Abstract
Objective
The objective of this study is to examine racial/ethnic differences in prevalence of chronic conditions and multimorbidities in the geriatric population of a state with diverse races/ethnicities.
Method
Fifteen chronic conditions and their dyads and triads were investigated using Hawaii Medicare 2012 data. For each condition, a multivariable logistic regression model was used to investigate differences in race/ethnicity, adjusting for subject characteristics.
Results
Of the 84,212 beneficiaries, 27.8% were Whites, 54.6% Asians, and 5.2% Hispanics. Racial/ethnic disparities were prevalent for most conditions. Compared with Whites, Asians, Hispanics, and Others showed significantly higher prevalence rates in hypertension, hyperlipidemia, diabetes, and most dyads or triads of the chronic conditions. However, Whites had higher prevalence rates in arthritis and dementia.
Discussion
Race/ethnicity may need to be considered when making clinical decisions and developing health care programs to reduce health disparities and improve quality of life for older individuals with chronic conditions.
Keywords: chronic condition, ethnicity, Medicare, multimorbidity, race
Introduction
Medical advancements in treatment and longevity have resulted in the growth of the older population living with chronic diseases in the United States. From a gerontological perspective, aging contributes to high susceptibility for development of multiple chronic conditions (Ward, Schiller, & Goodman, 2014). Multimorbidity, commonly defined as co-occurrence of two or more chronic conditions (Mercer, Smith, Wyke, O’Dowd, & Watt, 2009), creates an increasing burden for patients, their caregivers, and their health care professionals (Vogeli et al., 2007). Individuals with multimorbidity require closer medical attention or care and/or have poor functional status or health outcomes, resulting in rising health care expenses (Parekh, Goodman, Gordon, Koh, & Multiple, 2011). Prevalence of multimorbidity has rapidly increased over the past years among the older population nationally (Freid, Bernstein, & Bush, 2012). Approximately half of all U.S. adults have at least one chronic condition, and one in four adults has multimorbidity (Ward et al., 2014).
Several studies focused on prevalence estimates of co-occurring chronic conditions, especially dyads (i.e., combinations of two chronic conditions) and triads (i.e., combinations of three chronic conditions), among U.S. adults (Ashman & Beresovsky, 2013; Freid et al., 2012; Machlin & Soni, 2013; Steiner & Friedman, 2013; Steinman et al., 2012). However, fewer studies investigate state- or county-level chronic condition combinations (Lochner, Goodman, Posner, & Parekh, 2013; Lochner & Shoff, 2015; Rocca et al., 2014; St. Sauver et al., 2015). Rocca and colleagues (2014) studied multi-morbidity in Olmsted County, Minnesota, which illustrated a unique picture of multimorbidity in a local community and addressed the implications on clinical practice and etiologic research in the region. Posner and Goodman (2014) recommended analyzing specific patterns of multimorbidity in different regions, especially within local communities. The Centers for Medicare & Medicaid Services (CMS) standardized definition of chronic conditions allows for comparisons of multimorbidity patterns in geographically defined populations with national and/or worldwide patterns (Chronic Conditions Data Warehouse, 2016).
Despite several studies recognizing racial/ethnic differences in the prevalence of numerous chronic conditions at the national level (Freid et al., 2012; Rocca et al., 2014; St. Sauver et al., 2015; Ward et al., 2014), it is unknown whether specific racial/ethnic groups with high prevalence rates for specific combinations of chronic conditions among older adults exist. Racial/ethnic specific prevalence rates may have significant clinical and public health implications, especially for Hawaii, due to its diverse racial/ethnic population with the largest Asian population in the United States. Characterizing variations of multimorbidities at the state level would be very beneficial, as each state increasingly has a key role in the financing, regulating, and delivering health care services. In addition, Asians are the fastest growing population in the United States, with 43.3% increase from year 2000 to 2010 (Hoeffel et al., 2012); hence, studying multimorbidities in Hawaii would provide valuable insights into health care providers and federal health care policy makers.
The main objective of this study was to explore the racial/ethnic differences in the prevalence of 15 major chronic conditions and their combinations (dyads and triads) using Hawaii Medicare data to gain a better understanding of the prevalence of chronic conditions among Hawaii’s geriatric population and identify high-risk racial/ethnic subpopulations.
Method
Data Source and Study Population
This study utilized the Hawaii Medicare 2012 data. The University of Hawaii Institutional Review Board approved this study (Human Subject CHS #23362). Medicare is the national health insurance program for people aged 65 years or older and people with disabilities, end stage renal disease (ESRD), or amyotrophic lateral sclerosis. Our study population included Medicare Fee-for-Service (FFS) beneficiaries who were Hawaii residents, aged 65 years or older, and enrolled until death or at least 11 months in the year 2012. The beneficiaries with any Medicare Advantage plans, known as Part C or health maintenance organizations (HMOs), were excluded in the study as their claims data to identify chronic conditions were not available in Medicare database. Our study included 84,212 beneficiaries from year 2012.
Chronic Conditions and Multimorbidities
A chronic condition is defined as a condition that lasts or is expected to last 12 or more months and results in functional limitations and/or the need for ongoing medical care (Hwang, Weller, Ireys, & Anderson, 2001). CMS used this chronic condition definition to develop algorithms for identification of 27 chronic conditions using the International Classification of Diseases, 9th Revision (ICD-9), Clinical Modification (Chronic Conditions Data Warehouse, 2016). To be consistent with the annual CMS reports, we studied the following 15 major chronic conditions: Alzheimer’s disease or dementia, asthma, atrial fibrillation, cancer, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), depression, diabetes, heart failure, hyperlipidemia (i.e., high cholesterol), hypertension (i.e., high blood pressure), ischemic heart disease, osteoporosis, arthritis, and stroke.
A beneficiary is considered to have multimorbidity if the person has more than one chronic condition. For the 15 chronic conditions investigated, there are 105 possible dyads (combinations of any pairs of the 15 conditions) and 455 triads (combinations of any three of the 15 conditions).
Variables
Race/ethnicity was the primary independent variable. The small racial groups (i.e., Black, American Indian/American Alaskan) were combined into the “other” group, resulting in race/ethnicity categories of White, Asian/Pacific Islander (PI), Hispanic, and Other. Age was categorized into 65 to 74, 75 to 84, and 85 years or older. A Medicare beneficiary is dual eligible if the person also received full or partial Medicaid benefits in any month in the given year, which can be a potential indicator for socioeconomic status (SES). Residential area was categorized into island of Oahu and other islands based on beneficiary’s residential zip code, which can be an indication of one’s accessibility to medical or health care resources, as all the major acute care hospitals are all on the island of Oahu.
Statistical Analyses
Subject characteristics are presented in Table 1 using frequencies and percentages, stratified by race/ethnicity. Bivariate associations were conducted using chi-square tests to investigate racial/ethnic differences in subject characteristics. To remove confounding by age and allow more informative comparison between races/ethnicities, age-adjusted prevalence rates were calculated using the three age groups (65–74, 75–84, and ≥85 years). Chi-square test was first used to explore the association of chronic conditions with race/ethnicity, starting with a single condition, then for dyads and triads, and the resulting prevalence rates are presented in Table 2. For each chronic condition, a multivariable logistic regression model was utilized to evaluate racial/ethnic disparity in prevalence rates of the chronic conditions, adjusting for the subject characteristics (i.e., age, gender, dual eligibility, and residential area). In each model, the odds ratio (OR) and its 95% confidence interval (CI) were computed to assess the risk of chronic conditions for each race/ ethnicity group compared to Whites (see Table 3).
