Abstract
The medial shell region of the nucleus accumbens (msNAc) is a key center for the regulation of goal-directed behavior and is likely to be dysfunctional in neuropsychiatric disorders such as addiction, depression and schizophrenia. Nitric oxide (NO)-producing interneurons in the msNAc are potently modulated by dopamine (DA) and may play an important role in synaptic integration in msNAc networks. In this study, neuronal NO synthase (nNOS) activity was measured in anesthetized rats using amperometric microsensors implanted into the msNAc or via histochemical techniques. In amperometric studies, NO oxidation current was recorded prior to and during electrical stimulation of the ipsilateral fimbria. Fimbria stimulation elicited a frequency and intensity-dependent increase in msNAc NO efflux which was attenuated by systemic administration of the nNOS inhibitor NG-propyl-L-arginine. Parallel studies using NADPH-diaphorase histochemistry to assay nNOS activity produced highly complementary outcomes. Moreover, systemic administration of either a DA D1 receptor agonist or a DA D2 receptor antagonist potentiated nNOS activity in the msNAc elicited by fimbria stimulation. These observations demonstrate for the first time that NO synthesis in nNOS expressing interneurons in the msNAc is facilitated by robust activation of hippocampal afferents in a manner that is differentially modulated by DA D1 and D2 receptor activation.
Keywords: nucleus accumbens medial shell, nitric oxide synthase, nitric oxide, dopamine, electrochemistry, histochemistry
1.1 Introduction
The nucleus accumbens (NAc) lies in the rostral ventromedial region of the striatal complex and is thought to function as a “limbic-motor interface” which is critically involved in motivational processes, action selection, and suppression of inappropriate behaviors (Mogenson et al., 1980; Floresco, 2015; Dalley and Robbins, 2017). The medial shell of the nucleus accumbens (msNAc) in particular is thought to play a key role in facilitating the reinforcing properties of drugs of abuse and mediating goal directed behavior, while suppressing unrewarding or irrelevant behaviors (Basso and Kelley, 1999; Everitt et al, 1999; Sellings and Clarke, 2003; Burton et al, 2015). The msNAc processes diverse information arriving from the prefrontal cortex, mediodorsal thalamus, and various limbic centers (e.g., ventral hippocampus and basolateral amygdala) to enable control of NAc output, synaptic plasticity and goal-directed behavior (Groenewegen et al, 1999; Brog et al, 1993; Finch, 1996; O’Donnell and Grace, 1995; Floresco et al, 2001). The msNAc receives dopaminergic innervation from the posteromedial ventral tegmental area (Beckstead et al, 1979; Voorn et al, 1986; Ikemoto, 2007), which plays a central role in modulating excitatory synaptic drive onto NAc interneurons and projection neurons (O'Donnell, 2003; Goto and Grace, 2008). A better understanding of this processing is critical as dysregulation of NAc neurotransmission and synaptic plasticity is thought to occur in neuropsychiatric disorders such as addiction, depression, and schizophrenia (Grace, 2016; Wolf, 2016; Chuhma et al, 2017).
On the cellular level, the msNAc consists of a complex network of GABAergic medium-sized spiny projection neurons (MSNs) and a variety of distinct subclasses of interneurons (Meredith and Totterdell, 1999). Nitric oxide (NO) is a gaseous neurotransmitter synthesized in the msNAc by local interneurons containing neuronal nitric oxide synthase (nNOS) (Vincent, 2010; Hidaka and Totterdell, 2001; Hoque and West, 2012). nNOS expressing interneurons in the msNAc and striatal complex are robustly innervated by dopaminergic afferents (Fujiyama and Masuko, 1996; Meredith and Totterdell, 1999; Hidaka and Totterdell, 2001; Mitkovski et al, 2012) and express DA D1 receptor mRNA and D5 receptor protein (Le et al, 1991; Centonze et al, 2003; Rivera et al, 2002). However the co-localization of D2-like receptors or mRNA with peptidergic or other markers of nNOS interneurons has not been reported.
Our previous work in the dorsal striatum and NAc subregions indicates that DA D1 and D2 receptor activation produce opposite modulatory effects on nNOS activity (Sammut et al, 2006, Sammut et al, 2007a; Hoque et al, 2010; Hoque and West, 2012). For instance, electrical and chemical stimulation of the substantia nigra and systemic DA D1 receptor agonist administration were both observed to increase striatal NO efflux via nNOS and DA D1 receptor-dependent mechanisms (Sammut et al, 2006; Sammut et al, 2007; Park and West, 2009). These facilitatory effects of DA D1 receptor activation on striatal NO efflux were attenuated by systemic administration of DA D2 receptor agonist, whereas administration of DA D2 receptor antagonist augmented NO efflux evoked via electrical stimulation of the substantia nigra (Sammut et al, 2007). These observations are consistent with biochemical studies showing that DA D1/5 receptor activation elevated striatal tissue levels of cGMP (Altar et al, 1990; Di Stefano et al, 2005; Siuciak et al, 2006; Lin et al, 2010), whereas D1/5 antagonism decreased cGMP tone (Altar et al, 1990; Di Stefano et al, 2005). Conversely, DA D2 receptor activation decreased striatal tissue levels of cGMP (Di Stefano et al, 2005) while DA D2 receptor antagonism increased cGMP tone (Altar et al, 1990; Di Stefano et al, 2005). Furthermore, studies using NADPH-diaphorase (NADPH-d) staining as a readout of nNOS activity in the dorsal striatum and NAc have shown that DA D1 receptor antagonism decreased enzyme activity, whereas D2 receptor antagonism had the opposite effect (Morris et al, 1997; Hoque et al, 2010; Hoque and West, 2012). Our laboratory has also shown that NMDA receptor antagonists attenuate basal nNOS activity and activity elicited by systemic administration of DA D1/5 agonists and D2 antagonists in the NAc shell (Hoque and West, 2012), however, the source of the glutamatergic input responsible for driving NO synthesis and the impact of DA tone on this drive has not been determined. Given that nNOS interneurons in the NAc receive asymmetric glutamatergic contacts from the hippocampal ventral subiculum (French et al, 2005) and facilitate glutamate efflux evoked by fimbria/fornix stimulation (Kraus and Prast, 2002), we hypothesized that glutamate release from these afferents plays a key role in activating nNOS interneurons and that this synaptic drive is modulated by DA. The current study tested this hypothesis in intact rats using electrochemical recording techniques and NADPH-d histochemistry to investigate the impact of activation of the hippocampal fimbria on nNOS activity in the msNAc.
