Abstract
Endometrial carcinoma is a malignant tumor of the female genital tract. This study has been performed to evaluate the chemopreventive efficacy of vanillic acid (a bio flavonoid) on endometrial carcinoma (EC) by assessing the levels of thiobarbituric acid reactive substances (TBARS), lipid hydroperoxides (LOOH), cytochrome P450, antioxidants-superoxide dismutase (SOD), catalase (CAT), glutathione peroxides (GPx), reduced glutathione (GSH), vitamins C and E, matrix metalloproteinases (MMP-2 and 9) and cell cycle check point protein (cyclin D1) in N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) induced carcinogenic rats. EC provoked by intravaginal detention of MNNG (150 mg/kg b.w. for 90 days), lead to enhancement of the levels of TBARS, LOOH, cytochrome P450, and decrement in the levels of antioxidants (SOD, CAT, GPx, GSH, vitamins C and E) and upregulated expression of MMP-2 and 9 and cyclin D1 (by western blot analysis). The treatment of vanillic acid (100 mg/kg b.w.) to MNNG treated rats (1) normalized the histopathological alterations, (2) reduced the levels of TBARS, LOOH and cytochrome P450 (3) increased the levels of antioxidants (SOD, CAT, GPx, GSH, vitamins C and E) in plasma and uterus and (4) down regulated the expression of MMP-2, 9 and cyclin D1. The effect of vanillic acid is more predominant in pre-treatment group than co-treated rats. Our results designate that vanillic acid inhibits the EC by elevating antioxidants and by regulating the levels of metalloproteinase and cell cycle check point protein.
Keywords: Endometrial cancer, MNNG, Vanillic acid, MMP-2, MMP-9, Cyclin D1
Introduction
Endometrial carcinoma is the fourth most common gynecological cancer among women globally [1]. Understanding the roles of genetic and epigenetic processes associated with the development of this disease and evaluating the protective influence of phytochemicals with lower side effects is essential. In addition, little is recognized on molecular level variations that take place during endometrial carcinogenesis, for instance the actions of cell cycle regulating genes and apoptotic markers [2].
MNNG is a powerful and directly acting carcinogen that provokes tumor growth at the site of administration; it undergoes hydrolytic disintegration initially and alkylating moieties are released from MNNG which could bind with DNA. MNNG is metabolized by GSH and GSH transferase to produce S-nitrosyl-glutathione (GSNO) which mediates cytotoxic effects. MNNG also forms DNA adducts-normally, N7- and O6-methylguanine, which readily lead to mutations. However, O6-methylguanine-DNA methyltransferase (MGMT) could inhibit the toxic and mutagenic effects of MNNG. The oxidative stress is elevated and is connected to the pathogenesis of oxidative damage mediated tumorigenesis [3]. Antioxidant has an indirect action on animal carcinogenesis which is involved by both the initiation and promotion phases. Some antioxidant enzymes are implicated in key pathways that control the cell division and proliferation, platelet aggregation, detoxification, and inflammatory and immune responses [4]. Phase I enzyme is involved in the deactivation of carcinogen. In this reaction, hydroxylation occurs in the procarcinogens and they are catalyzed by microsomal cytochrome P450 dependant monooxygenases to form strong electrophiles which are able to interact with cellular nucleophiles such as DNA to form an additional compound which results in mutagenesis and neoplastic transformation [5].
Matrix metalloproteinases (MMPs) have been strongly implicated in the acquisition of invasive and metastatic stages. In stromal cells the MMPs are synthesized and are exclusively produced by cancer cells, and play key roles in the process of invasion and metastasis [6]. There are numerous studies which showed the association of polymorphisms in MMPs with malignant diseases. Cyclin D1 is an oncogene which promotes cell cycle progression through phosphorylation of the retinoblastoma protein and also prevents apoptosis in the cytoplasm which has been recognized as a potential therapeutic target in cancer [7].
There are more than 4000 naturally occurring flavonoids described so far. Due to their antioxidant properties in vitro and their inhibitory role on various stages of tumor growth in animal studies, flavonoids predominantly contribute to the protective effects of vegetables and fruits [8]. The antioxidant properties of flavonoids may protect tissues against oxygen derived free radicals and lipid peroxidation. Most flavonoids are effective free radical scavengers and exert protective action against cancer. Vanillic acid (oxidized form of vanillin) is abundantly present in vanilla beans, Angelica sinensis (a Chinese medicinal plant) and green tea. Vanillic acid exhibits numerous pharmacological actions, for instance, inhibiting snake venom activity [9], preventing carcinogenesis [10], inflammation and promoting apoptosis [11]. The aim of this study is to investigate the chemopreventive efficacy of vanillic acid against the MNNG induced endometrial carcinoma in rats on the levels of lipid peroxidation products, antioxidants, metalloproteinase and cell cycle check point proteins.
