Abstract
Cholesterol is an important modulator of membrane protein function and signaling in endothelial cells, thus making it an emerging target for anti-angiogenic agents. In this study, we employed a phenotypic screen that detects intracellular cholesterol distribution in endothelial cells (HUVEC) and identified 13 existing drugs as cholesterol trafficking inhibitors. Cepharanthine, an approved drug for anti-inflammatory and cancer management use, was amongst the candidates, which was selected for in-depth mechanistic studies to link cholesterol trafficking and angiogenesis. Cepharanthine inhibited the endolysosomal trafficking of free-cholesterol and low-density lipoprotein in HUVEC by binding to Niemann-Pick disease, type C1 (NPC1) protein and increasing the lysosomal pH. The blockade of cholesterol trafficking led to a cholesterol-dependent dissociation of mTOR from the lysosomes and inhibition of its downstream signaling. Cepharanthine inhibited angiogenesis in HUVEC and in zebrafish in a cholesterol-dependent manner. Furthermore, cepharanthine suppressed tumor growth in vivo by inhibiting angiogenesis and it enhanced the antitumor activity of the standard chemotherapy cisplatin in lung and breast cancer xenografts in mice. Altogether, these results strongly support the idea that cholesterol trafficking is a viable drug target for anti-angiogenesis and that the inhibitors identified among existing drugs, such as cepharanthine, could be potential anti-angiogenic and antitumor agents.
Keywords: Cholesterol trafficking, angiogenesis, tumor, lysosome, cepharanthine
1. Introduction
The tumor microenvironment, consisting of non-cancer cells, blood vessels and secreted proteins produced by those surrounding cells, has been recognized as a key factor influencing the growth of cancer [1]. One of the key events shaping the tumor microenvironment is the formation of new blood vessels into tumor tissues, termed tumor angiogenesis. The role of angiogenesis in cancer growth was first suggested by Judah Folkman in 1971 [2]. Since then, several angiogenesis inhibitors, including monoclonal antibody drugs and small molecule kinase inhibitors, have been developed and successfully introduced into the clinic for the treatment of cancer [3]. However, anticancer effects of the angiogenesis inhibitors varied across different cancer types and not all the treatment trials were successful. The difference in cancer sensitivity to angiogenesis inhibitors is likely to be attributable to development of resistance in cancer cells to angiogenesis inhibitors and tumor vascular heterogeneity [4]. Therefore, it is necessary to diversify drug targets and anti-angiogenic strategies to overcome such clinical challenges of current angiogenesis inhibitors.
Cholesterol is a fundamental component of cellular membranes, which regulates membrane permeability and fluidity. In addition to the structural support, it also functions in intracellular transport and cell signaling. Cholesterol can be synthesized in the endoplasmic reticulum (ER) or absorbed from extracellular space via low-density lipoprotein (LDL) receptor-mediated endocytosis [5]. Cellular cholesterol absorption and distribution requires appropriate intracellular transport of LDL. Upon internalization, LDL is delivered to early sorting endosomes and then to late endolysosomes where LDL and cholesteryl esters are hydrolyzed, after which the LDL receptor can be recycled back to the plasma membrane [6]. The Niemann-Pick type C (NPC) proteins, NPC1 and NPC2, in the late endolysosomes play an important role in hydrolyzing the cholesterol esters and delivering free cholesterol out of the endolysosomes [7]. Inhibition of NPC1 or NPC2 causes accumulation of cholesterol and glycolipids in the endolysosomes, a phenotype named NPC after the genetic disease of the same name [8]. Circulating cholesterol, in the form of LDL, has to enter or pass through the endothelium that lines blood vessels to be distributed to the whole body. Therefore, endothelial cells play an important role in whole body LDL penetration, accumulation and metabolism [9]. Conversely, a role of cholesterol in endothelial cell functions and angiogenesis has been increasingly suggested. In the early 2000s, several groups reported functional links between plasma cholesterol levels and angiogenesis based on the observation that cholesterol-lowering agents, statins (HMG-CoA reductase inhibitors) could modulate angiogenesis [10–12]. More direct evidence between endothelial cell cholesterol content and angiogenic signaling pathways has emerged recently. Depletion of plasma membrane cholesterol in endothelial cells by liver X receptor agonists or an over-expression of apolipoprotein A-I binding protein (AIBP) that promotes the efflux of cellular cholesterol to high-density lipoprotein (HDL) could lead to a reduction of lipid rafts and impaired vascular endothelial growth factor receptor-2 (VEGFR2) signaling pathways [13, 14]. In addition, ezetimibe, an approved cholesterol-uptake blocker that works by inhibiting NPC1-like 1 (NPCL1), significantly inhibited tumor angiogenesis in animal models [15].
In addition to the cholesterol contents, proper intracellular cholesterol trafficking was also suggested to play a critical for endothelial cell signaling and angiogenesis. We have previously shown that small molecule inhibitors of cholesterol trafficking, such as itraconazole and tamoxifen suppressed mTOR signaling and angiogenesis in vitro and in preclinical models [16–21]. Based on the promising preclinical data, itraconazole has entered Phase II clinical trials for the treatment of various types of cancer, including non-small cell lung cancer (NSCLC), basal cell carcinoma (BCC) and metastatic prostate cancer. Recently, positive clinical outcomes have been reported from the NSCLC, BCC and prostate cancer trials [22–24]. These results suggest that cholesterol trafficking is a viable drug target and that inhibitors of endothelial cell cholesterol trafficking could be a new class of anti-angiogenic agents. This notion prompted us to screen the Johns Hopkins Drug Library (JHDL) [25, 26] to identify existing drugs that can modulate endothelial cell cholesterol trafficking. In this study, we successfully identified cholesterol trafficking inhibitors among existing drugs, including an anti-inflammatory drug cepharanthine (CEP). CEP is a natural product derived from a plant Stephania cepharantha Hayata and was approved in Japan for the use in various disease conditions, such as inflammation and cancer management [27, 28]. Recent studies suggested potential effects of CEP on angiogenesis and cancer metastasis [29, 30]. However, precise molecular mechanisms behind the pharmacological effects of CEP have not been fully addressed. Our study shows that CEP inhibits angiogenesis by blocking cholesterol trafficking and provides a strong evidence that cholesterol trafficking inhibitors could be potential anti-angiogenic and anticancer agents.
2. Materials and methods
2.1. Cell culture
Human umbilical vein endothelial cells (HUVEC) were purchased from Thermo Fisher Scientific and grown in Medium 200 supplemented with low serum growth supplement (LSGS) (Thermo Fisher Scientific, Waltham, MA). A549, MDA-MB-231 and HEK293T cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). A549 cells were grown in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (Thermo Fisher Scientific). MDA-MB-231 and HEK293T cells were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. All the cells were maintained in a humidified incubator at 37°C adjusted to 5% CO2.
2.2. Reagents and antibodies
Cepharanthine (CEP) and cholesterol were purchased from Santa Cruz Biotechnology (Dallas, Texas). Methyl-β-cyclodextrin, filipin, itraconazole and cisplatin were bought from Sigma-Aldrich (St. Louis, MO). Primary antibodies for S6 kinase (S6K) (sc-8418, mouse monoclonal, 1:200), phospho-S6K (Thr421/Ser424) (sc-7984-R, rabbit polyclonal, 1:100), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sc-365062, mouse monoclonal, 1:1000) and lysosomal-associated membrane protein 1 (LAMP1) (sc-20011, mouse monoclonal, 1:100), and horseradish peroxidase (HRP)-conjugated secondary antibodies (sc-2005, goat anti-mouse IgG-HRP, 1:2500; sc-2004, goat anti-rabbit IgG-HRP, 1:2500) were purchased from Santa Cruz Biotechnology. Antibodies for eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) (9452S, rabbit monoclonal, 1:1000), protein disulfide isomerase (PDI) (3501S, rabbit monoclonal, 1:100) and mammalian target of rapamycin (mTOR) (2983S, rabbit monoclonal, 1:100) were from Cell Signaling Technology (Danvers, MA), for GM130 (610823, mouse monoclonal, 1:100), CD31 (550274, rat monoclonal, 1:50) and Ki67 (550609, mouse monoclonal, 1:100) were from BD Biosciences (San Jose, CA), and for NPC1 (13926-1-AP, rabbit polyclonal, 1:500) was from Proteintech (Chicago, IL). Secondary antibodies conjugated with Alexa Fluor 488 (A21202, donkey anti-mouse IgG conjugate, 1:1000; A21206, donkey anti-rabbit IgG conjugate, 1:1000; A11006, goat anti-rat IgG conjugate, 1:1000) and Alexa Fluor 647 (A21235, goat anti-mouse IgG conjugate, 1:1000; A21244, goat anti-rabbit IgG conjugate, 1:1000) were from Thermo Fisher Scientific.
