Abstract
Chronic ketamine use leads to cognitive and affective deficits including depression. Here, we examined sex differences and neural bases of depression in chronic ketamine users. Compared to non-drug using healthy controls (HC), ketamine-using females but not males showed increased depression score as assessed by the Center of Epidemiological Studies Depression Scale (CES-D). We evaluated resting state functional connectivity (rsFC) of the subgenual anterior cingulate cortex (sgACC), a prefrontal structure consistently implicated in the pathogenesis of depression. Compared to HC, ketamine users (KU) did not demonstrate significant changes in sgACC connectivities at a corrected threshold. However, in KU, a linear regression against CES-D score showed less sgACC connectivity to the orbitofrontal cortex (OFC) with increasing depression severity. Examined separately, male and female KU showed higher sgACC connectivity to bilateral superior temporal gyrus and dorsomedial prefrontal cortex (dmPFC), respectively, in correlation with depression. The linear correlation of sgACC-OFC and sgACC-dmPFC connectivity with depression was significantly different in slope between KU and HC. These findings highlighted changes in rsFC of the sgACC as associated with depression and sex differences in these changes in chronic ketamine users.
Keywords: ketamine, SUD, depression, fMRI, rsFC, vmPFC, sex difference
1. Introduction
First synthesized as a derivative of phencyclidine in 1960s, ketamine has been used as an anesthetic in medicine. Ketamine has powerful psychological effects and recent studies including many clinical trials have focused on its potential as an antidepressant. On the other hand, ketamine elicits euphoria and dissociation (“out-of-body” experiences) and has increasingly become one of the major substances of abuse in many parts of the world, including Asia (Huang et al., 2014; Jia et al., 2015; Liu et al., 2016; Sassano-Higgins et al., 2016; Singh et al., 2013; Tang et al., 2015). In animal studies, ketamine induces self-administration and conditioned place preference (Botanas et al., 2015; De Luca and Badiani, 2011; Guo et al., 2016a; Suzuki et al., 1999; van der Kam et al., 2009; Venniro et al., 2015; Winger et al., 2002; Young and Woods, 1981). The potential of ketamine abuse may have to do with its action on the dopaminergic systems (Hancock and Stamford, 1999; however, see Can et al., 2016). On the other hand, ketamine is an antagonist of N-methyl-D-aspartate receptor, and the neural bases underlying ketamine addiction likely involve more than the dopaminergic circuits.
Drug abuse leads to cognitive and affective dysfunction. Studies in humans have characterized deficits in attention, working memory and executive functions and changes in emotion and affective behavior in substance abusers. In particular, brain imaging has provided an important venue to investigate the neural bases of these cognitive and affective deficits (Li and Sinha, 2008). Resting state functional connectivity (rsFC), which captures the organization of functional brain networks, has been widely used to unravel changes in circuit functions in various neuropsychiatric conditions including addiction. Numerous studies implicated the subgenual anterior cingulate cortex (sgACC, or Brodmann area 25) in depression on the bases of functional imaging, lesioning, and electromagnetic stimulation (see Berlim et al., 2014; Dunlop and Mayberg, 2014; Savitz and Drevets, 2009 for a review). For instance, compared to controls, adolescents with depression demonstrated elevated connectivity between the sgACC and insula as well as amygdala, and decreased connectivity between the sgACC and precuneus in association with the severity of depression (Connolly et al., 2013). Compared with controls, unmedicated young adults with remitted depression demonstrated hyperconnectivity of the left sgACC to the right ventromedial prefrontal cortex and left hippocampus (Jacobs et al., 2016). Children at risk in developing major depression exhibited hyperconnectivity between the default mode network (DMN) and sgACC, and the magnitude of connectivity correlated positively with individual depression symptom scores (Chai et al., 2016). In a randomized sham-controlled trial, responders to repetitive transcranial magnetic stimulation treatment of depression showed significantly stronger anticorrelated rsFC between the sgACC and left superior medial prefrontal cortex at baseline (Baeken et al., 2014). Vasopressin, a modulator of mammalian social behavior reduces sgACC activity and its connectivity to the amygdala and other limbic regions implicated in emotional regulation (Zink et al., 2010). Together, these findings highlight sgACC connectivity as a neural marker of depression and response to depression treatment.
Many studies examined the effects of acute ketamine administration on rsFC in healthy volunteers and clinical populations (Abdallah et al., 2016; Li and Vlisides, 2016; Wong et al., 2016) as well as in non-human primates (Gopinath et al., 2016; Lv et al., 2016). In healthy humans, ketamine increased cortical/subcortical-hippocampal connectivity (Grimm et al., 2015; Khalili-Mahani et al., 2015) and thalamic connectivity to the somatosensory and temporal cortex (Hoflich et al., 2015). In contrast, ketamine decreased DMN connectivity to the dorsomedial prefrontal cortex (Scheidegger et al., 2012), fronto-temporal functional connectivity (Kraguljac et al., 2016) and sgACC connectivity with the hippocampus, parahippocampal gyrus, retrosplenial cortex, and thalamus (Wong et al. 2016). Increasing the depth of ketamine sedation suppressed anticorrelated activity between the DMN and other regions in healthy adults (Bonhomme et al., 2016). In rats the strongest ketamine effects were dose- and exposure-dependent increases in functional connectivity within the prefrontal cortex and in connectivities between the posterior hippocampus, retrosplenial cortex, and prefrontal regions (Gass et al., 2014). Task-based imaging studies have also demonstrated the effects of ketamine on regional responses in healthy participants (Kleinloog et al., 2015; Lehmann et al., 2016; Scheidegger et al., 2016; Steffens et al., 2016) and clinical populations (Becerra et al., 2015) in a variety of cognitive and affective paradigms. These findings together characterized a wide range of acute effects of ketamine on cerebral activity.
