie C, Heasman J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development. 1994;120(1):135–141. doi: 10.1242/dev.120.1.135. [DOI] [PubMed] [Google Scholar]
- Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod. 2009;80(3):449–457. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosfeld JL, Billmire DF. Teratomas in infancy and childhood. Current Problems in Cancer. 1985;9(9):1–53. doi: 10.1016/s0147-0272(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136(8):1295–1303. doi: 10.1242/dev.030619. [DOI] [PubMed] [Google Scholar]
- Gu Y, Runyan C, Shoemaker A, Surani MA, Wylie C. Membrane-bound steel factor maintains a high local concentration for mouse primordial germ cell motility, and defines the region of their migration. PLoS One. 2011;6(10):e25984. doi: 10.1371/journal.pone.0025984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–433. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Heaney JD, Anderson EL, Michelson MV, Zechel JL, Conrad PA, Page DC, Nadeau JH. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development. 2012;139(9):1577–86. doi: 10.1242/dev.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht I, Natan S, Zaritsky A, Levine H, Tsarfaty I, Ben-Jacob E. The motility-proliferation-metabolism interplay during metastatic invasion. Sci Rep. 2015;5:13538. doi: 10.1038/srep13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218(5136):22–28. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016 doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- Høyer PE, Byskov AG, Møllgård K. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol Cell Endocrinol. 2005;234(1–2):1–10. doi: 10.1016/j.mce.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Huszar D, Sharpe A, Jaenisch R. Migration and proliferation of cultured neural crest cells in W mutant neural crest chimeras. Development. 1991;112(1):131–141. doi: 10.1242/dev.112.1.131. [DOI] [PubMed] [Google Scholar]
- Juliano CE, Swartz SZ, Wessel GM. A conserved germline multipotency program. Development. 2010;137(24):4113–4126. doi: 10.1242/dev.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura M, Ikenishi K, Kotani M, Matsuno T. Observations on the migration and proliferation of gonocytes in Xenopus laevis. J Embryol Exp Morphol. 1976;36(1):197–207. [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130(8):1691–700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Kim S, Cook MS, Capel B, Zhu R, Matin A, Sarver AL, Parker KL, Griswold MD, Looijenga LH, Bardwell VJ, Zarkower D. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci U S A. 2009;106(52):22323–8. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar PS, Siekhaus DE, Lehmann R. In vivo migration: a germ cell perspective. Annu Rev Cell Dev Biol. 2006;22:237–265. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- Laguerre L, Soubiran F, Ghysen A, König N, Dambly-Chaudière C. Cell proliferation in the developing lateral line system of zebrafish embryos. Dev Dyn. 2005;233(2):466–472. doi: 10.1002/dvdy.20343. [DOI] [PubMed] [Google Scholar]
- Laird DJ, Altshuler-Keylin S, Kissner MD, Zhou X, Anderson KV. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet. 2011;7(12):e1002428. doi: 10.1371/journal.pgen.1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 2013;140(10):2075–2081. doi: 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K, Summersgill B, Yost S, Sultana R, Labreche K, Dudakia D, et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumors. Nature Communications. 2015;6:1–8. doi: 10.1038/ncomms6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Korc M. Cdk4/6 inhibition induces epithelial-mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther. 2012;11(10):2138–2148. doi: 10.1158/1535-7163.MCT-12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga LHJ, Van Agthoven T, Biermann K. Development of malignant germ cells - the genvironmental hypothesis. The International Journal of Developmental Biology. 2013;57(2–3–4):241–253. doi: 10.1387/ijdb.130026ll. [DOI] [PubMed] [Google Scholar]
- MacAuley A, Werb Z, Mirkes PE. Characterization of the unusually rapid cell cycles during rat gastrulation. Development. 1993;117:873–883. doi: 10.1242/dev.117.3.873. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, Hogan BL. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature. 1991;353:750–752. doi: 10.1038/353750a0. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, Barkoulas M, Zhang W, Chi Q, Sherwood DR. Invasive cell fate requires G1 cell-cycle arrest and histone deacetylase-mediated changes in gene expression. Dev Cell. 2015;35(2):162–174. doi: 10.1016/j.devcel.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop H. The Ancient Maya: New Perspectives (Understanding Ancient Civilizations.) 2004 Aug 19;:453. 2004. ABC-CLIO, 2004. [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Megosh HB, Cox DN, Campbell C, Lin H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr Biol. 2006;16:1884–1894. doi: 10.1016/j.cub.