2.3. Screening of cholesterol trafficking inhibitors
Total 3,131 drugs of the Johns Hopkins Drug Library (JHDL) arrayed in 96-well plates were diluted in sterile PBS at 100 μM (working dilution) and used to screen in HUVEC. The final drug concentration of 5 μM was used for the screening since screening assays for hit discovery are typically run at 1 – 10 μM compound concentration [31]. After 24 h incubation with the drugs, cells were fixed with 4% paraformaldehyde for 203min at room temperature and stained with filipin (503μg/ml) for 23h at room temperature. Cells were washed with phosphate buffered saline (PBS) and the fluorescent cholesterol images in each well were obtained using the Olympus IX81 fully automated fluorescence microscope (Olympus, Tokyo, Japan) equipped with Prior motorized stage. Each screening plate contains negative (dimethyl sulfoxide, DMSO) and positive (itraconazole, U18666A and imipramine) control compounds. All the captured images were manually assessed and hits were identified by comparing the cholesterol distribution patterns from each well with those treated with positive control compounds. The primary hits were further validated by confocal microscope analyses of intracellular cholesterol distribution. Briefly, HUVEC were seeded in a Nunc Lab-Tek II 8-Chamber Slide (Thermo Fisher Scientific) and treated with hit compounds for 83h. The concentrations of each hit compound used for confocal microscope analysis were chosen based on their IC50 values in HUVEC. After fixation and staining with filipin, cells were washed with PBS, mounted with Immu-mount (Thermo Fisher Scientific), and observed under the Carl Zeiss LSM 710 confocal microscope (Carl Zeiss, Thornwood, NY).
2.4. Immunofluorescence imaging of endothelial cells
HUVEC were seeded in a Nunc Lab-Tek II 8-Chamber Slide and treated with compounds for indicated time points. Cells were fixed with 4% paraformaldehyde for 203min at room temperature and then permeabilized with 0.5% Triton X-100 (for protein immunostaining) or 0.2% saponin (for co-staining with filipin) for 103min prior to blocking in a blocking buffer (3% bovine serum albumin (BSA) in PBS containing 0.1% Tween-20) for 13h. Cells were incubated with primary antibodies, including anti-LAMP1, anti-PDI, anti-mTOR and anti-GM130 in the blocking buffer overnight at 4°C, followed by the incubation with secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 647 for 13h at room temperature. The cellular nuclei were stained with Hoechst 33342 (Thermo Fisher Scientific). Cells were washed with PBS, mounted with Immu-mount, and observed under a Carl Zeiss LSM 710 confocal microscope. For the analysis of LDL trafficking, HUVEC were pretreated with DMSO or CEP for 8 h, and then 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate-labeled LDL (DiI-LDL) (Thermo Fisher Scientific) was added to the cells. The incubation was continued for 1 h or 6 h before cells were fixed and co-stained with filipin or primary antibodies against LAMP1, PDI and mTOR.
2.5. NPC1 competitive binding assay
The itraconazole photo-affinity probe was synthesized and the NPC1 pull-down experiments in live cells were conducted as described previously [17]. Briefly, A549 (for endogenous NPC1) or 293T cells overexpressing Myc-NPC1 [18] were grown in 6-cm dishes until confluent. Cells were pretreated with competitors (CEP and other cholesterol trafficking inhibitors) or DMSO for 30 min prior to the addition of 200 nM itraconazole photo-affinity probe. After incubation with the probe for 1 h, cells were washed with ice-cold PBS and placed under an ultraviolet (UV) lamp (365 nm) for 3 min on ice. After UV irradiation, cells were lysed with 0.4% sodium dodecyl sulfate (SDS) and sonication. The itraconazole photo-affinity labeled NPC1 in the cell lysate was captured by a click reaction with biotin-azide (Click Chemistry Tools, Scottsdale, AZ). Total proteins in the lysate were isolated by protein precipitation with four volumes of cooled acetone and the isolated protein pellet was resuspended in PBS containing 0.2% SDS. Finally, the itraconazole photo-affinity labeled NPC1 was pulled-down by streptavidin beads (Thermo Fisher Scientific) and the NPC1 in the pellet was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot as previously described [18].
2.6. Live cell fluorescence measurement of lysosomal pH
HUVEC were grown in confocal dishes overnight and treated with DMSO or CEP for indicated time points. Cell were then treated with the LysoSensor™ Yellow/Blue DND-160 (Thermo Fisher Scientific) for 5 min prior to the confocal analysis of the ratiometric measurement of lysosomal pH with the fixed laser excitation at 405 nm and the dual emission at 460 (blue) and 540 (yellow) nm. The relative intensity ratio of the blue and yellow fluorescence was obtained at a single cell level using the ZEN lite image analysis software (Carl Zeiss).
2.7. Cell proliferation assay
HUVEC were seeded at 2×103 cells/well in 96-well plates and then incubated overnight. The cells were treated with compounds with or without the cholesterol (8 μg/ml) and methyl-β-cyclodextrin (10%) complex for 723h. Metabolically active cells were analyzed with the incubation with 10% AlamarBlue reagent (Thermo Fisher Scientific) for 23h. The fluorescence signal at the bottom of the plate was read with the SpectraMax M5 fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) with an excitation wavelength of 5603nm and an emission wavelength of 5903nm.
2.8. Wound healing cell migration assay
HUVEC were seeded in a 96-well ImageLock Microplate (Essen BioScience, Ann Arbor, MI) at the density of 1×104 cells/well and then incubated overnight. Cells were then treated with CEP with or without the cholesterol-cyclodextrin complex for 6 h before the wound area was created by the WoundMaker™ (Essen BioScience). The cell plate was placed into the IncuCyte ZOOM (Essen BioScience) and the cell image was scanned every 2 h for 24 h. The cell migration was quantified by IncuCyte ZOOM Software based on the relative wound density.
2.9. Endothelial cell tube formation assay
A 15-well μ-Slide angiogenesis chamber (ibidi, Planegg, Germany) was coated with 103μL Matrigel (BD Biosciences). HUVEC pretreated with CEP with or without the cholesterol-cyclodextrin complex for 6 h were harvested by trypsinization and seeded on the Matrigel-coated chamber at a density of 1.5×103 cells/well. After incubation in a CO2 incubator for 183h, cells were washed carefully with PBS and stained with Calcein-AM (23μM, Thermo Fisher Scientific). The fluorescence-labeled tubular networks of the cells were observed under the Axio Observer fluorescence microscope (Carl Zeiss). The tube formation (total segments lengths) was quantified using the Angiogenesis Analyzer for Image J [32] and plotted using the GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA).
2.10. Zebrafish angiogenesis assay
A transgenic zebrafish line Tg(fli1a:EGFP)y1 was used to assess in vivo angiogenesis as previously described [33]. Briefly, zebrafish embryos were generated by natural pairwise mating and cultured in embryo water (0.2 g/L Instant Ocean Salt) at 28°C. Dead or unfertilized embryos were discarded after 8 hours post-fertilization (hpf). Working dilution of CEP (100 and 200 μM in PBS containing 1% DMSO) or cholesterol-cyclodextrin complex (800 μg/mL cholesterol in 10% methyl-β-cyclodextrin) was directly injected into embryos with Nanoject II Auto-Nanoliter Injector (Drummond Scientific Company, Broomall, PA) with an injection volume of 4.6 nL. The embryos were dechorionated manually at 24 hpf and N-phenylthiourea (PTU) (Sigma-Aldrich) at a final concentration of 3 mg/mL was added into the embryos. The embryos were incubated for 72 h and observed the formation of subintestinal vessels (SIVs) under a Carl Zeiss LSM 710 confocal microscope. The zebrafish angiogenesis was quantified by counting the number of SIV branch points.
2.11. Tumor xenograft mice experiments
All animal procedures were approved by the Animal Research Ethics Committee of the University of Macau. A549 lung cancer cells suspended in Matrigel were implanted subcutaneously into NOD/SCID (non-obese diabetic/severe combined immune deficiency) mice (1×107 cells/mouse). After tumors were palpable, the mice were treated with vehicle (sterile saline containing 5% DMSO, 5% tween-80 and 5% polyethylene glycol-400, daily), CEP (25 and 50 mg/kg, daily) or cisplatin (5 mg/kg, once a week), or drug combination for 32 days via i.p. injection. MDA-MB-231 breast cancer cells were implanted subcutaneously into BALB/c nude mice (2×106 cells/mouse). After tumors were palpable, the mice were treated with CEP (50 mg/kg, daily) or cisplatin (5 mg/kg, once a week), or drug combination for 46 days. Tumor size was measured periodically by calipers and the tumor volume was determined by the ellipsoid formula (L×W2×π/6). Mice body weight was measured regularly to assess drug toxicity.
2.12. Western blots and immunofluorescence analysis of tumor tissues
Tumor samples were cut into pieces and placed in a pre-cooled 5 mL tube filled with ice-cold radio-immunoprecipitation assay (RIPA) buffer (about 100 μL lysis buffer per 100 mg tumor, 25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with Complete Protease Inhibitor Cocktail and PhosSTOP Phosphatase Inhibitor Cocktail (Roche Life Sciences, Indianapolis, IN). The tumors were then homogenized with Polytron® PT 1200 E Manual Disperser (Kinematica, Bohemia, NY) on ice and the lysate was transferred to precooled 1.5 mL tube for centrifugation at 12,000 rpm for 15 min at 4°C. The supernatants containing tissue proteins were collected and measured for protein concentration. Approximately each 40 μg of protein sample was run on a SDS-PAGE and analyzed for Western blots. For immunofluorescence analysis tumor samples were embedded in OCT (optimum cutting temperature) compound (Sakura Finetek, Alphen aan den Rijn, Netherlands) and sectioned into 10 μm with Leica CM3050 S Cryostat (Leica Biosystems, Wetzlar, Germany). The tumor sections were fixed in cold acetone at −20°C for 20 min, permeabilized with 0.5% Triton X-100 for 203min and washed with PBS prior to blocking in 3% BSA in PBS containing 0.1% Tween 20 for 13h. The tumor sections were then incubated with the primary antibodies, including anti-CD31 (blood vessel marker) and anti-Ki67 (cell proliferation marker) at 4°C overnight followed by incubation with Hoechst 33342 and secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 647 for 13h. The samples were washed with PBS, mounted with Immu-mount, and observed under a Carl Zeiss LSM 710 confocal microscope.