On the other hand, few studies have examined changes in cerebral structure, activation and connectivity in chronic ketamine users (Hoflich et al., 2016; Liao et al., 2016; Wang et al., 2013), who frequently suffer comorbid depression (Chang et al., 2016). Women are more vulnerable than men to depression (Kessler, 2003). In a survey of over 1,600 chronic ketamine users females presented significantly more discontinuation symptoms such as anxiety, dysphoria, and tremors and reported more severe cognitive impairment compared with male users (Chen et al., 2014). Preclinical work also suggested sex differences in the behavioral effects of ketamine. For instance, female Sprague-Dawley rats appeared to be more sensitive to ketamine-induced conditioned place preference than male rats (Guo et al., 2016b). In another study male and female rats were exposed to a single intraperitoneal injection of ketamine of varying dosages and tested 30 minutes later on forced swim and novelty suppressed feeding (Carrier and Kabbaj, 2013). Compared to male rats, female rats demonstrated greater sensitivity to the antidepressant effects of ketamine, and the effects were contingent on female sex hormones. In a recent study both male and female rats showed depression-like behavior after chronic social isolation as well as synaptic and postsynaptic changes in the medial prefrontal cortex. However, a single ketamine injection reversed these changes in male but not female rats (Sarkar and Kabbaj, 2016). Together, these studies suggest important sex differences in the depression-related behavioral effects of ketamine.
Here, we combined clinical assessments and fMRI to explore changes in rsFC of the sgACC in relation to depression in ketamine users. We broadly hypothesized that female ketamine users will demonstrate more significant depression and altered sgACC connectivity in link with depression, as compared to male users.
2. Methods
2.1 Subjects and clinical assessments
The study was approved by the Research Ethics Committee of the China Medical University Hospital (CMUH103-REC2-052). Candidates were assured at screening that their decision to participate in the study or not would not affect their right to medical care, that all personal information would be kept confidential, and that they could withdraw from the study at any time. Each participant provided a written informed consent prior to data collection.
Ketamine users (KU) and healthy control (HC) participants were recruited through posters at hospitals and online advertisements in the greater area of Taichung City, Taiwan. After consenting to the study, participants completed a clinical interview, questionnaire assessment, behavioral test, and magnetic resonance imaging.
KU met International Statistical Classification of Diseases and Related Health Problems (ICD) criteria for ketamine use disorders and tested positive for ketamine in urine toxicology. A positive test result for other substances including methamphetamine, opioids, ecstasy, or marijuana, was an exclusion criterion. All HC participants denied use of any illicit substances and showed negative urine test results. None of the KU or HC participants had any major medical or neurological illnesses, history of brain concussion that resulted in loss of consciousness, or psychotic disorders. A total of 36 KU and 20 HC participated in this study. Table 1 summarizes the key clinical characteristics of the participants.
Table 1.
Clinical characteristics of the participants
| KU (M) | KU (W) | HC (M) | HC (W) | ANOVA p value | |||
|---|---|---|---|---|---|---|---|
| Group | Gender | Interaction | |||||
| Age | 25.2±5.8 | 27.5±5.7 | 25.3±4.5 | 25.1±4.2 | 0.45 | 0.50 | 0.44 |
| CES-D | 6.3±4.6 | 16.5±6.2 | 6.8±4.8 | 7.6±4.8 | 0.005 | 0.0004 | 0.02 |
| Ketamine use duration (months) | 59.4±37.0 | 59.0±40.0 | NA | NA | 0.98* | ||
| Cigarette in 30 days (day) | 24.5±11.1 | 30.0±0.0 | 1.5±2.5 | 0.0±0.0 | 3.6×10−16 | 0.39 | 0.13 |
| Cigarette in life (years) | 8.4±4.7 | 12.1±7.7 | 2.5±3.5 | 0.0±0.0 | 4.2×10−8 | 0.66 | 0.03 |
| Alcohol in 30 days (day) | 4.8±8.5 | 9.0±11.1 | 3.0±3.8 | 0.4±0.7 | 0.02 | 0.72 | 0.14 |
| Alcohol in life (years) | 4.3±4.3 | 6.7±6.2 | 5.2±6.2 | 1.9±3.4 | 0.18 | 0.76 | 0.05 |
All values are mean ± SD; KU: ketamine users; HC: healthy controls; CES-D: Center of Epidemiological Study-Depression score; M: men; W: women;
two sample t test
2.2 Magnetic resonance imaging: procedures and parameters
Participants underwent an MRI scan, consisting of 6- to10-minute resting-state fMRI and high-resolution structural imaging. MR image data were acquired using a 3-Tesla scanner (Signa HDx, GE, Milwaukee, USA) at the Department of Radiology, China Medical University Hospital, Taichung, Taiwan. The high-resolution structural images were acquired in transverse plane along the AC-PC line. A three-dimensional spoiled gradient-recalled protocol with inversion recovery pulse prepared (3D-SPGR-IrP) sequence was used (parameters: TE=minimal; prep time=450 ms; flip angle=12 degree; image matrix=224 × 224; FOV=224 mm × 224 mm; slice thickness=1 mm; NEX=1). The resting-state fMRI data were acquired using a gradient echo single-shot echo planar imaging sequence (parameters: TE=35 ms; TR=2000 ms; slice thickness=4.4 mm; slice number=32; image matrix=64 × 64; FOV=240 mm; total scan time=10 minutes). Four dummy scans acquired at the beginning of EPI were discarded.