Materials and Methods
Animals
Albino female (185–215 g) Wistar rats were acquired from the Central Animal House, Faculty of Medicine, Annamalai University, Tamil Nadu, India and kept at 26 ± 2 °C with 12 h light 12 h dark cycles. Commercially available pellet diet was given. This diet was fed to the rats during the experimental period and water was given ad libitum. Investigations were performed conferring to the ethical norms of the Institutional Animal Ethics Committee of Annamalai University (Approval No. 981, dated 07.02.2013). The rats were acclimatized for a week before starting the experiments.
Chemicals
Vanillic acid, thiobarbituric acid, nitrobluetetrazolium, phenazinemethosulphate glutathione and N-methyl-N′-nitro-N-nitrosoguanidine were procured from Sigma Chemical Co., St. Louis, MO, USA. Other chemicals with analytical grade were obtained from S.D Fine chemicals, Mumbai or Himedia laboratories Pvt. Ltd., Mumbai, India.
Experimental Protocol
After the acclimatization period (14 days), rats were separated into five groups. Group I rats served as controls. Rats in group II were given intravaginal detention of absorbent cottons dipped with MNNG (150 mg in 0.2 ml of olive oil) twice a week for 3 months (Fig. 1). Group III rats were administered with vanillic acid (100 mg/kg b.w.), orally for 3 months. Group IV rats were given with vanillic acid similar to group III starting one week before MNNG treatment and continued till the cessation of MNNG treatment. Group V rats received VA along with MNNG throughout the experimental period (90 days).
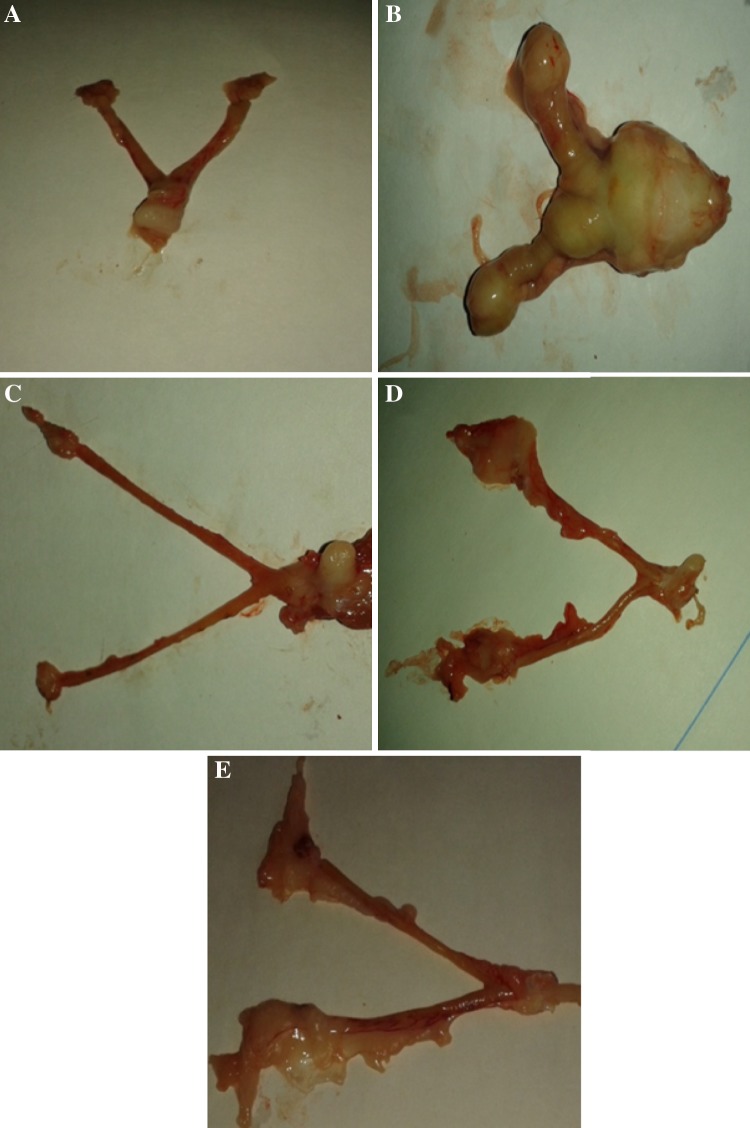
Fig. 1.