2.13. Statistical analysis
Dose-response curve and bar graphs were plotted with Prism 6 (GraphPad Software, La Jolla, CA). Statistical differences of the data between control and treatment groups were determined by two-sided Student’s t-test or One sample t-test. The P values less than .05 were considered significant.
3. Results
3.1. Screening and identification of cholesterol trafficking inhibitors in human endothelial cells
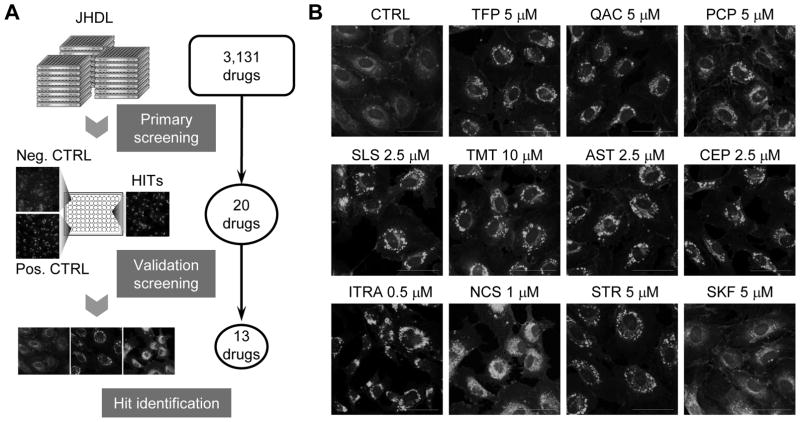
To identify cholesterol trafficking inhibitors, we screened the Johns Hopkins Drug Library (JHDL) consisting over 3,000 existing drugs we established previously [34]. Intracellular cholesterol in HUVEC was fluorescently labeled with filipin dye and the fluorescence images of intracellular cholesterol distribution were recorded and analyzed for abnormal cholesterol trafficking. Three known cholesterol trafficking inhibitors, including itraconazole [20], U18666A [35] and imipramine [20], were included in the library and were used as internal positive controls to identify hits. Through the primary and validation screenings, we identified 13 small molecule drugs as validated inhibitors of endothelial cholesterol trafficking (Fig. 1A and 1B). Based on structural properties, the hits were divided into four groups (Supplementary Fig. S1), including those that are structurally related to the selective estrogen receptor modulators (Class I), heterocyclic phenothiazine anti-psychotic drugs (Class II), steroidal alkaloid compounds (Class III) and others that are not structurally related each other (Class IV). Although all the 13 hits showed abnormal cholesterol distribution in endothelial cells, it was unclear whether the blockade of cholesterol trafficking would be a ‘causative effect’ or ‘passenger effect’ for the drug’s pharmacological activity. Therefore, we tested phenotypic reversal of the drug effects upon addition of cholesterol to identify true cholesterol trafficking inhibitors of which anti-cholesterol trafficking effect is the primary effect of the drug action. All the Class I and II drugs and 3 drugs from Class IV (CEP, sertraline and zolantidine) were likely the true cholesterol trafficking inhibitors as their anti-proliferative effects were reversed by cholesterol/cyclodextrin addition (Supplementary Fig. S2). From the structural aspect, Class I and II drugs have tricyclic rings and a nitrogen-containing side chain that are similar to the structural properties of the three positive control drugs, itraconazole, U18666A and imipramine. As unique small molecule structures often lead to a unique cellular target/mechanism of action, we were more interested in the Class IV drug CEP. CEP is an approved drug with a unique flexible cyclic chemical structure that does not appear in any of known cholesterol trafficking inhibitors. Therefore, we pursued CEP for mechanism of action in greater detail in the endothelial cells.
Figure 1.
Identification of cholesterol trafficking inhibitors in endothelial cells. (A) A schematic illustration of the screening procedure is shown. The John Hopkins Drug Library (JHDL) composed of 3,131 clinical drugs was used for screening to identify inhibitors of cholesterol trafficking in HUVEC. Finally, 13 hits were identified from primary and validation screenings. (B) HUVEC were treated with the hits for 8 h and observed under a confocal microscope after staining with filipin, a cholesterol tracer. CTRL, control; TFP, trifluoperazine; QAC, quinacrine; PCP, prochlorperazine; SLS, solasodine; TMT, tomatidine; ITRA, itraconazole; AST, astemizole; CEP, cepharanthine; NCS, niclosamide; STR, sertraline; SKF, zolantidine. Scale bar = 50 μm.
3.2. Effects of CEP on endothelial cell cholesterol distribution
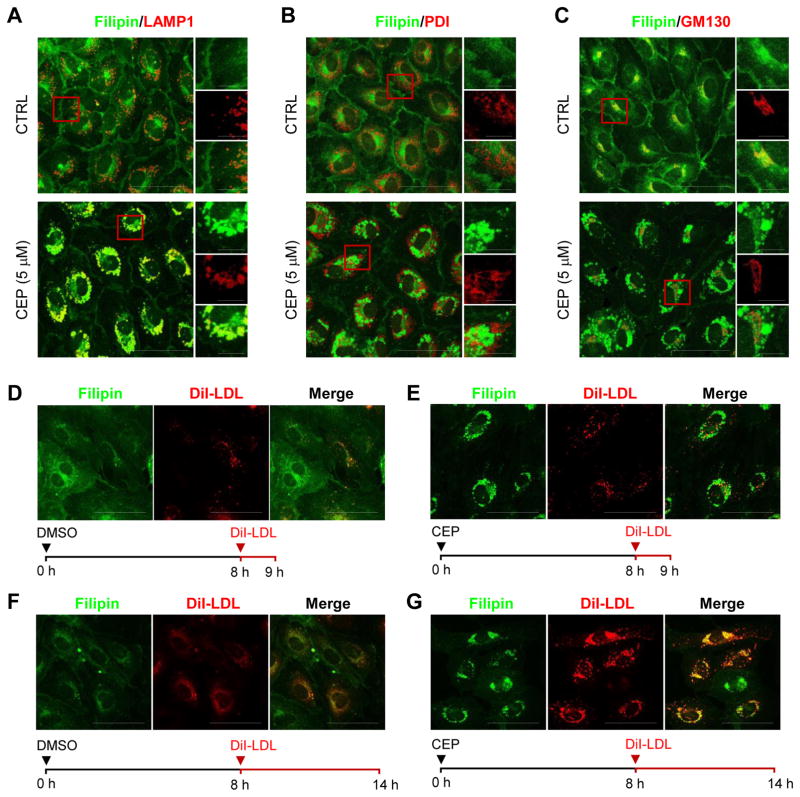
As CEP inhibited cholesterol trafficking causing an accumulation of intracellular cholesterol, we analyzed the subcellular location where cholesterol accumulated. HUVEC were treated with CEP and intracellular free cholesterol was fluorescently labeled with filipin. By co-immunostaining with LAMP1 (lysosomal-associated membrane protein 1, a marker of late endolysosomes), PDI (protein disulfide isomerase, a marker of the endoplasmic reticulum) or GM130 (cis-Golgi marker), we observed that CEP treatment induced a specific accumulation of cholesterol in late endolysosomes (Fig. 2A–C). The level of free cholesterol in the other cellular compartments, including the membrane and cytoplasm, was largely reduced.
Figure 2.
Effect of CEP on free cholesterol and LDL trafficking in endothelial cells. (A–C) HUVEC were treated with CEP (5 μM) or DMSO (CTRL or control) for 14 h and observed under a confocal microscope after immunostaining with filipin, LAMP1, a late endolysosome marker (A), PDI, an endoplasmic reticulum (ER) marker (B), and GM130, a Golgi marker (C). Scale bar = 50 μm. Images in the inlets (red square) were magnified and shown on the right side of each figure. Filipin is shown as green and each organelle marker is shown as red color. The bottom images are merged color images. Scale bar for the inlets = 10 μm. (D–G) HUVEC were treated with CEP (5 μM) or DMSO for 8 h and then the cells were incubated with DiI-LDL for additional 1 h (D and E) or 6 h (F and G), as indicated with the time frame shown at the bottom of each figure. Filipin is shown as green and DiI-LDL is shown as red color. Scale bar = 50 μm.
3.3. Effects of CEP on LDL trafficking
To explore the precise action point of CEP in cholesterol trafficking in endothelial cells, we next used a fluorescence-labeled LDL (DiI-LDL) and chased the LDL uptake and transport in HUVEC. Upon incubation, DiI-LDL was endocytosed and internalized (shown as punctae) in HUVEC within 1 hour (Fig. 2D). Pretreatment of HUVEC with CEP did not affect DiI-LDL endocytosis and internalization as shown in Figure 2E, while intracellular free cholesterol (pre-existing or de novo synthesized cholesterol shown by filipin) was largely accumulated in the late endolysosomes by the CEP pretreatment (Fig. 2E). After 6 hours of DiI-LDL incubation, most of DiI-LDL was located at the perinuclear region, but the fluorescence intensity became weaker and diffused over nearby area (Fig. 2F). This suggests that the LDL was finally transported into the endolysosomes and underwent degradation by lysosomal proteases. However in HUVEC pretreated with CEP, DiI-LDL fluorescence was heavily accumulated at the perinuclear region (Fig. 2G). The location of free cholesterol accumulation (filipin staining) and LDL accumulation (DiI-LDL punctae) was largely identical (Fig. 2G). These data suggest that the LDL was transported into the endolysosomes, but the degradation process was blocked in the cells treated with CEP. This was further confirmed with the subcellular organelle markers showing that DiI-LDL accumulation by CEP was preferentially colocalized with the LAMP1-positive late endolysosomes and was perfectly colocalized with mTOR-positive lysosomes (Fig. 3A–D). These data suggest that CEP acts at the endolysosomes to inhibit cholesterol trafficking in endothelial cells.