2.3 Imaging data pre-processing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). We followed standard procedures in image preprocessing, as in our recent work (Kann et al., 2016; Kline et al., 2016; Zhang et al., 2016a; Zhang et al., 2016b). Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Fair et al., 2007; Fox and Raichle, 2007; Fox et al., 2005; Rombouts et al., 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Cordes and colleagues suggested that BOLD fluctuations below a frequency of 0.1Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). Thus, we applied a temporal band-pass filter (0.009 < f < 0.08 Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies (Fair et al., 2007; Fox and Raichle, 2007; Fox et al., 2005; Lowe et al., 1998).
As extensively investigated in Van Dijk et al., 2012, micro head motion (> 0.1 mm) is an important source of spurious correlations in rsFC analysis (Van Dijk et al., 2012). Therefore, we applied a “scrubbing” method proposed by Power and colleagues (Power et al., 2012) and successfully applied in previous studies (Power et al., 2012; Smyser et al., 2010; Tomasi and Volkow, 2014) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r| α (t)| + r| β (t)| + r|γ(t)|, where (dx, dy, dz)and (α, β, γ) are the translational and rotational movements, respectively, and r (= 50 mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transform rotations into displacements (Power et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD (t) > 0.5 mm or DVARS(t) > 0.5% (Power et al., 2012; Tomasi and Volkow, 2014). On average, 1% of the time points were removed across subjects.
2.4 Seed based correlation and group analyses
Seed region of the sgACC (Brodmann area 25) was derived from a connectivity-based parcellation atlas (Neubert et al., 2015, Figure 1).
Figure 1.
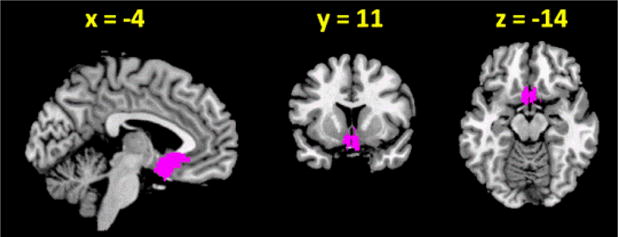
Seed region: subgenual anterior cingulate cortex (sgACC).
The BOLD time courses were averaged spatially across voxels over the sgACC seed. For individual subjects, we computed the correlation coefficient between the averaged time course of seed region and the time courses of all other brain voxels. To assess and compare rsFC, we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Berry and Mielke, 2000; Jenkins and Watts, 1968): z = 0.5loge[(1+r)/(1−r)]. The Z maps were used in group, random effect analyses. We performed a two-sample t test to compare the Z maps of HC and KU and one-sample t test on the Z maps of sgACC for HC and KU combined. We also performed analysis of variance with group and sex as two factors as well as covariance analysis to include variables of nicotine and alcohol use as covariates. Additionally, we performed whole brain simple regression analyses with CES-D as a regressor for KU. All results were examined with a voxel p<0.05, corrected for family-wise error (FWE) of multiple comparisons or a combination of uncorrected voxel p<0.001 and cluster p<0.05, FWE corrected, on the basis of Gaussian random field theory, in SPM, following current reporting standards (Eklund et al., 2016). Effect sizes of regions of interest were derived with MarsBar (http://marsbar.sourceforge.net/) for additional analyses.
3 Results
3.1 Clinical assessments
For all clinical measures, we conducted an analysis of variance (ANOVA) with group (KU vs. HC) and sex (men vs. women) as factors. The p values for main and interaction effects are shown in Table 1. Compared to HC, KU showed higher CES-D score (p = 0.005); women also showed higher CES-D score as compared to men (p = 0.0004). Further, there was a significant interaction effect (p = 0.002), with female KU showing a significantly higher CES-D score than female HC (16.5 ± 6.2 vs.7.6 ± 4.8, p=0.003, two-sample t test) but no difference in men (6.3 ± 4.6 vs. 6.8 ± 4.8, p=0.77). KU also showed significantly higher cigarette and alcohol use than HC (Table 1).
3.2 Resting state functional connectivity of the subgenual anterior cingulate cortex
In a two-sample t test we observed that KU and HC did not show differences in rsFC at a corrected threshold. Thus, we combined KU and HC in a one-sample t test of whole-brain connectivities of the subgenual anterior cingulate cortex (sgACC, Figure 2A). The sgACC showed positive connectivity to the rostral ACC/medial prefrontal cortex/medial orbitofrontal cortex, bilateral temporal poles/superior temporal gyri, bilateral caudate head/ventral striatum/anterior thalamus, and negative connectivity to bilateral occipital cortex, bilateral inferior frontal cortex/anterior insula (rIFC/AI), bilateral middle frontal gyrus and left angular gyrus.
Figure 2.