Table 1.
Subject Characteristics by Race/Ethnicity.
| Race/ethnicity, n (%)
|
||||||
|---|---|---|---|---|---|---|
| Variable | Total (N = 84,212) |
White (n = 23,392) |
Asian/PI (n = 46,018) |
Hispanic (n = 4,346) |
Other (n = 10,456) |
p value |
| Age | <.001 | |||||
| 65–74 years | 40,837 (48.5%) | 13,029 (55.7%) | 19,601 (42.6%) | 2,525 (58.1%) | 5,682 (54.3%) | |
| 75–84 years | 28,004 (33.3%) | 6,798 (29.1%) | 16,158 (35.1%) | 1,357 (31.2%) | 3,691 (35.3%) | |
| ≥85 years | 15,371 (18.3%) | 3,565 (15.2%) | 10,259 (22.3%) | 464 (10.7%) | 1,083 (10.4%) | |
| Gender | <.001 | |||||
| Male | 38,376 (45.6%) | 12,020 (51.4%) | 19,632 (42.7%) | 2,010 (46.2%) | 4,714 (45.1%) | |
| Female | 45,836 (54.4%) | 11,372 (48.6%) | 26,386 (57.3%) | 2,336 (53.8%) | 5,742 (54.9%) | |
| Residential area | <.001 | |||||
| Other island | 26,049 (30.9%) | 11,366 (48.6%) | 10,354 (22.5%) | 1,518 (34.9%) | 2,811 (26.9%) | |
| Oahu | 58,122 (69.1%) | 11,999 (51.4%) | 35,660 (77.5%) | 2,828 (65.1%) | 7,635 (73.1%) | |
| Dual eligibility | 8,127 (9.7%) | 1,879 (8.0%) | 4,613 (10.0%) | 793 (18.2%) | 842 (8.1%) | <.001 |
| Number of chronic conditions | <.001 | |||||
| 0–1 | 25,033 (29.7%) | 9,473 (40.5%) | 11,096 (24.1%) | 1,501 (34.5%) | 2,963 (28.3%) | |
| 2–3 | 30,578 (36.3%) | 7,343 (31.4%) | 17,931 (39.0%) | 1,393 (32.1%) | 3,911 (37.4%) | |
| 4–5 | 19,752 (23.5%) | 4,377 (18.7%) | 11,922 (25.9%) | 963 (22.2%) | 2,490 (23.8%) | |
| ≥6 | 8,849 (10.5%) | 2,199 (9.4%) | 5,069 (11.0%) | 489 (11.3%) | 1,092 (10.4%) | |
Note. Column percentage. Bivariate association was conducted using chi-square test. PI = Pacific Islander.
Table 2.
Age-Adjusted Prevalence Rates Stratified by Race/Ethnicity for Top 10 Chronic Conditions, Dyads, and Triads.
| Race/ethnicity, n (%)
|
||||||
|---|---|---|---|---|---|---|
| Chronic condition | Total, n (%)a | White (n = 23,392) |
Asian/PI (n = 46,018) |
Hispanic (n = 4,346) |
Other (n = 10,456) |
CMS rankb |
| Single | ||||||
| 1. Hypertension | 51,704 (61.4%) | 11,340 (49.9%) | 31,056 (64.8%) | 2,614 (68.4%) | 6,694 (63.2%) | 1: 59.2% |
| 2. Hyperlipidemia | 50,277 (59.7%) | 10,811 (46.7%) | 30,597 (61.9%) | 2,381 (70.4%) | 6,488 (59.4%) | 2: 48.1% |
| 3. Diabetes | 24,379 (28.9%) | 4,285 (18.7%) | 15,171 (33.4%) | 1,415 (32.7%) | 3,508 (32.4%) | 5: 27.5% |
| 4. Ischemic heart disease | 19,044 (22.6%) | 5,391 (24.1%) | 10,496 (22.3%) | 946 (22.2%) | 2,211 (21.8%) | 3: 31.2% |
| 5. Arthritis | 16,089 (19.1%) | 4,935 (21.7%) | 8,494 (18.2%) | 781 (17.3%) | 1,879 (19.0%) | 4: 30.5% |
| 6. Chronic kidney disease | 14,746 (17.5%) | 3,318 (15.1%) | 8,634 (19.1%) | 842 (17.4%) | 1,952 (19.0%) | 6: 16.2% |
| 7. Alzheimer’s or dementia | 9,708 (11.5%) | 2,448 (11.9%) | 5,944 (12.3%) | 360 (11.2%) | 956 (11.5%) | 9: 12.1% |
| 8. Heart failure | 8,839 (10.5%) | 2,582 (12.0%) | 4,709 (10.7%) | 474 (8.8%) | 1,074 (11.3%) | 7: 15.7% |
| 9. Osteoporosis | 8,273 (9.8%) | 1,465 (6.7%) | 5,626 (11.9%) | 272 (11.3%) | 910 (9.2%) | 13: 7.3% |
| 10. Cancer | 7,265 (8.6%) | 1,834 (8.1%) | 4,230 (8.3%) | 255 (9.4%) | 946 (8.3%) | 12: 9.1% |
| Dyad | ||||||
| 1. Hyperlipidemia– hypertension | 41,349 (49.1%) | 8,150 (35.7%) | 25,688 (52.3%) | 2,065 (58.3%) | 5,446 (50.4%) | 1: 54.4% |
| [69.9%] | ||||||
| 2. Diabetes–hypertension | 20,577 (24.4%) | 3,381 (14.8%) | 13,031 (28.5%) | 1,201 (28.1%) | 2,964 (27.5%) | 4: 31.5% |
| [34.8%] | ||||||
| 3. Diabetes–hyperlipidemia | 19,819 (23.5%) | 3,221 (14.0%) | 12,602 (27.0%) | 1,124 (28.0%) | 2,872 (26.0%) | 6: 25.6% |
| [33.5%] | ||||||
| 4. Hypertension–Ischemic heart disease | 15,774 (18.7%) | 4,063 (18.3%) | 9,015 (19.2%) | 813 (18.9%) | 1,883 (18.6%) | 2: 37.3% |
| [26.7%] | ||||||
| 5. Hyperlipidemia– Ischemic heart disease | 14,713 (17.5%) | 3,784 (16.8%) | 8,460 (17.6%) | 726 (18.3%) | 1,743 (16.8%) | 5: 30.6% |
| [24.9%] | ||||||
| 6. Chronic kidney disease– hypertension | 12,891 (15.3%) | 2,725 (12.4%) | 7,698 (17.1%) | 742 (15.5%) | 1,726 (17.0%) | 10: 18.3% |
| [21.8%] | ||||||