Figure 3.
Effect of CEP on NPC1 and lysosomal integrity. (A–D) HUVEC were pretreated with CEP (5 μM) or DMSO for 8 h prior to incubation with DiI-LDL for additional 6 h. The location of DiI-LDL accumulation was tested with confocal co-localization experiments with organelle markers, including LAMP1 for late endolysosomes (A), PDI for ER (B), mTOR for lysosomes (C) and GM130 for Golgi (D). Scale bar = 50 μm. Images in the inlets (red square) were magnified and shown on the right side of each figure. DiI-LDL is shown as red and each organelle marker is shown as green color. The bottom images are merged color images. Scale bar for the inlets = 10 μm. (E) 293T cells overexpressing myc-NPC1 and A549 cells (endogenous NPC1) were used for live-cell NPC1 competitive binding assays. NPC1 was pulled-down from the cells by itraconazole photo-affinity capture and the precipitated complex was subjected to Western blot (WB) analysis for NPC1. Input (1%) was used as an NPC1 Western blot control. ITRA, itraconazole; CEP, cepharanthine; AST, astemizole; PCP, prochlorperazine; QAC, quinacrine; STR, sertraline; TMX, tamoxifen; TFP, trifluoperazine. (F) HUVEC were treated with CEP (5 μM) or DMSO for 12 h and then immunostained with LAMP1 antibody (green) or Hoechst33342 (HO33342 for nuclei, blue). Scale bar = 50 μm. Images in the inlets (red square) were magnified and shown on the right upper corner of each figure. Scale bar for the inlets = 5 μm. (G–H) Lysosomal luminal pH was measured in HUVEC (control vs 5 μM CEP for 12 h) with a ratiometric pH indicator dye, LysoSensor Yellow/Blue DND-160. An increase in Blue/Yellow ratio represents an increase in the luminal pH of lysosomes (G). Scale bar = 10 μm. The ratio of the Blue/Yellow fluorescence intensity was calculated with Zen software and quantified (H). (I) HUVEC were treated with CEP (5 μM) in different time points and the lysosomal luminal pH was measured. The fold change of the Blue/Yellow ratio in CEP treated HUVEC to that in control is shown. *P < .05 and **P < .01 vs control.
3.4. Effects of CEP on NPC1, an endolysosomal membrane protein responsible for intracellular cholesterol trafficking
Endolysosomes are a central organelle for cholesterol trafficking as it mediates endosomal cholesterol transport and intracellular distribution of free cholesterol [6]. Once LDL is transported into the endolysosomes, cholesterol will be unesterified and released from LDL by the luminal enzymes, lysosomal acid lipase and NPC2. NPC1, an endolysosomal membrane protein, accepts the free cholesterol from NPC2 through its N-terminal cholesterol binding pocket, transfers cholesterol to the sterol-sensing domain (SSD) of the integral transmembrane portion and finally transports cholesterol out of the endolysosomes [7, 36]. Recently, two well-known cholesterol trafficking inhibitors, U18666A and itraconazole, have been found to directly bind to the SSD of NPC1 [18, 35]. The binding of the small molecules blocks the transfer of cholesterol to SSD of NPC1 and thus inhibits cholesterol transport out of endolysosomes, causing a heavy accumulation of free cholesterol in the organelle. Since we observed a high endolysosomal accumulation of free cholesterol by CEP, we wonder whether CEP inhibits the function of NPC1. We have previously developed a photoaffinity bioorthogonal probe of itraconazole that could successfully capture and pull-down NPC1 protein in living cells [18]. We used this itraconazole probe to test if CEP or other cholesterol trafficking inhibitors directly bind to the SSD of NPC1. Cultured 293T cells that overexpress myc-NPC1 were incubated with the itraconazole probe in the presence or absence of test compounds prior to the covalent linking of the probe to NPC1 by photoactivation and subsequent biotin-streptavidin pull down. Free itraconazole was used as a positive control compound for binding competition. As shown in Figure 3E, free itraconazole almost completely abrogated the binding of itraconazole probe to NPC1. Out of seven cholesterol trafficking inhibitors tested, only two compounds, including CEP and astemizole, significantly abrogated the binding of itraconazole probe to NPC1 (Fig. 3E). CEP and astemizole also showed a significant binding to the endogenous NPC1 in A549 cells. These data suggest that, like itraconazole, CEP directly binds to the SSD of NPC1 and inhibits endolysosomal cholesterol trafficking.
3.5. Effects of CEP on lysosomal function
Inhibition of the endolysosomal trafficking of free cholesterol by CEP can be explained by its binding to NPC1. However, it is unclear whether the accumulation of DiI-LDL in the endolysosomes is due to the inhibition of NPC1, because LDL degradation happens in the lumen of endolysosomes by lysosomal proteases. We have observed that, upon CEP treatment, LAMP1 or filipin staining always showed enlarged endolysosomes in endothelial cells. CEP treatment caused a greater than 5-fold increase in the size of LAMP1-positive endolysosomes (Fig. 3F). The enlargement of endolysosomes is not likely the result of cholesterol trafficking inhibition because no size change of endolysosomes was observed in the itraconazole treated cells (data not shown). We wondered whether the size change of the endolysosomes was associated with the change in the luminal pH of the organelle. A ratiometric measurement of endolysosomal pH showed that CEP significantly increased endolysosomal pH in endothelial cells (Fig. 3G and H). The increase in the endolysosomal pH occurred as early as 5 min of CEP treatment (Fig. 3I). Since the acidic environment in endolysosomes is critical for activities of lysosomal enzymes, the increase in the lysosomal pH by CEP would be detrimental for many acid hydrolases, such as lysosomal proteases and acid lipases. The effect of CEP on lysosomal size and pH was not likely due to the inhibition of NPC1 because the NPC1 inhibitor itraconazole did not change the endolysosomal pH (Supplementary Fig. S3). In addition, treatment of exogenous cholesterol had no effect on CEP-induced increase in lysosomal pH (Supplementary Fig. S3). These results suggest that CEP rapidly perturbs endolysosomal acidic environment, leading to an accumulation of LDL, which is independent event of its NPC1 inhibition and free cholesterol accumulation.