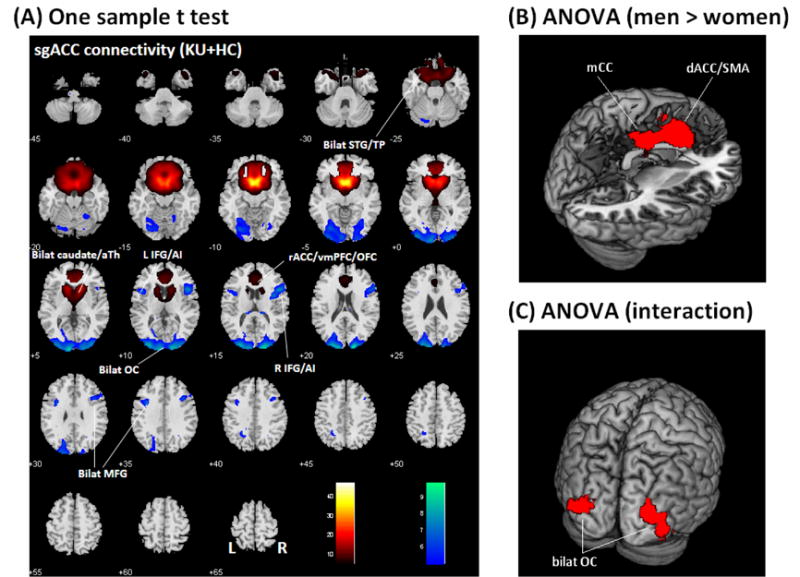
(A) One-sample t test of whole brain functional connectivity of the subgenual anterior cingulate cortex (sgACC), p<0.05 FWE, in all subjects. Positive and negative connectivities are shown each in warm and cool color. Scale represents voxel T value. Neurological orientation: R = right. ACC: anterior cingulate cortex; aTh: anterior thalamus; Bilat: bilateral; IFG/AI: inferior frontal gyrus/anterior insula; L: left; MFG: middle frontal gyrus; OC: occipital cortex; OFC: orbitofrontal cortex; R: right; STG: superior temporal gyrus; TP: temporal polar cortex; vmPFC: ventromedial prefrontal cortex. ANOVA showed (B) greater activation in the dorsal anterior cingulate cortex and supplementary motor area (dACC/SMA) and mid-cingulate cortex (mCC) in men, as compared to women; and (C) an interaction effect, with men showing greater connectivity than women in HC, as compared to KU, in bilateral occipital cortex (OC). Results of ANOVA were examined at a threshold of p<0.001 uncorrected, combined with cluster p<0.05, FWE corrected.
In an ANOVA of the z maps, we included group and sex as two factors. At a corrected threshold, KU and HC did not differ in sgACC connectivity, consistent with the results of two-sample t test. However, compared to women, men showed greater sgACC connectivity to the dorsal ACC/supplementary motor area (dACC/SMA, x = −6, y = 20, z = 31, voxel Z = 4.97) and mid-cingulate cortex (mCC, x = 6, y = −13, z = 34, voxel Z = 4.96) with a total volume of 21,411 mm3 (Figure 2B). Further, an interaction effect with KU (men – women) < HC (men – women) was observed in bilateral occipital cortices (x = −30, y = −88, z = 7, voxel Z = 4.46, 8,127 mm3 and x = 36, y = −73, z = −8, voxel Z = 4.21, 9,288 mm3), with men showing greater connectivity than women in HC, as compared to KU (Figure 2C).
3.3 Depression and sgACC connectivity
We performed whole brain simple regression of sgACC connectivity maps on CES-D scores in KU. At a combined threshold of uncorrected voxel p<0.001 and FWE-corrected cluster p<0.05, CES-D score negatively correlated with the sgACC connectivity to the right lateral orbitofrontal cortex (OFC, x = 36, y = 20, z = −14, voxel Z = 4.97; 3,618 mm3) and bilateral medial OFC (x = 3, y = 50, z = −11, voxel Z = 3.63; 6,156 mm3) for all KU. We also performed the analyses separately for men and women. At the same threshold, CES-D score positively correlated with sgACC connectivity to bilateral superior temporal gyrus (STG, x = 66, y = −37, z = 19, voxel Z = 3.97; 3,834 mm3; and x = −60, y = −61, z = 4, voxel Z = 3.68; 2,916 mm3) in male KU. CES-D score positively correlated with sgACC connectivity to dorsomedial prefrontal cortex (dmPFC, x = −6, y = 29, z = 52, voxel Z = 4.87; 3,267 mm3) in female KU.
We derived the effect sizes of connectivity of these three regions and tested for differences between KU and HC in the correlation with CES-D score (Figure 3). The results showed that, with men and women combined, sgACC connectivity to the OFC was negatively and significantly correlated with the CES-D (p = 0.00001, r = −0.66) in KU, but not in HC (p = 0.58, r = 0.13). Further, KU and HC significantly differed in the slope of the regressions (p = 0.001). For men, sgACC connectivity to the STG showed positive correlation with the CES-D both in KU (p = 0.00002, r = 0.74) and in HC (p = 0.009, r = 0.74). There was no difference between KU and HC in the slope of the regressions (p = 0.13). For women, sgACC connectivity to the dmPFC showed positive correlation with the CES-D in KU (p = 0.00003, r = 0.97) but not in HC (p = 0.69, r = −0.16); and the two groups differed significantly in the slope of the regressions (p = 0.03).
