Abstract
Depression is a well-known risk factor for developing relapse drinking, but the neuronal mechanisms underlying the interactions between depression and alcohol use disorders remain elusive. Accumulating evidence has associated depression with hyperactivity of the lateral habenula (LHb), an epithalamic structure in the brain that encodes aversive signals. Glutamate receptors contribute substantially to the excitability of LHb neurons. Glutamatergic synapses in LHb neurons largely express GluA1-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) that can be modulated by Ca2+/calmodulin-dependent protein II (CaMKII). In the current study, we tested the hypothesis that withdrawal from repeated cycles of ethanol drinking triggers an increase in LHb AMPAR and CaMKII activity concomitant with depression-like symptoms, and their inhibitions bring a reduction in depressive-like behaviors and alcohol consumption. Western blotting revealed a higher level of phosphorylated AMPAR GluA1 subunit at a CaMKII locus (GluA1-Ser831) in the LHb of ethanol-withdrawn rats than that of age-matched naïve counterparts. In ethanol-withdrawn rats, pharmacological inhibition of LHb AMPAR activity significantly mitigated the depressive-like behavior and ethanol drinking and seeking behaviors, but affected neither sucrose intake nor locomotor activity; and inhibition of LHb CaMKII activity, or chemogenetic inhibition of LHb activity produced similar effects. Conversely, activation of LHb AMPARs induced depressive-like behaviors in ethanol-naïve rats. These results demonstrate that CaMKII-AMPAR signaling in the LHb exemplifies a molecular basis for depressive-like symptoms during ethanol withdrawal and that inhibition of this signaling pathway may offer a new therapeutic approach to address the comorbidity of alcohol abuse and depression.
Keywords: Lateral habenula, ethanol intake, AMPA receptors, depressive-like behavior, CaMKII, forced swimming test, sucrose preference test
Graphical abstract
1. Introduction
Alcoholics frequently suffer from depression (Pettinati, 2004), which negatively impacts treatment outcomes and increases the probability of relapse (Pelc et al., 2002). The mechanisms underlying this comorbidity, however, are not well understood. Increasing evidence has linked depression with hyperactivity of the lateral habenula (LHb). The LHb has emerged as an important part of the reward circuit by providing negative reward signals to the dopamine and serotonergic neurons in the midbrain (Jhou et al., 2009; Kaufling et al., 2009; Matsumoto and Hikosaka, 2007; Omelchenko et al., 2009). Various aversion-related stimuli, such as stress, pain, fear, and reward omission, activate LHb neurons (Li et al., 2011a; Meye et al., 2015; Neumann et al., 2015; Wirtshafter et al., 1994; Zouikr et al., 2014). LHb neurons are hyperactive in the depressed state (Chourbaji et al., 2005; Proulx et al., 2014; Shumake et al., 2003; Zouikr et al., 2014). Conversely, inhibition of LHb neuronal activity suppresses depressive-like behaviors in animal models of depression (Li et al., 2011a; Li et al., 2013; Meye et al., 2015). However, the precise molecular targets responsible for LHb hyperactivity during ethanol withdrawal have not been fully unveiled.
Glutamate is the primary excitatory neurotransmitter in the CNS. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), a subclass of ionotropic glutamate receptors, play an essential role in regulating synaptic strength (Bredt and Nicoll, 2003), and are involved in several neurological, psychiatric and addictive disorders (Zhang and Abdullah, 2013), including alcohol use disorder (Kryger and Wilce, 2010). AMPARs are composed of four subunits (GluA1-4). The GluA1 subunit, in particular, plays an important role in alcohol reinforcement, drinking and seeking behaviors (Cannady et al., 2016; Salling et al., 2016; Wang et al., 2012). The LHb receives strong glutamatergic inputs and expresses mostly the GluA1 subunit (Meye et al., 2013). Enhanced glutamatergic transmission might activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Fink and Meyer, 2002), which then phosphorylates serine 831 (S831) on the GluA1 C-terminus, enhancing AMPAR activity (Hayashi et al., 2000; Malinow, 2003). A previous animal study found that increased expression of βCaMKII in the LHb causes more GluA1 insertion into synapses, resulting in increased synaptic efficacy and depressive-like behaviors (Li et al., 2013).
We have recently shown that increased glutamatergic transmission in the LHb contributes to the increased activity of LHb neurons in rats withdrawn from chronic ethanol consumption and that LHb inhibition by high frequency electrical stimulation reduces ethanol consumption (Li et al., 2016). However, it is unknown whether CaMKII-AMPAR signaling in the LHb is functionally relevant for depressive-like behavior and elevated ethanol drinking following a history of repeated cycles of drinking and withdrawal. In the current study, using a combination of behavioral, physiological, pharmacological, molecular, and chemogenetic approaches, we tested the hypothesis that during ethanol withdrawal, the activity of AMPARs and CaMKII in the LHb is increased, which contributes to depressive-like symptoms and elevated alcohol consumption; and that inhibition of the activity of LHb AMPAR and CaMKII ameliorates depressive-like behaviors and reduces alcohol consumption.
2. Materials and Methods
2.1. Animals and housing
All experiments were performed in accordance with the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Rutgers, The State University of New Jersey, Newark, New Jersey. Adult male Long-Evans rats (two months old at the start of the experiments, from Harlan Lab, NY) were individually housed in ventilated Plexiglas cages in a climate-controlled room (20–22°C) and acclimatized to the housing conditions and handling for at least 7 days before the start of the experiments. All rats were kept on a 12-h light/dark cycle: lights off at 11:00 a.m. Food and water were available ad libitum, or as indicated otherwise.
2.2. Ethanol drinking procedure
2.2.1. Intermittent access to ethanol in two-bottle free choice(IA2BC) drinking procedure
We trained rats to drink 20% ethanol in the IA2BC procedure as described previously (Li et al., 2011b; Li et al., 2016; Simms et al., 2008). Briefly, animals were given 24-h concurrent access to one bottle of 20% (v/v) ethanol in water and one bottle of water, starting at 11:00 a.m. on Monday. After 24 h, the ethanol bottle was replaced with a second water bottle that was available for the next 24 h. This pattern was repeated on Wednesdays and Fridays. On all other days, the rats had unlimited access to two bottles of water. On each ethanol-drinking day, the placement of the ethanol bottle was alternated to account for side preferences. The amount of ethanol or water consumed was determined by weighing the bottles before and after 24 hours of access. Animal body weight was measured weekly to monitor health and to calculate ethanol intake. Ethanol consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. The preference ratio of ethanol intake was calculated by the following formula: Preference ratio (%) = ethanol solution intake (ml) /total fluid intake (ml ethanol solution + ml water).
After eight-weeks in the IA2BC procedure, when a stable baseline drinking level (5.3 ± 1.2 ~ 6.2 ± 0.8 g/kg/24 h) had been achieved, rats were divided into 9 groups (S Table 1): (1) Forced swimming test (G1a, nrats=12), (2) Sucrose preference test (G1b, nrats=8), (3), Tissues containing the LHb were harvested at 24 h after the last ethanol session for electrophysiological recordings (G1c, nrats = 7), or for examination of AMPAR and CaMKII expression using Western blot (G1d, nrats = 6). Rats in groups 2–7 all received LHb cannulae implantation. Specifically, rats in group 2–5 were examined for the effects of DNQX through: forced swimming test (G2, nrats = 22), sucrose preference test (G3, nrats = 18), ethanol intake (G4, nrats = 9), in operant chamber testing self-administration of ethanol (G5, nrats=8). Rats in groups 6 and 7 were examined for the effects of CaMKII inhibitor KN-62 through: depressive-like behavior and ethanol intake (G6, nrats = 20), and GluA1 phosphorylation (G7, nrats = 12): rats in this group were sacrificed 10 minutes after intra-LHb infusion of KN-62 or aCSF, tissues containing the LHb were then collected for Western blot. Rats in group 8 received cannulae implantation to the mediodorsal thalamic nucleus (MD) to examine the effect of DNQX on ethanol intake (nrats = 7). Rats in group 9 received chemogenetic virus injection in the LHb to test whether chemogenetic inhibition of LHb changed ethanol intake and depressive like behaviors (nrats = 12).
