Abstract
Dengue is an arthropod-borne threat among tropical countries. Currently no effective means to treat the virus or to predict which patient will develop the severe form of the disease. Recently the relationship between oxidative/antioxidative response and dengue pathogenesis was suggested. Based on this the present study has analysed the expression of endogenous antioxidant genes: Catalase (CAT), Superoxide dismutase (MnSOD) and Glutathione peroxidase in patients with dengue compared to other febrile illness (OFI) and healthy controls. The study enrolled 88 dengue confirmed patients comprising 56 were patients with non-severe dengue, and 32 were severe dengue cases, 31 were patients with OFI, and 63 healthy controls were also involved. Peripheral blood mononuclear cells isolated from patients and controls during the day of admission and from the available cases on the day of defervescence were used to estimate the transcript levels by quantitative PCR. The expression levels of all the three genes were found to be down-regulated throughout the course of dengue infection (p < 0.05) and OFI cases compared to healthy controls. Within dengue group, no significant difference was observed in any of the parameters between severe and non-severe cases. Interestingly, a significant down-regulation of MnSOD expression was recorded in secondary dengue infection compared to primary during admission (p < 0.05). It was found that all the down-regulated study genes have positively correlated in all dengue cases during the day of admission (p < 0.01). But during defervescence, the same was found only between CAT and MnSOD. Down-regulated endogenous antioxidant enzymes during dengue infection could be the possible rationale of oxidative stress reported in dengue disease earlier. The present study markers could not distinguish dengue from OFI cases and severe from non-severe dengue cases. Mechanism of down-regulation has to be explored further which will pave the way for the therapeutic target in dengue disease.
Keywords: Dengue, CAT, MnSOD, GPx
Introduction
Dengue is a mosquito-borne acute viral disease affecting two-thirds of the world’s population predominantly in tropical and sub-tropical areas of the world [1]. Approximately 3.6 billion people are living in areas of risk, over two million cases of severe disease, and 21,000 deaths [1, 2]. Dengue virus infestation extends from self-limiting mild acute febrile illness, dengue fever (DF) to severe, life-threatening disease, dengue hemorrhagic fever (DHF) [3]. The mechanisms behind the pathogenesis of dengue remain elusive. The possible mechanisms attributed to the pathogenesis are antibody-dependent enhancement (widely accepted), virus virulence, DENV tropism, activation of the complement system, transient- autoimmunity, host genetic factors, cross-reactive T cell response, soluble factors, altered Th1 to Th2 response, high viral load, high numbers of non-protective T cells and the resulted inflammatory cytokine tsunami [4–6]. The role of the oxidative stress response realised in the pathogenesis of viral disease including HIV and hepatitis [7, 8]. We recently documented the involvement of oxidative stress in the regulation of immune response during dengue viral infection [9]. In the view of increasing evidence of oxidative stress during dengue infection speculate possible etiology of dengue pathogenesis [10]. The febrile period of dengue infection lasts for 4 or 5 days and is followed by defervescence. At defervescence, the disease will reveal it is severe or relatively benign in nature. During the acute febrile phase of infection, DF and DHF patients show similar clinical signatures. Therefore, a brisk differential DF/DHF diagnosis is essential for medical intervention to reduce the dengue mortality [11–13].
We have earlier reported that increased blood levels of potential oxidant-antioxidant markers in the circulation of dengue infected patients. Higher levels of protein carbonylations (PCOs), PCOs to PBSH (protein-bound sulphydryls) group ratio and decreased sialic acid and sulphydryl group levels were found to predict the severe dengue disease. An increased levels of lipid peroxidation observed in severe forms of dengue which speculate oxidative stress may be associated with thrombocytopenia [14–16].
During oxidative stress, the antioxidant defensive system tries to nullify ROS-mediated redox imbalance. Antioxidant enzymes like superoxide dismutase, catalase, and glutathione peroxidase come into the action synergistically to eradicate the free radicals [17, 18]. Earlier studies reported a lower level of antioxidant markers such as CAT, SOD, GPx, reduced glutathione (GSH) and paraoxonase in the peripheral circulation of dengue study subjects [19, 20].
Elevated enzyme levels of SOD and reduced GPx levels has also been reported in the circulation of paediatric dengue patients compared to control group [21]. However, to the best of our knowledge, no studies are available pertaining the gene expression profile of antioxidant enzymes in dengue patients. In the context of the pro-oxidant state in dengue infection, it is of considerable interest to examine whether the changes in antioxidant gene transcriptional levels may exist under the various clinical spectrum of dengue as well as to distinguish severe cases from non-severe at the earlier stage of infection are of prime importance. Therefore, we studied gene expression of CAT, MnSOD, and GPx in PBMCs isolated from dengue patients compared to OFI and control study subjects during the febrile period and around at the time of defervescence. These three genes chosen for the study considering their prime importance as effective endogenous enzymatic antioxidants [22]. Exogenous introduction of these antioxidant enzymes were reported to prevent RSV-induced ROS formation and activates NFkB which regulates the expression of pro-inflammatory genes [23]. Based on this the present study is designed to understand the expression pattern of these three transcripts during dengue viral infection. Oxidative stress is an established phenomenon in dengue, but the molecular mechanism behind the oxidative stress in dengue patients are unknown. The present work is the first step to unveil the same. The study found that the expression levels of all the three genes studied genes were down-regulated during admission and defervescence in dengue and OFI cases compared to healthy controls. However, none of the transcript levels were found to be significantly different between severe and non severe form of dengue, also between dengue and OFI group.