1.2 Materials and Methods
1.2.1 Chemicals
Urethane, the D1 receptor agonist R-(+)-SKF 81297 hydrobromide (SKF), the D2 receptor antagonist S-(−)-eticlopride hydrochloric acid (ETI), and reduced NADPH were purchased from Sigma-RBI (St. Louis, MO). The selective nNOS inhibitor NG-propyl-L-arginine (NPA) and NBT were purchased from Tocris (Ellisville, MO) All other reagents were of the highest grade commercially available.
1.2.2 Animals
Electrochemical and histochemical measurements were taken from a total of 52 adult male Sprague-Dawley (Harlan, Indianapolis, IN) rats weighing 229–400 grams. Rats were housed two-per cage under conditions of constant temperature (21–23°C) and maintained on a 12:12 hour light/dark cycle with food and water available ad libitum. All animal procedures were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee and adhere to the Guide for the Care and Use of Laboratory Animals published by the USPHS.
1.2.3 Subjects and Surgery
Prior to surgery, animals were deeply anesthetized with urethane (1.5–2 g/kg, i.p.) and placed in a stereotaxic apparatus. A burr hole (~2–3mm) was drilled in the skull and the dura mater were resected. The level of anesthesia was assessed via the hind limb compression reflex and maintained using supplemental administration of urethane. Body temperature was monitored using a rectal probe and maintained at 37–38°C with a heating pad. Concentric bipolar stimulating electrodes were implanted into the hippocampal fimbria using a micromanipulator (coordinates from Bregma: −1.3 mm anterior, 1.6mm lateral, and 4.0mm ventral). In a subset of animals, sham simulated control animals, a stimulating electrode was implanted as described but no stimulation was delivered. For electrochemical experiments, NO microsensors were implanted into the medial shell region of the nucleus accumbens ipsilateral to the stimulating electrode (coordinates from Bregma: 1.6 mm anterior, 0.7 mm lateral, and 7.8 mm ventral)(Paxinos and Watson, 1986). All experiments were initiated ~1–2 hours post-surgery.
1.2.4 Drug Preparation and Administration
All drugs were dissolved in physiological saline (SAL, 0.9% NaCl). Either SAL alone, the specific neuronal NOS inhibitor NPA (20 mg/kg), the D1 receptor agonist SKF (500μg/kg, i.p.) or D2 receptor antagonist ETI (100μg/kg, i.p.) were administered (i.p.) 20 min prior to initiation of fimbria stimulation. Effective doses and time courses for drug pretreatments were derived from the ranges previously reported by our lab and others (Floresco et al, 2001; Sammut et al, 2007; Hoque et al, 2010; Hoque and West, 2012).
1.2.5 Electrochemical detection of nitric oxide
Extracellular levels of NO were determined in the medial shell of the NAc using an NO selective, amperometric microsensor (amiNO-100, Innovative Instruments, Inc., Tampa, FL). NO is oxidized at the tip of the working electrode which keeps a constant potential of 0.85 V against a built in Ag/AgCl reference electrode. The oxidation current is detected by an amplifier (Apollo 4000) and recorded using software applications running on an IntelTM-based microcomputer. Prior to each experiment, the electrode was calibrated in a temperature controlled chamber using known solutions of the NO generating compound S-nitroso-N–acetyl-penicillamine (Sammut et al, 2006). Calibration curves were calculated prior to each experiment in order to determine the sensitivity of the electrode and confirm that the NO oxidation current exhibited a linear response to NO concentrations ranging from 0.6–48 nM. The lower detection limit of NO microsensors was approximately 0.1–0.5 nM NO. Stable NO oxidation signals were obtained in vivo for at least 150 seconds prior to electrical stimulation of the fimbria.
1.2.6 Electrical Stimulation
In electrochemical experiments, electrical stimuli consisted of trains (50 Hz, 500 ms train duration, 2.0 sec inter-train interval) of pulses (1000, 750 or 500 μA) or single pulses (5 or 0.5 Hz, 500 ms train duration, 1000, 750 or 500 μA) delivered for a single 20 second period (10 trains). NAc NO levels were monitored continuously during the stimulation period and for an additional 300–400 sec following cessation of the stimulation. Similar parameters were used in histochemical studies except stimulation was delivered at either 0.5 Hz or 50 Hz in 20 sec intervals over a 20 minute stimulus trial (see Fig 1).
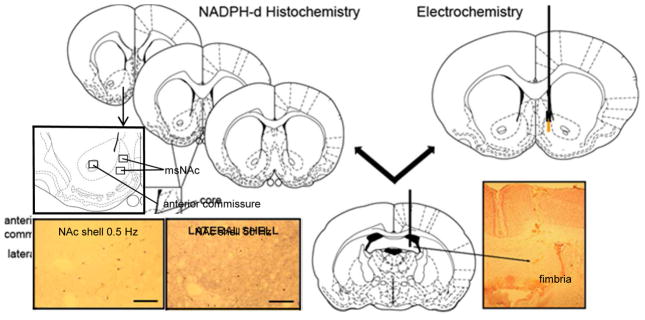
Figure 1. Techniques used to measure NOS activity in the msNAc.
