Abstract
Heart failure (HF) is a physiological state in which cardiac output is insufficient to meet the needs of the body. It is a clinical syndrome characterized by impaired ability of the left ventricle to either fill or eject blood efficiently. HF is a disease of multiple aetiologies leading to progressive cardiac dysfunction and it is the leading cause of deaths in both developed and developing countries. HF is responsible for about 73,000 deaths in the UK each year. In the USA, HF affects 5.8 million people and 550,000 new cases are diagnosed annually. Cardiac remodelling (CD), which plays an important role in pathogenesis of HF, is viewed as stress response to an index event such as myocardial ischaemia or imposition of mechanical load leading to a series of structural and functional changes in the viable myocardium. Protein kinase C (PKC) isozymes are a family of serine/threonine kinases. PKC is a central enzyme in the regulation of growth, hypertrophy, and mediators of signal transduction pathways. In response to circulating hormones, activation of PKC triggers a multitude of intracellular events influencing multiple physiological processes in the heart, including heart rate, contraction, and relaxation. Recent research implicates PKC activation in the pathophysiology of a number of cardiovascular disease states. Few reports are available that examine PKC in normal and diseased human hearts. This review describes the structure, functions, and distribution of PKCs in the healthy and diseased heart with emphasis on the human heart and, also importantly, their regulation in heart failure.
Keywords: Protein kinase C, Heart failure, Hypertrophy, Fibrosis, Cardiac remodelling
Heart failure
Cardiovascular diseases (CVDs) are composed of several different pathologies, including coronary ischemic heart disease, rheumatic heart disease, congenital cardiovascular defects, high blood pressure, heart failure, stroke, arrhythmias, myocardial infarction, and diseases of the arteries including endothelial dysfunction and atherosclerosis. Despite significant progress in the prevention and treatment of CVDs, statistics indicate that CVDs are the leading cause of deaths throughout the world [1]. The World Health Organization (WHO) estimates that CVDs are responsible for 17.5 million deaths in 2012, representing 31% of all global deaths. Of these, 7.4 million died of ischaemic heart disease and 6.7 million from stroke.
According to the American Heart Association [2], CVDs accounted for 31.3% (786,641) of all deaths (total of 2,515,458) in 2011. On the basis of 2011 death rate data, mortality owing to CVDs accounted an astounding 2150 people dying daily with an average of 1 death every 40 s.
Heart failure (HF) is a clinical syndrome characterized by impaired ability of the left ventricle to either fill or eject blood [3]. American Heart Association (AHA) statistical update in 2015 reported that 1 in 9 deaths has HF mentioned on the death certificate and data from 2011 revealed that HF any-mention mortality was 284,388 (129,635 males and 154,753 females). In 2012, total cost for HF was estimated to be $30.7 billion, of which a total of 68% was attributed to direct medical cost. Projection shows that by 2030, the total cost of HF will increase almost to $69.7 billion from 2012 in the USA [4].
HF can no longer be considered a simple contractile disorder or a disease of the heart alone. It is now accepted that as heart disease progresses into HF, heart size increases, cardiac function deteriorates, and symptoms of HF become evident. The aetiology of HF is diverse and it includes hypertension, myocardial infarction, arrhythmias, bacterial endocarditis, ischaemia, idiopathic and diabetic cardiomyopathy, coronary heart disease, and congenital cardiovascular defects. Of these aetiologies, coronary artery disease and myocardial infarction are the most common [5].
Protein kinase C
Discovery and structure
Protein kinases C (PKC) were identified over three decades ago, as kinases that are activated by proteolysis [6]. Initially identified as a nucleotide-independent, Ca2+-dependent serine kinase, PKCs are a family of serine/threonine kinases that are activated as a result of receptor-dependent activation of phospholipase C and the hydrolysis of membrane phosphoinositides [7]. PKCs are now known to be major mediators of signal transduction pathways and have been shown to regulate sets of biological functions as diverse as cell growth, differentiation, apoptosis, transformation, tumourigenicity, and others [8, 9].
According to differences in the binding capability of their regulatory domain, the presently known 13 members of the PKC family have been grouped into 3 classes: the classical PKCs (α, β1, β2, γ), novel PKCs (δ, ε, η, θ), and atypical PKCs (ζ, λ/ ι) [9, 10].