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262(2):303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284(44):30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B, Russell ES. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957;134(2):207–237. doi: 10.1002/jez.1401340202. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Jespersen A, Lutterodt MC, Yding Andersen C, Høyer PE, Byskov AG. Human primordial germ cells migrate along nerve fibers and Schwann cells from the dorsal hind gut mesentery to the gonadal ridge. Mol Hum Reprod. 2010;16(9):621–631. doi: 10.1093/molehr/gaq052. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130(18):4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The developing human: clinically oriented embryology. 7. Philadelphia: WB Saunders; 2003. [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42(2):211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416(6882):755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Murphey P, McLean DJ, McMahan CA, Walter CA, McCarrey JR. Enhanced Genetic Integrity in Mouse Germ Cells. Biology of Reproduction. 2013;88(1):6–6. doi: 10.1095/biolreprod.112.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci. 2007;86(10):2182–2193. doi: 10.1093/ps/86.10.2182. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125(17):3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pesce M, Farrace Mg, Piacentini M, Dolci S, De Felici M. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis) Development. 1993;118:1089–1094. doi: 10.1242/dev.118.4.1089. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Buckley C, Holley S, Menias CO. Teratomas: A Multimodality. Curr Probl Diagn Radiol. 2012;41(6):210–219. doi: 10.1067/j.cpradiol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Raz E. Guidance of primordial germ cell migration. Current Opinion in Cell Biology. 2004;16:169–173. doi: 10.1016/j.ceb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Rescorla FJ, Sawin RS, Coran AG, et al. Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. J Pediatr Surg. 1998;33(2):171–6. doi: 10.1016/s0022-3468(98)90426-2. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11(1):37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Molecular Endocrinology. 2000;14(7):1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133(24):4861–4869. doi: 10.1242/dev.02688. [DOI] [PubMed] [Google Scholar]
- Runyan C, Gu Y, Shoemaker A, Looijenga L, Wylie C. The distribution and behavior of extragonadal primordial germ cells in Bax mutant mice suggest a novel origin for sacrococcygeal germ cell tumors. The International Journal of Developmental Biology. 2008;52(4):333–344. doi: 10.1387/ijdb.072486cr. [DOI] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418(6895):293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development. 2007;134(14):2627–2638. doi: 10.1242/dev.005611. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C. The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development. 2003;130:6589–6597. doi: 10.1242/dev.00898. [DOI] [PubMed] [Google Scholar]
- Stebler J, Spieler D, Slanchev K, Molyneaux KA, Richter U, Cojocaru V, Tarabykin V, Wylie C, Kessel M, Raz E. Primordial germ cell migration in the chick and mouse embryo: the role of the chemokine SDF-1/CXCL12. Dev Biol. 2004;272(2):351–361. doi: 10.1016/j.ydbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Stepanik V, Dunipace L, Bae YK, Macabenta F, Sun J, Trisnadi N, Stathopoulos A. The migrations of Drosophila muscle founders and primordial germ cells are interdependent. Development. 2016;143(17):3206–3215. doi: 10.1242/dev.134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38(4):549–52. [PubMed] [Google Scholar]
- Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970;21:364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Stevens LC. Germ cell origin of testicular and ovarian teratomas. Transplant Proc. 1984;16:502–504. [PubMed] [Google Scholar]
- Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LH. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod. 2005;20(6):1466–1476. doi: 10.1093/humrep/deh800. [DOI] [PubMed] [Google Scholar]
- Su TT, Campbell SD, O'Farrell PH. The cell cycle program in germ cells of the Drosophila embryo. Dev Biol. 1998;196(2):160–170. doi: 10.1006/dbio.1998.8855. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5(1):71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tam PPL, Snow MHL. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Wikipedia contributors. Camino de Santiago. [accessed October 15, 2016];Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/w/index.php?title=Camino_de_Santiago&oldid=745696703.
- Wong TT, Collodi P. Inducible Sterilization of Zebrafish by Disruption of Primordial Germ Cell Migration. PLoS One. 2013;8(6):e68455. doi: 10.1371/journal.pone.0068455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet. 2014;23(4):920–928. doi: 10.1093/hmg/ddt486. [DOI] [PMC free article] [PubMed] [Google Scholar]