Left) The optical density of the region of interest (boxed area) in the right and left hemispheres of each of the three representative coronal sections was measured. The anterior commissure was used as a comparison area for the generation of measures of non-specific staining. Optical Density values measured in the msNAc subregions were subtracted from those measured in the anterior commissure to give a relative optical density value. Insets: photomicrographs demonstrating NADPH-d staining in the msNAc following 0.5 Hz (left) and 50 Hz (right) electrical stimulation of the fimbria. Right) Position of NO microsensor and stimulating electrode implants in the msNAc and hippocampal fimbria, respectively. Inset: photomicrograph demonstrating the target region of stimulation electrode in the hippocampal fimbria. Arrowhead demonstrates the site of termination of the electrode track. Diagrams are derived from the atlas of Paxinos and Watson, 1986.
1.2.7 Histological confirmation of stimulating electrode placement
In electrochemical microsensor experiments, rats were perfused transcardially with ice-cold saline followed by 10% phosphate buffered formalin. Brains were postfixed in sucrose/formalin solution (30%), then sectioned, mounted, and stained with Neutral red/Cresyl Violet (10:1). Slides were then rinsed, dehydrated and coverslipped. Slides from all rats were viewed under a microscope to confirm correct stereotactic placement of all stimulating and recording electrodes.
1.2.8 NADPH-diaphorase histochemistry
NADPH-d staining was performed as described previously (Hoque et al. 2010; Hoque and West, 2012). Briefly, brains were extracted and immersed in a 30% sucrose/10% formalin solution for 24 hours, then frozen over dry ice and stored at −80°C. Next, brains were cut with a cryostat (Microm HM 550) to obtain 40μm thick coronal sections. Serial, coronal sections were then mounted on gelatinized slides, rinsed 3 times in 0.1 mol/L phosphate buffered SAL, washed in phosphate buffered SAL containing 0.25% Triton X-100, and incubated at room temperature on a rotary shaker for 5 min, followed by incubation at 37°C for 60 min in phosphate buffered SAL/Triton solution containing 0.2 mg/mL NADPH and 0.25 mg/mL nitroblue tetrazolium. Slides were then rinsed, dehydrated and coverslipped.
1.2.9 Data Analysis
In electrochemical experiments, data are expressed as concentration NO (nM) as extrapolated from in vitro calibration curves or percent control as indicated (Sammut et al. 2006). The NO oxidation current recorded over the last 30 seconds of the pre-stimulation period was averaged. The baseline mean was then subtracted from the mean NO oxidation current recorded during the last 10 seconds of the 20 second stimulation periods, to yield the stimulation induced change in NO efflux (Sammut et al, 2006; 2007). The average NO concentration observed over 2–3 stimulation trials was considered the control effect to which responses recorded following drug administration were compared.
In histochemical experiments, the optical density of standardized areas of the msNAc subregion containing nNOS cell bodies, dendrites and axons was measured using Image J software (NIH; Hoque and West, 2012). The optical density of the msNAc was measured on a scale ranging from 0 to 255 (0 representing the darkest labeling). Average background staining was measured in the white matter of the anterior commissure and average msNAc optical density values were subtracted from these background values. Optical density measures were obtained from the msNAc within both the right and left hemispheres of all three coronal sections and averaged to give a single value. Prior to combining the data from the right and left hemispheres, we confirmed that no difference in staining was present between the two hemispheres assessed in SAL treated animals (data not shown, one-way ANOVA, p>0.05). In addition, slides from each treatment group were tested for uniform light transmission through the blank portions of the slide to ensure consistency within measures across groups. The intensity of staining in msNAc was expressed as the mean relative optical density ± SEM. The statistical significance of stimulation and drug-induced changes in measures of NO efflux and NADPH-d staining was determined using a one-or two-way ANOVA with a Tukey or Dunnett’s post-hoc test, or t-test as indicated.
1.3 Results
1.3.1 Electrode placements
All stimulating electrode tips were localized within the hippocampal fimbria between 0.92 and 1.8 mm posterior to bregma, 1.1 and 2.6 mm lateral to the midline, and 3.0 and 4.5 mm ventral to the surface of the skull. All identified placements for NO electrodes implanted into the msNAc were localized between 0.7 mm and 1.7 mm anterior to bregma, 0.5 and 1.25 mm lateral to the midline, and 6.0 and 8.25 mm ventral to the surface of the skull (Paxinos and Watson, 1986).