Effect of vanillic acid and MNNG on morphological features of rat uterus. Uterus of control and VA alone supplemented rats shows no evidence of tumour (a and c). Uterus of MNNG and MNNG + VA (100 mg/kg b.w.) pre and co-treatment rats show varying degrees of tumours incidence. Rats supplemented with VA (100 mg/kg b.w.) throughout the study period shows no macroscopically evident tumours
After 90 days, animals were sacrificed by cervical dislocation under anesthesia (treatment with ketamine hydrochloride i.p., 30 mg/kg b.w.), after overnight fast. Subsequently, the plasma and tissues (uterus) were collected. The uterus was dissected out and cleaned by saline and sliced into pieces, homogenized with phosphate buffer saline (PBS) and centrifuged at 1300×g for 15 min at 5 °C. Biochemical estimations were performed in the supernatant collected.
Histopathology and Lipid Peroxidation Markers
For histopathological examination, uterus tissue dissected from all groups were washed with saline and then fixed in 10 % neutral buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (H&E) and observed under a trinocular microscope. Plasma TBARS was estimated [12] by their action with thiobarbituric acid (TBA) in acidic conditions to form a pink-colored chromophore, which has absorbance peak at 530 nm. The estimation of lipid hydroperoxides (HP) in plasma and tissue was performed [13]. In this method, reaction of ferrous ion in acidic conditions forms xylenol orange (a chromophore), which was read at 560 nm.
Cytochrome P450
Cytochrome P450 was assayed [14]. To the mixture containing 1.0 ml of buffer, 0.1 ml of tissue homogenate, a few milligrams of solid sodium dithionate were added. CO was gently bubbled for approximately 1 min and the samples were read at 400 nm.
Antioxidants
The activity of superoxide dismutase (SOD) in the plasma and tissue was measured [15]. Superoxide radical reacts with nitroblue tetrazolium in condensed nicotinamide adenine dinucleotide and forms formazan blue product. SOD arrests the generation of formazan blue. The strength of the color is proportionate to the action of the enzyme which was read at 560 nm. The action of catalase in plasma and tissue was assayed [16]. In this method, dichromate in acetic acid forms perchromic acid which is converted to chromic acetate when heated with hydrogen peroxide. The chromic acetate formed was read at 620 nm. A reduced glutathione (GSH) level in plasma and tissue was estimated [17]. This method is established by the formation of yellow color, owing to the reaction of dithionitro benzoic acid with mixtures having sulfhydryl clutches which was read at 412 nm. Glutathione peroxidase (GPx) action was measured. By addition of sample, the enzyme reacts with H2O2 and GSH and color was measured [18]. Plasma vitamin C was determined [19]. Plasma α-tocopherol was estimated [20] and the method is based on the conversion of ferric ions to ferrous ions by α-tocopherol and the development of a red-colored color with 2,2′-dipyridyl read at 520 nm.
Western Blot Analysis
The expression of MMP-2, 9 and cyclin D1 in uterus was investigated by western blotting. The uterus tissue sections were homogenized in chilled RIPA buffer [Triton (1 %), SDS (0.1 %), 0.5 % deoxycholate (0.5 %), EDTA (1 mmol), Tris (20 mM, pH 7.5), NaCl (150 mmol), NaF (10 mmol), and 0.1 mmol/l phenylmethylsulfonyl fluoride (PMSF, 0.1 mmol)] and centrifuged at 14,000 rpm for 10 min at 5 °C. Samples encompassing 50 µg of proteins were electrophoresed on a 10 % sodium dodecyl sulfate–polyacrylamide gel. The resolved proteins were blot transferred on to PVDF membrane (Millipore). The membranes were kept with blocking buffer containing BSA (5 %) for 2.5 h to diminish non-specific binding. The membranes were then incubated with primary antibodies [in Tris-buffered saline and Tween-20 (0.04 %, TBST)], recognizing MMP-2, 9, cyclin D1 and β-actin (1:1000 dilution), in a quaking platform overnight at 5 °C. Then, the membranes were incubated with the related secondary antibodies (anti-rat IgG conjugated to horseradish peroxidase) for 2 h. The membranes were washed thrice with TBST for 20 min. The protein bands were visualized by an enhanced chemiluminescence method using an ECL kit (GenScript ECL kit, USA), scanned and analysed by Image J software (Bethesda, USA).
Statistical Analysis
Data were examined by one-way analysis of variance (ANOVA) and a considerable variation between the treatment groups were assessed by Duncan’s Multiple Range Test (DMRT). A p value of <0.05 was considered significant (SPSS 12.0 software package, Tokyo, Japan).
Results
Body Weight
Decreased body weight in MNNG treated rats was noticed as compared with control group (Table 1). After the administration of vanillic acid (only) the body weight was significantly increased. Growth rate (the variation between initial and final body weight divided by the total treatment time) was significantly improved in group II compared with groups IV and V. In control and vanillic acid (only) treated group tumors were not observed. In the case of animals given MNNG (group II), the tumour incidence was 100 %. During pre-treatment of vanillic acid to MNNG administered rats (group IV) the tumour incidence was decreased to 30 % and is decreased to 50 % in co-treated rats.