| 7. Arthritis–hypertension | 12,200 (14.5%) | 3,259 (14.6%) | 6,788 (14.5%) | 642 (13.9%) | 1,511 (15.2%) | 3: 31.6% |
| [20.6%] | ||||||
| 8. Arthritis–hyperlipidemia | 11,491 (13.6%) | 2,959 (13.0%) | 6,548 (13.8%) | 567 (13.8%) | 1,417 (13.8%) | 7: 23.9% |
| [19.4%] | ||||||
| 9. Chronic kidney disease– hyperlipidemia | 11,247 (13.4%) | 2,322 (10.5%) | 6,765 (14.8%) | 642 (14.0%) | 1,518 (14.6%) | 18: 12.5% |
| [19.0%] | ||||||
| 10. Diabetes–ischemic heart disease | 8,691 (10.3%) | 1,783 (7.9%) | 5,220 (11.5%) | 503 (11.0%) | 1,185 (11.2%) | 9: 18.9% |
| [14.7%] | ||||||
| Triad | ||||||
| 1. Diabetes–hyperlipidemia– hypertension | 17,927 (21.3%) | 2,828 (12.4%) | 11,491 (24.7%) | 1,029 (25.4%) | 2,579 (23.6%) | 2: 31.6% |
| [40.2%] | ||||||
| 2. Hyperlipidemia– hypertension–ischemic heart disease | 13,248 (15.7%) | 3,229 (14.5%) | 7,737 (16.2%) | 677 (16.6%) | 1,605 (15.5%) | 1: 35.8% |
| [29.7%] | ||||||
| 3. Chronic kidney disease–hyperlipidemia– hypertension | 10,508 (12.5%) | 2,089 (9.5%) | 6,388 (14.0%) | 605 (13.1%) | 1,426 (13.7%) | 7: 18.6% |
| [23.6%] | ||||||
| 4. Arthritis–hyperlipidemia– hypertension | 9,752 (11.6%) | 2,341 (10.4%) | 5,651 (11.9%) | 513 (11.9%) | 1,247 (12.2%) | 3: 28.8% |
| [21.9%] | ||||||
| 5. Diabetes–hypertension– ischemic heart disease | 7,865 (9.3%) | 1,543 (6.9%) | 4,790 (10.5%) | 453 (10.1%) | 1,079 (10.2%) | 4: 21.5% |
| [17.7%] | ||||||
| 6. Diabetes– hyperlipidemia–ischemic heart disease | 7,413 (8.8%) | 1,452 (6.5%) | 4,527 (9.8%) | 417 (9.9%) | 1,017 (9.4%) | 8: 18.5% |
| [16.6%] | ||||||
| 7. Chronic kidney disease– diabetes–hypertension | 7,232 (8.6%) | 1,176 (5.3%) | 4,522 (10.5%) | 474 (9.1%) | 1,060 (10.1%) | 14: 13.9% |
| [16.2%] | ||||||
| 8. Chronic kidney disease– diabetes–hyperlipidemia | 6,585 (7.8%) | 1,069 (4.8%) | 4,138 (9.4%) | 425 (8.5%) | 953 (9.0%) | 23: 11.1% |
| [14.8%] | ||||||
| 9. Chronic kidney disease– hypertension–ischemic heart disease | 6,074 (7.2%) | 1,390 (6.4%) | 3,530 (8.0%) | 358 (6.9%) | 796 (8.0%) | 12: 16.1% |
| [13.6%] | ||||||
| 10. Chronic kidney disease– hyperlipidemia–ischemic heart disease | 5,671 (6.7%) | 1,439 (6.4%) | 3,182 (8.0%) | 316 (6.9%) | 734 (8.0%) | 16: 12.7% |
| [12.7%] | ||||||
Note. All conditions were significantly associated with race/ethnicity by chi-square tests (p value < .05). Age-adjusted prevalence rate was calculated using the three age groups (65–74, 75–84, and ≥85 years). PI = Pacific Islander; CMS = Centers for Medicare & Medicaid Services; FFS = Fee-for-Service.
The numbers in the bracket were prevalence rates computed based on Hawaii Medicare FFS beneficiaries aged 65 years or older who had at least 2 and 3 of the chronic conditions with respect to dyads and triads.
The prevalence rates and national ranks of the beneficiaries aged ≥65 years were obtained from 2012 CMS annual report (accessed from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/CC_Main.html). The age-adjusted prevalence rates for dyads and triads were computed based on the U.S. Medicare FFS beneficiaries aged 65 years or older who have at least 2 and 3 of the chronic conditions, respectively.
Table 3.
OR [and 95% CI] of Race/Ethnicity for Top 10 Chronic Conditions, Dyads, and Triads.
| Race/ethnicity, OR [95% CI]
|
|||
|---|---|---|---|
| Chronic condition | Asian/PI vs. White | Hispanic vs. White | Other vs. White |
| Single | |||
| 1. Hypertension | 2.06 [1.99, 2.13] | 1.65 [1.54, 1.76] | 1.92 [1.83, 2.02] |
| 2. Hyperlipidemia | 2.21 [2.14, 2.29] | 1.44 [1.35, 1.54] | 1.80 [1.72, 1.89] |
| 3. Diabetes | 2.19 [2.10, 2.28] | 2.10 [1.95, 2.26] | 2.25 [2.14, 2.38] |
| 4. Ischemic heart disease | 0.96 [0.92, 1.00] | 0.97 [0.89, 1.05] | 0.95 [0.90, 1.01] |
| 5. Arthritis | 0.78 [0.74, 0.81] | 0.81 [0.74, 0.88] | 0.80 [0.75, 0.85] |
| 6. Chronic kidney disease | 1.24 [1.18, 1.29] | 1.44 [1.32, 1.56] | 1.40 [1.32, 1.49] |
| 7. Alzheimer’s or dementia | 0.89 [0.84, 0.95] | 0.67 [0.59, 0.76] | 0.91 [0.83, 0.99] |