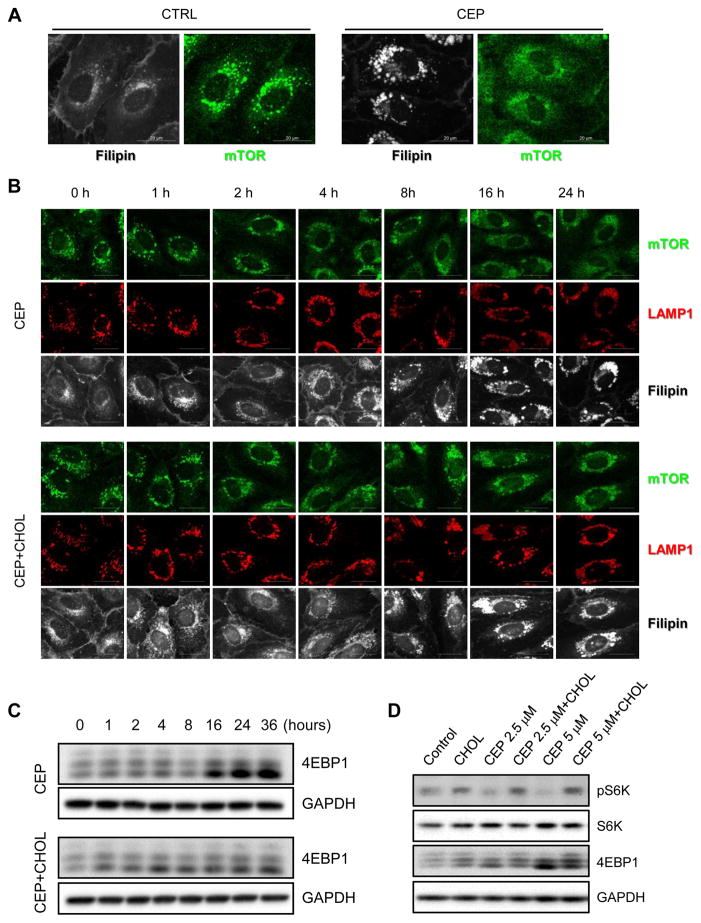
3.6. Effects of CEP and cholesterol on lysosomal localization of mTOR
It has been suggested that proper cholesterol trafficking is required for mTOR activation in endothelial cells, although it is unclear how cholesterol trafficking contributes to mTOR activation [20]. In order to explore the involvement of mTOR signaling in the cellular phenotype of the cholesterol trafficking inhibitor, we tested the effect of CEP on mTOR localization and signaling pathways. When active, mTOR is mainly located on the surface of lysosomes (shown as punctae in the control of Fig. 4A) in HUVEC. The treatment of cells with CEP caused diffused mTOR localization throughout the cytosol (Fig. 4A, CEP), suggesting that mTOR was dissociated from the lysosomes. We have tested this in further detail with a lysosomal marker and an addition of cholesterol at different time points. CEP treatment caused dissociation of mTOR from LAMP1-positive lysosomes over time, and this dissociation was correlated in time with the accumulation of free cholesterol in the lysosomes (Fig. 4B, CEP). However, co-treatment with cholesterol partially reversed CEP-induced mTOR dissociation from the lysosomes (Fig. 4B, CEP+Cholesterol). While cholesterol co-treatment did not alter the CEP-induced free cholesterol accumulation, it did replenish cholesterol in the membrane and cytoplasmic region where cholesterol was depleted upon CEP treatment (Fig. 4B). The dissociation of mTOR from the lysosomes led to the inhibition of mTOR downstream signaling pathways. eIF4E-binding protein 1 (4EBP1) is one of the well-known down-stream substrates of mTOR, which has multiple phosphorylation sites. Among the multiple bands of 4EBP1 in Western blots, the upper bands correspond to the hyper-phosphorylated form and the lower bands represent the hypo-phosphorylated form. The ratio of the hypo/hyper-phosphorylated form of 4EBP1 was increased upon CEP treatment over time and this effect was significantly reversed by co-treatment with cholesterol (Fig. 4C). CEP also showed a dose-dependent inhibition of the phosphorylation of S6 kinase, another substrate of mTOR, as well as 4EBP1, and this effect was reversed by a cholesterol treatment (Fig. 4D). These data suggested that CEP caused an accumulation of intracellular cholesterol in the lysosomes, leading to a depletion of plasma/organellar membrane cholesterol and, in turn, dissociation of mTOR from the lysosomes in a cholesterol-dependent manner. To further verify the depletion of cholesterol in the organelle membrane by CEP, we analyzed a localization of the membrane cholesterol sensor, SREBP1 (sterol regulatory element-binding protein 1). When cells have high level of cholesterol, SREBP1 is tightly retained in the ER membrane through sterol-mediated SCAP (SREBP cleavage-activating protein)-INSIG (ER resident protein) interaction [37]. When cells lack cholesterol, SREBP1-SCAP complex dissociates from the ER membrane and moves to the Golgi apparatus where SREBP1 matures and then is released to nucleus to activate transcription of genes involved in cholesterol uptake and biosynthesis [38]. We observed that SREBP1 was largely translocated into the nucleus upon CEP treatment and this effect was partially reversed by addition of cholesterol (Supplementary Fig. S4), again verifying that CEP depleted membranous cholesterol in endothelial cells.
Figure 4.
Effect of CEP on mTOR subcellular distribution and signaling. (A) HUVEC were treated with CEP or DMSO for 24 h and then were immunostained with filipin (gray) and mTOR antibody (green). mTOR in control HUVEC is shown as punctae. Scale bar = 20 μm. (B) HUVEC were treated with CEP (5 μM) in the presence or absence of cholesterol-cyclodextrin complex (CHOL) in different time points and then immunostained with mTOR antibody (green), LAMP1 antibody (red) and filipin (gray). Scale bar = 20 μm. (C) HUVEC were treated with CEP (5 μM) in the presence or absence of cholesterol-cyclodextrin complex (CHOL) in different time points and then analyzed with Western blots for an mTOR substrate, 4EBP1. (D) HUVEC were treated with CEP (2.5 and 5 μM) in the presence or absence of cholesterol-cyclodextrin complex (CHOL) for 24 h and then analyzed with Western blots for 4EBP1, phospho-S6 kinase (pS6K) and total S6 kinase (S6K). GAPDH was used as an internal loading control.
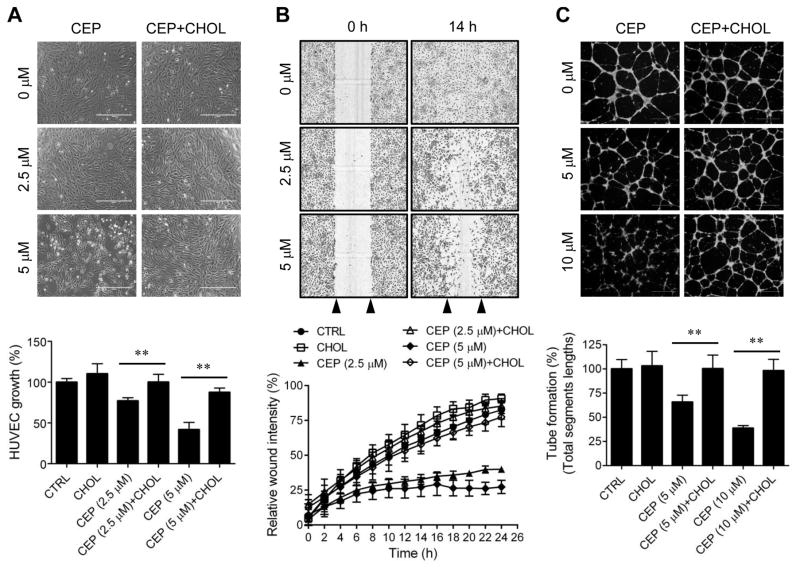
3.7. Effects of CEP and cholesterol on angiogenesis in vitro and in vivo
Since CEP inhibited mTOR signaling in endothelial cells in a cholesterol-dependent manner, we next examined the effect of CEP on angiogenesis and its reversal by exogenous cholesterol. For in vitro angiogenesis, we analyzed endothelial cell proliferation, migration and tube formation. CEP inhibited HUVEC proliferation in a dose- and time-dependent manner with an IC50 value of 3 μM at 72 h treatment (Supplementary Fig. S5). The inhibition of the cell proliferation by CEP was significantly reversed by cholesterol (Fig. 5A). Next, a real-time wound-healing assay was performed to measure HUVEC migration. Since CEP is likely a slow-acting inhibitor in endothelial cells based on mTOR inhibition and HUVEC proliferation, cells were pretreated with CEP for 6 h before subjected to the migration assay. CEP at 2.5 and 5 μM dose dependently inhibited HUVEC migration and this effect was also significantly reversed by cholesterol (Fig. 5B). In the tube formation assay we used CEP at 5 and 10 μM concentrations because this assay where endothelial cells are not attached on the bottom in general requires higher compound concentrations for the inhibition. Pretreatment of HUVEC with 5 and 10 μM CEP for 6 h significantly inhibited HUVEC tube formation on Matrigel and exogenous cholesterol successfully reversed the inhibition at both 5 and 10 μM CEP (Fig. 5C). Cyclodextrin was used as a carrier of cholesterol for intracellular delivery and we confirmed that cyclodextrin itself had no effect on any of CEP effects above (data not shown).
Figure 5.
Effects of CEP and cholesterol reversal on angiogenesis in vitro. (A) HUVEC were treated with indicated concentrations of CEP for 72 h in the presence or absence of cholesterol-cyclodextrin complex (CHOL) and the cell proliferation was assessed with AlamarBlue assay. Upper panel is the representative cell images and lower panel is the quantitation of the cell proliferation. Scale bar = 400 μm. (B) HUVEC were pretreated with indicated concentrations of CEP for 6 h in the presence or absence of cholesterol-cyclodextrin complex (CHOL) and the scratch-induced cell migration was measured in real-time using the IncuCyte ZOOM. Upper panel is the representative cell images and lower panel is the quantitation of the cell migration. Arrow heads indicate the wound region. Scale bar = 600 μm. (C) HUVEC were pretreated with indicated concentrations of CEP for 6 h in the presence or absence of cholesterol-cyclodextrin complex (CHOL) and the tube formation was done on Matrigel. Upper panel is the representative tube images (fluorescently stained with Calcein-AM) and lower panel is the quantitation of the tube formation with the Angiogenesis Analyzer for Image J. Scale bar = 600 μm. **P < .01 between two indicated groups.