Figure 3.
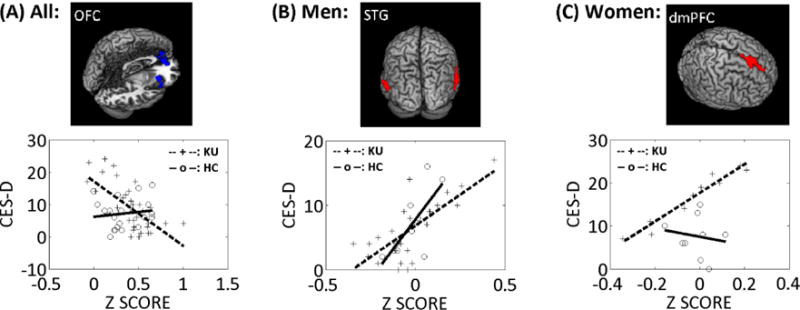
sgACC connectivity in relation to depression. (A) sgACC connectivity with the orbitofrontal cortex (OFC) was negatively correlated with CES-D score in male and female KU combined (blue clusters). (B) sgACC connectivity with bilateral superior temporal gyrus (STG) was positively correlated with CES-D score in male KU (red clusters). (C) sgACC connectivity with dorsomedial prefrontal cortex (dmPFC) was positively correlated with CES-D score in female KU (red cluster).
3.4 sgACC connectivity: cigarette and alcohol use as potential confounds
KU and HC differed in the extent of cigarette and alcohol use (Table 1). We examined whether the findings described above were related to cigarette and alcohol use. We correlated the effect size of sgACC connectivity of the OFC, STG, and dmPFC with days of cigarette use in the past month, years of cigarette use in life, days of alcohol use in the past month, and years of alcohol use in life. The results showed that there were no significant correlations (all p’s > 0.14) except for a trend-level negative correlation between the effect size of STG and days of cigarette use in the prior month (p = 0.05, r = −0.39) as well as years of smoking (p = 0.07, r = −0.36) in men. None of these correlations were significant when examined at a corrected threshold of p<0.05/12=0.0042).
We also conducted analysis of covariance for whole brain sgACC connectivity with group and sex as two factors and alcohol and nicotine use variables as covariates. The results were nearly identical to those from ANOVA (Section 3.2). There were sex main effect and group by sex interaction effect. Compared to women, men showed greater sgACC connectivity with the dACC/SMA, x = −6, y = 23, z = 31, voxel Z = 4.91) and mid-cingulate cortex (mCC, x = 6, y = −13, z = 34, voxel Z = 4.83) with a total volume of 19,980 mm3. There was an interaction effect in bilateral occipital cortices (x = −30, y = −88, z = 7, voxel Z = 4.24, 4,617 mm3; and x = 36, y = −73, z = −8, voxel Z = 3.98, 7,263 mm3).
4 Discussion
Compared to non-drug using controls (HC), ketamine users (KU) did not demonstrate significant changes in sgACC connectivity, at a corrected threshold. However, in KU, a linear regression of sgACC connectivity against CES-D demonstrated less connectivity to the right lateral orbitofrontal cortex (lOFC) and bilateral medial OFC (mOFC) in association with depression severity. Examined separately, men and women showed higher sgACC connectivity to bilateral superior temporal gyrus (STG) and dorsomedial prefrontal cortex (dmPFC), respectively, in correlation with depression. The sgACC connectivity to the OFC and dmPFC in relation to depression significantly differed between KU and HC. These findings are discussed in the below.
4.1 sgACC connectivity and depression in ketamine users
In KU the severity of depression is associated with decreased sgACC connectivity with lateral and medial OFC in men and women combined. The lOFC is involved in affective regulation and emotional experience. For instance, ratings of subjective emotion were correlated with activation in the right lOFC during viewing of negative versus neutral emotional images (Garrett and Maddock, 2006). The lOFC appeared to be specifically involved in reappraisal of negative emotions (Golkar et al., 2012). Higher neuroticism and introversion was associated with activation in the lOFC during losses in a card choice task to maximize profits (Fujiwara et al., 2008). Individual differences in negative urgency (impulsive behavior under negative emotional states) was associated with lOFC activation during exposure to negative as compared to neural emotional images (Cyders et al., 2015). The mOFC plays an important role in determining the affective tone of unconstrained thoughts (Tusche et al., 2014) and processing stimuli of positive affective value and those learned to be associated with stimuli of positive affective value (Shenhav et al., 2013). Being in a congruent emotional state, irrespective of the valence of emotion, activates the mOFC (Kuhn et al., 2011). The medial and lateral OFC were each related to counterfactual thinking and regret in economic decisions (Sommer et al., 2009). Thus, lOFC and mOFC appears to respond to distinct emotional states. The finding of altered sgACC connectivity to both lOFC and mOFC suggest the possibility of dysregulation of both positive and negative emotions in chronic ketamine users.