| 8. Heart failure | 0.85 [0.80, 0.89] | 0.99 [0.89, 1.10] | 1.03 [0.95, 1.11] |
| 9. Osteoporosis | 1.61 [1.51, 1.71] | 0.92 [0.80, 1.05] | 1.26 [1.15, 1.38] |
| 10. Cancer | 1.13 [1.07, 1.20] | 0.78 [0.68, 0.89] | 1.16 [1.07, 1.26] |
| Dyad | |||
| 1. Hyperlipidemia–hypertension | 2.26 [2.19, 2.34] | 1.75 [1.64, 1.87] | 1.99 [1.89, 2.08] |
| 2. Diabetes–hypertension | 2.34 [2.24, 2.44] | 2.23 [2.06, 2.40] | 2.36 [2.23, 2.49] |
| 3. Diabetes–hyperlipidemia | 2.38 [2.28, 2.49] | 2.18 [2.01, 2.35] | 2.35 [2.22, 2.49] |
| 4. Hypertension–ischemic heart disease | 1.13 [1.08, 1.18] | 1.14 [1.05, 1.25] | 1.12 [1.05, 1.19] |
| 5. Hyperlipidemia–ischemic heart disease | 1.15 [1.11, 1.21] | 1.10 [1.00, 1.20] | 1.09 [1.02, 1.16] |
| 6. Chronic kidney disease–hypertension | 1.35 [1.29, 1.42] | 1.54 [1.41, 1.69] | 1.51 [1.41, 1.62] |
| 7. Arthritis–hypertension | 0.97 [0.92, 1.02] | 1.07 [0.97, 1.17] | 1.03 [0.96, 1.10] |
| 8. Arthritis–hyperlipidemia | 1.06 [1.01, 1.11] | 1.04 [0.94, 1.14] | 1.04 [0.97, 1.11] |
| 9. Chronic kidney disease–hyperlipidemia | 1.40 [1.33, 1.48] | 1.57 [1.42, 1.72] | 1.52 [1.42, 1.64] |
| 10. Diabetes–ischemic heart disease | 1.57 [1.48, 1.66] | 1.60 [1.44, 1.78] | 1.62 [1.50, 1.75] |
| Triad | |||
| 1. Diabetes–hyperlipidemia–hypertension | 2.42 [2.32, 2.54] | 2.26 [2.09, 2.45] | 2.36 [2.23, 2.51] |
| 2. Hyperlipidemia–hypertension–ischemic heart disease | 1.23 [1.17, 1.29] | 1.21 [1.11, 1.33] | 1.18 [1.10, 1.26] |
| 3. Chronic kidney disease– hyperlipidemia–hypertension | 1.55 [1.47, 1.63] | 1.73 [1.57, 1.91] | 1.66 [1.54, 1.78] |
| 4. Arthritis–hyperlipidemia–hypertension | 1.15 [1.09, 1.21] | 1.19 [1.08, 1.32] | 1.17 [1.09, 1.26] |
| 5. Diabetes–hypertension–ischemic heart disease | 1.64 [1.55, 1.74] | 1.72 [1.54, 1.92] | 1.68 [1.55, 1.82] |
| 6. Diabetes–hyperlipidemia–ischemic heart disease | 1.67 [1.57, 1.77] | 1.66 [1.48, 1.86] | 1.67 [1.54, 1.82] |
| 7. Chronic kidney disease–diabetes– hypertension | 2.00 [1.87, 2.13] | 2.39 [2.13, 2.67] | 2.17 [1.99, 2.37] |
| 8. Chronic kidney disease–diabetes– hyperlipidemia | 2.02 [1.88, 2.17] | 2.33 [2.07, 2.62] | 2.13 [1.94, 2.33] |
| 9. Chronic kidney disease– hypertension– ischemic heart disease | 1.23 [1.16, 1.32] | 1.53 [1.35, 1.73] | 1.38 [1.26, 1.52] |
| 10. Chronic kidney disease–hyperlipidemia– ischemic heart disease | 1.03 [0.97, 1.10] | 1.27 [1.12, 1.45] | 1.21 [1.10, 1.32] |
Note. OR and 95% CI were obtained by multivariable logistic models adjusted for race/ethnicity, age, gender, dual eligibility, and residential area.
The bold-faced values indicate significantly different from Whites. OR = odds ratio; CI = confidence interval; PI = Pacific Islander.
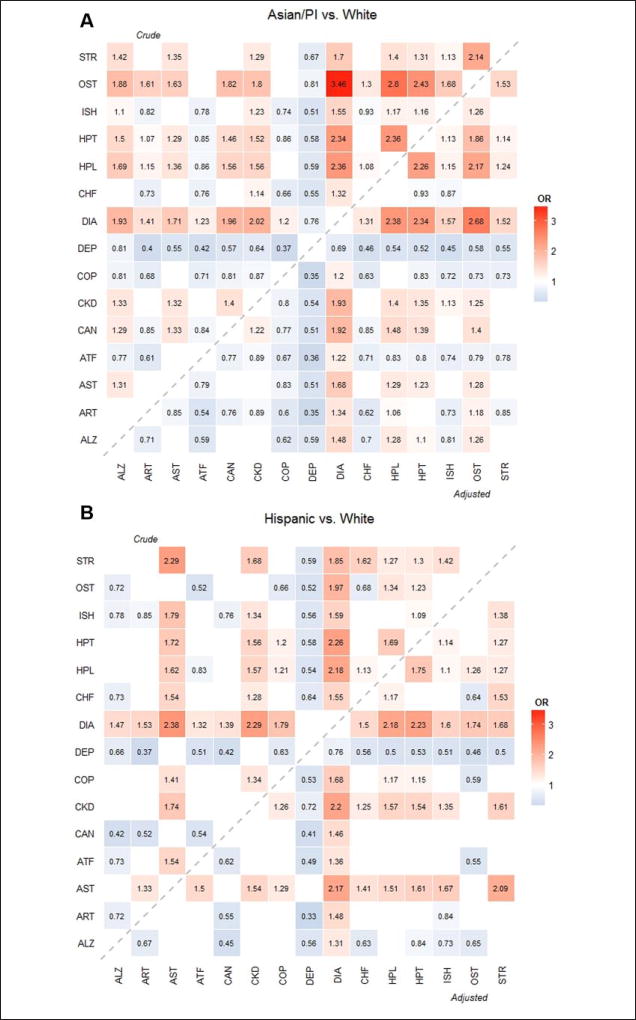
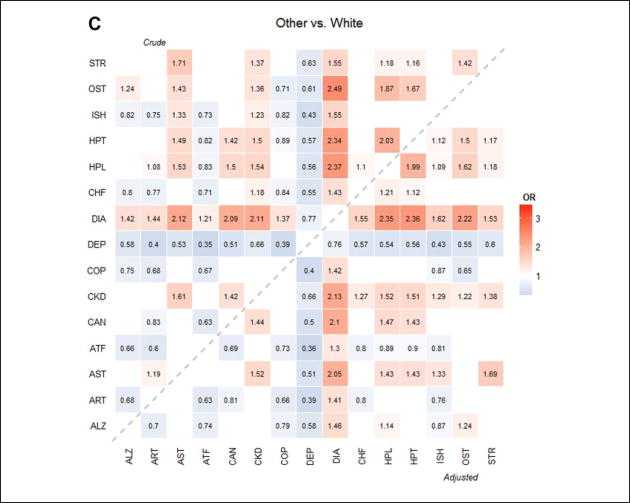
In addition, heat maps were generated to depict racial/ethnic differences in prevalence among all the dyads and are presented in Figure 1. The number in each cell indicates the OR corresponding to the dyad between the column and row chronic conditions comparing one race/ethnicity group against Whites. Crude ORs (in the upper triangular region) were obtained from a logistic regression with race/ethnicity only and adjusted ORs (in the lower triangular region) were obtained from the multivariable logistic regression with race/ ethnicity, adjusting for the subject characteristics. For simplicity and easy comparison, we only reported those ORs that were significantly different from Whites. The blank cells are the ORs that were not significantly different. The hotter cells (more reddish in color) indicate that the prevalence rate of the race/ethnicity group is higher than Whites (i.e., OR > 1), and the cooler cells (more bluish in color) indicate that the prevalence rate of the group is lower than Whites (i.e., OR < 1). A p value <.05 was considered statistically significant. All analyses were conducted in SAS version 9.4 and heat maps were generated using R version 3.0.2.
Figure 1.
Heat map of the OR of dyads by race/ethnicity.