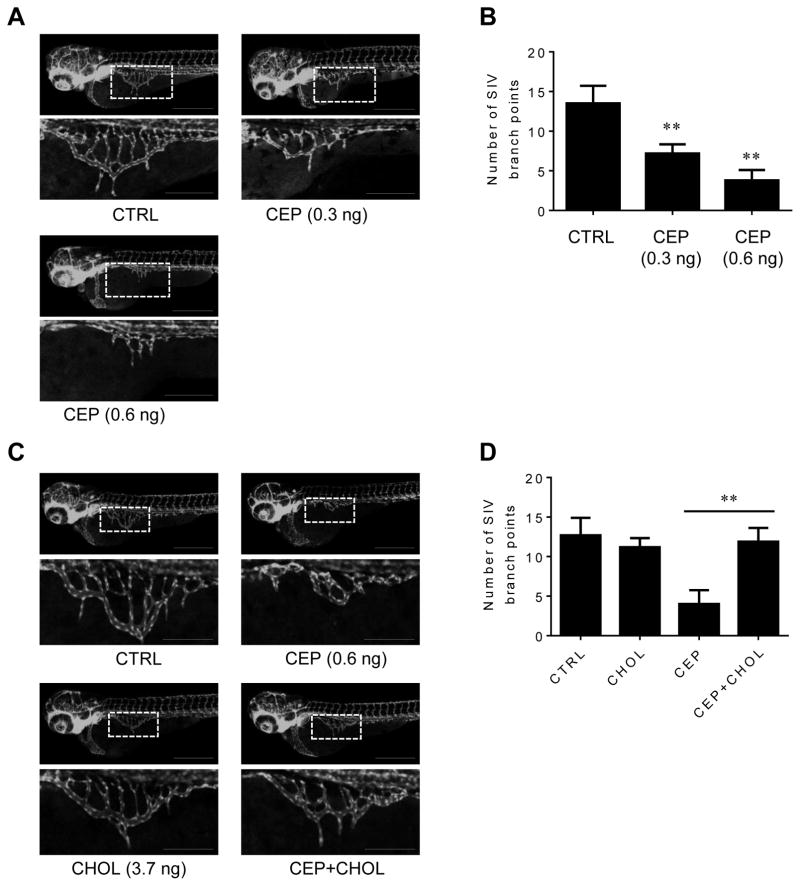
In order to further validate cholesterol-dependent anti-angiogenic activity of CEP, we employed a zebrafish angiogenesis model using the Tg(fli1a:EGFP)y1 transgenic line, which express EGFP in the vasculature under the control of the fli1 promoter, an endothelial cell marker in zebrafish embryos. During the zebrafish embryo development, intersegmental vessels (ISV) are formed at earlier stage (12–48 hpf) and, subsequently, subintestinal vessels (SIV) are formed at around 48–72 hpf [39, 40]. At 72 hpf, the SIV was developed as smooth basket-like structure (Fig. 6A) [40]. CEP significantly inhibited the SIV formation in the zebrafish embryos, while it had a minimal effect on ISV formation (Fig. 6A and B). This was likely because ISV formed at an earlier stage during development and CEP (injected at 8 hpf) acted slowly in inhibition of angiogenesis, hence inhibiting mainly SIV formation in zebrafish at 72 hpf. CEP did not show any apparent toxicity in zebrafish observed up to 96 hpf (data not shown). The co-injection of cholesterol together with CEP successfully reversed the anti-angiogenic activity of CEP (Fig. 6C and D). Cholesterol alone has no significant effect on the zebrafish angiogenesis and cyclodextrin itself did not affect CEP activity in zebrafish (data not shown). These data demonstrated that CEP inhibits angiogenesis in a cholesterol-dependent manner.
Figure 6.
Effects of CEP and cholesterol reversal on zebrafish angiogenesis. (A and C) Transgenic zebrafish (Tg(fli1a:EGFP)y1) embryos at 8 hpf were injected with working dilutions of CEP (final dose: 0.3 or 0.6 ng per injection each embryo) with or without cholesterol-cyclodextrin complex (CHOL, final cholesterol dose: 3.7 ng in 10% cyclodextrin per injection each embryo) and the SIV angiogenesis was observed at 72 hpf. Scale bar = 500 μm. Images in the inlets (white square) designating SIV were magnified and shown at the bottom of each figure. Scale bar for the inlets = 200 μm. (B and D) The zebrafish angiogenesis was quantified by counting the number of SIV branch points. **P < .01 vs control (B); **P < .01 between two indicated groups (D).
3.8. Effects of CEP alone or combination with a standard chemotherapy drug cisplatin on lung and breast cancer growth and tumor angiogenesis in mice
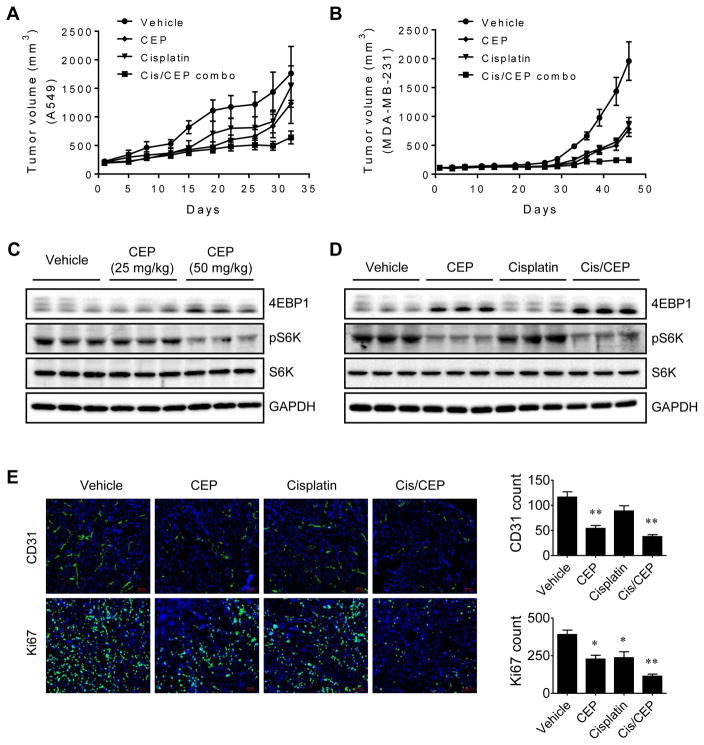
Since tumor growth is dependent on angiogenesis, we tested whether CEP alone or the combination of CEP with a standard chemotherapy drug has antitumor activity in mice tumor models. Two different human tumor xenograft models, A549 non-small cell lung cancer and MDA-MB-231, a triple negative breast cancer were employed to assess in vivo antitumor and antitumor-promoting effects of CEP. Daily administration of CEP alone inhibited the growth of tumors by 30% in A549 and 60% in MDA-MB-231 xenografts (Fig. 7A and B). A standard chemotherapy drug cisplatin administered once a week showed an antitumor activity similar to that seen in CEP treatment. Co-administration of CEP significantly enhanced the antitumor effect of cisplatin (64% inhibition vs 20% by cisplatin alone in A549 and 87% inhibition vs 56% by cisplatin alone in MDA-MB-231 xenografts). In this drug treatment condition, we did not observe any significant signs of toxicity or body weight changes in mice (Supplementary Fig. S6 and S7). To assess mTOR signaling in vivo, the tumors were isolated from the mice and analyzed for phosphorylation status of 4EBP1 and S6K. CEP dose dependently reduced the level of phosphorylation of 4EBP1 and S6K in the tumors (Fig. 7C). This effect was specific to CEP since cisplatin had no effect on mTOR signaling in the tumor (Fig. 7D). Lastly, we examined in vivo antiangiogenic activity of CEP in the tumor tissues. The tumor sections were processed for immunohistochemistry of a blood vessel marker CD31 and a cell proliferation marker Ki67. CEP significantly reduced the number of blood vessels in the tumor sections, whereas cisplatin did not show meaningful effect on tumor angiogenesis. In Ki67 staining, both CEP and cisplatin partially reduced the tumor cell proliferation, and the drug combination significantly reduced the tumor proliferation (Fig. 7E). These data suggested that CEP inhibited angiogenesis in mice, thereby blocking tumor growth.
Figure 7.
Antitumor and antitumor-promoting effect of CEP on lung and breast cancer xenografts. (A and B) Tumor volume measurements of A549 lung cancer (A) and MDA-MB-231 breast cancer (B) xenografts in mice are shown. CEP (50 mg/kg, daily), cisplatin (5 mg/kg, weekly), or combination of CEP and cisplatin (Cis/CEP combo) was administered intraperitoneally into the xenograft mice. (C and D) The tumor tissues from mice were isolated and analyzed with Western blots for 4EBP1 and S6K phosphorylation to assess the mTOR down-stream signaling in the tumor. (E) Tumor tissues were processed for immunofluorescence staining of blood vessel marker, CD31 and cell proliferation marker, Ki67. Representative immunofluorescence images are shown on the left. Scale bar = 100 μm. The number of blood vessels and Ki67-positive tumor cells were quantified and shown on the right. *P < .05 and **P < .01 vs vehicle control.
4. Discussion
In this study, we screened the Johns Hopkins Drug Library (JHDL) for cholesterol trafficking inhibitors in human endothelial cells and systematically analyzed the screening hits with structural features and cholesterol dependence of their pharmacological effects. Amongst the identified cholesterol trafficking inhibitors, we selected CEP for further mechanistic and pharmacological studies because of its structural uniqueness and future clinical considerations as it is a human approved drug with a proven safety record [28]. Subcellular distribution and trafficking analyses of free cholesterol and LDL showed that CEP inhibited cholesterol trafficking in the lysosomes with at least two distinct target points. First, CEP directly binds to the SSD of NPC1 on the lysosome membrane. U18666A, itraconazole and cholesterol bind to the SSD in a mutually exclusive manner, suggesting that these small molecules could compete with cholesterol for the binding to the SSD. With the itraconazole photo-affinity binding experiments, we observed that CEP and itraconazole shared a binding site within the SSD of NPC1 and the binding was mutually exclusive (Fig. 3E). Our results suggest that, like itraconazole and U18666A, CEP is likely to block the cholesterol transfer to the SSD of NPC1, causing lysosomal accumulation of free cholesterol. The second target point of CEP is the direct effect on lysosomal acidic environment. LDL trafficking experiments showed that CEP blocked LDL degradation in the lysosomes (Fig. 2D–G), which is likely to be attributable to the dysfunction of lysosomal acidification. Lysosomal proteases and lysosomal acid lipases responsible for LDL degradation and cholesterol release require an acidic environment for their activities. Rapid inhibition of lysosomal acidification by CEP (as early as 5 min) (Fig. 3I) suggests that, as an amphiphilic cation, it may directly enter the lysosomes and modulate pH, an effect similar to that seen with lysosomotropic agents [41]. This possibility has been proposed previously [42]. The dysfunction of lysosomal acidification and an increase in the organelle size is not likely due to the inhibition of NPC1 as another NPC1 inhibitor itraconazole did not change lysosomal size or pH in endothelial cells (Supplementary Fig. S3). Given the critical role of NPC1 and lysosomal microenvironment in cholesterol trafficking, we strongly believe that these two are causative targets for the inhibition of cholesterol trafficking by CEP. However, we cannot completely rule out the possible existence of another target of CEP as it is possible that inhibition of acidification of the lysosome by CEP might indirectly prevent the itraconazole probe from binding to NPC1.