1.3.2 NO efflux elicited in the msNAc during electrical stimulation of the fimbria is frequency- and intensity-dependent
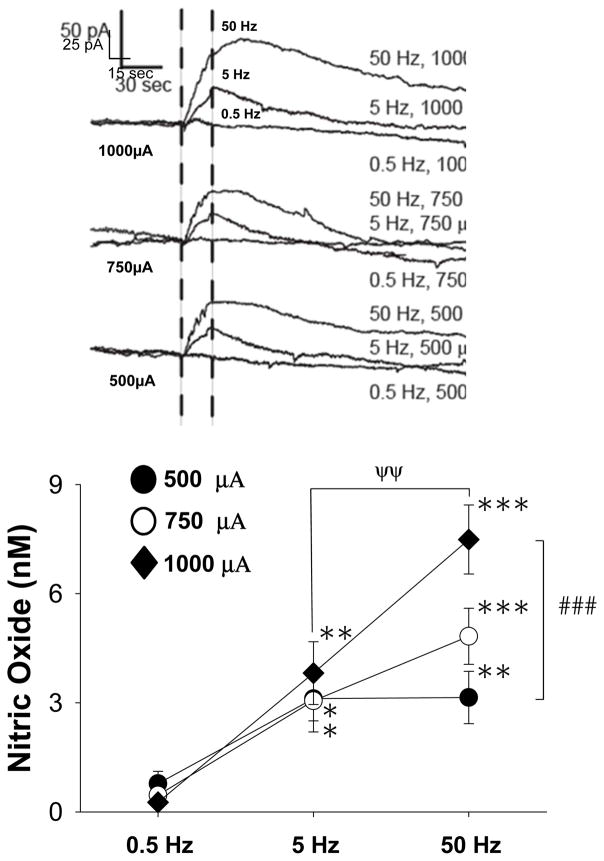
In order to explore the impact of fimbria stimulation on nNOS activity in the NAc, we tested a series of distinct electrical stimulation protocols designed to activate this area. Fimbria-evoked NO efflux in the msNAc was strongly dependent on the frequency of electrical stimulation (Fig 2, F(2,20)= 27.828, p<0.001; two way RM-ANOVA). Evoked NO efflux in the msNAc was also found to be dependent on a modest but significant interaction between frequency and intensity of fimbria stimulation (Fig 2, F(4,20)= 2.869, p=0.05; two way RM-ANOVA). Post-hoc comparisons revealed that NO efflux evoked during higher frequencies of fimbria stimulation (5 and 50Hz) was significantly greater than NO efflux observed during low frequency pulses (0.5Hz) to the fimbria (Fig 2, Tukey post-hoc test). The effect of intermediate (5Hz) fimbria stimulation on msNAc NO efflux was not dependent on the intensity of the stimulation (Fig 2, p>0.05, Tukey post-hoc test). However, high frequency train stimulation (50Hz) elicited a robust increase in NO efflux that was found to be dependent on the current intensity (1000μA > 500μA) of stimulation (Fig 2, Tukey post-hoc test). Lastly, the effect of high (50Hz) frequency stimulation using 1000μA current amplitudes was found to be larger than the effects of this same current amplitude delivered using intermediate (5Hz) fimbria stimulation frequencies (Fig 2, Tukey post-hoc test).
Figure 2. NO efflux elicited in the msNAc via electrical stimulation of the fimbria is dependent on both frequency and intensity of stimulation.
Top: Representative traces demonstrating the impact of fimbria stimulation delivered across a range of different frequencies (0.5–50 Hz) and intensities (0.5–1.0 mA) on NO efflux recorded in the msNAc using NO selective electrodes. Dashed vertical lines indicate the initiation and termination of the stimulation period (20 sec). Bottom: High frequency train stimulation (50 Hz, 20 sec, 0.5 ms pulse duration, 2 s inter-train interval) significantly increased msNAc NO efflux over that evoked during low frequency stimulation (0.5 Hz, 20 sec, 0.5 ms pulse duration) delivered using stimulus pulse intensities of 500, 750 or 1000 μA (**p<0.01, ***p<0.001). Intermediate frequency stimulation (5 Hz) also significantly increased msNAc NO efflux over that evoked during low frequency (0.5 Hz) stimulation at all tested stimulus intensities (*p<0.05; **p<0.01). Greater NO efflux was evoked at the 1000 μA stimulus intensity when delivered At 50 Hz as compared to 5 Hz (ψψp<0.01). The magnitude of NO efflux evoked by robust fimbria stimulation (50 Hz, 1000 μA) was also significantly greater than that evoked by pulses having lower stimulus intensities (50 Hz, 500 μA) (###p<0.001). Results are expressed as the mean ± SEM and are derived from n=8 rats.
1.3.3 Systemic administration of the selective nNOS inhibitor NPA attenuates NO efflux elicited in the msNAc during high frequency train stimulation of the fimbria
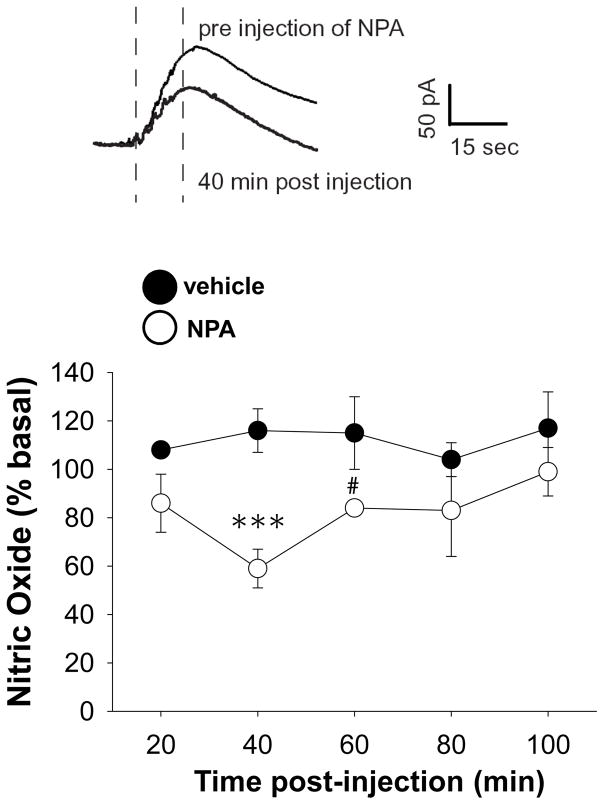
To assess the contribution of nNOS activation to fimbria induced NAc NO efflux, train stimulation (50Hz, 750μA) was delivered after systemic administration of SAL or the selective and potent nNOS inhibitor NPA (20 mg/kg, i.p.). Fimbria-evoked NO efflux in the msNAc was strongly dependent on nNOS activity as the magnitude of evoked NO efflux was reduced in NPA treated rats as compared to SAL treated controls (Fig 3, F(1,27)=6.694, p<0.05, two way RM-ANOVA). Post-hoc comparisons revealed that NO efflux evoked in the msNAc by fimbria stimulation was strongly attenuated 40 min post-NPA administration, and a trend towards a decreased was still evident 60 min post-drug (Fig 3, Tukey post-hoc test). These results indicate that fimbria-evoked NO efflux in the NAc is largely dependent on activation of the nNOS isoform.