Note. ORs were used to compare Asians/PIs, Hispanics, and Others to Whites. Crude ORs were obtained from logistic regressions adjusting for race/ethnicity only. Adjusted ORs were obtained from multivariable logistic regressions adjusting for race/ethnicity, age, gender, residential area, and dual eligibility status. The upper triangular region shows crude ORs and lower triangular region shows adjusted ORs compared with Whites. Hotter (reddish) color indicates larger OR (greater than 1; that is, prevalence rate of the race/ethnicity is higher than Whites) and colder (bluish) color indicates lower OR (less than 1; that is, the prevalence or mortality rate of the race/ethnicity rate is lower than Whites). Insignificant ORs were not presented in the figures. OR = odds ratio; PI = Pacific Islander; ALZ = Alzheimer’s disease or dementia; ART = arthritis; AST = Asthma; ATF = atrial fibrillation; CAN = cancer; CKD = chronic kidney disease; COP = chronic obstructive pulmonary disease; DEP = depression; DIA = diabetes; CHF = heart failure; HPL = hyperlipidemia; HPT = hypertension; ISH = ischemic heart disease; OST = osteoporosis; STR = stroke.
Results
Table 1 summarizes the characteristics of the study population, 23,392 beneficiaries were White (27.8%), 46,018 were Asian/PI (54.6%), and 4,346 were Hispanic (5.2%). In all, 18.3% of the beneficiaries were age 85 years or older, 45.6% were male, 69.1% lived in Oahu, and 9.7% were enrolled in both Medicare and Medicaid. Among the 15 chronic conditions examined, the prevalence of multimorbidity was high, with 70.3% having two or more chronic conditions and 10.5% having six or more chronic conditions. Race/ ethnicity showed significant differences for all of the subject characteristics: More people age 85 years or older were Asians/PIs, more males were White, more Whites and Hispanics lived on the other islands, and more Hispanics were dual eligible than the other race/ethnic groups. More Whites (40.5%) had none or one chronic condition than the other racial/ethnic groups (Asians/ PIs: 24.1%, Hispanics: 34.5%, and Others: 28.3%).
Table 2 shows age-adjusted prevalence rates of the 10 most common chronic conditions, dyads, and triads in the Hawaii study population. The most common single conditions were hypertension (61.4%), followed by hyperlipidemia (59.7%), diabetes (28.9%), ischemic heart disease (22.6%), arthritis (19.1%), CKD (17.5%), Alzheimer’s disease or dementia (11.5%), heart failure (10.5%), osteoporosis (9.8%), and cancer (8.6%). The top prevalent 10 dyads and triads included hyperlipidemia, hypertension, or diabetes in the chronic condition combinations. The most frequent dyad and triad were the pair of hyperlipidemia and hypertension (49.1%) and the combination of diabetes, hyperlipidemia, and hypertension (21.3%).
Compared with the national ranks, many of the common conditions in the CMS (Centers for Disease Control and Prevention [CDC], 2015) also appeared in the top 10 prevalent condition lists for Hawaii (see Table 2). However, there are some interesting differences. For example, the prevalence rate of hyperlipidemia in Hawaii was much higher than the United States (59.7% vs. 48.1%) while the prevalence rate of arthritis and hypertension dyad was much lower than the United States (20.6% vs. 31.6%) among beneficiaries with at least two chronic conditions. More interestingly, the triad of hyperlipidemia, ischemic heart disease, and arthritis ranked 5th in the United States was not in the 10 most common triads in our study.
Significant differences among racial/ethnic groups were detected for age-adjusted prevalence rates of all the conditions (Table 2). For most conditions including dyads and triads, Asians/PIs, Hispanics, and Others showed higher prevalence rates than Whites. However, Whites had slightly higher prevalence rates in arthritis and heart failure than the other race/ ethnicity groups. Females had higher prevalence rates in hypertension, hyperlipidemia, arthritis, Alzheimer’s disease or dementia, osteoporosis, and dyad or triad combinations with arthritis than males, but males had higher rates in diabetes, ischemic heart disease, CKD, cancer, and dyad or triad combinations with ischemic heart disease, diabetes, or CKD (see Supplement Table 1).
Table 3 shows the magnitude and significance of ORs for chronic conditions. For hypertension, hyperlipidemia, and diabetes, and most of their dyad or triad combinations, Whites had a lower prevalence rate than all the other racial/ethnic groups. Interestingly, the prevalence rate of Alzheimer’s disease or dementia was higher in Asians/PIs than Whites in Table 2 (12.9% vs. 10.5%), but after adjusting for the subject characteristics, the prevalence was reversed (OR = 0.89, 95% CI = [0.84, 0.95]). All of subject characteristics were statistically significant. Specially, the older beneficiaries were more likely to have Alzheimer’s disease or dementia than beneficiaries aged between 65 and 74 years (age 85+ vs. 65–74: OR = 19.8, 95% CI = [18.4, 21.3]; age 75–84 vs. 65–74: OR = 4.93, 95% CI = [4.59, 5.30]), and dual eligible beneficiaries were 3.68 times more likely to have Alzheimer’s disease or dementia (95% CI = [3.46, 3.92]). Compared with Whites, Asians/PIs were less likely to have heart failure (OR = 0.85, 95% CI = [0.80, 0.89]) but more likely to have osteoporosis (OR = 1.61, 95% CI = [1.51, 1.71]) and cancer (OR = 1.13, 95% CI = [1.07, 1.20]). Hispanics, however, showed lower prevalence in cancer than Whites (OR = 0.78, 95% CI = [0.68, 0.89]).
Figure 1 illustrates the prevalence of all 105 dyads by racial/ethnic group compared to Whites. The crude and adjusted ORs were mostly similar although some significant differences exist (e.g., Alzheimer’s disease or dementia with CKD). For all racial/ethnic groups, blue bands (OR < 1) appeared for depression, indicating that the prevalence of the dyad combinations with depression in Whites were higher than all the other racial/ ethnic groups. For all racial/ethnic groups, red bands (OR > 1) appeared for diabetes except for the dyad with depression, indicating that the prevalence of the dyad combinations with diabetes of the racial/ethnic groups were higher than Whites. Of note, a blue band appeared for only Asian/PI group for COPD except for the two dyads of “COPD and diabetes” (OR > 1) and “COPD and hyperlipidemia” (insignificant OR), which indicates that the prevalence rates of the dyads with COPD in Asians/PIs were lower than Whites. No obvious blue band appeared in the Hispanic group, but rather red cells appeared in five dyads with COPD: asthma, CKD, diabetes, hyperlipidemia, and hypertension. In addition, dyads with stroke showed distinctive patterns in race/ethnicity. Compared with Whites, Asians/PIs had lower prevalence rate for stroke with arthritis and atrial fibrillation, and Hispanics and others had higher prevalence for stroke with asthma and CKD.
Discussion
The health care needs and concomitant medical and societal burdens have been rising for older adults with chronic conditions and particularly those with multimorbidities. In 2010, the Department of Health and Human Services (HHS) developed strategic frameworks to improve the health status of individuals with multimorbidity (U.S. Department of HHS, 2010), such as identifying populations with morbidity and subgroups with specific clusters of conditions and focusing care models on the subgroups at high risk of poor health outcomes. To address this need, our study explored the age-adjusted prevalence of chronic conditions and multimorbidity among older adults in a geographically defined region with diverse racial/ethnic groups. In this study, we identified the most common conditions including dyads and triads and compared their prevalence patterns among racial/ethnic groups using Hawaii Medicare data. Although multimorbidity has been reported in studies using nation-, state-, or local county-level data (Centers for Medicare & Medicaid Services, 2012; Freid et al., 2012; Goodman et al., 2016; Lochner & Cox, 2013; Lochner et al., 2013; Rocca et al., 2014; Schneider, O’Donnell, & Dean, 2009), our study provides a unique depiction of chronic conditions and multimorbidity in the state with the most diverse races and ethnicities. The findings have major implications to the other regions of the United States where multiple racial and ethnic groups exist.