Methods
Patient Recruitment and Sample Collection
The study subjects were recruited from Jawaharlal Institute of Post Graduate Medical Education and Research (JIPMER) Hospital, Puducherry, India. The prospective cohort study consist of 88 dengue patients, 31 other febrile illness (OFI) subjects and 63 controls (20 non-febrile illness subjects (NFI) and 43 healthy volunteers). Out of all 88 dengue cases 38 dengue cases who reported till defervescence stage were involved in the study. Remaining 50 cases were not willing to participate in the study or discharged against medical advice. Similarly, 13 OFI cases who shown up till defervescence were involved. The subjects recruited for the study were categorised into three groups. Group I included 88 dengue infected cases (59 adults and 29 children, with ages varying from 1 to 72 years) whose infection was confirmed by NS1 antigen detection by dengue early ELISA kit or dengue IgM Capture ELISA Kit, (Pan Bio, Inverness Medical Innovations, Australia). To classify the patients into primary and secondary infection dengue IgG capture ELISA kit was used (Pan Bio, Inverness Medical Innovations, Australia). A dengue virus infection has been defined as primary if the capture IgM/IgG ratio is greater than 1.2, or as secondary, if the ratio is less than 1.2 [24]. The samples were collected from the patients from the regions of Puducherry and Tamilnadu, India during the dengue fever outbreak in the year of 2012–2014. The World Health Organization grading system was used to classify dengue patients like our previous studies [25, 26]. Dengue fever divided into ‘dengue without warning sign, dengue with the warning sign and severe dengue’. Dengue without warning sign consists of fever with two of the following criteria: ‘nausea, vomiting, rash, aches and pains, leukopenia and Positive tourniquet test.’ In addition to the above dengue with warning sign includes any of the following clinical condition: ‘abdominal pain, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy, restlessness, liver enlargement >2 cm, increase in hematocrit concurrent with rapid decrease in platelet count’. The classification of SD was considered for cases with any of the following criteria: ‘severe plasma leakage leading to shock or fluid accumulation with respiratory distress, severe bleeding evaluated by clinician and severe organ involvement such as Liver (AST or ALT ≥ 1000), CNS (impaired consciousness), failure of heart and other organs’. In the present study patients classified as dengue with or without warning signs, were considered as a single group, non-severe dengue (NSD).
Group II included 31 age and sex-matched patients with other febrile illness (OFI). OFIs would be defined as those patients with short febrile illness (<2 weeks) who are negative for dengue NS1 antigen, anti-IgM/IgG dengue antibodies, and no obvious bacterial, rickettsial or protozoan etiology [27]. Group III is control group further divided into two groups. First is non-febrile illness (NFI) which is defined as patients belongs to pediatric age group (1–12 years) with illness other than febrile illness who come to JIPMER for minor surgical, skin or eye problem without systemic disease [28]. This group served as controls for comparison of pediatric cases. The second one is healthy adult Volunteers of age > 13 years. Subjects excluded from the study groups are patients with the history of smoking, alcohol intake, malignancy, inflammatory disorders, on steroids therapy and antioxidant supplementation since the above conditions can affect the level of study parameters.
Ethical Approval
The study permitted by the institutional ethics committee (Human studies) meeting of the year 2012 held on 03/11/2012 (Ref No.IEC/SC/2012/4/101). After taking written informed consent, 3 ml of blood sample collected within 24 h of admission (Febrile period), and another sample during the follow-up coinciding with the day of defervescence; the day which fever is subsiding indicated by a decline in body temperature which commences nearly 3rd or 4th day of admission and at the same time points for OFI patients. Clinical, haematological and biochemical parameters recorded for all patients recruited in the study.
PBMC Isolation, RNA Extraction and cDNA Synthesis
PBMCs were separated by using HiSep LSM 1077 (Himedia, Mumbai, India), washed twice with PBS, stored in 1 ml RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan) and kept at −80°. Total RNA was extracted using RNA easy minikit (Qiagen, GmbH, Hilden, Germany) based on the manufacturer’s protocol. The purity and concentration of RNA were assessed using a NanoDrop spectrophotometer (ThermoScientific, Waltman, MA, USA). The complementary DNA (cDNA) was synthesized from 1 µg of RNA using high capacity cDNA reverse transcription Kit with RNase inhibitor (Applied Biosystems, Foster City, CA, USA). The cDNAs thus obtained were tested for integrity by amplification of β-actin transcripts in a 40-cycle PCR. Standard curves were constructed using the mean cycle threshold (Ct) value.