The next question was how cholesterol trafficking affects mTOR signaling in endothelial cells. mTORC1 is mainly located on the surface of lysosomes when active. Nutrients, such as amino acids, stimulate a Rag-dependent lysosomal localization of mTOR [43]. When nutrient levels are low, mTOR is dissociated from the lysosomes and located in the cytoplasm, becoming inactive. In addition to nutrient sensing, mTOR was also suggested to be involved in sensing membrane cholesterol levels [20]. When cholesterol trafficking was inhibited by itraconazole, mTOR signaling became inactive in endothelial cells, and this effect was rescued by addition of exogenous cholesterol, although the mechanism behind the inhibition of mTOR signaling was elusive [20]. Similar to itraconazole, CEP dramatically reduced membrane and cytoplasmic cholesterol level, with a large accumulation of cholesterol in the lysosomes (Fig. 2A). In our mTOR localization experiments in normal endothelial cells, mTOR was mainly located on the lysosomes, shown as punctae. Upon CEP treatment, mTOR punctae were progressively diffused over the cytoplasm, suggesting that mTOR was dissociated from the lysosomes (Fig. 4A and B). At the time when mTOR was significantly dissociated from the lysosomes, the phosphorylation levels of S6K and 4EBP1 were reduced (Fig. 4C and D). Addition of exogenous cholesterol in the cells treated with CEP replenished the membrane and cytoplasmic cholesterol, which was shown with filipin staining (Fig. 4B). While the addition of cholesterol did not affect CEP-induced cholesterol accumulation in the lysosomes, it significantly reversed mTOR localization back to the lysosomes. The cholesterol reversal effect on mTOR localization was positively correlated with the reversal of S6K and 4EBP1 phosphorylation. These data suggest that: (i) inhibition of cholesterol trafficking depleted membrane cholesterol, including organelles and plasma membranes, and (ii) this effect abrogated surface localization of mTOR on the lysosomes. This data further support the idea that mTOR may play an important role in sensing membrane cholesterol.
mTOR signaling is a central signaling pathway that regulates protein synthesis, cell cycle progression, actin cytoskeleton formation, cell migration and angiogenic sprouting in endothelial cells, thus becoming an important drug target for angiogenesis inhibitors [44–46]. We observed that CEP inhibited endothelial growth, migration and tube formation in a cholesterol dependent manner, which was well correlated with its effects on mTOR signaling pathway (Fig. 5). Very recently, Paudel et al reported that CEP inhibited vascular smooth muscle cell proliferation and migration, which was accompanied by the inhibition of matrix metalloproteinase-9 (MMP-9) expression [47]. mTOR substrate proteins, S6K and 4EBP1 are known to regulate the expression and activity of MMP-9 during the cell migration [48]. mTOR signaling is also known to contribute to the cell migration by regulating dynamic actin cytoskeleton that drives membrane protrusion [49]. We observed that, like an mTOR kinase inhibitor torin-1, CEP blocked the formation of actin stress fiber in HUVEC by disrupting actin-paxillin crosslink (data not shown). These results suggest that inhibition of cell migration and angiogenesis by CEP is likely due to its effect on mTOR signaling. We further showed the cholesterol-dependent anti-angiogenic activity of CEP in a transgenic zebrafish angiogenesis model, demonstrating that the inhibition of cholesterol trafficking is a primary mechanism of anti-angiogenic action of CEP (Fig. 6). Finally, we showed that CEP alone or in combination with cisplatin inhibited the growth of tumor xenografts in mice. Along with the tumor reduction, mTOR signaling and tumor angiogenesis were significantly inhibited in the presence of CEP, suggesting that CEP is an in vivo inhibitor of mTOR signaling (Fig. 7).
CEP is an approved drug in Japan that has been widely used for the treatment of illnesses such as inflammation, venom-induced hemolysis, bronchial asthma and leukopenia induced by cancer chemotherapy [50]. Since its long-term use in Japan, CEP has received considerable attention from cancer researchers for its beneficial effects on cancer patients [51]. Kato and Suzumura reported that CEP could potentiate antitumor activity of vincristine and adriamycin in leukemia cells [52]. Subsequent exploratory clinical studies showed that the combination of CEP with vinblastine and adriamycin exhibited a marked antitumor effect in patients with metastatic renal cell carcinoma [53, 54]. In addition, following in vitro and preclinical studies of CEP on cancer further demonstrated a potential anticancer and anti-metastatic effect of CEP [29, 30]. However, the mechanism of action of CEP in its anticancer and anti-metastatic effect has not been fully elucidated. It has been proposed that CEP is a membrane interacting and stabilizing agent, and this effect may modulate the function of membrane proteins such as V-type ATPase on the lysosomes [55]. In support of this notion, CEP inhibited lysosome acidification in cancer cells and potentiated the effects of weak-base chemotherapy drugs by preventing sequestration of the drugs in the lysosomes [42]. In addition to its anticancer potentiating activity, CEP also showed several beneficial effects on cancer management. A retrospective analysis of the prostate cancer patients who underwent radiotherapy showed that CEP exhibited significant protective effect on the acute and late toxicity associated with the radiation therapy [28]. In patients with head and neck cancer who underwent chemo-radiotherapy, co-administration of CEP alleviated taste disorder and oral discomfort associated with the chemo-radiotherapy [56]. These data suggest that CEP is indeed a safe drug which may further improve clinical outcomes on cancer patients when combined with standard chemotherapy drugs.
In addition to CEP, some other drugs we found from the screening (Supplementary Fig. S1), such as an antihistamine drug astemizole [57, 58], an antipsychotic trifluoperazine [59], and an anti-protozoal quinacrine [60], are also approved drugs that have been reported to show anti-angiogenic and anticancer properties. Although precise mechanisms of these drugs for anti-angiogenic effects remain elusive, we believe that inhibition of cholesterol trafficking is likely the primary mechanism of action as evidenced by the cholesterol rescue effects. These results in turn strongly support the idea that cholesterol trafficking is a viable drug target for angiogenesis and cancer. As exemplified with CEP, approved cholesterol trafficking inhibitors, especially those non-cancer drugs, have a good safety profile and a great potential for prompt development as anti-angiogenic agents in clinical settings.
Supplementary Material
Highlights.
A phenotypic screen identified 13 existing drugs, including cepharanthine, as cholesterol trafficking inhibitors.
Cepharanthine inhibited lysosomal cholesterol trafficking by binding to NPC1 protein and increasing the lysosomal pH.
The blockade of cholesterol trafficking led to a cholesterol-dependent dissociation of mTOR from the lysosomes.
Cepharanthine inhibited angiogenesis in HUVEC and in zebrafish in a cholesterol-dependent manner.
Cepharanthine treatment enhanced the antitumor activity of cisplatin in lung and breast cancer xenografts in mice.