Our study showed that multimorbidity was common (70.3%) among the older adults in Hawaii, similar to the CDC report (69.1%; Lochner & Cox, 2013). Multimorbidity was the highest among Asians/PIs (75.9%) and lowest among Whites (59.5%). The prevalence rates for six or more chronic conditions were highest among Hispanics (11.3%), followed by Asians/PIs (11.0%), Others (10.4%), and Whites (10.5%). The most common chronic conditions were hyperlipidemia and hypertension and combinations of chronic conditions with either hyperlipidemia or hypertension. Although the prevalence rates for most of the chronic conditions and their dyads were lower in Whites than in all the other racial/ethnic groups, some single chronic conditions or dyads were less common in Asians/PIs (e.g., heart failure) and Hispanics (e.g., cancer) with other conditions more prevalent in Whites (e.g., arthritis, Alzheimer’s disease, or dementia).
The heat maps illustrated the distinct patterns of prevalence in dyads for each racial/ethnic group compared with Whites. Some of the findings from the heat maps could be potential areas for further research in racial/ethnic disparities. First, the prevalence rates for all the dyads with depression were higher in Whites as observed in previous studies (Akincigil et al., 2012; Quinones et al., 2014). The lower prevalence rates of depression in all the other racial/ethnic groups could be due to underestimation as suggested by the previous studies. Various factors such as access barriers (e.g., limited use of psychotherapy, geographic-level differences in the accessibility of mental health services) could contribute to the racial/ethnic differences. More research is needed to elucidate the reasons for this disparity and to explore utilization of psychotherapy and mental health services.
Another interesting finding from the heat maps was the racial/ethnic difference in dyads with COPD. COPD was originally considered a disease disproportionally affecting White males or smokers. Our study showed that five dyads with COPD in Hispanics had higher prevalence rates than Whites. Three dyads of COPD were co-occurring with major metabolic conditions (i.e., hyperlipidemia, hypertension, and diabetes) and the two other pairs were with CKD and asthma. The higher prevalence of COPD dyads in Hispanics may potentially be due to a high prevalence in the single chronic conditions involved (e.g., CKD). These differences may result from the effects of migration and acculturation (Brehm & Celedón, 2008), differences in health care access and utilization (Hasegawa, Tsugawa, Tsai, Brown, & Camargo, 2014), different SES, or cultural and lifestyle differences (Cruz-Flores et al., 2011).
Dyads including stroke also showed distinctive racial/ethnic patterns. Regional and racial/ethnic differences in prevalence and mortality rates in stroke patients have been reported (Cruz-Flores et al., 2011; Howard, 2013). In our study, Asians/PIs had lower prevalence in stroke with arthritis and with atrial fibrillation compared with Whites, even though Asians/PIs had higher prevalence in the major risk factors for stroke such as diabetes, hypertension, hyperlipidemia, and aging. The lower prevalence rates in these dyads may be affected by lower rates in the single chronic conditions (see Table 2), or they might be caused by disparities in stroke care and knowledge about strokes. As addressed by Cruz-Flores et al. (2011), racial/ethnic disparity in stroke might be due to the lack of awareness of stroke symptoms and signs and the need for urgent treatment among Asians/PIs.
Our study showed that Hawaii has a unique distribution in prevalence rates of chronic conditions. This might be caused by various reasons such as the effect of migration and acculturation, regional uniqueness (e.g., tropical climate), SES, cultural lifestyle, different types of health care access or utilization (e.g., acupuncture, alternative medicine, etc.), and genetic differences. For example, the prevalence of osteoporosis in Hawaii is the highest among the United States. This might be due to the larger Asian population in the state. Asian women are known to be at high risk for developing osteoporosis due to potential ethnic genetic background (e.g., bone area and geometry; Lei, Chen, Xiong, Li, & Deng, 2006). Furthermore, the prevalence rates of chronic conditions are not homogeneous in terms of race/ethnicity across the United States (CDC, 2015). This may indicate that not only race/ethnicity but also geographical region can contribute to the health disparities. Future studies should be conducted to examine the reasons for disparities in utilization and prevalence.
With respect to age, consistent with other studies (Ashman & Beresovsky, 2013; Centers for Medicare & Medicaid Services, 2012; Clerencia-Sierra et al., 2015; Fabbri et al., 2015), prevalence of all the chronic conditions was lower among beneficiaries aged 65 to 74 years than those aged 75 years or older. The prevalence rates of some chronic conditions increased with age while other chronic conditions decreased from age 75–84 to age 85 or older. The recognition of these chronic conditions and their combinations among the high-risk age groups may have a crucial role in directing resources and health care delivery. Specifically, escalation in prevalence rate of Alzheimer’s disease or dementia is dramatic. Currently, there are only a few proven ways to prevent Alzheimer’s disease or dementia. Ongoing research hopefully will help identify high-risk individuals, which could improve the prevention of the disease by lowering risk factors such as hypertension, hyperlipidemia, and diabetes and by increasing physical activity.
Our study findings can help support clinical practice and research on chronic conditions and multimorbidities. First, this study highlights the vulnerable racial/ethnic subgroups with specific chronic condition combinations. In particular, Asians, Native Americans, and other PIs are often ignored or combined to “other” racial/ethnic group due to the small sample sizes. Asians are, however, the most rapidly increasing racial group in the United States and are estimated to reach 11.7% (Asian alone or in combination) of the total U.S. population, with more than 45 million people by year 2060 (Colby & Ortman, 2015). Nevertheless, Asian Americans have been facing many challenges in SES and health such as living below the poverty level, high rates of limited English proficiency, lower education (National Commission on Asian American and Pacific Islander Research in Education [CARE], 2008), and higher incidence rates for cancer (Miller, Chu, Hankey, & Ries, 2008), chronic hepatitis B, and diabetes (King et al., 2012). Although the reasons for these distinctive patterns are uncertain, race/ethnicity may need to be considered when making clinical decisions and developing health care programs. The patterns identified can guide future research with racial/ ethnic subgroups that may help clinicians develop race/ethnic specific treatments and self-management plans for delivering high quality health care.
As seen in other studies, regardless of race/ethnicity, we found high prevalence rates in chronic conditions, dyads, and triads related to metabolic conditions (e.g., hyperlipidemia, hypertension). From a clinical perspective, interventions aimed at changing lifestyles and behaviors related to metabolic conditions can prevent or improve many chronic conditions. A holistic health care approach for patients with multiple chronic conditions with similar or dissimilar risk profiles can improve the management of their conditions. As a starting point, there have been efforts to make the similar/dissimilar risk profiles in some chronic conditions including diabetes and asthma (Bokhour et al., 2008; Magnan et al., 2015). The similar risk profiles, so-called “concordant” patterns, share care goals between the chronic conditions (e.g., hypertension and hyperlipidemia) or are caused by similar pathogenesis, but “discordant” patterns do not share care goals (e.g., arthritis and hypertension). We observed that discordant patterns were more prevalent in females than males (see Supplement Table 1), which may indicate genetic difference in gender or different self-care management for chronic conditions. Building concordant-discordant framework and developing clinical guidelines from the framework should be considered for all chronic conditions. Recognizing discordant or concordant patterns for each chronic condition can be the foundation for both health care professionals and patients to improve health status of the patients such as identifying the optimal treatment plan and medications.