The first PKCs to be identified and cloned were α, β, and γ isozymes, initially isolated from brain complementary DNA (cDNA) libraries [11]. Low-stringency screening of brain cDNA libraries with probes derived from the α, β, and γ isozymes yielded three additional PKCs, the PKC-δ, PKC-ε, and PKC-ζ isozymes [12], and further low-stringency screens of other tissue cDNA libraries led to identification of PKC-η [13], PKC-θ [14], and PKC-ι (the mouse ortholog of λ in humans) [15]. At present, there are over 450 protein kinases in the human genome [16].
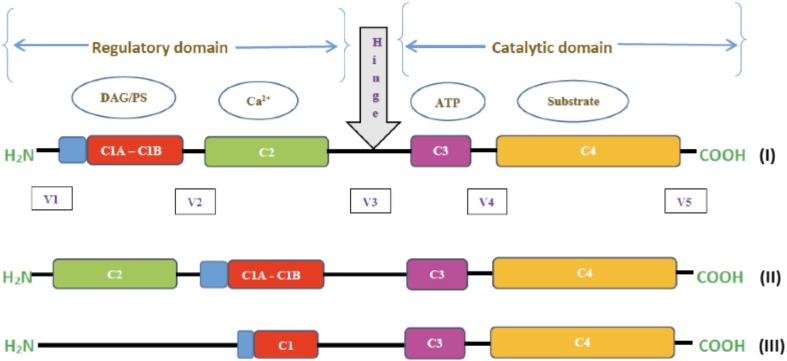
All PKCs have a common general structure composed of a single polypeptide chain with two principal modules including a NH2-terminal regulatory domain that contains the membrane-targeting motifs and a COOH-terminal catalytic domain that binds ATP and substrates (see Fig. 1). Initial research in 1986 by Caussens et al. [11] revealed that throughout the primary sequence of the enzymes, there are four conserved (C1–C4) regions, with each region being a functioning module, and are flanked by variable (V) regions.
Fig. 1.
Schematic representation of the primary structure of PKC gene family. PKC isoenzymes are composed of single polypeptide chains that consist of regulatory and catalytic domains. Indicated are a series of conserved (C1–C4) regions and variable regions (V1–V5). The C1 region (red) consist of a cys-rich motifs, C2 (green) is the calcium binding region, C3 (purple) comprises the ATP binding lobe, and C4 (gold) is the substrate binding lobe. Also indicated is the pseudosubstrate domain (blue) in the V1 region. The regulatory and catalytic domains are separated by V3 (hinge). Structure (I) represents cPKC: α, β1, β11, γ, structure (II) represents nPKC: δ, ε, η, θ, and structure (III) represents aPKC: ζ, λ/
cPKCs (α, β1, β2, γ)
The classical PKC consists of five variable and four conserved regions (C-regions). The catalytic central part is found in the C4 region; the C3 region contains the ATP binding site. The C2 region contains the recognition site for acidic lipids and also, it is responsible for binding (Ca2+), while the C1 region is responsible for diacylglycerol or phorbolester (e.g. phorbol-12,13-myristate-acetate (PMA)) binding and consists primarily of two cysteine-rich ‘zinc-finger-like’ regions. The activity of this group depends on Ca2+ and on the presence of phospholipids (DAG) and phosphatidylserine.
nPKCs (δ, ε, η, θ)
For the novel PKCs, they are structurally similar to the conventional cPKCs. However, the C2 region does not have functional groups to mediate Ca2+ binding and thus, it does not depend on Ca2+, but requires dioleoylglycerol and phatidylserine for their activation.
aPKCs (ζ, λ/ι)
The atypical PKCs are the third group of isozymes and these differ significantly in structure from the previous two groups. The C1 region contains only one of the cysteine-rich motif and the C2 region is absent. These isozymes, therefore, do not depend on Ca2+ for activation and they also lack sensitivity to dioleolglycerol/phorbolesters. Research has further shown that these isozymes are targets of lipid-derived secondary messengers [17] and may be activated by lipids such as arachidonic acid and phosphatidylinositol 3,4,5-triphosphate. Initial studies by Nishizuka [9] revealed that protein kinase C was involved in lipid signalling for sustained cellular responses. The catalytic and regulatory halves in PKCs are separated by a hinge region that is proteolytic [18] which results in a constitutively active kinase [6]. Further detailed works on PKC structure are described in other studies [19–22].