Table 1.
Effect of vanillic acid on body weight and tumour incidence in normal and MNNG treated rats
| Groups | Body weight (g) | Tumor incidence | |||
|---|---|---|---|---|---|
| Initial | Final | No. of animals | No. of animals bearing tumor | Average | |
| Normal | 175.03 ± 8.75a | 193.18 ± 13.5c | 6 | – | 0 |
| MNNG | 190.32 ± 15.2b | 160.26 ± 8.01a | 6 | 6 | 100 % |
| VA | 167.42 ± 10.02a | 185.42 ± 12.97b | 6 | – | 0 |
| VA + MNNG (pre-treatment) | 190.32 ± 15.2b | 180.35 ± 10.82b | 6 | 1 | 30 % |
| MNNG + VA (co-treatment) | 200.14 ± 12.02c | 170.24 ± 11.91d | 6 | 3 | 50 % |
Values are means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT)
Histological Observation of Uterus
The occurrence and absence of tumors in different groups are shown (Fig. 1). The uterus in the control rat showed normal glandular structure. The MNNG induced rats showed irregular proliferation of atypical glands in the endometrium and tumour cell invasion. Vanillic acid (only) treated rats showed normal glandular structure. The MNNG rats treated with vanillic acid (pre-treatment) showed proliferation of atypical glands found in the endometrium, without invasion into the muscularis. The tumour bearing rats treated with vanillic acid (co-treatment) showed the diffused proliferation of atypical glands in the endometrium (Fig. 2).
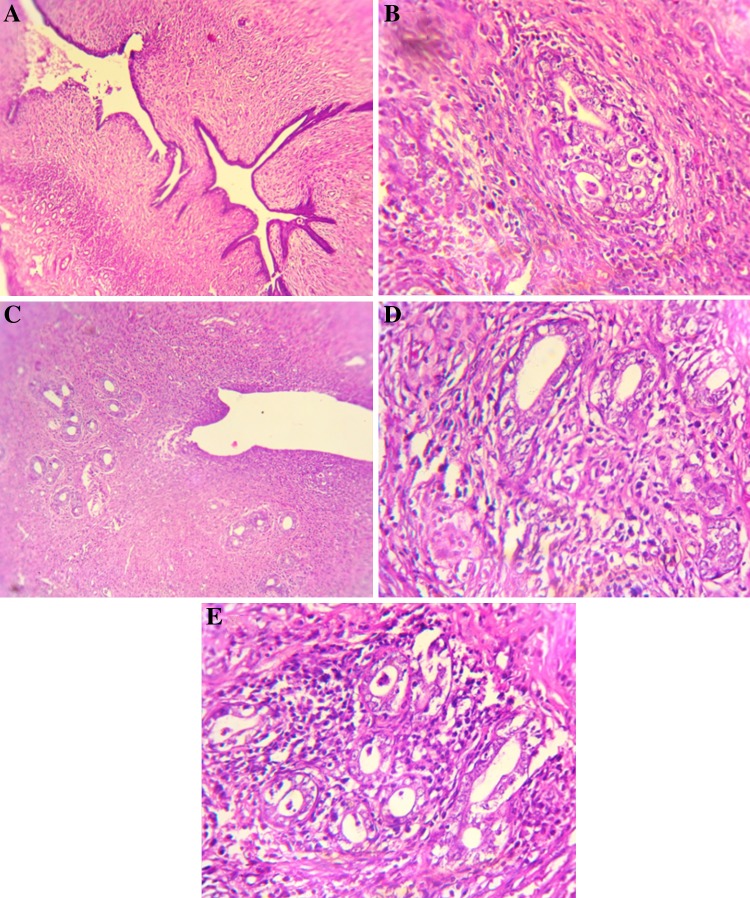
Fig. 2.
Histopathological changes in endometrium haematoxylin and eosin (20X). a Control: Normal glandular structure. b MNNG: Irregular proliferation of atypical glands in the endometrium and tumor cell invasion, multiple groups of adenocarcinoma. c VA: Showing normal glandular structure. d Pre-treatment (VA + MNNG): Proliferation of atypical glands is found in the endometrium without invasion into the muscularis. e Co-treatment (MNNG + VA): Diffuse proliferation of atypical glands in the endometrium
Lipid Peroxidation Products and Cytochrome P450
Levels of lipid peroxidation products and cytochrome P450 in plasma were significantly elevated in MNNG induced rats, whereas, the levels of lipid peroxidation in uterus is decreased. Oral treatment of vanillic acid to MNNG treated (pre and co-treatment) animals considerably diminished the levels of TBARS, LOOH and cytochorme P450 in plasma and increased the TBARS and LOOH levels in uterus (Table 2). However, in pre-treatment group the effect was more predominant.