Figure 3. Systemic administration of the nNOS inhibitor NPA attenuated NO efflux elicited in the msNAc during electrical train stimulation of the fimbria.
Top: Representative recordings showing the effects of train stimulation (20 sec, 50 Hz, 750 μA, 0.5 ms) of the fimbria delivered prior to, and 40 min after, systemic administration of NPA (20 mg/kg, i.p.). Dashed vertical lines indicate the initiation and termination of the stimulation period. Bottom: The mean ± S.E.M. increase in NO efflux evoked by train stimulation was significantly reduced 40 min after NPA administration (***p<0.005). A trend towards a decrease in NO efflux evoked by train stimulation of the fimbria was still evident 60 min post-drug (#p=0.067). Results are expressed as the mean ± SEM of data normalized to pre-vehicle/NPA values and are derived from n=4–5 rats per group.
1.3.4 NADPH-d staining elicited in the msNAc via electrical stimulation of the fimbria is dependent on stimulation frequency and nNOS activity
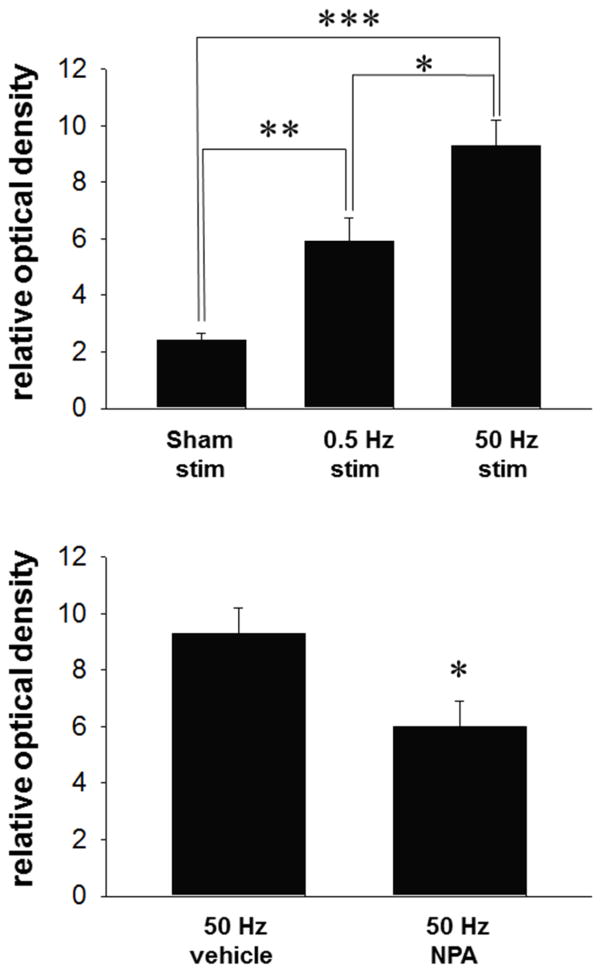
In parallel experiments, NADPH-d histochemistry was used to further assess the impact of fimbria stimulation on nNOS activity measured in the msNAc. As observed in the amperometry studies, electrical stimulation (0.5 Hz and 50 Hz, 750 μA) of the hippocampal fimbria elicited a stimulus frequency-dependent increase in NADPH-d staining as compared to sham-treated controls (Fig 4 top, F(2,14)=23.148, p<0.001, one way ANOVA). Post-hoc comparisons revealed that low frequency (0.5 Hz) electrical stimulation activated nNOS in the msNAc as compared to sham-simulated animals (Fig 4 top, Tukey post-hoc test). 50 Hz train stimulation of the fimbria produced a robust increase in nNOS activity in the msNAc as compared to animals receiving sham stimulation, an effect which was significantly greater than that observed in the 0.5 Hz stimulation group (Fig 4 top, Tukey post-hoc test).
Figure 4. Increases in NADPH-d staining elicited in the msNAc via fimbria stimulation is dependent on frequency of electrical stimulation and nNOS activity.
Top: Low frequency stimulation (0.5 Hz for 20 minutes duration) increased staining in the msNAc compared to sham simulated control animals (electrode implanted with no stimulation) (**p<0.01). High frequency stimulation (50 Hz at 750μA, 0.5 ms, 2 s ITI for 20 minutes duration) significantly increased NADPH-d staining in the msNAc compared to both sham simulated controls (***p<0.001) and animals receiving low frequency (0.5 Hz) stimulation (*p<0.05). Results are expressed as the mean ± S.E.M. from n=5–6 rats per group. Bottom: Staining elicited by high frequency stimulation (50 Hz at 750μA, 0.5 ms, 2 s ITI for 20 minutes duration) was significantly reduced in the msNAc after NPA administration (*p<0.05). Results are expressed as the mean ± SEM from n= 5 rats per group.
We next assessed whether the increase in msNAc NADPH-d staining induced by fimbria stimulation was dependent on nNOS activation. Train stimulation (50Hz, 750μA) was delivered to the fimbria 20 min after systemic administration of SAL or NPA (20 mg/kg, i.p.). Administration of NPA attenuated staining in the msNAc elicited by fimbria stimulation (Fig 4 bottom, t-test). These results support the amperometry studies indicating that the increase in msNAc NOS activity induced by fimbria stimulation is at least partially dependent on activation of the nNOS isoform.