4.2 Sex differences in sgACC connectivity in KU
Increased sgACC connectivity with the dorsomedial prefrontal cortex (dmPFC) was associated with higher CES-D score in women but not in men. Previous studies have shown increased sgACC connectivity with the dmPFC in patients with major depressive disorder (Davey et al., 2012; Hamilton et al., 2011; Sheline et al., 2010). Increased sgACC connectivity with the dmPFC may be related to depression symptoms such as self-reproach and guilty rumination (Davey et al., 2012), in relation to the role of the dmPFC in autobiographical recall and self-referential analyses (Bado et al., 2014). Importantly, guilty rumination figured more prominently in women than in men with depression (Nolen-Hoeksema, 2012; Shors et al., 2017). A study combining fMRI and magnetic resonance spectroscopy revealed a significant negative correlation between GABA concentration in the dmPFC and BOLD signals in sgACC during exposure to sad face in contrast to control images (Stan et al., 2014). In individuals with treatment-refractory depression undergoing a 4-week course of repetitive transcranial magnetic stimulation of the dmPFC, higher baseline sgACC to dmPFC connectivity was associated with better treatment outcomes (Salomons et al., 2014). The latter finding support the importance of sgACC-dmPFC connectivity as a clinical biomarker of depression. However, as suggested by the current findings of sex difference, more work is warranted to elucidate whether and how sgACC-dmPFC connectivity influences cognitive and affective functions differently in men and women with depression.
The superior temporal gyrus (STG) along with the dmPFC is involved in evaluating emotional facial and vocal expressions (Dricu and Fruhholz, 2016). Adults with childhood maltreatment exhibited increased STG activity during emotional processing (Hein and Monk, 2016). A meta-analysis associated an inter-connected network of brain regions including the STG during experiences of guilt (Gifuni et al., 2016). Individuals with social anxiety disorder demonstrated increased activation in the STG, insula, and medial frontal cortex during exposure to facial emotions (Binelli et al., 2014). An earlier study reported changes in sgACC activity and connectivity to a swath of cortical and subcortical structures including the STG and dmPFC during facial emotional processing in individuals exposed to mood deterioration induced by typhoid vaccination (Harrison et al., 2009). Algorithm of machine learning showed that sgACC connectivity to the STG may represent a diagnostic marker of major depression (Zeng et al., 2014). However, as with work on the dmPFC, none of these studies described how men and women differed in STG responses or sgACC-STG connectivity during emotional processing. As men and women showed differences in sgACC connectivity with a wide limbic circuit that supports nociception (Wang et al., 2014) and with the saliency network in association with shyness (Yang et al., 2015), it is important to examine sex differences in sgACC activity and connectivity in association with emotion regulation and depression.
4.3 Limitations of the study and conclusions
A number of limitations are worth considering. First, we did not include a group of non-substance abusing individuals with depression for comparison, so it remained unclear whether the current findings were specific to chronic ketamine users or related broadly to depression. Second, although regression analyses largely ruled out an effect of alcohol and cigarette use on the current findings, it remained unclear how these comorbidities may influence sgACC connectivity, particularly in terms of the interacting effects with sex differences. That is, while the analyses did not reveal a significant relationship between imaging findings and smoking/drinking variables, we could not conclude that these findings are specific to ketamine misuse. Third, we did not assess history of childhood trauma, which can dispose individuals to depression, and this issue may be most relevant in the context of sex differences. Fourth, a few subjects underwent resting state fMRI for only 6 minutes; a longer scan duration would be needed to minimize within-subject variability in connectivity metrics (Tomasi et al., 2016). Finally, we targeted the sgACC in the current study, but other regions of the limbic circuit need to be investigated in future work for their role in the pathogenesis of depression in chronic ketamine users (Downey et al., 2016). In conclusion, we demonstrated altered sgACC connectivity in association with depression and sex differences in the altered patterns of connectivity in chronic ketamine users. These findings add to a growing literature of the addiction neuroscience of ketamine misuse.
Highlights.
Chronic ketamine misuse is associated with depression in women but not men.
sgACC shows altered connectivity in link with depression in chronic ketamine users.
Women and men differed in sgACC connectivity in accord with features of depression.
Findings add to extant literature that has focused on the acute effects of ketamine.
Acknowledgments
This study was supported by the Ministry of Science and Technology (MOST 104-2410-H-003-012) and Ministry of Health and Welfare (1031700961) of Taiwan and NIH grants K02DA026990 and K25DA040032. The funding agencies were otherwise not involved in study design, data analysis, or the decision to publish the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bado P, Engel A, de Oliveira-Souza R, Bramati IE, Paiva FF, Basilio R, et al. Functional dissociation of ventral frontal and dorsomedial default mode network components during resting state and emotional autobiographical recall. Hum Brain Mapp. 2014;35:3302–3313. doi: 10.1002/hbm.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeken C, Marinazzo D, Wu GR, Van Schuerbeek P, De Mey J, Marchetti I, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15:286–297. doi: 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, et al. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2015;16:2368–2385. doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31–38. doi: 10.1016/j.jad.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87:1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Binelli C, Subira S, Batalla A, Muniz A, Sugranyes G, Crippa JA, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64:205–217. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno MA, Jaquet O, Bahri MA, et al. Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology. 2016;125:873–888. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, de la Pena JB, Dela Pena IJ, Tampus R, Yoon R, Kim HJ, et al. Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: Evidence of its abuse potential. Pharmacol Biochem Behav. 2015;133:31–36. doi: 10.1016/j.pbb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, et al. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther. 2016;359:159–170. doi: 10.1124/jpet.116.235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. Altered Intrinsic Functional Brain Architecture in Children at Familial Risk of Major Depression. Biol Psychiatry. 2016;80:849–858. doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Huang MC, Chen LY. Major Depressive Disorder Induced by Chronic Ketamine Abuse: A Case Report. Prim Care Companion CNS Disord. 2016;18 doi: 10.4088/PCC.15l01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Huang MC, Lin SK. Gender differences in subjective discontinuation symptoms associated with ketamine use. Substance Abuse Treatment Prevention and Policy. 2014;9 doi: 10.1186/1747-597X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi KA, Kareken DA. Negative Urgency Mediates the Relationship between Amygdala and Orbitofrontal Cortex Activation to Negative Emotional Stimuli and General Risk-Taking. Cereb Cortex. 2015;25:4094–4102. doi: 10.1093/cercor/bhu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- De Luca MT, Badiani A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology (Berl) 2011;214:549–556. doi: 10.1007/s00213-010-2062-x. [DOI] [PubMed] [Google Scholar]
- Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol. 2016;26:994–1003. doi: 10.1016/j.euroneuro.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Dricu M, Fruhholz S. Perceiving emotional expressions in others: Activation likelihood estimation meta-analyses of explicit evaluation, passive perception and incidental perception of emotions. Neurosci Biobehav Rev. 2016;71:810–828. doi: 10.1016/j.neubiorev.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16:479–490. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui K. Personality-dependent dissociation of absolute and relative loss processing in orbitofrontal cortex. Eur J Neurosci. 2008;27:1547–1552. doi: 10.1111/j.1460-9568.2008.06096.x. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Maddock RJ. Separating subjective emotion from the perception of emotion-inducing stimuli: an fMRI study. Neuroimage. 2006;33:263–274. doi: 10.1016/j.neuroimage.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, et al. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology. 2014;39:895–906. doi: 10.1038/npp.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifuni AJ, Kendal A, Jollant F. Neural mapping of guilt: a quantitative meta-analysis of functional imaging studies. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9606-6. [DOI] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012;7:e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Maltbie E, Urushino N, Kempf D, Howell L. Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology (Berl) 2016;233:3673–3684. doi: 10.1007/s00213-016-4401-z. [DOI] [PubMed] [Google Scholar]
- Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, et al. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl) 2015;232:4231–4241. doi: 10.1007/s00213-015-4022-y. [DOI] [PubMed] [Google Scholar]
- Guo R, Tang Q, Ye Y, Lu X, Chen F, Dai X, et al. Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regul Toxicol Pharmacol. 2016a;77:263–274. doi: 10.1016/j.yrtph.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Guo R, Tang QX, Ye Y, Lu X, Chen F, Dai XH, et al. Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regulatory Toxicology and Pharmacology. 2016b;77:263–274. doi: 10.1016/j.yrtph.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA. Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth. 1999;82:603–608. doi: 10.1093/bja/82.4.603. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Monk CS. Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J Child Psychol Psychiatry. 2016 doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Ganger S, et al. Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1291-0. [DOI] [PubMed] [Google Scholar]
- Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Windischberger C, et al. Ketamine-Induced Modulation of the Thalamo-Cortical Network in Healthy Volunteers As a Model for Schizophrenia. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Lin SK, Chen CK. A roller coaster from methamphetamine to ketamine. Taiwanese Journal of Psychiatry. 2014;28:217–231. [Google Scholar]
- Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. 2016;46:1055–1067. doi: 10.1017/S0033291715002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. Holden-Day; San Francisco: 1968. [Google Scholar]
- Jia Z, Liu Z, Chu P, McGoogan JM, Cong M, Shi J, et al. Tracking the evolution of drug abuse in China, 2003–10: a retrospective, self-controlled study. Addiction. 2015;110(Suppl 1):4–10. doi: 10.1111/add.12769. [DOI] [PubMed] [Google Scholar]
- Kann S, Zhang S, Manza P, Leung HC, Li CR. Hemispheric Lateralization of Resting-State Functional Connectivity of the Anterior Insula: Association with Age, Gender, and a Novelty-Seeking Trait. Brain Connect. 2016;6:724–734. doi: 10.1089/brain.2016.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. Journal of Affective Disorders. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, et al. Ketamine interactions with biomarkers of stress: a randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage. 2015;108:396–409. doi: 10.1016/j.neuroimage.2014.12.050. [DOI] [PubMed] [Google Scholar]
- Kleinloog D, Rombouts S, Zoethout R, Klumpers L, Niesters M, Khalili-Mahani N, et al. Subjective Effects of Ethanol, Morphine, Delta(9)-Tetrahydrocannabinol, and Ketamine Following a Pharmacological Challenge Are Related to Functional Brain Connectivity. Brain Connect. 2015;5:641–648. doi: 10.1089/brain.2014.0314. [DOI] [PubMed] [Google Scholar]