Table 2.
Effect of vanillic acid on lipid proxidation markers, cytochrome P450, enzymic and non-enzymic antioxidants in normal and MNNG induced rats
| Groups | Control | MNNG | VA | VA + MNNG (pre-treatment) | MNNG + VA (co-treatment) |
|---|---|---|---|---|---|
| TBARS (plasma) (mmol/dl) |
2.08 ± 0.18a | 5.34 ± 0.45d | 2.02 ± 0.15a | 2.85 ± 0.17b | 3.55 ± 0.30c |
| TBARS (endometrium) (mmol/mg tissue) |
144.98 ± 7.41c | 111.32 ± 9.87a | 135.13 ± 7.08c | 138.45 ± 8.23c | 124.09 ± 9.74b |
| LOOH (plasma) (mmol/dl) |
0.8 ± 0.06a | 2.22 ± 0.12e | 0.09 ± 0.06b | 1.34 ± 0.07c | 2.03 ± 0.14d |
| LOOH (endometrium) (mmol/mg tissue) |
97.04 ± 7.83a | 145.89 ± 11.05d | 101.56 ± 5.54a | 119.56 ± 6.40b | 134.67 ± 7.53c |
| Cytochrome p450 Ua/mg protein |
9.32 ± 0.50a | 20.23 ± 1.28c | 11.25 ± 0.73a | 12.41 ± 5.23a | 16.09 ± 0.89b |
| SOD (plasma) mg/dl |
9.49 ± 0.59e | 4.48 ± 0.32a | 6.30 ± 0.46c | 7.39 ± 0.39d | 5.65 ± 0.41b |
| SOD (endometrium) Ub/mg protein |
12.61 ± 0.64e | 7.72 ± 0.39a | 11.82 ± 0.65d | 10.45 ± 0.54c | 8.79 ± 0.50b |
| CAT (plasma) mg/dl |
0.82 ± 0.06c | 0.54 ± 0.04a | 0.78 ± 0.06c | 0.71 ± 0.05b | 0.65 ± 0.06b |
| CAT (endometrium) Uc/mg protein |
2.71 ± 0.15d | 0.98 ± 0.06a | 2.08 ± 0.12c | 1.96 ± 0.10c | 1.75 ± 0.12b |
| GPx (plasma) mg/dl |
0.92 ± 0.05c | 0.57 ± 0.03a | 0.85 ± 0.05c | 0.87 ± 0.06c | 0.79 ± 0.07b |
| GPx (endometrium) Ua/mg protein |
25.21 ± 1.52c | 18.63 ± 1.01a | 24.08 ± 1.69c | 22.14 ± 1.40b | 20.39 ± 1.23d |
| GSH (plasma) mg/dl |
53.32 ± 2.89d | 20.74 ± 1.56a | 46.03 ± 3.22c | 47.01 ± 3.29c | 39.56 ± 2.34b |
| GSH (endometrium) mg/100 g of tissue |
1.54 ± 0.11c | 0.91 ± 0.06a | 1.60 ± 0.11c | 1.34 ± 0.09b | 1.29 ± 0.07d |
| Vitamin C (plasma) mg/dl |
3.43 ± 0.19d | 1.25 ± 0.09a | 3.24 ± 0.19c | 3.04 ± 0.21b,c | 2.84 ± 0.22d |
| Vitamin C (endometrium) mg/100 g of tissue |
10.54 ± 0.67d | 5.09 ± 0.32a | 10.12 ± 0.60d | 8.77 ± 0.70c | 7.95 ± 0.55b |
| Vitamin E (plasma) mg/dl |
2.50 ± 0.15d | 0.89 ± 0.05a | 2.95 ± 0.17e | 1.94 ± 0.11c | 1.12 ± 0.07b |
| Vitamin E (endometrium) mg/100 g of tissue |
8.43 ± 0.57d | 4.09 ± 0.24a | 9.84 ± 0.59e | 7.49 ± 0.39c | 6.14 ± 0.41b |
Ua = µg of GPx utilized/min. Ub = enzyme concentration required to inhibit the NBT to 10 % reduction in 1 min. Uc = µmole of H2O2 consumed/min. Values are means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT)
Enzymic and Non-enzymic Antioxidants
The actions of enzymic and non-enzymic antioxidants were considerably reduced in plasma and uterus of MNNG treated rats. On the other hand supplementing vanillic acid (pre and co-treatments) significantly elevated the activity of antioxidants in plasma and uterus (Table 2). The elevation of these levels was more predominant in pre-treated rats.