Analysis of CAT MnSOD and GPx Gene Expression Using qPCR
The primers used for the study (Table 1) were designed using oligo analyzer software from IDT (Coral ville, IA). A total of 20 μl of the reaction sample consisted of 2 μl cDNA (1:30 dilutions), 10 μl of SYBR Premix Ex TaqII (Takara Bio Inc., Shiga, Japan), 0.4 μl of each primers at 0.2 μM (VBC-Biotech Service GmbH, Vienna, Austria), and 7.2 μl of nucleic acid free water (Sigma- Aldrich St. Louis, MO, United States). Amplification were monitored using CFX96 Real-Time PCR Detection System (Bio- Rad Laboratories, USA). Reaction conditions for catalase, MnSOD were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s. Reaction condition for GPx was 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 55 °C for 30 s. The melting curve analysis was used to determine the specificity of each primer set. Specific PCR products confirmed by melting curve analsis. PCRs were performed in triplicate, and the reproducibility of the SYBR Green real-time PCR was assessed by running control samples independently on different days. The relative expression level of CAT, MnSOD and GPx were determined using the comparative Ct method, also known as 2−∆(Ct) method [29]. Quantitative PCR method enables to analyze the expression of genes relative to the expression of the housekeeping gene β-actin. The relative copy number (RCN) of the target gene allows direct comparison of mRNA expression across individuals, time points, and groups standardized against constitutively expressed housekeeping genes.
Table 1.
Primers for CAT, MnSOD, GPx & β-actin
| Gene primers | Sequence |
|---|---|
| CAT forward primer | 5′-CTCCACTGTTGCTGGAGAAT-3′ |
| CAT revere primer | 5′-CGAGATCCCAGTTACCATCTTC-3′ |
| MnSOD–forward primer | 5′-GGAGATGTTACAGCCCAGATAG-3′ |
| MnSOD–reverse primer | 5′GTTAGGGCTGAGGTTTGT-3′ |
| GPx forward primer | 5′-GCTGTGGAAGTGGATGAAGAT-3′ |
| GPx reverse primer | 5′-TCGATGAGGAACTGTGGAGA-3′ |
| β-actin–forward primer | 5′-ATCTGGCACCAACACTTCTACA-3′ |
| β-actin–reverse primer | 5′-GTTTCGTGGATGCCACA-3′ |
Statistical Analysis
Variables analysed by descriptive statistics to evaluate the clinical characteristics of the cases. Normality assessed with the Shapiro–Wilk test. Results of study parameters presented either mean (SD) or median with interquartile range. Kruskal–Wallis test or Oneway analysis of variance was used to estimate the presence of any statistical differences between the study variables. Wilcoxon signed rank test used for comparing febrile and following defervescence day of gene expression analysis. Pearson’s coefficient correlation analysis was carried out for gene expression analysis of dengue cases between the study variables during the day of admission and day of defervescence. All statistical analysis were performed using SPSS 21 (SPSS, Chicago, IL).
Results
Clinical, Haematological and Biochemical Characteristics
Out of 182 study subjects, the average age of controls, dengue infected patients, and OFI cases were 20.87 ± 26, 24.13 ± 15.90 and 23.65 ± 15.66 respectively (Table 2). Age of all the study group was comparable (p = 0.91). All dengue infected cases were classified based on the WHO 2009 criteria in which 56 patients were categorised as non-severe dengue and 32 under severe dengue. Clinical characteristics such as abdominal pain, hepatosplenomegaly, third space collection, were considerably varied between the study groups as shown in Table 2. Haematological parameters like haematocrit, platelet count, total leucocyte count, % of neutrophil, lymphocyte, and biochemical parameter AST significantly differed between the study groups (Table 2).
Table 2.