Regulations
PKCs are central enzymes in the regulation of cell growth and hypertrophy and play a major role in signal transduction in the heart. Initial work, mostly using phorbol esters, showed that PKC is a critical enzyme in regulation of cell growth and differentiation [23], in the phosphorylation of substrates [24], in stimulation of other proteins such as kinases [25], in the regulation of ion channel and receptors [26], and altered gene expression [27]. It has been reported that PKC activation plays a critical role in the development of delayed preconditioning by translocating to the perinuclear region to induce gene expression or by activating mitogen-activated protein kinases (MAPK). Although these initial studies were significant, phorbol esters are not izozyme-selective and therefore, it was not possible to identify which isozymes regulate a given function.
Intracellular events, associated with response to circulating hormones, trigger activation of PKC. These events can influence various physiological processes in cardiovascular system, resulting in chronotropic and inotropic effects [28]. Numerous studies based on animal models have implicated PKC activation with a number of cardiac diseases and heart failure, with much of the initial focus being placed on cardiac ischaemia [29–32]
PKC isozymes expression in the heart and various tissues
PKC isozymes are ubiquitously expressed in all tissues at all times of development. Extensive experimental research indicates that different PKC isoforms serve distinct biological functions [27, 33–35]. Interestingly, it has been observed that PKC isoforms differ in their tissue distribution. Analysis, using Northern blotting immune-blotting techniques, revealed that many isozymes are widely expressed in a variety of tissues, while others are only expressed in a few tissues. In addition to their tissue distribution, PKC isoforms have been shown to differ with respect to substrate specificity [27, 36–40] and their susceptibility to downregulate upon phorbol ester treatment [9]. Several studies have revealed that there exist distinct individual functions in vitro studies among PKC isoforms. Examples of such isoforms include PKC-α and PKC-β phosphorylate histone IIIS strongly, while the other isozymes do so weakly, if at all [27]. Johnson et al. [34] investigated the spontaneous rate of contraction of neonatal rats and found that myocytes can be inhibited by activation of PKC-ε, but not by PKC-α, PKC-β, PKC-δ, or PKC-ζ. Studies relating to in situ binding of individual PKCs to specific intercellular proteins have not been well investigated.
Knowledge of the expression of PKCs in tissues is an important factor to help in understanding which PKC isozymes are involved in specific cardiovascular functions. PKCs have demonstrated to have sometimes opposing roles in both normal and diseased states [41], and Basu et al. [42] have shown that depending on stimulation, they can have opposing roles in the same cell. The relative content of each isozyme in the heart has been a controversial issue since it was found as different in different species. Numerous studies have investigated PKC expression pattern in cardiac tissues from various mammalian species including rats [43–52], rabbits [53–55], guinea pig [56], hamster [57], and dog [58].
Initial research by Hug et al. [59] showed that PKC-α, PKC-β, PKC-δ, PKC-ε, PKC-λ, and PKC-ζ were found to be widely distributed in many tissues, including the muscle, brain, lung, skin, and heart. Studies indicate [14] that PKC-θ is mainly expressed in the skeletal muscle, platelets, haematopoietic cells, and endothelium. In one of the first reports characterizing the expression of PKC isoenzymes in the heart, PKC-ε was described as the principal, if not the only PKC isoenzyme to be expressed in the rat heart [51]. Khalil et al. [60] and Liou et al. [61] reported the presence of PKC-(α, β, δ, ε, ζ) in vascular smooth muscle, while these isoenzymes, in addition to PKC-η and PKC-θ, were found to be expressed in endothelium platelets. Later, many studies identified the presence of PKC-α, PKC-δ, PKC-ε, PKC-η, and PKCs-ζ in rat-cultured cardiomyocytes [62–64], and even PKC-γ that was considered to be present only in the nervous system and adrenal tissues was found in the rabbit heart [32, 54]. Abundant expression of both βI and βIIPKC in human cardiomyocytes has also been reported [38, 65–68]. However, with the vast amount of studies on animal hearts, there exist only few reports available that examined the expression of PKCs in human hearts.
Bowling et al. [66] identified expression of PKC-α, βI, βII, and ε in human heart tissues using antibodies by Western blot analysis. Work done by Shin et al. [68] represented the first comprehensive study of PKC isoform expression in human ventricle, utilizing antibodies directed against all known PKC isoforms. The findings from their work, performed by Western analysis and immune-histochemistry, revealed that all isoforms, except PKC-ϒ and PKC-θ, were detected, indicating that in human ventricular myocytes, PKC expression is remarkably diverse. The findings of another study carried out by Simonis et al. [67], using polyclonal antibodies/monoclonal antibodies by Western blot analyses technique, revealed that in the human heart, six isoforms of PKC are expressed. These are PKC-α, PKC-β, PKC-δ, PKC-ε, PKC-λ, and PKC-ζ. PKC-γ and PKC-θ were not present in the human heart, consistent with previous finding. This study also highlighted the importance in relative distribution between atria and ventricles. PKC-ζ and PKC-δ are primarily expressed in the atria, while PKC-α, PKC-βI, and PKC-βII, which are all Ca2+-dependent, reside predominantly in the ventricle. PKC-ε and PKC-λ are evenly distributed in both atria and ventricles.