2.2.2. Operant self-administration after intermittent access to ethanol
The operant chamber (30 cm wide, 29 cm high) was encased within a larger sound-attenuating chamber and had two levers that were located against the right wall, 7 cm from the floor and 1 cm from the right or left edge of the right wall. A 2.5-cm white stimulus light was located above each lever. A rectangular recess (3 cm in diameter) was located between the 2 levers, 3 cm above the floor. Syringe pumps delivered fluid into a fluid receptacle within this recess (ethanol port). A house light, located on the right wall 14 cm from the floor, was on for the duration of each behavioral session. In addition, the operant chamber contained an infrared head poke detector that recorded how many times an animal’s head entered the ethanol reward port. All behavioral equipment (MED Associates, St. Albans, VT) was computer-controlled via software (MED Associates) that also recorded the responses and reinforcer deliveries during behavioral sessions.
This experiment was conducted similarly to our previous studies (Fu et al., 2016; Li et al., 2012). Briefly, a group of rats (n = 8) under the IA2BC paradigm for 16 to 20 sessions received 3 overnight (12 to 14 hours) sessions with 0.1 ml of 20% ethanol available on a fixed-ratio-1 (FR1) schedule after responses to the active lever. After shaping, subjects received daily 45-minute sessions, 5 days a week. One week later, the response requirement was increased to a FR3 schedule in 30-minute sessions for 2 weeks.
2.3. Brain slice preparation and electrophysiological recordings
Rats at 24 h withdrawal from ethanol after eight weeks in the IA2BC paradigm or ethanol-naïve counterparts were sacrificed under anesthesia of ketamine/xylazine (80 mg/10 mg/kg, i.p). Brain slices were prepared as described (Zuo et al., 2013). Briefly, the brain was rapidly removed and placed in ice-cold-glycerol-based artificial cerebrospinal fluid (GaCSF) containing the following (in mM): 252 glycerol, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 0.3 L-ascorbate, and 10 glucose, and oxygenated with 95% O2 / 5% CO2 (carbogen). Coronal slices (300 µm thick) were cut with a Compresstome VF-200 slicer (Precisionary Instruments Inc., Greenville, NC, USA), and then transferred to a holding chamber and incubated for at least 1 h at room temperature (24°C–25°C) in carbogenated regular artificial cerebrospinal fluid (aCSF) of almost the same composition as GaCSF, with the exception being that 252 mM glycerol was replaced with 126 mM NaCl.
Electrophysiological recordings were conducted as described (Kang et al., 2017; Zuo et al., 2013). Electrophysiological events were recorded with an Axon 200B amplifier, a Digidata 1440A A/D converter, and Clampfit 10.6 software (Molecular Devices Co., Union City, CA, USA). Throughout the experiments, the bath was continually perfused with warm (33°C) carbogenated aCSF (1.5–2.0 ml/min). Access resistance was monitored by a step of −10 mV (0.1 Hz) and experiments were discarded if the access resistance increased >20%. Neuronal excitability was examined in the whole-cell mode, and 10-pA current steps (from −30 to 50 pA) were applied, from which V-I plots were determined (the number of action potential vs current). The internal solution contained (in mM): 140 K-gluconate, 5 KCl, 2 MgCl2, 10 HEPES, 2 MgATP, and 0.2 GTP. The pH was adjusted to 7.2 with Tris-base and the osmolality to 310 mOsmol/L with sucrose for all internal solutions. EPSCs were evoked through a bipolar stainless steel electrode placed 200 µm from the recording site. The internal solution contained (in mM): CsCl 130, NaCl 4, MgCl2 2, EGTA 1.1, HPEPS 5, Na2ATP 2, Na+-creatine-phosphate 5, Na3GTP 0.6, and spermine 0.1. All experiments were carried out in the presence of picrotoxin (100 µM) and AMPAR EPSCs were pharmacologically isolated by application of the NMDA antagonist DL-2-amino-5-phosphono-valeric acid (AP5, 50 µM). The NMDAR components were calculated by subtraction of the EPSCs measured in the presence of AP-5 from those in the absence of AP5. AMPA/NMDA ratios of evoked-EPSCs were obtained by AMPA-EPSC −70 mV/NMDA-EPSCs at +50 mV, using the late component of the EPSC at 30 ms after the onset. Rectification indexes were calculated by the formula (AMPA-EPSC −70 mV/AMPA-EPSC +50 mV)/1.75 in the presence of AP5 and intracellular spermine as described (Maroteaux and Mameli, 2012).
2.4. Stereotaxic surgery and microinjection procedure
2.4.1. Stereotaxic surgery
Cannulae implantation was performed using a stereotaxic apparatus (Kopf, USA) under isoflurane (Ohio Medical Products) anesthesia. A bilateral guide cannula (C235G-3.0/Spc, 22 gauge; Plastics One, Roanoke, VA) was aimed dorsal to the LHb (3.8 mm posterior to bregma, +/− 0.75 mm mediolateral, 4.2 mm ventral to the skull surface) or the MD (1.8 mm posterior to bregma, +/− 0.75 mm mediolateral, 4.0 mm ventral to the skull surface), according to Paxinos and Watson (Paxinos and Watson, 2007). One week after recovery from surgery, subjects resumed ethanol drinking in home cages or in the operant chambers, or to 2% sucrose drinking.
When ethanol (or sucrose) intake was stable, microinjections began. Before microinjection, to reduce the stress of subjects and habituate them to the microinjection procedures, animals were taken from the colony, brought to the experimental room, and handled for 5 min every day for 3 days. During this phase, animals became accustomed to the experimenter, the experimental room, and to the manipulation procedure. Histological verification of the placements was done at the end of behavioral test as described (Li et al., 2011b). Supplemental figure 1 indicates the placement of cannulae tips for animals used in this study. Data from animals with misplaced cannulae were not shown and were excluded from analysis.
2.4.2. Microinjection procedure
AMPA, DNQX and KN-62 (Sigma, St. Louis, MO), or vehicle (aCSF) were administered through a 28-gauge internal cannulae (Plastics One, Roanoke, VA, USA) connected to a Hamilton 1.0 µl syringe driven by a syringe pump (Harvard Instruments, South Natick, Mass., USA). Microinjections were given 10 min prior to the behavioral tests. Obstructers were removed and injectors were inserted bilaterally to a depth of 1 mm beyond the end of the guide cannula. The drug or aCSF (200 nl) was infused over 60 seconds into the LHb of a gently restrained rat. The injectors were left in place for an additional 60 seconds to allow for diffusion. After removal of the injectors, new sterile obstructers were inserted. For measuring ethanol drinking and seeking behavior, the order of drug treatments was partially counterbalanced such as that aCSF was given first, followed by drug injection, with doses in random order. There was a minimum of seven days for recovery between successive drug injections.
2.4.3. Intra-LHb chemogenetic virus injection
After rats were trained to drinking ethanol under the IA2BC paradigm for eight weeks (24 sessions), the engineered human muscarinic receptor, the inhibitory hM4D (hM4Di), was introduced by injecting AAV5-CaMKIIa-hM4Di-mCherry, or control AAV5-CaMKIIa-eGFP (titers of 10e12–10e13 vg/ml, UNC Vector Core, Chapel Hill, NC) bilaterally into the LHb on the coordination described in 2.5.1, except that 5.4 mm, instead of 4.0 mm ventral to the skull surface. A volume of 500 nl per side was delivered at a rate of 70 nl min−1. One week after recovery from surgery, subjects resumed ethanol drinking in the IA2BC procedure. Three weeks after virus injection, CNO (10 mg/kg, i.p.) was given 30 min before accessing to ethanol or forced swimming test (Kang et al., 2017).