- Kline RL, Zhang S, Farr OM, Hu S, Zaborszky L, Samanez-Larkin GR, et al. The Effects of Methylphenidate on Resting-State Functional Connectivity of the Basal Nucleus of Meynert, Locus Coeruleus, and Ventral Tegmental Area in Healthy Adults. Front Hum Neurosci. 2016;10:149. doi: 10.3389/fnhum.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Frolich MA, Tran S, White DM, Nichols N, Barton-McArdle A, et al. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Muller BC, van der Leij A, Dijksterhuis A, Brass M, van Baaren RB. Neural correlates of emotional synchrony. Soc Cogn Affect Neurosci. 2011;6:368–374. doi: 10.1093/scan/nsq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Seifritz E, Henning A, Walter M, Boker H, Scheidegger M, et al. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc Cogn Affect Neurosci. 2016;11:1227–1235. doi: 10.1093/scan/nsw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci. 2016;10:612. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu J, Xie A, Yang M, Johnson M, et al. Decreased Thalamocortical Connectivity in Chronic Ketamine Users. PLoS One. 2016;11:e0167381. doi: 10.1371/journal.pone.0167381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, Zhou W. Ketamine abuse potential and use disorder. Brain Res Bull. 2016;126:68–73. doi: 10.1016/j.brainresbull.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, et al. Large-Scale Persistent Network Reconfiguration Induced by Ketamine in Anesthetized Monkeys: Relevance to Mood Disorders. Biol Psychiatry. 2016;79:765–775. doi: 10.1016/j.biopsych.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Sallet J, Rushworth MF. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci U S A. 2015;112:E2695–2704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: the role of gender. Annu Rev Clin Psychol. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20:1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–498. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biological Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A Review of Ketamine Abuse and Diversion. Depress Anxiety. 2016;33:718–727. doi: 10.1002/da.22536. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Henning A, Walter M, Lehmann M, Kraehenmann R, Boeker H, et al. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Hum Brain Mapp. 2016;37:1941–1952. doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Barrett LF, Bar M. Affective value and associative processing share a cortical substrate. Cogn Affect Behav Neurosci. 2013;13:46–59. doi: 10.3758/s13415-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Millon EM, Chang HY, Olson RL, Alderman BL. Do sex differences in rumination explain sex differences in depression? J Neurosci Res. 2017;95:711–718. doi: 10.1002/jnr.23976. [DOI] [PubMed] [Google Scholar]
- Singh D, Chawarski MC, Schottenfeld R, Vicknasingam B. Substance Abuse and the HIV Situation in Malaysia. J Food Drug Anal. 2013;21:S46–S51. doi: 10.1016/j.jfda.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Peters J, Glascher J, Buchel C. Structure-function relationships in the processing of regret in the orbitofrontal cortex. Brain Struct Funct. 2009;213:535–551. doi: 10.1007/s00429-009-0222-8. [DOI] [PubMed] [Google Scholar]
- Stan AD, Schirda CV, Bertocci MA, Bebko GM, Kronhaus DM, Aslam HA, et al. Glutamate and GABA contributions to medial prefrontal cortical activity to emotion: implications for mood disorders. Psychiatry Res. 2014;223:253–260. doi: 10.1016/j.pscychresns.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Steffens M, Becker B, Neumann C, Kasparbauer AM, Meyhofer I, Weber B, et al. Effects of ketamine on brain function during smooth pursuit eye movements. Hum Brain Mapp. 2016;37:4047–4060. doi: 10.1002/hbm.23294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Aoki T, Kato H, Yamazaki M, Misawa M. Effects of the 5-HT(3) receptor antagonist ondansetron on the ketamine- and dizocilpine-induced place preferences in mice. Eur J Pharmacol. 1999;385:99–102. doi: 10.1016/s0014-2999(99)00762-1. [DOI] [PubMed] [Google Scholar]
- Tang M, Ching CK, Tse ML, Ng C, Lee C, Chong YK, et al. Surveillance of emerging drugs of abuse in Hong Kong: validation of an analytical tool. Hong Kong Med J. 2015;21:114–123. doi: 10.12809/hkmj144398. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi DG, Shokri-Kojori E, Volkow ND. Temporal Evolution of Brain Functional Connectivity Metrics: Could 7 Min of Rest be Enough? Cereb Cortex. 2016 doi: 10.1093/cercor/bhw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A, Smallwood J, Bernhardt BC, Singer T. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. Eur J Pharmacol. 2009;606:94–101. doi: 10.1016/j.ejphar.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Mutti A, Chiamulera C. Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats. Psychopharmacology (Berl) 2015;232:4505–4514. doi: 10.1007/s00213-015-4077-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Zheng D, Xu J, Lam W, Yew DT. Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat. 2013;7:23. doi: 10.3389/fnana.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Erpelding N, Davis KD. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain. 2014;155:755–763. doi: 10.1016/j.pain.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Wong JJ, O’Daly O, Mehta MA, Young AH, Stone JM. Ketamine modulates subgenual cingulate connectivity with the memory-related neural circuit-a mechanism of relevance to resistant depression? PeerJ. 2016;4:e1710. doi: 10.7717/peerj.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang S, Kendrick KM, Wu X, Yao L, Lei D, et al. Sex differences in intrinsic brain functional connectivity underlying human shyness. Soc Cogn Affect Neurosci. 2015;10:1634–1643. doi: 10.1093/scan/nsv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther. 1981;218:720–727. [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp. 2014;35:1630–1641. doi: 10.1002/hbm.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Li CS. Resting-State Functional Connectivity of the Locus Coeruleus in Humans: In Comparison with the Ventral Tegmental Area/Substantia Nigra Pars Compacta and the Effects of Age. Cereb Cortex. 2016a;26:3413–3427. doi: 10.1093/cercor/bhv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Fucito LM, Luo X, Mazure CM, Zaborszky L, et al. Resting-State Functional Connectivity of the Basal Nucleus of Meynert in Cigarette Smokers: Dependence Level and Gender Differences. Nicotine Tob Res. 2016b doi: 10.1093/ntr/ntw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