1.3.5 DA D1 agonist and D2 antagonist administration potentiates the effects of fimbria stimulation on NADPH-diaphorase staining in the msNAc
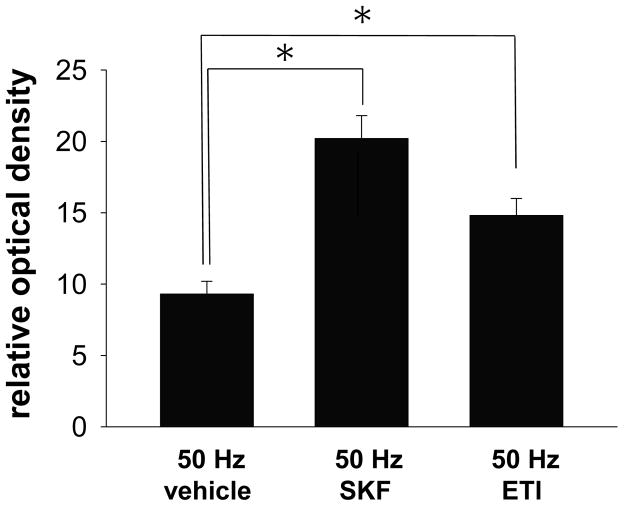
In order to examine the potential role of DA D1 and D2 receptor activation in modulating the effects of hippocampal fimbria afferents on nNOS activity, either SAL, the D1 agonist SKF (500μg/kg, i.p.), or the D2 antagonist ETI (100μg/kg, i.p.) was administered 20 min prior to electrical train stimulation (50Hz, 750μA) of the fimbria pathway. The facilitatory effect of high frequency train stimulation of the hippocampal fimbria on NADPH-d staining was potentiated in the msNAc following systemic administration of both SKF and ETI (Fig 5, F(2,15)=16.386, p<0.001, one way ANOVA). Post-hoc comparisons revealed that the increase in msNAc NADPH-d staining induced by fimbria stimulation was potentiated by both SKF and ETI pretreatment (Fig 5, Dunnett’s post-hoc test), indicating that nNOS activity in the msNAc is facilitated by robust activation of hippocampal afferents in a manner that is elevated by DA D1 receptor stimulation and attenuated by DA D2 receptor activation.
Figure 5. Systemic administration of either a DA D1 agonist or a D2 antagonist potentiates NADPH-d staining elicited in the msNAc during electrical train stimulation of the fimbria.
A) The increase in the intensity of NADPH-d staining induced in the msNAc during high frequency stimulation (50 Hz at 750μA, 0.5 ms, 2 s ITI for 20 minutes duration) of the fimbria was potentiated by systemic administration of either the DA D1 agonist SKF (500μg/kg, i.p.) or the DA D2 antagonist ETI (100μg/kg, i.p.) as compared to saline treated controls (*p<0.05). Results are expressed as the mean ± S.E.M. from n=5–7 rats per group.
1.4 Discussion
We have found that glutamatergic hippocampal afferent projections to the msNAc stimulate nNOS activity and NO production in a manner that is dependent on both the frequency and intensity of stimulation. Furthermore, our results showed that systemic administration of either a DA D1 receptor agonist or a D2 receptor antagonist potentiates nNOS activity in the msNAc elicited by stimulation of the hippocampal fimbria. These studies demonstrate for the first time that NO synthesis in nNOS expressing interneurons in the msNAc is driven by activation of hippocampal afferents in a manner that is differentially regulated by DA D1 and D2 receptor activation. These findings have implications for our understanding of the role of NO and DA interactions in the generation of motivational processes in via the msNAc and related circuitry.
1.4.1 Hippocampal afferents to the msNAc stimulate nNOS activity
The msNAc receives glutamatergic projections from the prefrontal cortex, basolateral amygdala, midline and intralaminar thalamic nuclei, and the hippocampal formation (Brog et al, 1993; Finch, 1996). The ventral subiculum of the hippocampus projects mainly to the medial NAc via the fimbria (Kelley et al, 1982; French et al, 2005). The current studies show that electrical stimulation of the ipsilateral fimbria transiently increased NO efflux in the msNAc in a stimulus intensity- and frequency- dependent manner. The stimulation parameters utilized in the current study were designed to mimic the phasic and tonic firing patterns of units observed in ventral subiculum circuits (Menendez de la Prida et al, 2003). Furthermore, we show that fimbria stimulation alters the intensity of NADPH-d staining in a frequency-dependent manner that is consistent with observations made using NO microsensors. Low frequency, tonic stimulation (0.5 Hz) of the fimbria resulted in an increase in NADPH-d staining in the msNAc as compared to sham-stimulated animals. Studies using the nNOS inhibitor NPA, revealed that both NO efflux and NADPH-d staining in the msNAc are robustly increased during intense, phasic activation of hippocampal inputs in a manner which is at least partially dependent on neuronal sources of NO. These studies are consistent with microdialysis studies showing that activation of the hippocampal fornix increases the release of GABA and NO via the activation of local interneurons in the NAc (Kraus and Prast, 2002). More recent studies by Prast and colleagues have showed that local field potentials evoked in the NAc during low frequency electrical stimulation of the parafascicular thalamus are attenuated by pretreatment with the non-selective NOS inhibitor L-NAME (Kraus et al, 2014), indicating that thalamic inputs may also drive NOS interneurons to synthesize and release NO in the msNAc. The activation of nNOS interneurons is most likely mediated via a direct effect of glutamatergic afferents as anatomical studies have shown that these neurons express NMDA receptor mRNA (Price et al, 1993) and protein (Gracy and Pickle, 1997). Recent studies by Kalivas and colleagues have also shown that selective activation of metabotropic glutamate receptor 5 also stimulates NO release from nNOS interneurons in the NAc core (Smith et al, 2017). Taken together, these studies indicate that graded, phasic activation of afferents from the fimbria stimulate NO production via glutamatergic activation of nNOS activity.