2.5. Forced swimming test
To measure general depressive-like behaviors in rats, we adapted a commonly used modified forced swim task (Slattery and Cryan, 2012) in a transparent plastic tube (diameter = 24.5 cm, height = 51 cm), filled to 30 cm with water at 23–25 °C. Before the forced swimming test, to reduce the stress of and habituate subjects to the test room, animals were brought to the test room for 30 minutes each day for 3 consecutive days. Thus, the animals became accustomed to the experimenter and the environment. The pre-test was performed 24 h after ethanol withdrawal, where the rats were placed individually into the forced swimming test apparatus for 15 minutes. The test was performed 24 h after the pre-test (i.e., at 48 h after ethanol withdrawal), where the rats were placed individually into the forced swimming test apparatus for 5 minutes, and their behaviors were recorded with video recorders. Two individuals, blind to the animal treatment history, later analyzed the videos and scored the following behaviors: immobility, swimming, and climbing by using a time-sampling technique in each 5-second period of the 5-minute test. Immobility is defined as the rat floating in the water without struggling and only making movements necessary to keep its head above water. Swimming is defined as the rat making horizontal movements throughout the swim cylinder, which also includes climbing.
2.6. Sucrose Preference Test
The depressive-like behavior was also examined using the sucrose preference test (Zhou et al., 2017). Briefly, rats were trained to drink ethanol under the IA2BC paradigm for eight weeks (24 sessions), before they were habituated to two bottles of 1% sucrose solution for 24 h. They then were subjected to food deprivation for 20 h before getting access to one bottle of 1% sucrose solution and one bottle of water for 3 h. The preference ratio of 1% sucrose was calculated by the following formula: Preference ratio (%) = 1% sucrose solution intake (ml/3 h) /total fluid intake (ml/3 h sucrose solution + ml/3 h water).
2.7. Measurements of locomotion activity
A 60-minute locomotion test was done 10 minutes after intra-LHb injection of DNQX (500 ng/0.2 µl/side) or aCSF (0.2 µl/side). Movements were recorded automatically in locomotor chambers (TruScan Photobeam Activity Monitors, 16×16×16 inches) using TruScan 2.0 software, as described (Li et al., 2012).
2.8.Western blot analysis
Tissue containing the LHb of both hemispheres was harvested and homogenized on ice in radio-immunoprecipitation assay buffer (Sigma-Aldrich, St. Louis, MO). Proteins were quantified using the Bradford assay (Bio-Rad Laboratories) and 10 µg per sample was loaded on a SDS-PAGE gel and transferred to a nitrocellulose membrane. Western blot analysis was performed through the use of the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories). The levels of total GluA1, CaMKII, phospho-GluA1 (Ser831), phospho-GluA1 (Ser 845) and GAPDH in the LHb were detected by anti-GluA1 (1:1000), anti-CaMKII (1:5000), anti-phospho-GluA1 (Ser831), anti-phospho-GluA1 (Ser845) (Millipore, 1:2,000), and anti-GAPDH antibody (1:2,000, Sigma-Aldrich), respectively. We quantified the Western blots in two steps. First, we compared protein loading and relative protein expression to GAPDH levels in each lane by quantitatively analyzing the optical densities of bands using ImageJ version 1.38 (NIH, Bethesda, MD). Then, we presented changes in protein levels in rats experiencing ethanol withdrawal or KN-62 treatment as a percentage of those in the control group (set to 100).
2.9. Drugs
We purchased 6, 7-dinitroquinoxaline-2, 3-dione (DNQX), DL-2- amino-5-phosphono-valeric acid (DL-AP5), H-89, KN-62, and other common salts from Sigma (St Louis, MO); ethanol, from grains, 190 proof, stored in glass bottles, from Pharmco Products (Brookfield, CT). Clozapine-N-oxide (CNO) was from NIDA Drug supply program (NIH, Bethesda, MD).
2.10. Statistical analysis
Drinking data during 30-min or 24-h access periods as well as lever pressing and reward port entry data from ethanol self-administration in the operant chambers were subjected to a one-way or two-way repeated measures analysis of variance (RM ANOVA) to extract significant main effects and interactions with Bonferroni post hoc comparisons. Data from forced swimming test, sucrose preference test, locomotion tests, and Western blots were subject to Student’s t test. Statistical significance was declared at p <0.05.
3. Results
3.1. Depressive-like behaviors in rats withdrawn from repeated cycles of ethanol consumption
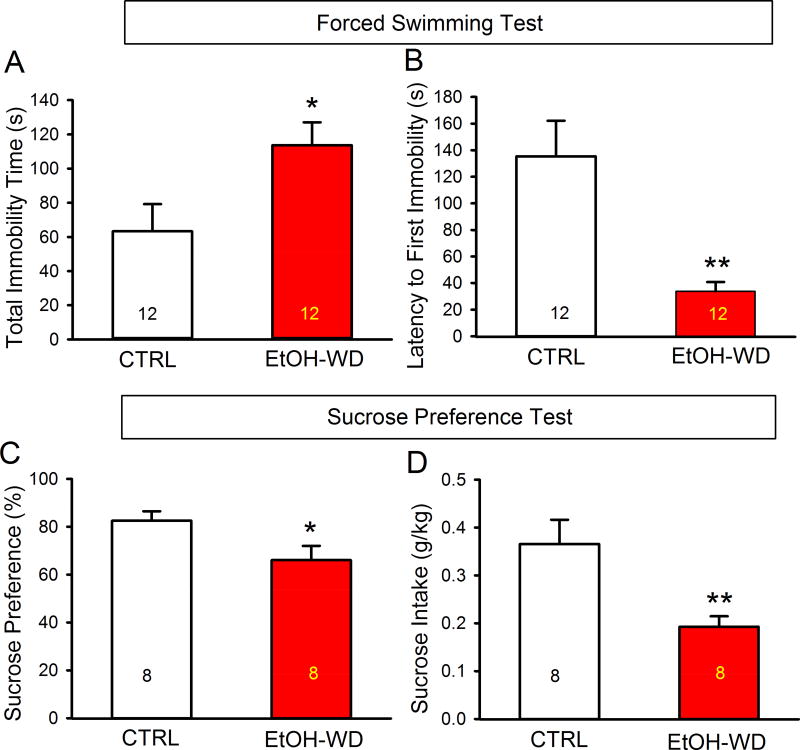
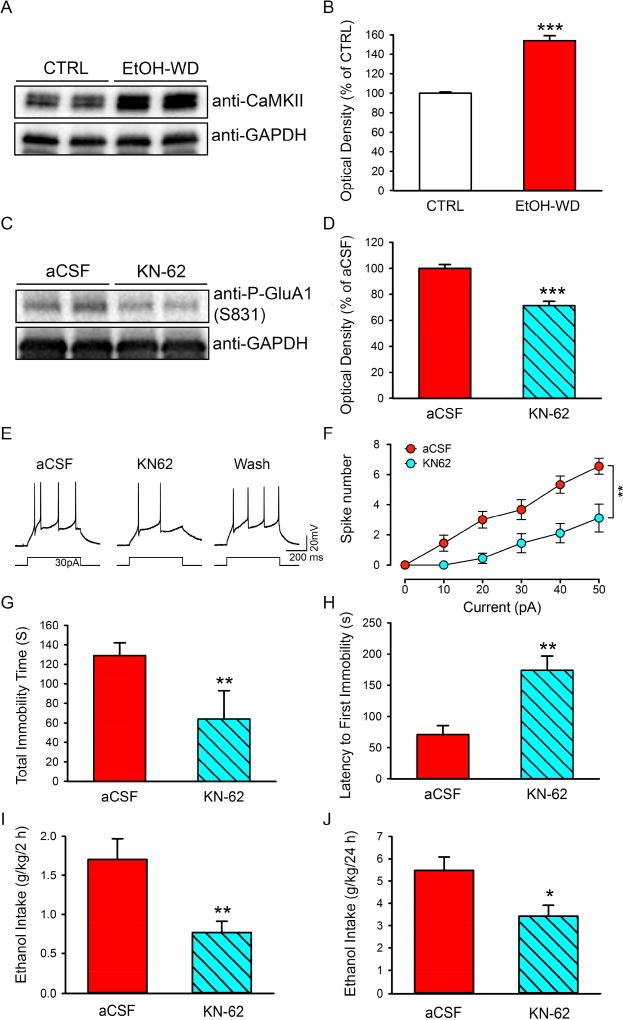
We examined depressive-like behaviors in rats that had been in the IA2BC paradigm for 2 - months and at 48 h withdrawal from the last ethanol session. These rats displayed a longer immobility time (Fig. 1A, EtOH-WD; t = 2.43, P = 0.023) and a shorter latency to the first immobility (Fig. 1B, EtOH-WD; t = 3.59, P = 0.002) compared to ethanol naïve (CTRL) rats in the forced swimming test. Ethanol withdrawn rats also showed less sucrose preference (Fig. 1C, EtOH-WD; t = 2.36, P = 0.032) and sucrose intake (Fig. 1D, EtOH-WD; t = 2.99, P = 0.009), compared to ethanol naïve (CTRL) rats in the sucrose preference test.