Clinical, Hematological and Biochemical characteristics of the study groups
| Characteristics | OFI (n = 31) | ADC (n = 88) | NSD (n = 56) | SD (n = 32) |
|---|---|---|---|---|
| Gender | ||||
| Male N (%) | 13 (58.06) | 51 (57.95) | 29 (51.79) | 22 (68.75) |
| Female N (%) | 18 (41.94) | 37 (42.05) | 27 (48.21) | 10 (31.25) |
| Age in years mean (SD) | 24.13 (15.90) | 23.65 (15.66) | 25.43 (15.07) | 20.69 (16.39) |
| Pulse rate mean (SD) | 101.6 (19.77) | 90.98 (19.38) | 88.82 (18.77) | 93.35 (20.24) |
| Systolic BP mean (SD) mmHg | 110 (20.92) | 105.02 (14.19) | 105.42 (12.97) | 104.57 (15.78) |
| Diastolic BP mean (SD) mmHg | 69.76 (12.29) | 70.44 (11.07) | 70.83 (11.39) | 70 (10.95) |
| Positivity for dengue | ||||
| NS1 Ag N (%) | 0 | 45 (51.14) | 32 (57.14) | 13 (40.63) |
| Anti-dengue IgM antibody N (%) | 0 | 85 (96.59) | 54 (96.43) | 31 (96.88) |
| Anti-dengue IgG antibody N (%) | 0 | 66 (75.00) | 38 (67.86) | 28 (87.50) |
| Clinical characteristics | ||||
| Third space collection (%) | 2 (6.45) | 18 (20.45) | 0 | 18 (56.25)### |
| Abdominal pain N (%) | 9 (29.03) | 40 (45.45) | 16 (28.57) | 24 (75.00)### |
| Myalgia N (%) | 16 (51.61) | 38 (43.18) | 27 (48.21) | 11 (34.38) |
| Headache N (%) | 13 (41.94) | 36 (40.90) | 23 (41.07) | 13 (40.63) |
| Retro-orbital pain N (%) | 5 (16.13) | 16 (18.18) | 11 (19.64) | 5 (15.63) |
| Arthralgia N (%) | 2 (6.45) | 13 (14.77) | 11 (19.64) | 2 (6.25) |
| Maculopapular rash N (%) | 1 (3.23) | 14 (15.90) | 7 (12.5) | 7 (21.88) |
| Gingival bleeding N (%) | 3 (9.68) | 12 (13.64) | 4 (7.14) | 8 (25.00) |
| Vomiting N (%) | 17 (54.84) | 43 (48.86) | 26 (46.43) | 17 (53.13) |
| Hepato-spleno-megaly N (%) | 6 (19.35) | 4 (4.54)* | 2 (3.57) | 2 (6.25) |
| Haematological parameters | ||||
| Total count mean (SD) cells/mm3 | 7234.07 (3207.20) | 5137.19 (2508.31)* | 4976.84 (2443.95) | 5371.53 (2630.37) |
| Neutrophils mean (SD) % | 63.84 (14.62) | 47.40 (13.33)*** | 47.34 (11.30) | 47.48 (16.19) |
| Lymhocytes mean (SD) % | 31.64 (13.57) | 47.56 (12.82)*** | 47.95 (10.66) | 46.96 (15.77) |
| Hct mean (SD) | 36.10 (5.62) | 42.09 (7.57)* | 40.85 (5.36) | 44.05 (9.96) |
| Platelet count mean (SD) cells/mm3 | 139,576.92 (84,038.64) | 67,906.25 (62,866.63)*** | 82,923.08 (67,858.36) | 44,480 (46,320.54)## |
| Biochemical parameters | ||||
| Albumin mean (SD) g/dl | 3.26 (0.49) | 3.46 (0.56) | 3.56 (0.44) | 3.23 (0.71) |
| Total protein mean (SD) g/dl | 6.34 (0.95) | 6.376 (1.10) | 6.58 (0.84) | 5.92 (1.47) |