The prevalence of morbidity varies by state (Lochner & Shoff, 2015). Charactering such state-level variations of multimorbidities would be valuable, as each state increasingly has a key role in the financing, regulating, and delivering of health care. The racial/ethnic differences in chronic condition patterns will also have significant clinical and public health implications, especially for a state like Hawaii with diverse racial/ethnic subpopulations. According to Hawaii State Plan on Aging 2015 (Hawaii Department of Health, 2015), the gerontology population will continue to increase in Hawaii, which already has a large older adult population. In spite of having a large proportion of older adults, Hawaii has the highest healthy life expectancy in the nation for older adults (CDC, 2013). However, our study showed the healthier older population could be race/ethnicity specific. The prevalence rates in the most common conditions in Whites were lower in Hawaii than the national rates. Thus, comparative research among Whites in Hawaii with other states can provide discernments for effective health care management plans for other states. In addition, the state of Hawaii could develop intervention programs for chronic conditions and multimorbidities targeting race/ethnic groups such as Asian/PI to reduce racial/ethnic disparities.
Although the current study was based on a state-level data, our approach of studying health disparities can be extended to studies at the city-, state-, or nation-level data. Results of such studies can assist policy makers and health care professionals to develop and implement various prevention measures and health care policies. For those common chronic conditions in the United States (e.g., hypertension and hyperlipidemia), policy makers or stakeholders can consider developing tools or web applications to provide disease specific information on health care, public health, and social services for individuals with the conditions; to help maximize self-care management services; and to reduce barriers to patient-centered decision making.
A few limitations of our study should be presented. First, we limited our study sample to Medicare FFS Beneficiaries who were enrolled at least 11 months in 2012. Therefore, beneficiaries who did not enroll in 2012 or not for both Parts A and B were excluded. In Hawaii, about 47% of beneficiaries have HMOs. Although the Medicare FFS population was comparable with the Hawaii population (Supplement Table 2), estimation of prevalence rates of chronic conditions without this subpopulation can produce a selection bias, and our results may not be generalizable to the geriatric population in Hawaii. Second, we could only explore racial/ethnic disparities based on the available race/ethnicity categories. Asian and PI, two of the common racial groups in Hawaii (2010 Census Hawaii: Asian 38.6%, and Native Hawaiian and other PIs 10.0%), were collapsed into the Asian/PI race variable in Medicare data. As Hawaii is the most racially and ethnically diverse state and studies showed significant differences in health outcomes with more detailed race/ethnicity (Lim et al., 2015; Nakagawa, Lim, Harvey, Miyamura, & Juarez, 2016), having more racial/ethnic categories would allow for more meaningful comparisons. Third, due to the nature of Medicare data, we could not assess whether beneficiaries had chronic conditions before they enrolled in the Medicare plan, which could lead to a potential underestimation. Fourth, similar to the limitations in all administrative claims databases, disease misclassification could occur as the chronic conditions are based on ICD-9 codes in which physician coding or data entry errors may lead to discrepancies in classifications of diseases and treatments provided. This misclassification may lead to over or under diagnosis of chronic conditions and therefore their prevalence estimates could be unreliable. Fifth, SES was not adjusted in our multivariable logistic models. SES is known to be associated with chronic diseases such as stroke, diabetes, hypertension, and heart disease (Dalstra et al., 2005), but SES is not available in administrative data such as Medicare data. To overcome this, we included dual eligibility status in our analyses. Medicaid is a social health care program for families and individuals with limited resources to pay for health care or people with certain disabilities. Although we believe dual eligibility can serve as an indicator for SES, future studies are needed to confirm our findings as poverty alone does not qualify people to receive Medicaid benefits. Sixth, we did not adjust significance levels for multiple testing. The purpose of the study was not to test hypotheses but to investigate potential racial/ethnic disparities. Future studies are needed to confirm our findings under certain hypotheses (e.g., the prevalence rates for depression are equivalent among racial/ethnic groups). Last, the prevalence rates presented in this study are specific to the state of Hawaii, and, as a result, our findings may not be generalizable to older populations in other states.
Nevertheless, our study offers a starting point toward identifying the older population risk groups with specific chronic conditions and their multimorbidity. These findings contribute to expanding knowledge about chronic conditions and their multimorbidity in a variety of populations which will encourage additional research in the identification of effective chronic condition prevention and management approaches.
Conclusion
Despite extensive effort on the management of geriatric chronic conditions, the prevalence rates are still increasing. Multimorbidity has a huge impact on quality of life for the geriatric population and poses a challenge to the U.S. health care system. The variation of multimorbidity across racial/ethnic groups underscores the importance of race and ethnicity in chronic disease management and self-care. Race/ethnicity may need to be integrated in developing health care programs aimed to reduce health disparities and to improve quality of life for individuals with chronic conditions.
Supplementary Material
Acknowledgments
The authors thank Dr. Jill Miyamura of the Hawaii Health Information Corporation for providing access to the Hawaii Medicare database, Mr. Yang Rui for his technical support, and Ms. Rosa Castro for reviewing the draft of the article.
The content is solely the responsibility of the authors and does not necessarily reflect the official opinion of the National Institutes of Health (NIH).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was partially supported by grants U54MD007584, P20GM103466, G12MD007601, and U54GM104944 from the National Institutes of Health (NIH).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Akincigil A, Olfson M, Siegel M, Zurlo KA, Walkup JT, Crystal S. Racial and ethnic disparities in depression care in community-dwelling elderly in the United States. American Journal of Public Health. 2012;102:319–328. doi: 10.2105/ajph.2011.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman JJ, Beresovsky V. Multiple chronic conditions among US adults who visited physician offices: Data from the National Ambulatory Medical Care Survey, 2009. Preventing Chronic Disease. 2013;10:E64. doi: 10.5888/pcd10.120308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhour BG, Cohn ES, Cortés DE, Yinusa-Nyahkoon LS, Hook JM, Smith LA, Lieu TA. Patterns of concordance and non-concordance with clinician recommendations and parents’ explanatory models in children with asthma. Patient Education & Counseling. 2008;70:376–385. doi: 10.1016/j.pec.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JM, Celedón JC. Chronic obstructive pulmonary disease in Hispanics. American Journal of Respiratory and Critical Care Medicine. 2008;177:473–478. doi: 10.1164/rccm.200708-1274PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Healthy life expectancies at age 65 highest in Hawaii, lowest in Mississippi. 2013 Retrieved from http://www.cdc.gov/media/releases/2013/p0718-life-expectancy.html.
- Centers for Disease Control and Prevention. Medicare chronic conditions dashboard: State level. Comparison of geographic areas by chronic conditions, 2014. 2015 Retrieved from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/Chronic-Conditions-State/CC_State_Dashboard.html.
- Centers for Medicare & Medicaid Services. Chronic conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Baltimore, MD: Author; 2012. [Google Scholar]
- Chronic Conditions Data Warehouse. CCW chronic condition algorithms. 2016 Retrieved from https://www.ccwdata.org/web/guest/condition-categories.