1.4.2 Dopaminergic modulation of nNOS activity
The msNAc receives dopaminergic projections primarily from the ventral tegmental area (Beckstead et al, 1979). The msNAc sends efferents to the ventral pallidum, which in turn, projects to the mediodorsal thalamus, ultimately connecting to the prefrontal cortex (Ikemoto, 2007). Observations that robust stimulation of the hippocampal fimbria increases NOS activity in a manner which is potentiated by DA D1 receptor activation suggest that nNOS expressing interneurons in the msNAc may act to integrate coincidental input from DA neurons in the VTA and excitatory afferents arising from limbic regions such as the hippocampus. These observations are consistent with numerous studies showing that systemic administration of DA D1 receptor agonist increased NO efflux in the dorsal striatum via a nNOS and D1 receptor-dependent manner (Sammut et al, 2006; Sammut et al, 2007; Park and West, 2009). Other laboratories have reported that DA D1 receptor activation elevated striatal tissue levels of cGMP (Altar et al, 1990; Di Stefano et al, 2005; Siuiak et al, 2006), whereas D1 antagonism produced the opposite effect (Altar et al, 1990; Di Stefano et al, 2005). In addition, bath application of the DA D1 receptor agonist SKF 38393 produced a strong depolarization of the membrane of nNOS interneurons recorded in striatal brain slices in the presence of tetrodotoxin (Centonze et al, 2002; 2003). This indicates that DA D1 receptor agonist induced activation of striatal nNOS interneurons/NO synthesis is most likely due to a direct effect on the nNOS interneuron. The effects of these drug manipulations are likely to translate to the ventral striatum including the msNAc, as they are consistent with observations from the current study.
Although nNOS interneurons in the dorsal striatum are thought to express DA D1-like (probably D5) receptors (Le et al, 1991; Centonze et al, 2003; Rivera et al, 2002), to our knowledge, no evidence has been advanced to show that DA D2 receptors colocalize with any of the known markers of nNOS interneurons (e.g., somatostatin, neuropeptide Y, nNOS, and NADPH-d staining). These reports suggest instead that the DA D2 receptor-mediated effects observed herein are indirect in nature (e.g., driven by suppression of excitatory glutamatergic or cholinergic inputs to nNOS interneurons). Our results, do however, confirm and extend our previous reports of the role of DA D2 receptor activation in suppressing nNOS activity in the dorsal striatum (Sammut et al, 2007; Hoque et al, 2010) and NAc (Hoque and West, 2012), as in the current study ETI administration potentiated NADPH-d staining evoked via fimbria stimulation in the msNAc. Using NO selective microsensors, our lab previously has shown that DA D2 antagonism increases NO efflux in the dorsal striatum evoked via electrical stimulation of the substantia nigra. Conversely, DA D2 receptor stimulation decreases evoked NO efflux (Sammut et al, 2007). Thus, under non-stimulated conditions, endogenous DA tonically suppresses basal nNOS activity via DA D2 receptor activation. When nNOS activity is robustly stimulated by excitatory inputs, further DA D2 receptor activation, mediated by phasic DA transmission, will oppose afferent drive. Therefore, DA D2 receptor antagonism induced following ETI administration is likely to remove this inhibition and allow for unchecked potentiation of nNOS activity by excitatory inputs.
1.5 Conclusions
Although the msNAc is considered a part of the ventral striatum, it has a distinct functional role in the integration of cognitive and motivational processes (Mogenson et al 1980; Floresco, 2015; Dalley and Robbins, 2017). Mogenson et al (1980) described the NAc as the “limbic-motor interface”. This original concept has driven considerable research into the functional properties of NAc neuronal cell populations. Studies have shown that the NAc serves as a point of convergence, integrating affective, cognitive and motor information from a variety of inputs (O’Donnell, 2002; Floresco, 2015; Grace, 2016). In the striatal complex, excitatory glutamatergic projections synapse both on MSNs and local circuit neurons (Fujiyama and Masuko 1996; Hidaka and Totterdell 2001; French et al, 2005). Interestingly, the glutamatergic hippocampal projections and DAergic VTA inputs have been shown to converge on the nNOS interneurons (Meredith and Totterdell, 1999; French et al, 2005). Given this central role in the integration of glutamatergic and DAergic transmission, nitrergic interneurons within the NAc may be vital to the coordination of the “limbic-motor interface” described by Mogenson et al, (1980). Therefore, pharmacotherapies designed to manipulate NO signaling and/or transmission across hippocampal-NAc synapses may be efficacious for the treatment of neuropsychiatric disorders such as schizophrenia, depression, and addiction.
Highlights.
Electrical train stimulation of the hippocampal fimbria elicited a frequency and intensity-dependent increase in nitric oxide efflux in the medial shell of the nucleus accumbens as measured using a nitric oxide selective electrochemical microsensor.
Evoked nitric oxide efflux was attenuated by administration of a selective nitric oxide synthase inhibitor (NG-propyl-L-arginine).
Parallel histochemical measures of nitric oxide synthase activity reproduced the above outcomes observed with electrochemistry.
Additional histochemical experiments demonstrated that fimbria-evoked stimulation of nitric oxide synthase activity is facilitated by dopamine D1 receptor agonism and D2 receptor antagonism.
Acknowledgments
The authors thank Dr. Gloria E. Meredith for her insightful input during the design of these studies. This work was supported by the Chicago Medical School and United States Public Health grant NS 047452 (ARW).