| ALT mean (SD) IU/ml | 67 (41.94) | 92.02 (67.90) | 91.78 (63.32) | 92.53 (78.90) |
| AST mean (SD) IU/ml | 91.13 (88.13) | 154.87 (98.97)** | 158.14 (105.96) | 147.94 (84.85) |
* p < 0.05; ** p < 0.01; *** p < 0.001 when compared OFI
# p < 0.05; ## p < 0.01 when compared to NSD
OFI other febrile illness, ADC all dengue cases, NSD non-severe dengue, SD severe dengue, Hct hametocrit, ALT alanine amino transferase, AST aspartate amino transferase
Gene Expression of Study Variables in Dengue Cases and OFI
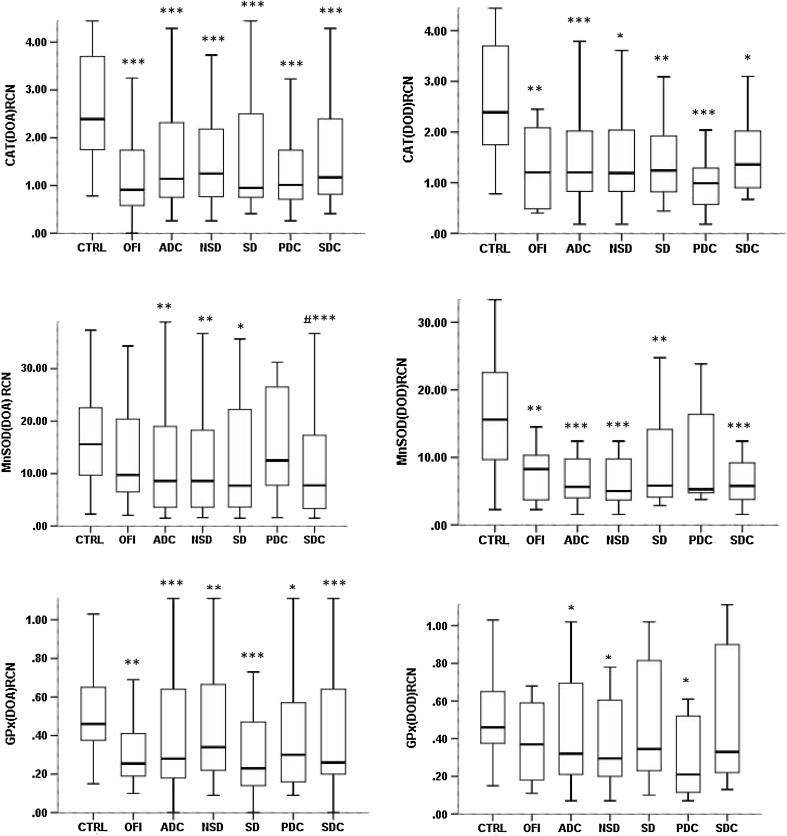
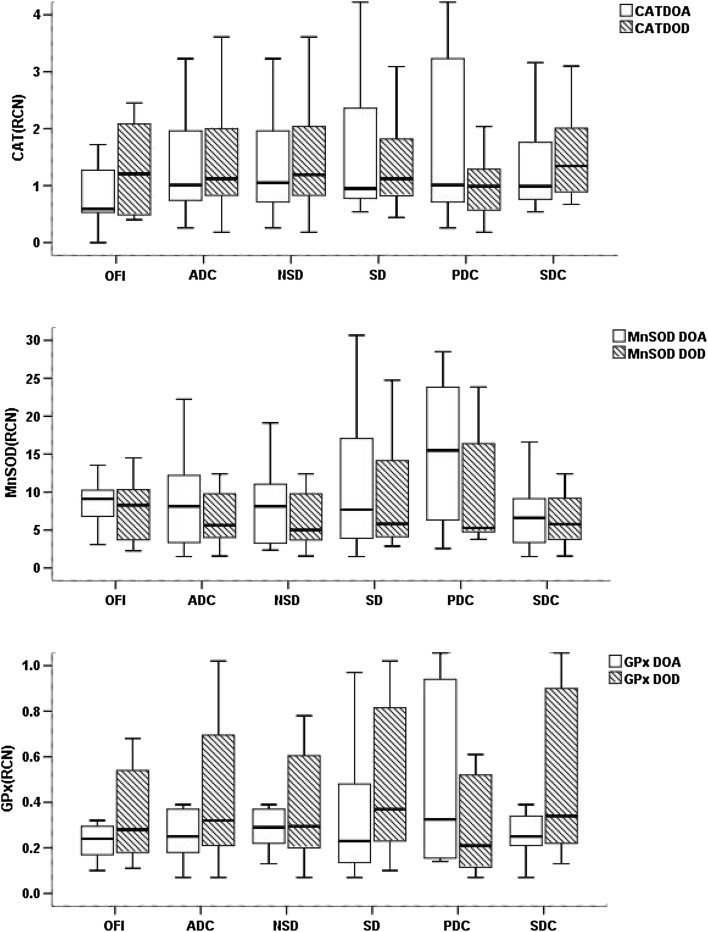
Expression levels of antioxidant genes were down-regulated generally in all the dengue groups including OFI cases during the day of admission as well as the day of defervescence as shown in Fig. 1. Decreased expression of catalase, MnSOD and GPx found in all dengue study groups significantly (p < 0.05) compared to controls. There was no significant difference found among various dengue groups and OFI between the day of admission (DOA) and day of defervescence (DOD) (Fig. 2).
Fig. 1.
Gene expression of CAT, MnSOD and GPx in all the study groups at the day of admission and day of defervescence. The amount of mRNA of each tested gene was measured by qPCR assay and expressed as relative copy number (RCN) with median and interquartile range. *** p < 0.001, **p < 0.01, *p < 0.05 when compared to controls. DOA day of admission; DOD day of defervescence, CTRL controls, OFI other febrile illness, ADC all dengue cases; NSD non-severe dengue, SD severe dengue, PDC primary dengue cases, SDC secondary dengue cases. DOA: CTRL (n = 63), OFI (n = 31), ADC (n = 88), NSD (n = 56), SD (n = 32), PDC (n = 29), SDC (n = 59). DOD: CTRL (n = 63), OFI (n = 13), NSD (n = 22), SD (n = 16), ADC (n = 38), PDC (n = 9), SDC (n = 29). Statstics : Kruskal–Wallis test
Fig. 2.
Comparison of gene expression levels of CAT, MnSOD and GPx in various study groups between the day of admission (DOA) and the day of defervescence (DOD). DOA day of admission, DOD day of defervescence, OFI other febrile illness, ADC all dengue cases, NSD non-severe dengue, SD severe dengue, PDC primary dengue cases, SDC secondary dengue cases. OFI (n = 13), NSD (n = 22), SD (n = 16), ADC (n = 38), PDC (n = 9), SDC (n = 29). Statistics: Wilcoxon signed rank test
Pattern of Gene Expression Between SD and NSD Cases
There were no statistically significant variation of study parameters observed between SD and NSD cases during the study period (Figs. 1, 2).