Figure 1.
Depressive-like behaviors in ethanol-withdrawal rats. Rats at 48-hour withdrawal from repeated cycles of voluntary ethanol drinking (EtOH-WD) had a significantly prolonged total immobility time (A) and a shortened latency to the first immobility in the forced swim test (B), a reduced sucrose preference (C) and intake (D) in the sucrose preference test, compared with ethanol naïve control (CTRL). *p < 0.05, **p < 0.01. Data are expressed as mean ± SEM.
3.2. Increased neuronal excitability, AMPA/NMDA ratios and GluA1 phosphorylation in the LHb of rats withdrawn from repeated cycles of ethanol consumption
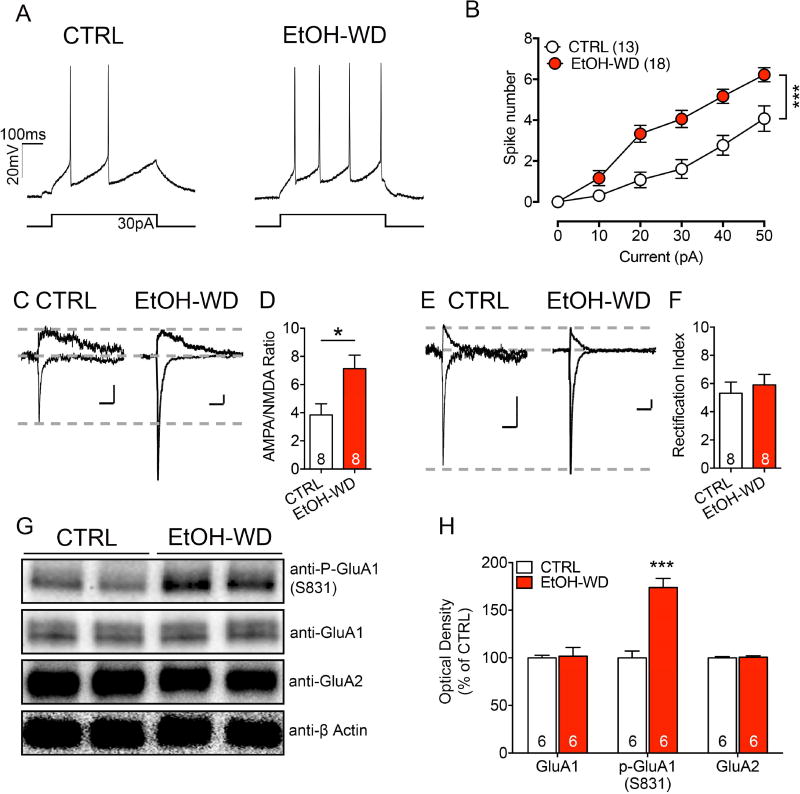
To examine the changes in the LHb at the cellular and molecular levels, we measured the neuronal excitability, which was substantially higher in the LHb neurons of brain slices from rats at 24h withdrawal from ethanol than those from the ethanol-naive group (Two-way RM ANOVA, F1,29 = 16.65, p < 0.001). The AMPA/NMDA ratio of LHb neurons was also significantly increased in withdrawal rats (Fig. 2C, D), indicating a postsynaptic strengthening of excitatory transmission. No substantial change in EPSC rectification index was noted (Fig. 2E, F), suggesting that the overall AMPAR subunit composition remained similar during withdrawal, although the withdrawal induced the insertion of new AMPARs at the synapse.
Figure 2.
Withdrawal from repeated cycles of ethanol consumption significantly increases excitability, AMPA/NMDA ratio, and GluA1 phosphorylation of LHb neurons. (A) Current injections elicit significantly more spikes in LHb neurons of ethanol-withdrawn rats (EtOH-WD) than in those of naïve control rats (CTRL). (B) Number of spikes evoked over a wide range of current pulses was consistently higher in EtOH-WD than in CTRL group. ***p < 0.001. The recorded cell numbers are indicated. (C) Representative traces of AMPA and NMDA-EPSCs recorded in LHb neurons in slices from CTRL and EtOH-WD groups. (D) Pooled data indicating increased AMPA/NMDA ratio of LHb neurons from EtOH-WD group relative to that from CTRL group (ncells = 8/group); CTRL versus EtOH-WD, t = 2.643, df = 13.49, p = 0.019. (E) Sample traces of AMPA-EPSCs recorded at −70 and +50 mV of LHb neurons in slices from CTRL and EtOH-WD groups. (F) No significant changes in the Rectification Index of LHb neurons in slices from CTRL and EtOH-WD group (ncells = 8 in each group); CTRL versus EtOH-WD, t = 0.5428 df = 13.96, p = 0.595. Representative Western blot image (G) and analysis (H) to quantify expression levels of phosphor-GluA1 at residue 831 (S831) and total GluA1 in the LHb in naïve control (CTRL) rats and ethanol-withdrawn (EtOH-WD) rats at 24 h after removal of the ethanol bottle (nrats = 6). Data are expressed as mean ± SEM. ***p < 0.001.
Using Western blot analysis, we sought to determine whether the GluA1 expression in the LHb had changed during ethanol withdrawal. Quantification analysis revealed a marked increase in phosphorylated GluA1 at Ser831 (Fig. 2G, H) and Ser845 (Supplementary figure 2A, B) in the LHb of ethanol-withdrawn rats. However, the total GluA1 and GluA2 expression was not significantly changed (Fig. 2G, H).
3.3. Intra-LHb infusion of DNQX attenuates depressive-like behaviors in ethanol withdrawn rats
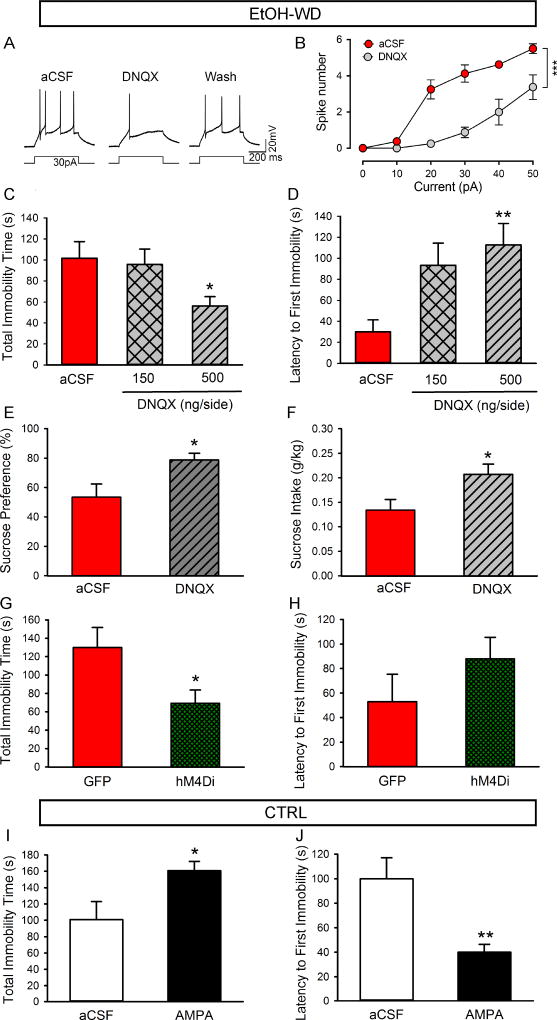
To determine the role of AMPARs in the LHb on neuronal activity, we examined the effect of the AMPAR antagonist DNQX. Bath application of DNQX (20 µM) significantly reduced excitability of LHb neurons of ethanol-withdrawn rats (Fig. 3A, B; Two-way RM ANOVA, F1,14 = 36.43, p < 0.001). Then, to determine the role of LHb AMPARs in the depressive-like behaviors, we bilaterally infused DNQX (150 or 500 ng/200 nl/side) into the LHb of ethanol-withdrawn rats 10 min before the forced swimming test. Histological verification revealed that the cannula tips in 22 of the 24 rats were within the LHb (Supplementary figure1). Intra-LHb DNQX at the dose of 500 ng but not 150 ng, substantially reduced immobility time (Fig. 3C, P < 0.05) and prolonged the latency to the first immobility in the forced swimming test (Fig. 3D, P = 0.002), compared to the aCSF infusion. Intra-LHb DNQX (500 ng) also significantly increased sucrose preference (Fig. 3E, P = 0.03) and intake (Fig. 3F, P = 0.01) in the sucrose preference test. Chemogenetic inhibition of LHb activity by CNO (10 mg/kg, i.p.) also substantially reduced immobility time (Fig. 3G, P < 0.05), without changing the latency to the first immobility in the forced swimming test (Fig. 3H, P > 0.05). To determine whether enhancing LHb neuronal activity in ethanol naïve (CTRL) rats can lead to depressive-like behaviors, we bilaterally infused AMPA (11 ng/200 nl/side) into the LHb, which significantly prolonged the total immobility time (Fig. 3I, P < 0.05) and shortened the latency to the first immobility (Fig. 3J, P < 0.01).