- Clerencia-Sierra M, Calderon-Larranaga A, Martinez-Velilla N, Vergara-Mitxeltorena I, Aldaz-Herce P, Poblador-Plou B, Prados-Torres A. Multimorbidity patterns in hospitalized older patients: Associations among chronic diseases and geriatric syndromes. PLoS ONE. 2015;10:14. doi: 10.1371/journal.pone.0132909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. 2015 Retrieved from http://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf.
- Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Valderrama AL. Racial-ethnic disparities in stroke care: The American experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- Dalstra J, Kunst A, Borrell C, Breeze E, Cambois E, Costa G, Mackenbach J. Socioeconomic differences in the prevalence of common chronic diseases: An overview of eight European countries. International Journal of Epidemiology. 2005;34:316–326. doi: 10.1093/ije/dyh386. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: New tasks, priorities, and frontiers for Integrated Gerontological and Clinical Research. Journal of the American Medical Directors Association. 2015;16:640–647. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freid VM, Bernstein AB, Bush MA. Multiple chronic conditions among adults aged 45 and over: Trends over the past 10 years (NCHS Data Brief No. 100) Hyattsville, MD: National Center for Health Statistics; 2012. Jul, [accessed October 24, 2016]. Retrieved from www.cdc.gov/nchs/products/databriefs/db100.htm. [PubMed] [Google Scholar]
- Goodman RA, Ling SM, Briss PA, Parrish RG, Salive ME, Finke BS. Multimorbidity patterns in the United States: Implications for research and clinical practice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2016;71:215–220. doi: 10.1093/gerona/glv199. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Tsugawa Y, Tsai CL, Brown DF, Camargo CA., Jr Frequent utilization of the emergency department for acute exacerbation of chronic obstructive pulmonary disease. Respiratory Research. 2014;15:40. doi: 10.1186/1465-9921-15-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawaii Department of Health. Hawaii State Plan on Aging For Older American’s Act Title III and Title VII Programs. Honolulu, HI: Hawaii State Department of Health; 2015. Dec, [Google Scholar]
- Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian population: 2010 (Census 2010 Briefs, No. C2010BR-11) Washington, DC: U.S. Census Bureau; 2012. [accessed October 24, 2016]. Retrieved from www.census.gov/prod/cen2010/briefs/c2010br-11.pdf. [Google Scholar]
- Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126–S128. doi: 10.1161/STROKEAHA.111.000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Weller W, Ireys H, Anderson G. Out-of-pocket medical spending for care of chronic conditions. Health Affairs (Millwood) 2001;20:267–278. doi: 10.1377/hlthaff.20.6.267. [DOI] [PubMed] [Google Scholar]
- King GL, McNeely MJ, Thorpe LE, Mau MLM, Ko J, Liu LL, Chow EA. Understanding and addressing unique needs of diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35:1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei SF, Chen Y, Xiong DH, Li LM, Deng HW. Ethnic difference in osteoporosis-related phenotypes and its potential underlying genetic determination. Journal of Musculoskeletal and Neuronal Interactions. 2006;6:36–46. [PubMed] [Google Scholar]
- Lim E, Cheng Y, Reuschel C, Mbowe O, Ahn HJ, Juarez DT, Chen JJ. Risk-adjusted in-hospital mortality models for congestive heart failure and acute myocardial infarction: Value of clinical laboratory data and race/ethnicity. Health Services Research. 2015;50(Suppl. 1):1351–1371. doi: 10.1111/1475-6773.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare Beneficiaries, United States, 2010. Preventing Chronic Disease. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner KA, Goodman RA, Posner S, Parekh A. Multiple chronic conditions among Medicare beneficiaries: State-level variations in prevalence, utilization, and cost, 2011. Medicare & Medicaid Research Review. 2013;3(3):E1–E18. doi: 10.5600/mmrr.003.03.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner KA, Shoff CM. County-level variation in prevalence of multiple chronic conditions among Medicare Beneficiaries, 2012. Preventing Chronic Disease. 2015;12:3. doi: 10.5888/pcd12.140442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlin SR, Soni A. Health care expenditures for adults with multiple treated chronic conditions: Estimates from the Medical Expenditure Panel Survey, 2009. Preventing Chronic Disease. 2013;10:8. doi: 10.5888/pcd10.120172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnan EM, Gittelson R, Bartels CM, Johnson HM, Pandhi N, Jacobs EA, Smith MA. Establishing chronic condition concordance and discordance with diabetes: A Delphi study. BMC Family Practice. 2015;16 doi: 10.1186/s12875-015-0253-6. Article 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer SW, Smith SM, Wyke S, O’Dowd T, Watt GCM. Multimorbidity in primary care: Developing the research agenda. Family Practice. 2009;26:79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Lim E, Harvey S, Miyamura J, Juarez DT. Racial/ ethnic disparities in the association between preeclampsia risk factors and preeclampsia among women residing in Hawaii. Maternal and Child Health Journal. 2016;20:1814–1824. doi: 10.1007/s10995-016-1984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Commission on Asian American and Pacific Islander Research in Education. Asian Americans and Pacific Islanders. 2008 Retrieved from http://www.api-asf.org/research/2008_CARE_Report.pdf.
- Parekh AK, Goodman RA, Gordon C, Koh HK HHS Interagency Workgroup on Multiple Chronic Conditions. Managing multiple chronic conditions: A strategic framework for improving health outcomes and quality of life. Public Health Reports. 2011;126:460–471. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner SF, Goodman RA. Multimorbidity at the local level: Implications and research directions. Mayo Clinic Proceedings. 2014;89:1321–1323. doi: 10.1016/j.mayocp.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Quinones AR, Thielke SM, Beaver KA, Trivedi RB, Williams EC, Fan VS. Racial and ethnic differences in receipt of antidepressants and psychotherapy by veterans with chronic depression. Psychiatric Services. 2014;65:193–200. doi: 10.1176/appi.ps.201300057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Boyd CM, Grossardt BR, Bobo WV, Rutten LJF, Roger VL, St. Sauver JL. Prevalence of multimorbidity in a geographically defined American population: Patterns by age, sex, and race/ethnicity. Mayo Clinic Proceedings. 2014;89:1336–1349. doi: 10.1016/j.mayocp.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Sauver JL, Boyd CM, Grossardt BR, Bobo WV, Rutten LJF, Roger VL, Rocca WA. Risk of developing multimorbidity across all ages in an historical cohort study: Differences by sex and ethnicity. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006413. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health and Quality of Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner CA, Friedman B. Hospital utilization, costs, and mortality for adults with multiple chronic conditions, nationwide inpatient sample, 2009. Preventing Chronic Disease. 2013;10:19. doi: 10.5888/pcd10.120292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MA, Lee SJ, Boscardin WJ, Miao YH, Fung KZ, Moore KL, Schwartz JB. Patterns of multimorbidity in elderly veterans. Journal of the American Geriatrics Society. 2012;60:1872–1880. doi: 10.1111/j.1532-5415.2012.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Multiple chronic conditions—A strategic framework: Optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC: Author; 2010. [Google Scholar]
- Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. Journal of General Internal Medicine. 2007;22:391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: A 2012 update. Preventing Chronic Disease. 2014;11:E62. doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.