PKC isozymes in cardiac diseases and heart failure
In addition to roles in regulations, alterations in PKC levels are associated with normal cardiac development. PKC-α, PKC-β, PKC-ε, and PKC-ζ expressions are high in foetal and neonatal hearts but decrease in expressions in adult hearts [69]. However, it was shown [66] that during the process of heart failure in humans, the levels of PKC-α, and PKC-β isozymes increase.
Mounting evidence suggests, and it has also been observed that individual or multiple PKCs are involved in cardiac diseases and heart failure [69]. These include, but not limited to, atherosclerosis [70], myocardial infarction, acute ischaemic [30, 55, 71–73], cardiac hypertrophy [29, 74], cardiac arrhythmia [75], heart failure [76], and cardiac fibrosis [77].
Myocardial infarction and ischaemia preconditioning
Ischaemic heart disease continues to be the leading cause of death in Western countries. Over the past two decades, significant effort, especially with preliminary work done by Ytrehus et al. [78], has been devoted in understanding the role of specific PKCs in cardiac diseases. Preconditioning can be described as a natural cardiac-protective mechanism, and it involves subjecting the heart to brief periods of ischaemia and reperfusion prior to a longer ischemic period. Preconditioning protects the heart from ischaemia and reperfusion-induced damage [79] by inducing myocardial adaptation to the ensuing prolonged ischemic event. In other words, a brief period of ischaemia followed by reperfusion renders the heart more resistant to injury from a subsequent longer ischaemic insult instead of accentuating the injury. These results, using a canine heart, were some of the first to highlight the fact that direct PKC activation prior to ischaemic event provides cardiac protection. Based upon seminal observations from these experiments, the term ischemic preconditioning (IPC) was used to describe this phenomenon. IPC was subsequently shown to be effective in other species including rats [80], sheep [81], rabbits [82], and pigs [83].
The role of PKCs in ischemic preconditioning is now well established in a variety of mammalian models including rats [84–86], rabbits [78, 87], and canine [88] where different specific PKCs have been found in various animal species. However, although the complex choreography of activation or inhibition of various isoforms of PKC with ischaemia and reperfusion has been worked out in animal models over the past 30 years, there has been no success in translating this knowledge into useful therapy in humans.
Research work by Yellon et al. [89] was one of the first studies of IPC in humans where they examined whether a preconditioning protocol protects the myocardium from prolonged ischaemia. Their study showed that preconditioning ultimately leads to a preservation of ATP levels in preconditioned human hearts in contrast to non-preconditioned hearts. Subsequent studies by Yellon et al. [90–93] and others [94–96] provided further evidence for PKC involvement in human IPC.
The mechanism of preconditioning is still a subject of debate. One of the earlier favoured hypotheses for preconditioning suggests that endogenous ligands such as adenosine initiate an intracellular pathway by acting on G protein-linked receptors leading to the activation of PKC via diacylglycerol [78]. After which, activated PKC then phosphorylates a secondary effector protein, which is thought to induce protection.
There have been supportive [97] and conflicting reports [98, 99] with respect to the role of PKCs in ischaemic preconditioning. It was even suggested [100] that PKC might rather be a ‘spectator’ rather than a ‘player’, that is, it seems likely that PKC activation is an epiphenomenon rather than a mandatory or exclusive means of preconditioning the heart. Subsequent studies suggest that cardiac preconditioning inhibits both apoptosis and necrosis [101]. Earlier conflicting data were related to the initial use of non-selective individual PKC activators/inhibitors such as diacylglycerol (DAG), indolocarbazole, and bisindolymaleimides [102, 103] that exhibited poor selectivity for PKC isozyme. Subsequent studies, using selective isozyme-specific inhibitors and activators (6–10 amino acids in length), helped to explain earlier reported uncertainty.
Translating ischemic conditioning from animal models to human
While on the topic of IPC, it is important to briefly discuss challenges of translation of cardioprotection, its limitations in human studies, and need for PKC manipulation in ischaemia/reperfusion. Translation of cardioprotection can be characterized as a four-step process from (1) reductionist animal studies to (2) more clinically relevant animal studies, to (3) clinical proof-of-concept studies with surrogate end points such as infarct size, and to finally (4) clinical outcome trials [104].