Figure 3.
Role of AMPA receptors in LHb neuronal excitability and depressive-like behaviors. (A–H, in ethanol-withdrawn rats, WD). Representative traces (A) and the plot of spike number verses current (B) show that bath perfusion of the AMPA receptor antagonist DNQX (20 µM) significantly decreased the number of spikes induced by a current step in LHb neurons of ethanol-withdrawn (WD) rats (ncells = 8). (C, D) Intra-LHb injection of DNQX at the dose of 500 ng/200 nl/side, but not 150 ng significantly shortened the total immobility time (C) and increased the latency to the first immobility (D) in the forced swim test (nrats = 11). (E, F) Intra-LHb DNQX (500 ng/200 nl/side) increased preference for sucrose (E) and sucrose intake (F) in the sucrose-preference test (nrats = 8). Chemogenetic inhibition of the LHb by CNO (10 mg/kg, i.p.) significantly shortened the total immobility time (G), without significant change in the latency to the first immobility (H) in rats infected with hM4Di in the LHb, compared with rats infected with GFP only in forced swimming test (nrats = 6). (I–J, in ethanol-naïve rats). Intra-LHb injection of AMPA (11 ng/200 nl/side) in ethanol-naïve rats significantly prolonged the total immobility time (I) and shortened the latency to the first immobility (J) in forced swimming test (nrats= 7). The values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with aCSF.
3.4. Intra-LHb infusion of DNQX selectively reduces alcohol drinking and seeking
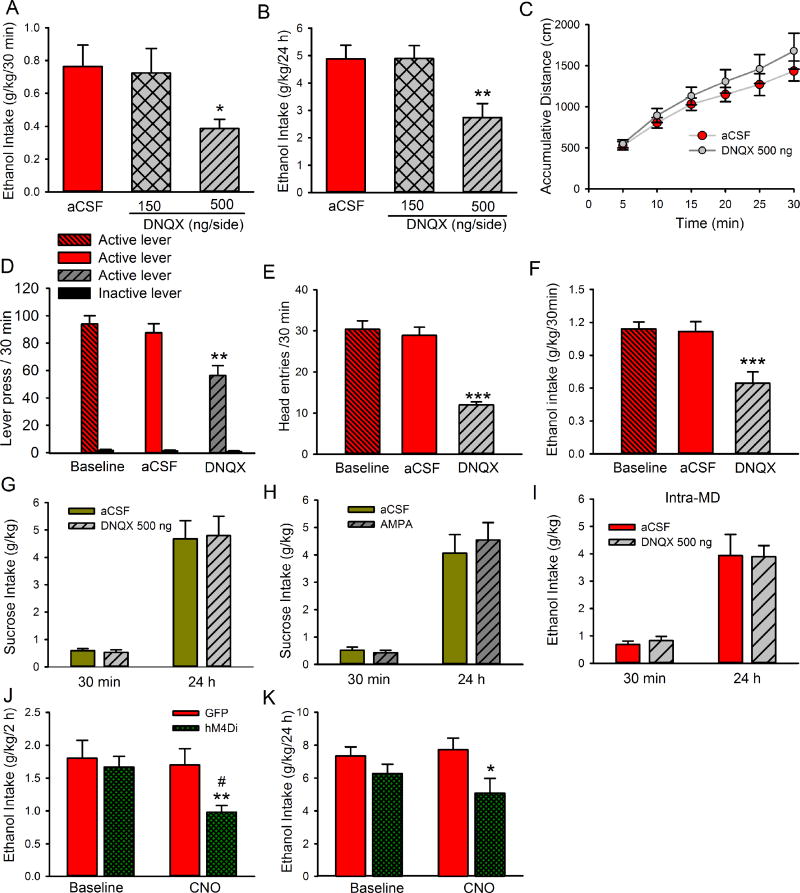
To test whether inhibition of LHb AMPARs could alter ethanol consumption, we bilaterally infused DNQX or aCSF into the LHb 10 minutes before access to ethanol, and measured ethanol and water intake were at 30 min and 24 h after access. Histological verification revealed that the cannula tip placements for all animals were within the LHb (Supplementary figure1). One-way RM ANOVA analysis revealed a significant decrease in ethanol consumption measured at 30 min (F2,26 = 4.67, P = 0.03, Fig. 4A). Post-hoc analysis revealed that the ethanol intake of rats that received DNQX infusion at the dose of 500 ng, but not of 150 ng, was significantly lower than that of rats that received intra-LHb infusion of aCSF (Fig. 4A, P < 0.01). One-way RM ANOVA also revealed a significant decrease in ethanol consumption at 24 h after ethanol access (F2, 26 = 6.29, p = 0.006, Fig.4B). Additionally, intra-LHb infusion of 500 ng DNQX significantly reduced ethanol preference (F2, 26 = 3.82, p = 0.03, Table 1) in rats 24 h after ethanol access, but did not alter water consumption or total fluid intake (Table 1) at both 30 min and 24 h time points. Ethanol intake and preference at 48 h and 72 h after intra-LHb infusion of 500 ng DNQX did not change compared to the aCSF infusion (Table 1).
Figure 4.
Intra-LHb infusion of DNQX reduces alcohol drinking and seeking without changing sucrose intake in ethanol-withdrawn rats. DNQX was bilaterally injected into the LHb 10 min before the start of the drinking or operant session. DNQX at the dose of 500 ng/200 nl/side, but not 150 ng significantly decreased ethanol consumption at 30 min (A) and 24 h (B) after the onset of access to ethanol compared with aCSF infusion, nrats = 9/arm. (C) DNQX (500 ng/200nl/side) did not affect total distance travelled (cm) across the first 30-min session after intra-LHb infusion compared with aCSF infusion (nrats = 6). (D) Intra-LHb DNQX (500 ng/200nl/side) decreased the number of active lever presses, but not inactive lever presses during the 30-minute test session (nrats = 8). DNQX also reduced the number of head entries into the ethanol port (E) as well as ethanol intake (F) during the 30-minute test session (nrats = 8). (G), Intra-LHb DNQX (500 ng/200nl/side) did not affect sucrose consumption (nrats = 10) under the IA2BC procedure. (H) Intra-LHb infusion of AMPA (11 ng/200 nl/side) did not change sucrose consumption under the IA2BC procedure (nrats = 9). (I), Bilateral injection of DNQX (500 ng/200nl/side) into the mediodorsal thalamic nucleus (MD) did not affect ethanol intake at 30 min or 24 h after the onset of access to ethanol (nrats = 7). Chemogenetic inhibition of LHb by administration of CNO (10 mg/kg, i.p.) significantly decreased ethanol intake at 2 h (J) and 24 h (K) after access to ethanol compared with rats infected with GFP only (nrats = 6). The values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with aCSF, #p < 0.05 compared with baseline.
Table 1.