MMP-2, 9 and Cyclin D1
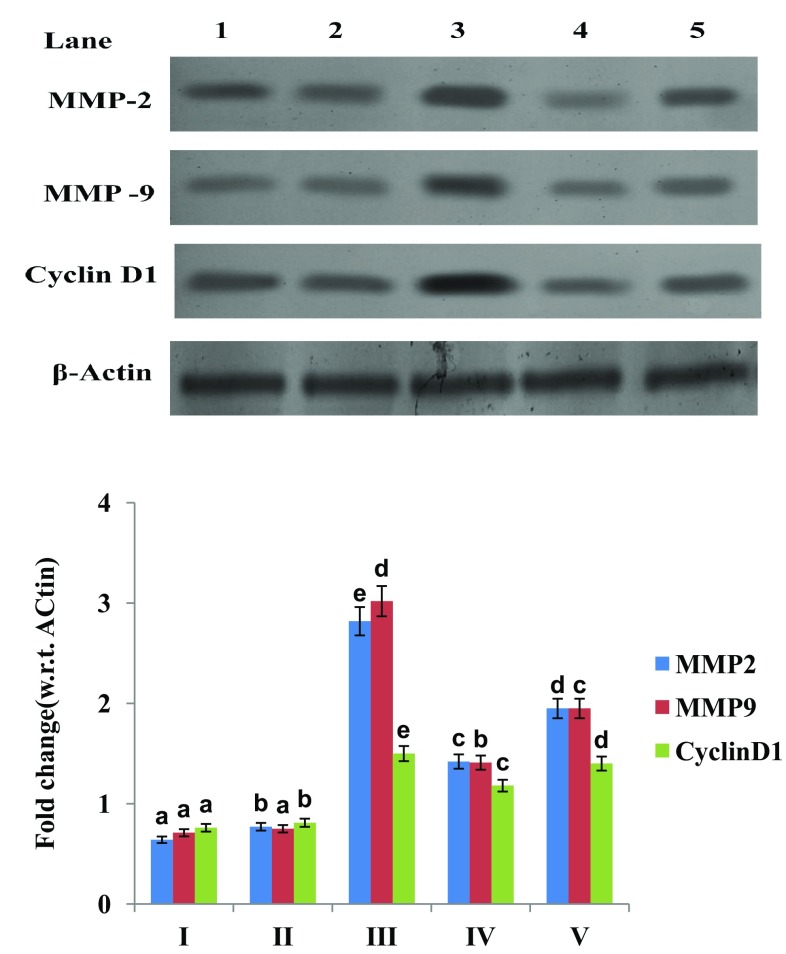
The expression of MMP-2, 9 and cyclin D1 was significant upregulated in MNNG treated rats compared to control. Pre and co-treatment of vanillic acid to MNNG treated rats downregulated the expression of MMP-2, 9 and cyclin D1 compared to MNNG treated rats. Vanillic acid (only) administered rats have not showed any changes in the expression of these markers compared to control (Fig. 3).
Fig. 3.
Effect of vanillic acid on MMP-2, 9 and cyclin D1 protein expression in endometrium of control and MNNG induced rats. MMP-2, 9, cyclin D1 and β-actin expression by western blot. Lane 1 Control rats. Lane 2: VA. Lane 3: MNNG. Lane 4: VA + MNNG pretreated. Lane 5: MNNG + VA co-treatment. Band intensity scanned by densitometer. Group I Control rats; Group II VA; Group III MNNG; Group IV VA + MNNG (pre-treatment); Group V MNNG + VA (co-treatment). Histograms from densitometric analysis expressed as ratio and given as mean ± SD from six rats in each group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT)
Discussion
MNNG, an alkylating carcinogen used in the present study is known to methylate all the oxygen and nitrogen atoms of DNA. MNNG has been found to cause DNA strand breaks, point mutations and sister chromatid exchanges which if not adequately repaired, may result in activation of oncogenes leading to carcinoma [21]. Our study demonstrated that the body weight was decreased due to improper nutritional status and metabolic functions in MNNG treated group [22]. After the administration of vanillic acid (pre and co-treatment) the body weights of the animals seem to be increased. It might be due to its antioxidant and anticancer effects. MNNG induced rats showed 100 % of tumour incidence and vanillic acid treated rats significantly reduced the tumour incidence rate.