Gene Expression in Primary and Secondary Dengue Cases
During the day of admission, MnSOD expression was down regulated significantly compared to primary dengue cases (p < 0.05 and Fig. 1). But this dynamic change was lost during defervescence (Fig. 1). There was no significant change of genes expression found between primary and secondary dengue between the day of admission (DOA) and day of defervescence (DOD) (Fig. 2).
Correlation Analysis of Gene Expression Between the Study Variables During the Day of Admission and Day of Defervescence
It was found that all the down-regulated study genes have positively correlated in all dengue cases during the day of admission as shown in Table 3 (p < 0.01). But during defervescence, the same was found only between CAT and MnSOD.
Table 3.
Correlation analysis of gene expression in dengue cases between the study variables during the day of admission and day of defervescence
| Day of admission (DOA) | Day of defervescence (DOD) | |||
|---|---|---|---|---|
| Correlation coefficient (ρ) | p value | Correlation coefficient (ρ) | p value | |
| CAT versus MnSOD | 0.593 | <0.001 | 0.756 | <0.001 |
| MnSOD versus GPx | 0.334 | 0.002 | −0.066 | 0.709 |
| GPx versus CAT | 0.591 | <0.001 | 0.001 | 0.996 |
DOA ADC (n = 88), DOD ADC (n = 38)
Statistics: Pearson’s coefficient correlation analysis
Discussion
The mechanism that triggers dengue severity is currently not understood completely. A study documented the involvement of oxidative stress in regulating antiviral properties during dengue viral infection [30] and the involvement of oxidative or nitrosative strain in dengue infection has reviewed recently [10]. Microarray-based transcriptional studies have resulted in the differential expression of immune responsive genes, oxidative and anti-oxidative responsive genes among severe and non-severe dengue cases compared to healthy controls [31, 32]. To further understand the anti-oxidant response during dengue viral infection, in the present study we validated the gene expression level of three selected antioxidant transcripts in dengue cases compared to other febrile illness and controls (healthy volunteers/NFI). The results are discussed on three bases: All dengue versus other febrile controls; severe versus non-severe and finally between primary and secondary cases.
Firstly the expression levels were found to be down-regulated for all three genes studied in all study both dengue as well as OFI compared to healthy controls (Fig. 1). Reduced expression of antioxidant enzymes may be the cause of redox imbalance in dengue disease as well as the same reigning in OFI also. Earlier we have reported a positive correlation existing between pro-inflammatory cytokine and oxidative stress [9]. So the down-regulation of antioxidant genes could able to exacerbate oxidative stress mediated cytokine injury. The mechanism underlying the down-regulation of antioxidant enzymes has to be explored which will help to discover therapeutic interventions, diagnostic and prognostic markers for dengue disease.
Secondly, within dengue cases, the study did not find any significant variation between severe and non-severe cases. Our earlier studies on oxidative parameters like MDA, protein carbonylation, protein carbonylations (PCOs), PCOs to PBSH (protein-bound sulphydryls) group ratio, sialic acid and sulphydryl groups residues have clearly distinguished severe dengue cases during the febrile period of infection [14, 15]. But the current study of gene expression transcript neither able to differentiate between severe and non-severe dengue cases speculate the role of viral hijacking on the regulation of host genes. It has been reportedly hijacks Nrf2 dependent pathway by Marburgvirus [33]. But further studies have required to explore this phenomenon in dengue infection. Kalessan et al. reported that persisting levels of MDA levels up to 7 days of dengue infection [28] and this may partly explain our findings of undistinguishable gene expression during the day of defervescence among dengue cases. Despite a longer follow-up study is required further to get more knowledge about the transformation of the observed trend.
Finally, Secondary dengue infection by a different DENV serotype has established as a risk factor for severe dengue disease [4]. During DOA, we found that MnSOD expression significantly downregulated in secondary dengue compared to primary dengue infection (Fig. 2). There is no study reported about the alteration of oxidative stress or apoptosis response in the presence or absence of cross-reactive non-neutralising antibody. So the factor underlying the MnSOD gene expression pattern in primary/secondary infection during DOA remains unknown. However, the changes in antioxidant gene expression in secondary cases would be considered important since secondary cases are highly prone to get into severity than primary infection.