Water and total fluid intake, and ethanol preference after intra-LHb or MD infusion of DNQX in IA2BC rats
| 30 min | 24 h | 48–72 h | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Regions | Groups | Water intake (ml) |
Total fluid (ml) |
Preference for Ethanol (%) |
Water Intake (ml) |
Total fluid (ml) |
Preference for Ethanol (%) |
Ethanol intake (g/kg/24h) |
| Intra-LHb | aCSF (n = 9) | 1.0 ± 0.3 | 3.1 ± 0.5 | 73.6 ± 7.0 | 13.2 ± 2.1 | 26.8 ± 1.6 | 51.8 ± 5.4 | 5.1 ± 0.7 |
| 150 ng DNQX (n = 9) | 1.6 ± 0.5 | 3.5 ± 0.7 | 53.7 ± 11.2 | 17.3 ± 2.1 | 29.4 ± 1.7 | 42.8 ± 5.3 | ||
| 500 ng DNQX (n = 9) | 1.4 ± 0.5 | 3.8 ± 0.5 | 62.5 ± 9.5 | 18.4 ± 3.2 | 25.8 ± 3.0 | 30.8 ± 5.3* | 4.3 ± 0.8 | |
|
| ||||||||
| Intra-MD | aCSF (n = 7) | 1.1 ± 0.4 | 3.2 ± 0.6 | 73.0 ± 8.0 | 14.7 ± 1.8 | 26.6 ±1.6 | 42.7 ± 7.2 | |
| 500 ng DNQX (n = 7) | 1.2 ± 0.5 | 3.7 ± 0.5 | 68.8 ± 10.1 | 19.7 ± 1.9 | 31.3 ± 1.8 | 38.4 ± 3.9 | ||
The values are expressed as mean ± SEM.
P < 0.05 compared with aCSF.
To exclude the possibility that the decrease in ethanol intake induced by intra-LHb DNQX infusion was a result of any impairment in locomotion, we measured spontaneous locomotion in the locomotor chamber 30 mins after DNQX infusion. Intra-LHb infusion of DNQX (500 ng/200 nl/side) did not significantly alter locomotor activity (Fig. 4C). Two-way ANOVA showed a significant increase in the cumulative distance traveled over time [F5,50 = 14.86, p < 0.001] with no significant main effect of DNQX treatment or treatment - time interaction.
Next, we tested the effect of intra-LHb DNQX on ethanol seeking behavior in the operant chambers, as previously described (Fu et al., 2016). Briefly, the delivery of the 20% ethanol reward was contingent on responses of the active lever under an FR3 schedule (see Materials and Methods). No reward was received if the rats pressed the inactive lever, and the event was merely recorded as a measure of nonspecific behavioral activity. When the rats maintained a stable level of responding over 20 sessions (3 weeks) on the FR3 schedule, DNQX (500 ng/200 nl/side) or aCSF was infused into the LHb 10 minutes before the session. Histological verification revealed that the cannula tips in 8 of the 10 rats were within the LHb (Supplementary figure1) for analysis. Figure 3D–F depicts operant responding for ethanol by rats after intra-LHb DNQX infusion. Two-way RM ANOVA revealed significant main effects of drug treatment (F2, 14 = 13.0, P < 0.001) and lever pressing (F1, 7 = 260.2, P < 0.001) with a significant interaction between the two main factors (F2, 14 = 11.5, P < 0.001). Post hoc analysis revealed that intra-LHb DNQX decreased the number of active lever presses compared to aCSF injection (P < 0.01) or baseline (P < 0.001) (Fig. 4D). There was no significant difference in the number of active lever presses between the baseline and aCSF (vehicle) treatment. There were also no significant changes in inactive lever responding. Furthermore, intra-LHb DNQX infusion produced a significant decrease in both the number of head entries into the ethanol port (P < 0.0001, Fig. 4E) and operant ethanol consumption (P < 0.001, Fig. 4F) compared to vehicle treatment.
Having found that blocking LHb AMPARs decreases ethanol intake, we next examined whether this effect was selective for ethanol by measuring the intake of a natural reward, 2% sucrose. Briefly, a group of ethanol-naïve rats were implanted with cannula into the LHb. One week after surgery, they were trained to drink 2% sucrose using the intermittent access to two-bottle choice-drinking procedure, similar to the IA2BC procedure for ethanol. Two weeks after sucrose drinking, when a stable drinking level has achieved, these rats received bilateral intra-LHb infusion of drugs. As shown in Fig. 4G, intra-LHb infusion of DNQX (500 ng/200 nl/side) had no effects either on the intake of the sucrose solution at both 30 min and 24 h time points after the onset of drinking, or on sucrose preference or water consumption (p > 0.05) (data not shown). Bilateral intra-LHb infusion of AMPA (11 ng/200 nl/side) also did not affect the intake of the 2% sucrose solution at both 30 min and 24 h time points (Fig. 4H).
To determine whether the effect of DNQX is brain region-specific, we injected DNQX into the mediodorsal thalamic nucleus (MD), a neighboring region of the LHb. Histological verification revealed that the cannula tip placements for all animals were within the MD (Supplemental Figure 1). A bilateral intra-MD infusion of DNQX (500 ng/200 nl/side) had no effect on ethanol intake either at the 30 min [t1, 26 = 0.74, p = 0.47] or the 24 h time point [t1, 26 = 0.04, p = 0.96] after ethanol access (Fig. 4I). Also, no effect was observed on ethanol preference, water intake, or total fluid (Table 1).
Next, we tested whether temporal inhibition of LHb neurons can change ethanol intake. As expected, systemic administration of CNO (10 mg/kg, i.p.) to animals infected with hM4Di-AAV 3 weeks prior significantly decreased ethanol intake at 2 h (t = 3.53, p = 0.004) (Fig. 4J) and 24 h (t = 2.32, p = 0.04) (Fig. 4K) after ethanol access. However, no effect was observed on ethanol preference, water intake, or total fluid at both time point (data not shown). Rats injected with AAV-GFP did not show any difference in baseline levels in the ethanol intake (Fig. 4J, K).
3.5. Inhibition of CaMKII in the LHb blunts GluA1 phosphorylation, decreases neuronal activity, attenuates depressive-like behaviors, and reduces ethanol intake
It has been suggested that activated CaMKII plays a crucial role in catalyzing AMPAR phosphorylation at GluA1 residue serine 831, which leads to enhanced AMPAR activity (Barria et al., 1997b). To determine whether CaMKII levels in the LHb of ethanol-withdrawn rats had changed, we used Western blot analysis. The results showed that the CaMKII levels in the LHb were significantly higher in rats at 24h withdrawal from ethanol than in ethanol naïve rats (Fig. 5A, B).
Figure 5.
Effects of the CaMKII inhibitor KN-62 on GluA1 phosphorylation, excitability of LHb neurons, depressive-like behaviors, and ethanol consumption in ethanol-withdrawn rats. Representative Western blot image (A) and analysis to quantify expression levels of CaMKII in the naïve control (CTRL) and ethanol withdrawn rats (EtOH-WD) (B). (B) (***P<0.001) (nrats = 5). KN-62 or aCSF was injected into the LHb 10 min before tissue harvest, forced swimming test, or access to ethanol. Representative Western blot image (C) and analysis (D) to quantify expression levels of phosphor-GluA1 at the site of 831 (S831) after intra-LHb infusion of aCSF or KN-62(30 ng/300 nl/side) (nrats = 5). Representative traces (E) and the plots of spike numbers vs current (F) show that KN-62 (10 µM) significantly decreased the number of spikes induced by a current step in LHb neurons of ethanol-withdrawn rats. Intra-LHb infusion of KN-62 (30 ng/300 nl/side) substantially shortened the total immobility time (G) and prolonged the latency to the first immobility (H) in the forced swimming test (nrats = 10), and reduced ethanol intake at 2 h (I), and 24 h (J) after the onset of access to ethanol (nrats = 10). The values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with aCSF.