There is an inverse connection among the concentrations of lipid peroxides and the rate of cell proliferation, i.e. the increased amount of lipid peroxidation in the cells with decreased concentration of cell division in endometrial tumor tissue [23]. Enriched levels of lipid peroxides, besides the lowering levels of SOD, CAT actions, denotes increased oxidative stress owing to MNNG induced malignancy. The microsomal cytochrome P450 enzyme plays an essential role in activating the oxidative damage, thus by promoting the detoxification of carcinogens and also by controlling the duration and strength of their toxicity [24]. Studies showed that in contrast to decreased lipid peroxidation in tumor tissues, enhanced lipid peroxidation was observed in the circulation of tumor bearing animals. In our present work, we noticed increased levels of TBARS, LOOH and cytochrome P450 in MNNG induced rats by generating the free radicals which could lead to the formation of oxidative damage. After the treatment of vanillic acid the levels of lipid peroxidation products and phase I enzyme were decreased which might be due to the free radical scavenging property of vanillic acid.
Cells are able to detoxify many reactive molecules before they react with susceptible cellular targets. The enzymes SOD and CAT and the glutathione play a key role in the cellular defense against free radical damage [25]. Reports indicate that animal tumor cells lack the complex enzyme systems, which normally exert a protection by scavenging the toxic oxygen species such as superoxide radical, hydrogen peroxide and lipid hydroperoxides [23]. The significant decrease in enzymatic and non enzymatic antioxidant levels in MNNG treated rats might be due to the increased lipid peroxidation. Our data suggest that the antioxidant property of vanillic acid may be responsible for protecting the cells against oxidative stress, possibly by increasing the endogenous defensive capacity of the uterus to combat oxidative stress induced by MNNG. It may be noted, however, that the pre-treatment of vanillic acid was found to be more effective than the other treatment regimens.
Apoptosis evasion and the development of the cancerous cells in a tumour are often accompanied by the progress of vascularisation, which plays a vital role in the development of distant metastasis in malignant tumour. The regulation of MMP expression can be linked to a number of inducing agents, including growth factors, cytokines, oncogenes, and ECM-derived signals [26]. As such in the degradation of ECM, the MMPs process and release a variety of molecules, which are regulators of vascular growth or function, including fibroblast growth factor receptor type I, tumor necrosis factor-α, and heparin binding epidermal growth factor [27]. Thus the metalloproteinases are not limited to simply degrading structural proteins that surround the cell, but have a more generalized role in the interactions of cells with their matrix environment, affecting basic cellular processes such as differentiation, proliferation and apoptosis. In the current study, the carcinogen MNNG, upregulates MMP-2 and MMP-9 which would lead to metastatic dissemination of tumour cells and thus stimulate tumour invasion and metastasis [28]. Downregulation of MMP-2 and MMP-9 by vanillic acid seen in this study is associated with inhibition of tumour angiogensis, invasion and metastasis as a result of prevention of endometrial cancer induced by MNNG.
Cell cycle transition between G1 and S phase was mediated by cyclin D1. Numerous reports suggest that cyclin D1 is overexpressed in tumours and mediates chemoresistance and radioresistance [29]. The destruction of cyclin D1 expression in cancer cells did not inhibit the cell growth but increased the growth-inhibitory effect of cisplatin and fluoropyrinidine compounds. This finding suggested that cyclin D1 could exhibit a defensive effect against drug induced cytotoxicity, and implies a condition for cyclin D1, in the maintenance of chemoresistance in the cells [30]. Our results revealed that, there are many changes in MNNG induced endometrial carcinoma which leads to upregulation of cyclin D1. In particular, changes in cyclin D1 play a crucial role in the transformation of the normal uterus into the malignant phenotype [29]. The ability of vanillic acid to modulate the downregulated expression of cyclin D1 supports the notion that this phytochemical displays chemopreventive efficacy by reversing gene expression signature associated with tumorigenesis.