Komaravelli et al. reported down-regulation of antioxidant enzymes in Respiratory syncytial virus infection due to virus-induced deacetylation, ubiquitination, and degradation of Nrf2 (NF-E2-related factor 2) a transcription factor which induces the expression of antioxidant enzymes [34]. Monica et al. reported that during Hepatitis C virus infection delocalization of sMAF proteins caused the formation of Nrf-2-sMAF complex which in turn blocked Nrf2 translocation and ultimately caused transcriptional inactivation of detoxifying enzymes [35]. It has been reported that SOD and catalase expression were negatively regulated in the presence of cytokines such as TNF-α, and IFN-γ which elevated during dengue infection [36]. All these virological factors may contribute the down-regulation of antioxidant enzymes in dengue infection, and OFI cases ultimately lead to oxidative-mediated pathogenesis. Involvement of regulatory molecule like nuclear factor erythroid 2–related factor 2 (Nrf2), that regulates multiple antioxidant genes during redox imbalance, may be a possible cause of positively correlated down-regulation of study gene transcripts during the day of admission and defervescence seen in the current study [37]. Even though the same observation was lost during the day of defervescence except between CAT vs. MnSOD. Al-Alimi et al. [38] reported that deficiency of glucose 6 phosphate dehydrogenase enzyme which required for maintaining glutathione in the reduced state leads to the production of nitric oxide, superoxide radicles and viral persistence in DENV-2 infected monocytes. Our study results were in agreement with observation on earlier reports on antioxidants levels which were diminished in dengue study subjects [19, 20]. The study subjects belong to Puducherry and Tamilnadu region which were reported for the prevalence of all four dengue serotypes earlier [39]. But the study did not investigate the levels of gene expression based on the dengue serotype. Besides the study undergoes some of the limitations like a discrete data analysis was not performed for juvenile or adult cohort because of the limited number of pediatric dengue cases. Because of the limited sample volume, we could not able to study the levels of protein in the circulation, even though the study corroborates the earlier reports of disrupted antioxidant defence in dengue patients [19, 20].
In summary, expression levels of all study genes were down-regulated throughout the course of dengue infection and OFI cases compared to healthy controls. Within dengue group, no significant difference was observed in any of the parameters between severe and non-severe cases. Interestingly, a significant down-regulation of MnSOD expression was recorded in secondary dengue infection compared to primary during admission. Down-regulated endogenous antioxidant enzymes during dengue infection could be the possible rationale of oxidative stress reported in dengue disease earlier. Mechanism of down-regulation has to be explored further which will pave the way for the therapeutic target in dengue disease. Therefore, further prospective studies with various viral serotypes from endemic countries may be warranted to validate the role of oxidative stress-mediated pathogenesis in the course of dengue disease.
Acknowledgements
Authors would like to acknowledge Indian council of medical research (ICMR), Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) for their financial support.
Contributor Information
Agieshkumar Balakrishna Pillai, Phone: 0413-2615620, Email: agiesh.b@gmail.com.
Soundravally Rajendiran, Phone: 0413-2296171, Email: soundy27@gmail.com.
References
- 1.WHO|Global Strategy for dengue prevention and control, 2012–2020 [Internet]. WHO. [cited 2016 Mar 22]. Available from: http://www.who.int/denguecontrol/9789241504034/en/.
- 2.Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis. 2010;4(11):e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28(3):183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 6.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29(42):7221–7228. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Aquaro S, Scopelliti F, Pollicita M, Perno CF. Oxidative stress and HIV infection: target pathways for novel therapies? Future HIV Ther. 2008;2(4):327–338. doi: 10.2217/17469600.2.4.327. [DOI] [Google Scholar]
- 8.Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, et al. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soundravally R, Hoti SL, Patil SA, Cleetus CC, Zachariah B, Kadhiravan T, et al. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2014;18:68–72. doi: 10.1016/j.ijid.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Castro R, Pinzón HS, Alvis-Guzman N. A systematic review of observational studies on oxidative/nitrosative stress involvement in dengue pathogenesis. Colomb Médica Cali Colomb. 2015;46(3):135–143. [PMC free article] [PubMed] [Google Scholar]
- 11.Halstead SB, Lum LC. Assessing the prognosis of dengue-infected patients. F1000 Med Rep. 2009;1. [DOI] [PMC free article] [PubMed]
- 12.CDC–Clinical and Laboratory Guidance–Dengue [Internet]. [cited 2016 Mar 22]. Available from: http://www.cdc.gov/dengue/clinicalLab/.