To determine the role of CaMKII in drinking and depressive-like behaviors, we infused CaMKII inhibitor KN-62 (30 ng/300nl/side) bilaterally into the LHb 24h after ethanol withdrawal. Histological verification showed that 10 of 12 rats had the correct cannula tip placements in the LHb (Supplementary figure1). Western blot analysis revealed that KN-62 infusion significantly decreased the density of the band of phosphorylated GluA1 (Ser831) in the LHb (Fig. 5C, D) compared to aCSF infusion. Also, bath application of KN-62 (10 µM) could reduce the neuronal excitability in the LHb of alcohol withdrawn rats (Fig. 5E, F; Two-way RM ANOVA, F1,16 = 14.86, p < 0.01).
Remarkably, intra-LHb infusion of KN-62, compared with aCSF, substantially reduced the total immobility time (Fig. 5G, p = 0.019) and prolonged the latency to the first immobility in forced swimming test (Fig. 5H, P = 0.002), compared to the aCSF infusion. Furthermore, intra-LHb KN-62 significantly decreased ethanol consumption at 2h (Fig. 5I, P = 0.006) and 24h (Fig. 5J, P = 0.02) after the onset of ethanol access, with a strong tendency to decrease ethanol preference (2h: P = 0.07; 24h: p = 0.09, Table 2), without changing the water consumption at both time points measured. However, intra-LHb KN62 induced a significant decrease in total fluid intake at 2h (P = 0.02) and a strong tendency to decrease total fluid intake at 24h (P = 0.08) after access to ethanol (Table 2), without affecting ethanol intake between 48 and 72h (Table 2).
Table 2.
Water intake and total fluid intake, and ethanol preference after intra-LHb infusion of KN-62 in IA2BC rats
| 2 h | 24 h | 48–72 h | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Intra-LHb | Water intake (ml) |
Total fluid (ml) |
Preference for Ethanol (%) |
Water Intake (ml) |
Total fluid (ml) |
Preference for Ethanol (%) |
Ethanol intake (g/kg/24h) |
| aCSF (n = 10) | 4.6 ± 1.0 | 9.6 ± 1.3 | 54.2 ± 7.6 | 20.3 ± 3.4 | 36.5 ± 3.4 | 46.4 ± 5.7 | 5.3 ± 1.2 |
| KN-62 (n = 10) | 3.8 ± 0.6 | 6.0 ± 1.7* | 36.8 ± 5.1 | 20.4 ± 2.3 | 30.4 ± 2.4 | 33.4 ± 4.4 | 4.9 ± 0.9 |
The values are expressed as mean ± SEM.
P < 0.05 compared with aCSF.
4. Discussion
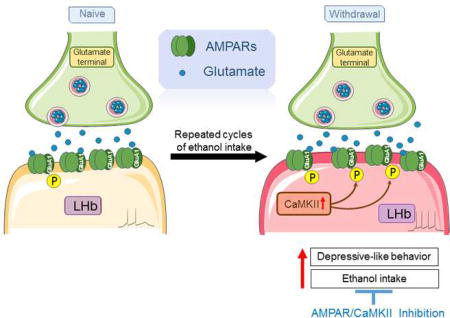
Depression occurs more often during alcohol abstinence and is a major risk factor for relapse. However, the neurobiological mechanisms underlying the association between alcohol use disorders and depression remain elusive. Using an established drinking model (Li et al., 2011b; Li et al., 2016; Simms et al., 2008), we observed depressive-like behaviors in rats during withdrawal, as evidenced by an increase in the total immobility time and a decrease in the latency to the first immobility in the forced swimming test, as well as a reduction in sucrose intake and preference. This was concomitant with an increase in phosphorylation of AMPAR GluA1 and in the expression and activity of CaMKII in the LHb. Intra-LHb infusion of the AMPAR antagonist DNQX alleviated depressive-like behaviors and reduced ethanol intake in both the intermittent access and operant self-administration procedures, but did not affect locomotion or sucrose intake. Chemogenetic inhibition of LHb activity produced similar effects. Moreover, pharmacological activation of LHb AMPAR activity induced depressive-like behaviors in ethanol-naïve rats. Finally, intra-LHb infusion of CaMKII inhibitor KN-62, which reduced phosphorylation of AMPAR GluA1 in the LHb, rescued depressive-like behaviors and reduced ethanol intake.
The forced swimming test is based on the observation that when rodents are placed in an inescapable cylinder filled with water, after initial intense escape-directed behavior, such as swimming and climbing, they stop struggling and show despair immobility behavior that disengages the animal from active forms of stress coping (Slattery and Cryan, 2012). In the current study, we observed an increased total immobility time and a shortened latency to immobility in ethanol withdrawn rats. These rats also displayed anhedonia, as indicated by lower preference and intake for 1% sucrose. These data suggest that depressive-like behaviors occur in rats during ethanol withdrawal.
Previous studies have indicated that LHb neurons are hyperactive when rodents are in a depressed state, and that suppression of LHb neurons reduces depressive-like behaviors in animal models of depression (Li et al., 2011a; Li et al., 2013; Meye et al., 2015). In a recent rat study, we found that at 24 h withdrawal from chronic voluntary ethanol drinking, the activity of LHb neurons was increased, and the amplitude of excitatory postsynaptic currents was increased in the LHb neurons, suggesting that the activity of postsynaptic AMPARs is increased (Li et al., 2016). We further demonstrated that the increased glutamate transmission contributes to the increased activity of LHb neurons (Zuo et al., 2017). AMPARs have been shown to play a key role in the regulation of reinforcement and the reward process of ethanol (Stuber et al., 2008; Wang et al., 2012). Chronic intermittent access to ethanol increases surface concentrations of GluA1 in the striatum, which may promote alcohol self-administration by altering the reinforcement processes (Wang et al., 2012). Surface GluA1 in the amygdala is also upregulated after chronic intermittent alcohol vapor exposure (Christian et al., 2012). Furthermore, phosphorylation of GluA1 at serine 831 plays a crucial role in increasing AMPAR activity in various regions of the brain (Malinow, 2003). Consistent with this finding, we observed that during withdrawal from voluntary ethanol consumption, GluA1 S831 phosphorylation in the LHb was significantly increased. This result mirrors the observation of increased GluA1 S831 phosphorylation in the amygdala in animals after both voluntary alcohol drinking (home-cage) and low-dose operant alcohol self-administration (Salling et al., 2016). These data suggest that AMPARs in specific brain regions that regulate alcohol drinking and seeking behavior are important targets of alcohol. This idea is further supported by our data that shows intra-LHb infusion of the AMPAR antagonist DNQX selectively reduced ethanol consumption in the home cage and the operant chamber. Intra-LHb DNQX also mitigated depressive-like behaviors. These results suggest that LHb AMPARs could be an important molecular target in depression. This is further supported by our data that activation of LHb AMPARs can induce depressive-like behaviors in ethanol naïve rats. Given that depression occurs more often in alcoholics (Grant et al., 2015), and that aberrant activity of the LHb contributes to depression-like symptoms (Proulx et al., 2014), we propose that improvement in depressive-like behaviors induced by the inhibition of LHb AMPARs may contribute to the decrease in ethanol drinking and seeking.
Interestingly, the literature demonstrates a differential role for the LHb in ethanol-directed behaviors. For instance, studies found that rats drink more alcohol if the LHb was lesioned before the start of drinking (Haack et al., 2014; Tandon et al., 2017), indicating that the LHb plays a brake-like role during the acquisition phase. Conversely, we found that temporal inhibition of the LHb by DNQX or chemogenetic tool reduced drinking in rats that have been drinking ethanol for two months, when the animals may have already become ethanol dependent (Li et al., 2011b).