Our results suggest that administration of vanillic acid during the experimental period significantly improved apoptosis, decreased circulatory lipid peroxidation and enhanced enzymic and non-enzymic antioxidant concentrations by reversing the changes produced by MNNG. Vanillic acid in pre-treated group is more effective in the prevention of endometrial carcinoma than the co-treatment group.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Yasa C, Takmaz O, Dural O, Akhan SE. The value of tumor markers in endometrial carcinoma: review of literature. J Cancer Ther. 2013;4:966–970. doi: 10.4236/jct.2013.45110. [DOI] [Google Scholar]
- 2.Janiec-Jankowska A, Konopka B, Goluda C, Najmoła U. Tp53 mutations in endometrial cancers: relation to PTEN gene defects. Int J Gynecol Cancer. 2010;20:196–202. doi: 10.1111/IGC.0b013e3181c83675. [DOI] [PubMed] [Google Scholar]
- 3.Edara S, Kanugula S, Pegg AE. Expression of the inactive C145A mutant human O6-alkylguanine-DNA alkyltransferase in E. coli increases cell killing and mutations by N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis. 1999;20:103–108. doi: 10.1093/carcin/20.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Cerutti PA. Oxy-radicals and cancer. Lancet. 1994;344:862–863. doi: 10.1016/S0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 5.Subapriya R, Kumaraguruparan R, Chandramohan KVP, Nagini S. Chemopreventive effects of ethanolic neem leaf extract against MNNG-induced oxidative stress. Pharmazie. 2003;58:512–517. [PubMed] [Google Scholar]
- 6.Puljiz M, Puljiz Z, Vucemilo T, Ramić S, Knezević F, Culo B, et al. Prognostic significance of matrix metalloproteinases 2 and 9 in endometrial cancer. Coll Antropol. 2012;4:1367–1372. [PubMed] [Google Scholar]
- 7.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D1 as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 8.Ghasemzadeh A, Omidvar V, Jaafar HZE. Polyphenolic content and their antioxidant activity in leaf extract of sweet potato (Ipomoea batatas) J Med Plants Res. 2012;6:2971–2976. [Google Scholar]
- 9.Dhananjaya BL, Nataraju A, Raghavendra Gowda CD, Sharath BK, D’Souza CJ. Vanillic acid as a novel specific inhibitor of snake venom 5′-nucleotidase: a pharmacological tool in evaluating the role of the enzyme in snake envenomation. Biochemistry. 2009;74:1315–1319. doi: 10.1134/s0006297909120037. [DOI] [PubMed] [Google Scholar]
- 10.Vetrano AM, Heck DE, Mariano TM, Mishin V, Laskin DL, Laskin JD. Characterization of the oxidase activity in mammalian catalase. J Biol Chem. 2005;280:35372–35381. doi: 10.1074/jbc.M503991200. [DOI] [PubMed] [Google Scholar]
- 11.Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a induced liver injury. Biol Pharm Bull. 2009;32:1215–1219. doi: 10.1248/bpb.32.1215. [DOI] [PubMed] [Google Scholar]
- 12.Yagi K. Lipid peroxides and human disease. Chem Phys Lipids. 1987;45:337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 13.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-N. [DOI] [PubMed] [Google Scholar]
- 14.Omura T, Sato R. The carbon monoxide binding pigment of liver. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 15.Kakkar PS, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 16.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:72–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 19.Roe JM, Kuether CA. Detection of ascorbic acid in whole blood, and urine through 2,4-DNPH derivative of dehydroascorbic acid. J Biol Chem. 1943;147:399–407. [Google Scholar]
- 20.Baker H, Frank O, De Angelis B, Feingold S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Rep Int. 1980;21:531–536. [Google Scholar]
- 21.Kodama Y, Ozaki K, Sano T, Matsuura T, Narama I. Enhanced tumorigenesis of forestomach tumors induced by N-methyl-N′-nitro-N-nitrosoguanidine in rats with hypoinsulinemic diabetes. Cancer Sci. 2010;101:1604–1609. doi: 10.1111/j.1349-7006.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizadeh AM, Afrouzan H, Dinparast-Djadid N, Sawaya AC, Azizian S, Hemmati HR, et al. Chemoprotection of MNNG-initiated gastric cancer in rats using Iranian Propolis. Arch Iran Med. 2015;18:18–23. [PubMed] [Google Scholar]
- 23.Bandebuche S, Melinkeri RR. Oxidative stress and antioxidant status in patients of ovarian cancer. Biomed Res. 2011;22:196–200. [Google Scholar]
- 24.Sathiyapriya J, Rajeswari K, Annamalai G, Sivakumar K, Suresh K. Chemopreventive and antioxidant efficacy of fisetin in experimental oral carcinogenesis. Int J Rec Sci Res. 2013;4:313–319. [Google Scholar]
- 25.Ravid A, Rocker D, Machlenkin A, Rotem C, Hochman A, Essler- Icekson G, et al. 1, 25-Dihydroxyvitamin D3 enhances the susceptibility of breast cancer cells to doxorubicin induced oxidative damage. Cancer Res. 1999;59:862–867. [PubMed] [Google Scholar]
- 26.Liu N, Huang J, Sun S, Zhou Z, Zhang J, Gao F, et al. Expression of matrix metalloproteinase-9, cyclooxygenase-2 and vascular endothelial growth factor are increased in gastrointestinal stromal tumors. Int J Clin Exp Med. 2015;8:6495–6501. [PMC free article] [PubMed] [Google Scholar]
- 27.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2010;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 28.Small AR, Neagu A, Amyot F, Sackett D, Chernomordik V, Gandjbakhche A. Spatial distribution of VEGF isoforms and chemotactic signals in the vicinity of a tumour. J Theor Biol. 2008;252:593–607. doi: 10.1016/j.jtbi.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Diel JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;2020:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YJ, Li X, Hydbring P. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]