Abbreviations
- DA
dopamine
- msNAc
medial shell of the nucleus accumbens
- MSNs
medium-sized spiny projection neurons
- NAc
nucleus accumbens
- NADPH-d
NADPH-diaphorase
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Boyar WC, Kim HS. Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol. 1990;181:17–21. doi: 10.1016/0014-2999(90)90240-7. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta JH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175(19):191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–78. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, Roesch MR. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem. 2015;117:51–59. doi: 10.1016/j.nlm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Kalmbach A, Yetnikoff L, Rayport S. Heterogeneity in dopamine neuron synaptic actions across the striatum and its relevance for schizophrenia. Biol Psych. 2017;81(1):43–51. doi: 10.1016/j.biopsych.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosc. 2017;18(3):158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Sozio P, Cacciatore I, Cocco A, Giorgioni G, Costa B, Montali M, Lucacchini A, Martini C, Spoto G, Di Pietrantonio F, Di Matteo E, Pinnen F. Preparation and pharmacological characterization of trans-2-amino-5(6)-fluoro-6(5)-hydroxy-1-phenyl-2,3-dihydro-1H-indenes as D2-like dopamine receptor agonists. J Med Chem. 2005;48:2646–2654. doi: 10.1021/jm040889k. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward the role of amygdala-ventral striatal subsystems. Ann NY Acad Sci. 1999;877(1):412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6(5):495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Ann Rev Psych. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of Hippocampal and Amygdalar-Evoked Activity of Nucleus Accumbens Neurons by Dopamine: Cellular Mechanisms of Input Selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Ritson GP, Hidaka S, Totterdell S. Nucleus accumbens nitric oxide immunoreactive interneurons receive nitric oxide and ventral subicular afferents in rats. Neurosci. 2005;135:121–131. doi: 10.1016/j.neuroscience.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Masuko S. Association of dopaminergic terminals and neurons releasing nitric oxide in the rat striatum: An electron microscopic study using NADPH-diaphorase histochemistry and tyrosine hydroxylase immunohistochemistry. Brain Res Bull. 1996;40:121–127. doi: 10.1016/0361-9230(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–8. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural localization and comparative distribution of nitric oxide synthase and N-methyl-D-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res. 1997;747:259–272. doi: 10.1016/s0006-8993(96)01249-8. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877(1):49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Han X, Jing MY, Zhao TY, Wu N, Song R, Li J. Role of dopamine projections from ventral tegmental area to nucleus accumbens and medial prefrontal cortex in reinforcement behaviors assessed using optogenetic manipulation. Metab Brain Dis. 2017;48:1–12. doi: 10.1007/s11011-017-0023-3. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Totterdell S. Ultrastructural features of the nitric oxide synthase-containing interneurons in the nucleus accumbens and their relationship with tyrosine hydroxylase-containing terminals. J Comp Neurol. 2001;431:139–154. doi: 10.1002/1096-9861(20010305)431:2<139::aid-cne1061>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hoque KE, Indorkar RP, Sammut S, West AR. Impact of dopamine–glutamate interactions on striatal neuronal nitric oxide synthase activity. Psychopharmacol. 2010;207:571–581. doi: 10.1007/s00213-009-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque KE, West AR. Dopaminergic modulation of nitric oxide synthase activity in subregions of the rat nucleus accumbens. Synapse. 2012;66:220–231. doi: 10.1002/syn.21503. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neurosci. 1982;10:2321–35. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kraus MM, Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbria. Neurosci. 2002;112:331–343. doi: 10.1016/s0306-4522(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Kraus MM, Prast H, Philippu A. Influence of parafascicular thalamic input on neuronal activity within the nucleus accumbens is mediated by nitric oxide—An in vivo study. Life Sci. 2014;102:49–54. doi: 10.1016/j.lfs.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Le MC, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Fretier P, Jiang C, Vincent SR. Nitric oxide signaling via cGMP-stimulated phosphodiesterase in striatal neurons. Synapse. 2010;64:460–466. doi: 10.1002/syn.20750. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Suarez F, Pozo MA. Electrophysiological and morphological diversity of neurons from the rat subicular complex in vitro. Hippocampus. 2003;13(6):728–44. doi: 10.1002/hipo.10123. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S. Microcircuits in the nucleus accumbens’ shell and core involved in cognition and reward. Psychobiology. 1999;27(2):165–168. [Google Scholar]
- Mitkovski M, Padovan-Neto FE, Raisman-Vozari R, Ginestet L, Da-Silva CA, Del-Bel EA. Investigations into potential extrasynaptic communication between the dopaminergic and nitrergic systems. Front Phys. 2012;3:372–387. doi: 10.3389/fphys.2012.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM. Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacol. 1997;36:1589–1599. doi: 10.1016/s0028-3908(97)00159-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17(3):429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: Hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DJ, West AR. Regulation of striatal nitric oxide synthesis by local dopamine and glutamate interactions. J Neurochem. 2009;111:1457–1465. doi: 10.1111/j.1471-4159.2009.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Price RH, Jr, Mayer B, Beitz AJ. Nitric oxide synthase neurons in rat brain express more NMDA receptor mRNA than non-NOS neurons. Neuroreport. 1993;4:807–10. doi: 10.1097/00001756-199306000-00053. [DOI] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la CA, Moratalla R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bray KE, West AR. Dopamine D(2) receptor-dependent modulation of striatal NO synthase activity. Psychopharmacol. 2007;191(3):793–80. doi: 10.1007/s00213-006-0681-z. [DOI] [PubMed] [Google Scholar]
- Sammut S, Dec A, Mitchell D, Linardakis J, Ortiguela M, West AR. Phasic dopaminergic transmission increases NO efflux in the rat dorsal striatum via a neuronal NOS and a dopamine D(1/5) receptor-dependent mechanism. Neuropsychopharmacol. 2006;31:493–505. doi: 10.1038/sj.npp.1300826. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23(15):6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, III, Boger HA, Kalivas PW. Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci. 2017;37(4):742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, Stock JL, McNeish JD, Strick CA, Menniti FS, Schmidt CJ. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: Evidence for altered striatal function. Neuropharmacol. 2006;51:374–385. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Vincent SR. Nitric oxide neurons and neurotransmission. Prog Neurobiol. 2010;90(2):246–255. doi: 10.1016/j.pneurobio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Voorn P, Jorritsma-Byham B, Van Dijk C, Buijs RM. The dopaminergic innervation of the ventral striatum in the rat: A light- and electron-microscopy study with antibodies against dopamine. J Comp Neurol. 1986;251:84–99. doi: 10.1002/cne.902510106. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]