CaMKII is a Ca2+-activated enzyme that plays a crucial role in many cellular responses, including the long-term potentiation of synaptic transmission (Lisman et al., 2002). CaMKII activation has been thought to enhance glutamatergic synapses by the phosphorylation of the Ser831 residue of the GluA1 subunit of AMPARs (Barria et al., 1997a) and by indirectly promoting AMPAR insertion in the synapse (Hayashi et al., 2000). CaMKII is rich in the LHb and its activation is required for depressive-like behaviors (Li et al., 2013). In the current study, we sought to determine whether an ethanol drinking history alters the protein expression and activity of CAMKII in the LHb. Our results indicated that withdrawal from chronic intermittent ethanol drinking significantly increased the expression and phosphorylation of CaMKII in the LHb. This is consistent with a recent study showing that the expression and phosphorylation of CaMKII is increased in the amygdala in mice that lever-pressed for alcohol (Salling et al., 2016). Since CaMKII plays a critical role in associative learning and memory (Mayford et al., 1996), findings from the current study and others suggest that ethanol-drinking induced upregulation of CaMKII expression and activity in brain regions that regulate positive and negative reinforcement of ethanol may reflect the increased learning behavior associated with drug stimuli. It is important to note that intra-LHb injection of the CaMKII inhibitor KN-62 inhibited GluA1 phosphorylation, mitigated depressive-like behaviors, and reduced ethanol intake. These findings are consistent with a recent mouse study that shows microinjection of CaMKII inhibitor KN-93 in the amygdala decreases ethanol positive reinforcement (Salling et al., 2016). Since CaMKII phosphorylation of GluA1 (Ser831) in the LHb is required for synaptic plasticity and depressive-like behaviors (Li et al., 2013), the findings in the current study suggest that the CaMKII-AMPAR signaling pathway may be a candidate target for the treatment of depressive-like behavior that characterizes alcohol use disorder.
The increased anxiety level of alcoholics observed during abstinence is believed to be another important factor contributing to relapse drinking. Importantly, LHb hyperactivity facilitates anxiogenesis (Pobbe and Zangrossi, 2008). We recently reported that during withdrawal from repeated cycles of ethanol consumption, rats showed pronounced anxiety-like behaviors (Kang et al., 2017). Therefore, inhibition of LHb CaMKII-AMPAR signaling may also mitigate anxiety-like behaviors in ethanol-withdrawn rats. Additionally, we observed significant increases in phosphorylation of the AMPAR GluA1 subunit at both S831 and S845 in the LHb of ethanol-withdrawn rats. Intra-LHb infusion of CaMKII KN-62 inhibited GluA1 phosphorylation, reduced ethanol intake, and mitigated depressive-like behaviors, while intra-LHb infusion of protein kinase A (PKA) inhibitor H-89 did not affect ethanol consumption. These data suggest that the CaMKII-GluA1-S831 mechanism in the LHb plays a more important role in alcohol drinking and alcohol-related behaviors than phosphorylated GluA1-S845. Overall, our findings are in general agreement with a recent rodent study, which reported that chronic alcohol consumption does not affect GluA1 Ser845 phosphorylation in the spinal cord, whereas a mutation at the GluA1 Ser831 can completely block the prolongation of postsurgical pain produced by 4-week ethanol consumption (Liu et al., 2017). However, these findings are divergent from a previous study that reported that cocaine increased both glutamate transmission and excitability in the LHb, which was due to phosphorylation of S845 instead of S831 (Meye et al., 2015). Taken together, these data suggest that the CaMKII-GluA1-S831 mechanism is unique to the effects of alcohol. A future study on phospho-GluA1 mutant mice would be beneficial to further assess this possibility.
In summary, the most salient finding of this study is that withdrawal from chronic voluntary ethanol drinking leads to significant depressive-like symptoms and a substantial increase in CaMKII-AMPAR activity in the LHb. Pharmacological inhibition of AMPARs or CaMKII activity in the LHb alleviates depressive-like behaviors and reduces ethanol consumption in rats. This is the first evidence demonstrating the role of the LHb CaMKII-AMPAR signaling in depressive-like behaviors and relapse-like drinking after withdrawal from chronic intermittent ethanol consumption. Our data suggest that drugs targeting the CaMKII-AMPAR signaling pathway may be beneficial for alcohol use disorders, especially for individuals who elicit comorbidities like depression.
Supplementary Material
Depressive-like behavior is observed in ethanol-withdrawn rats
Excitability of LHb neurons is increased in withdrawn rats
Phosphorylated AMPAR and CaMKII activity is increased in the LHb of withdrawn rats
Inhibition of LHb AMPAR, CaMKII, and neurons rescues depressive-like behavior
Inhibition of LHb AMPAR, CaMKII, and neurons reduces ethanol intake
Acknowledgments
This work was supported by NIH-NIAAA AA021657, AA022292, and a grant from New Jersey Health Foundation. Clozapine-n-oxide (CNO) was from NIDA Drug supply program (NIH, Bethesda, MD). The authors thank Dr. Rose Paulose for editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contribution
J. H. Y., J.L., S.K. elaborated the study design. J.L., S.K. R.F., L.W., W.W., H.L., D.G. and W.Z. collected the data. J.L. and J. H. Y drafted the article. All authors critically reviewed the content and approved the final version for publication.
Disclosure/Conflict of Interest
The authors declare no conflicts of interest, financial or otherwise.
References
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997a;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997b;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361– 379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Graham C, Crayle J, Besheer J, Hodge CW. Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addict Biol. 2016 doi: 10.1111/adb.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Meyer T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol. 2002;12:293–299. doi: 10.1016/s0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Fu R, Zuo W, Gregor D, Li J, Grech D, Ye JH. Pharmacological Manipulation of the Rostromedial Tegmental Nucleus Changes Voluntary and Operant Ethanol Self-Administration in Rats. Alcohol Clin Exp Res. 2016;40:572–582. doi: 10.1111/acer.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361–371. doi: 10.1016/j.neuroscience.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011a;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011b;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J Pharmacol Exp Ther. 2012;341:196–204. doi: 10.1124/jpet.111.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zuo W, Fu R, Xie G, Kaur A, Bekker A, Ye JH. High Frequency Electrical Stimulation of Lateral Habenula Reduces Voluntary Ethanol Consumption in Rats. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, 3rd, Hu H. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhao Z, Guo Y, Shu H, Li C, Tang Y, Xing Y, Tao F. Spinal AMPA Receptor GluA1 Ser831 Phosphorylation Controls Chronic Alcohol Consumption-Produced Prolongation of Postsurgical Pain. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J Neurosci. 2012;32:12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front Hum Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Valentinova K, Lecca S, Marion-Poll L, Maroteaux MJ, Musardo S, Moutkine I, Gardoni F, Huganir RL, Georges F, Mameli M. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nat Neurosci. 2015;18:376–378. doi: 10.1038/nn.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PA, Ishikawa M, Otaka M, Huang YH, Schluter OM, Dong Y. Increased excitability of lateral habenula neurons in adolescent rats following cocaine self-administration. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc I, Ansoms C, Lehert P, Fischer F, Fuchs WJ, Landron F, Pires Preto AJ, Morgan MY. The European NEAT program: an integrated approach using acamprosate and psychosocial support for the prevention of relapse in alcohol-dependent patients with a statistical modeling of therapy success prediction. Alcohol Clin Exp Res. 2002;26:1529–1538. doi: 10.1097/01.ALC.0000029584.62149.22. [DOI] [PubMed] [Google Scholar]
- Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Zangrossi H., Jr Involvement of the lateral habenula in the regulation of generalized anxiety- and panic-related defensive responses in rats. Life Sci. 2008;82:1256–1261. doi: 10.1016/j.lfs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2016;79:430–442. doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon S, Keefe KA, Taha SA. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion. J Physiol. 2017;595:1393–1412. doi: 10.1113/JP272994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Abdullah JM. The role of GluA1 in central nervous system disorders. Rev Neurosci. 2013;24:499–505. doi: 10.1515/revneuro-2013-0021. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang M, Wang X, Liu K, Wan Y, Li M, Liu L, Zhang C. Abnormal Expression of MicroRNAs Induced by Chronic Unpredictable Mild Stress in Rat Hippocampal Tissues. Mol Neurobiol. 2017 doi: 10.1007/s12035-016-0365-6. [DOI] [PubMed] [Google Scholar]
- Zouikr I, James MH, Campbell EJ, Clifton VL, Beagley KW, Dayas CV, Hodgson DM. Altered formalin-induced pain and Fos induction in the periaqueductal grey of preadolescent rats following neonatal LPS exposure. PLoS One. 2014;9:e98382. doi: 10.1371/journal.pone.0098382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–189. doi: 10.1016/j.neuropharm.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Wang L, Chen L, Krnjevic K, Fu R, Feng X, He W, Kang S, Shah A, Bekker A, Ye JH. Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula. Neuropharmacology. 2017;113:178–187. doi: 10.1016/j.neuropharm.2016.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.