Acknowledgments
This work was supported by the Science and Technology Development Fund (FDCT) of Macau SAR (FDCT/119/2013/A3 and FDCT/024/2015/A1 to J.S.S); Multi-Year Research Grant of the University of Macau (MYRG2015-00181-FHS and MYRG2017-00176-FHS to J.S.S); NRF grant (2015K1A1A2028365 and 2015M3A9C4076321 to H.J.K); PhRMA Foundation Fellowship in Pharmacology/Toxicology (to S.A.H); National Cancer Institute Grant (R01CA184103 to J.O.L); the Flight Attendant Medical Research Institute (to J.O.L); Prostate Cancer Foundation (to J.O.L); and the Johns Hopkins Institute for Clinical and Translational Research, which is funded in part by Grant UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DW. Cholesterol biosynthesis and metabolism. Cardiovasc Drugs Ther. 1992;6:103–110. doi: 10.1007/BF00054556. [DOI] [PubMed] [Google Scholar]
- 6.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peake KB, Vance JE. Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett. 2010;584:2731–2739. doi: 10.1016/j.febslet.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 9.van Hinsbergh VW, Havekes L, Emeis JJ, van Corven E, Scheffer M. Low density lipoprotein metabolism by endothelial cells from human umbilical cord arteries and veins. Arteriosclerosis. 1983;3:547–559. doi: 10.1161/01.atv.3.6.547. [DOI] [PubMed] [Google Scholar]
- 10.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 11.Feleszko W, Balkowiec EZ, Sieberth E, Marczak M, Dabrowska A, Giermasz A, Czajka A, Jakobisiak M. Lovastatin and tumor necrosis factor-alpha exhibit potentiated antitumor effects against Ha-ras-transformed murine tumor via inhibition of tumor-induced angiogenesis. Int J Cancer. 1999;81:560–567. doi: 10.1002/(sici)1097-0215(19990517)81:4<560::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Dulak J, Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–594. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noghero A, Perino A, Seano G, Saglio E, Lo Sasso G, Veglio F, Primo L, Hirsch E, Bussolino F, Morello F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- 14.Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, Wiesner P, Taleb A, Deer E, Pattison J, Torres-Vazquez J, Li AC, Miller YI. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174:1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 17.Head SA, Shi W, Zhao L, Gorshkov K, Pasunooti K, Chen Y, Deng Z, Li RJ, Shim JS, Tan W, Hartung T, Zhang J, Zhao Y, Colombini M, Liu JO. Antifungal drug itraconazole targets VDAC1 to modulate the AMPK/mTOR signaling axis in endothelial cells. Proc Natl Acad Sci U S A. 2015;112:E7276–7285. doi: 10.1073/pnas.1512867112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Head SA, Shi WQ, Yang EJ, Nacev BA, Hong SY, Pasunooti KK, Li RJ, Shim JS, Liu JO. Simultaneous targeting of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition of mTOR signaling and angiogenesis. ACS Chem Biol. 2017;12:174–182. doi: 10.1021/acschembio.6b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim JS, Li RJ, Lv J, Head SA, Yang EJ, Liu JO. Inhibition of angiogenesis by selective estrogen receptor modulators through blockade of cholesterol trafficking rather than estrogen receptor antagonism. Cancer Lett. 2015;362:106–115. doi: 10.1016/j.canlet.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci U S A. 2010;107:4764–4769. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudin CM, Brahmer JR, Juergens RA, Hann CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P, Liu JO. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2013;8:619–623. doi: 10.1097/JTO.0b013e31828c3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, Tang JY. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 24.Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS, Zhao M, Mendonca J, Kachhap SK, Rudek MA, Carducci MA. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18:163–173. doi: 10.1634/theoncologist.2012-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014;10:654–663. doi: 10.7150/ijbs.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv J, Shim JS. Existing drugs and their application in drug discovery targeting cancer stem cells. Arch Pharm Res. 2015;38:1617–1626. doi: 10.1007/s12272-015-0628-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Hu G, Wang C, Xu H, Chen X, Qian A. Cepharanthine, an alkaloid from Stephania cepharantha Hayata, inhibits the inflammatory response in the RAW264.7 cell and mouse models. Inflammation. 2014;37:235–246. doi: 10.1007/s10753-013-9734-8. [DOI] [PubMed] [Google Scholar]
- 28.Nomoto S, Imada H, Ohguri T, Yahara K, Kato F, Morioka T, Korogi Y. Effect of Cepharanthin in preventing radiation induced normal tissue damage in prostate cancer. Gan To Kagaku Ryoho. 2004;31:1063–1066. [PubMed] [Google Scholar]
- 29.Harada K, Ferdous T, Itashiki Y, Takii M, Mano T, Mori Y, Ueyama Y. Cepharanthine inhibits angiogenesis and tumorigenicity of human oral squamous cell carcinoma cells by suppressing expression of vascular endothelial growth factor and interleukin-8. Int J Oncol. 2009;35:1025–1035. doi: 10.3892/ijo_00000417. [DOI] [PubMed] [Google Scholar]
- 30.Uthaisar K, Seubwai W, Srikoon P, Vaeteewoottacharn K, Sawanyawisuth K, Okada S, Wongkham S. Cepharanthine suppresses metastatic potential of human cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 2012;13(Suppl):149–154. [PubMed] [Google Scholar]
- 31.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Tong Y, Han XQ, Kwok HF, Yue GG, Lau CB, Ge W. Anti-angiogenic activity of Herba Epimedii on zebrafish embryos in vivo and HUVECs in vitro. Phytother Res. 2013;27:1368–1375. doi: 10.1002/ptr.4881. [DOI] [PubMed] [Google Scholar]
- 34.Shim JS, Rao R, Beebe K, Neckers L, Han I, Nahta R, Liu JO. Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir. J Natl Cancer Inst. 2012;104:1576–1590. doi: 10.1093/jnci/djs396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu FR, Liang QR, Abi-Mosleh L, Das A, De Brabander JK, Goldstein JL, Brown MS. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife. 2015;4:e12177. doi: 10.7554/eLife.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Wang J, Coutavas E, Shi H, Hao Q, Blobel G. Structure of human Niemann-Pick C1 protein. Proc Natl Acad Sci U S A. 2016;113:8212–8217. doi: 10.1073/pnas.1607795113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: Sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama C, Wang XD, Briggs MR, Admon A, Wu J, Hua XX, Goldstein JL, Brown MS. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 39.Yue GG, Lee JK, Kwok HF, Cheng L, Wong EC, Jiang L, Yu H, Leung HW, Wong YL, Leung PC, Fung KP, Lau CB. Novel PI3K/AKT targeting anti-angiogenic activities of 4-vinylphenol, a new therapeutic potential of a well-known styrene metabolite. Sci Rep. 2015;5:11149. doi: 10.1038/srep11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He ZH, Ge W, Yue GG, Lau CB, He MF, But PP. Anti-angiogenic effects of the fruit of Alpinia oxyphylla. J Ethnopharmacol. 2010;132:443–449. doi: 10.1016/j.jep.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Villamil Giraldo AM, Appelqvist H, Ederth T, Ollinger K. Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem Soc Trans. 2014;42:1460–1464. doi: 10.1042/BST20140145. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda R, Che XF, Yamaguchi T, Ushiyama M, Zhang CL, Okumura H, Takeda Y, Shibayama Y, Nakamura K, Jeung HC, Furukawa T, Sumizawa T, Haraguchi M, Akiyama S, Yamada K. Cepharanthine potently enhances the sensitivity of anticancer agents in K562 cells. Cancer Sci. 2005;96:372–376. doi: 10.1111/j.1349-7006.2005.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 45.Mead H, Zeremski M, Guba M. mTOR Signaling in Angiogenesis. Cancer Drug Discov Dev. 2010:49–74. [Google Scholar]
- 46.Farhan MA, Carmine-Simmen K, Lewis JD, Moore RB, Murray AG. Endothelial Cell mTOR Complex-2 Regulates Sprouting Angiogenesis. PLoS One. 2015;10:e0135245. doi: 10.1371/journal.pone.0135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paudel KR, Karki R, Kim DW. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol In Vitro. 2016;34:16–25. doi: 10.1016/j.tiv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Parent CA. TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito R, Tsuchiya S, Ishizuka T, Fueki N, Ezawa K, Minato K, Nakano H, Takise A, Kurihara M, Fueki R. Clinical effects of cepharanthin (Ceph.) on leukopenia by chemotherapy in lung cancer patients. Nihon Gan Chiryo Gakkai Shi. 1989;24:2587–2593. [PubMed] [Google Scholar]
- 51.Rogosnitzky M, Danks R. Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions. Pharmacol Rep. 2011;63:337–347. doi: 10.1016/s1734-1140(11)70500-x. [DOI] [PubMed] [Google Scholar]
- 52.Kato T, Suzumura Y. Potentiation of antitumor activity of vincristine by the biscoclaurine alkaloid cepharanthine. J Natl Cancer Inst. 1987;79:527–532. [PubMed] [Google Scholar]
- 53.Kakehi Y, Yoshida O, Segawa T, Kanematsu A, Hiura M, Shichiri Y, Arai Y. Intraarterial chemotherapy for metastatic renal cell carcinomas: combination with MDR-overcoming agents. Hinyokika Kiyo. 1994;40:925–929. [PubMed] [Google Scholar]
- 54.Tsunemori H, Kitamura Y, Taniguchi S, Okazoe H, Taoka R, Inui M, Sugimoto M, Kakehi Y. Experience using intra-arterial chemotherapy in combination with MDR-reversing agent for bone and soft-tissue metastases from renal cell carcinoma. Nishi Nihon Hinyokika. 2008;70:245–249. [Google Scholar]
- 55.Shinozawa S, Araki Y. Comparative studies on the chemical modifications of Ehrlich ascites tumor cell membranes by hydrophobic drugs (cepharanthine, papaverine and cholesterol) Nihon Yakurigaku Zasshi. 1979;75:207–214. doi: 10.1254/fpj.75.207. [DOI] [PubMed] [Google Scholar]
- 56.Shimazu R, Tanaka G, Tomiyama R, Kuratomi Y, Inokuchi A. Cepharanthin effect on radiation-induced xerostomia and taste disorder in patients with head and neck cancer. Nihon Jibiinkoka Gakkai Kaiho. 2009;112:648–655. doi: 10.3950/jibiinkoka.112.648. [DOI] [PubMed] [Google Scholar]
- 57.Downie BR, Sanchez A, Knotgen H, Contreras-Jurado C, Gymnopoulos M, Weber C, Stuhmer W, Pardo LA. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem. 2008;283:36234–36240. doi: 10.1074/jbc.M801830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Quiroz J, Camacho J. Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agent Med Chem. 2011;11:307–314. doi: 10.2174/187152011795347513. [DOI] [PubMed] [Google Scholar]
- 59.Pulkoski-Gross A, Li J, Zheng C, Li YY, Ouyang NT, Rigas B, Zucker S, Cao J. Repurposing the antipsychotic trifluoperazine as an antimetastasis agent. Mol Pharmacol. 2015;87:501–512. doi: 10.1124/mol.114.096941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobo MR, Green SC, Schabel MC, Gillespie GY, Woltjer RL, Pike MM. Quinacrine synergistically enhances the antivascular and antitumor efficacy of cediranib in intracranial mouse glioma. Neuro Oncol. 2013;15:1673–1683. doi: 10.1093/neuonc/not119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.