- 13.Calzavara-Silva CE, Gomes ALV, Maia RCC, Acioli-Santos B, Gil LHVG, Marques Jr ETA. Early molecular markers predictive of dengue hemorrhagic fever. An Acad Bras Ciênc. 2009;81(4):671–677. doi: 10.1590/S0001-37652009000400006. [DOI] [PubMed] [Google Scholar]
- 14.Rajendiran S, Lakshamanappa HS, Zachariah B, Nambiar S. Desialylation of plasma proteins in severe dengue infection: possible role of oxidative stress. Am J Trop Med Hyg. 2008;79(3):372–377. [PubMed] [Google Scholar]
- 15.Soundravally R, Sankar P, Bobby Z, Hoti SL. Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidation. Platelets. 2008;19(6):447–454. doi: 10.1080/09537100802155284. [DOI] [PubMed] [Google Scholar]
- 16.Soundravally R, Sankar P, Hoti SL, Selvaraj N, Bobby Z, Sridhar MG. Oxidative stress induced changes in plasma protein can be a predictor of imminent severe dengue infection. Acta Trop. 2008;106(3):156–161. doi: 10.1016/j.actatropica.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Young IS, Tate S, Lightbody JH, McMaster D, Trimble ER. The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 1995;18(5):833–840. doi: 10.1016/0891-5849(94)00202-U. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen HE, Prieme H, Loft S. Role of oxidative DNA damage in cancer initiation and promotion. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 1998;7(1):9–16. [PubMed] [Google Scholar]
- 19.Chandrasena LG, Peiris H, Kamani J, Wanigasuriya P, Jayaratne SD, Wijayasiri WA, et al. Antioxidants in patients with dengue viral infection. Southeast Asian J Trop Med Public Health. 2014;45(5):1015–1022. [PubMed] [Google Scholar]
- 20.Rasool M, Malik A, Khan KM, Qureshi MS, Shabbir B, Zahid S, et al. Assessment of biochemical and antioxidative status in patients suffering from dengue fever. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2015;35(3):411–418. doi: 10.1007/s11596-015-1446-x. [DOI] [PubMed] [Google Scholar]
- 21.Ray G, Kumar V, Kapoor AK, Dutta AK, Batra S. Status of antioxidants and other biochemical abnormalities in children with dengue fever. J Trop Pediatr. 1999;45(1):4–7. doi: 10.1093/tropej/45.1.4. [DOI] [PubMed] [Google Scholar]
- 22.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32(8):595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 23.Hosakote YM, Komaravelli N, Mautemps N, Liu T, Garofalo RP, Casola A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L991–L1000. doi: 10.1152/ajplung.00192.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurram M, Qayyum W, Hassan SJU, Mumtaz S, Bushra HT, Umar M. Dengue hemorrhagic fever: comparison of patients with primary and secondary infections. J Infect Public Health. 2014;7(6):489–495. doi: 10.1016/j.jiph.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Soundravally R, Sherin J, Agieshkumar BP, Daisy MS, Cleetus C, Narayanan P, et al. Serum levels of copper and iron in dengue fever. Rev Inst Med Trop São Paulo. 2015;57(4):315–320. doi: 10.1590/S0036-46652015000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TDR|Dengue–Guidelines for diagnosis, treatment, prevention and control [Internet]. WHO. [cited 2016 Mar 24]. Available from: http://www.who.int/tdr/publications/training-guideline-publications/dengue-diagnosis-treatment/en/.
- 27.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179(4):755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 28.Klassen P, Biesalski HK, Mazariegos M, Solomons NW, Fürst P. Classic dengue fever affects levels of circulating antioxidants. Nutr Burbank Los Angel Cty Calif. 2004;20(6):542–547. doi: 10.1016/j.nut.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Olagnier D, Peri S, Steel C, van Montfoort N, Chiang C, Beljanski V, et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014;10(12):e1004566. doi: 10.1371/journal.ppat.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons CP, Popper S, Dolocek C, Chau TNB, Griffiths M, Dung NTP, et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis. 2007;195(8):1097–1107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolfvenstam T, Lindblom A, Schreiber MJ, Ling L, Chow A, Ooi EE, et al. Characterization of early host responses in adults with dengue disease. BMC Infect Dis. 2011;11:209. doi: 10.1186/1471-2334-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page A, Volchkova VA, Reid SP, Mateo M, Bagnaud-Baule A, Nemirov K, et al. Marburgvirus hijacks nrf2-dependent pathway by targeting nrf2-negative regulator keap1. Cell Rep. 2014;6(6):1026–1036. doi: 10.1016/j.celrep.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Komaravelli N, Tian B, Ivanciuc T, Mautemps N, Brasier AR, Garofalo RP, et al. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic Biol Med. 2015;88(1):391–403. doi: 10.1016/j.freeradbiomed.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvajal-Yepes M, Himmelsbach K, Schaedler S, Ploen D, Krause J, Ludwig L, et al. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. 2011;286(11):8941–8951. doi: 10.1074/jbc.M110.186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung-man Ho J, Zheng S, Comhair SA, Farver C, Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61(23):8578–8585. [PubMed] [Google Scholar]
- 37.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 38.Al-Alimi AA, Ali SA, Al-Hassan FM, Idris FM, Teow S-Y, Mohd Yusoff N. Dengue virus type 2 (DENV2)-induced oxidative responses in monocytes from glucose-6-phosphate dehydrogenase (G6PD)-deficient and G6PD normal subjects. PLoS Negl Trop Dis. 2014;8(3):e2711. doi: 10.1371/journal.pntd.0002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers RM, Varkey MJ, Reuben R, Jesudass ES. Dengue outbreak in Vellore, southern India, in 1968, with isolation of four dengue types from man and mosquitoes. Indian J Med Res. 1970;58(1):24–30. [PubMed] [Google Scholar]