Since the first clinical study conducted to test external application of an IPC stimulus in patients undergoing coronary artery bypass graft (CABG) surgery, more than 150 clinical trials have been conducted and thousands of experimental animal studies on mechanical and pharmacological conditioning and cardioprotective interventions. However, the concept on the translation of cardioprotection strategies to clinical practice continues to disappoint. There is yet no single randomized clinical trial, which has explicitly demonstrated a better clinical outcome for patients experiencing an acute myocardial infarction or undergoing cardiovascular surgery when receiving an adjunct cardioprotective.
In the field of cardioprotection, substantial gaps still remain between experimental studies aiming at the identification of novel mechanisms and studies providing robust preclinical data that are worth of being tested in humans.
The critical time frame for adjunct cardioprotection that depends on factors such as (1) species (2) heart rate, and (3) residual blood flow still constitutes a major problem [105]. Systematic animal studies on the time frame for adjunct cardioprotection, in interaction with the above listed variables, are lacking while the exact time frame for adjunct cardioprotection in humans is not really clear.
A very important fact when extrapolating from animal models to humans is that it is vital to understand the differences between animal models and patients. Most animal experiments, including larger mammals that are closer to humans in their anatomy and physiology, are performed in young and healthy animals that lack the risk factors. Compare this to older individuals with cardiovascular disease who participate in clinical trials, with comorbidities such as diabetes, hypertension, kidney disease, and are taking medications [106]. Secondly, the effectiveness of ischemic-conditioning strategies in humans seems to be less profound than reported in the animal literature, with some randomized clinical trials showing no significant benefit [107, 108]. These disparities are keys to understanding why ischemic-conditioning strategies fail to translate from animals to humans.
The results of large, multi-centre, randomized, controlled clinical trials of ischemic conditioning on clinical outcomes after cardiac surgery have highlighted the challenges in translating cardioprotection into clinical practice. In future, it is recommended that only results that have been proven robust in multi-centre approaches be worth tested for translation to patients.
With respect to PKC kinases and translation cardioprotection, investigation of signalling pathways underlying ischemic conditioning has identified molecular targets for pharmacological manipulation—a therapeutic strategy termed ‘pharmacological cardioprotection’. The PKC family of kinases plays essential roles in CVDs and has been linked as playing an important role in the reperfusion injury salvage kinase (RISK) pathway in IPC mechanism. Since this technique of pharmacological manipulation was realized, there has been much excitement on the role of kinases in PKC manipulations in IPC. However, over time, it has been revealed that there does not appear to be any translational-clinical science benefit on the horizon for manipulation of PKC in ischaemia/reperfusion. This currently disappointing situation has led many clinicians to prematurely give up on attempts of PKC pharmacological cardioprotection beyond rapid reperfusion with more focus being placed on long-term cardiovascular therapies.
PKC-δ and PKC-ε in myocardial infarction, ischemic reperfusion, and preconditioning
Although they are members of the same subgroup (the so-called novel group), PKC-δ (commonly referred to as pro-death kinase) and PKC-ε (commonly referred to as pro-survival kinase) mediate contrasting and even opposing effects. They are both activated in the ischaemic human heart [109] where they play a key role in ischaemic preconditioning. However, the mechanism and exact role of PKC in the survival of cardiac cells remain unknown and controversial with research confirmed that these two related PKC isozymes have both parallel and opposing effects in the heart, indicating the danger in the use of therapeutics with non-selective isozyme inhibitors and activators [110]. Studies by Hassouna et al. [65], using various specific PKC inhibitors, investigated which PKCs were involved in IPC of the human atrial myocardium sections using the temporal relationship to the opening of mitoKATP channels. The results, with reference to PKC-δ and PKC-ε, showed that PKC-ε inhibitors blocked IPC of the human myocardium and is upstream of mitoKATP channels while PKC-δ inhibitors did not blocked IPC.
Ischaemia and reperfusion cardiac damages have shown [111, 112] to be dependent on translocation of PKC-δ into the mitochondria where cytochrome c is released resulting in inhibition of mitochondrial functions. It has been suggested that oxidative stress seems to trigger PKC-δ into the mitochondria [113]. PKC-δ activation results in phosphorylation steps [114] and inhibition of ATP regeneration. Cardiac mitochondrial inhibition now triggers higher reactive oxygen species (ROS) production and built up of reactive aldehydes (e.g. 4-hydroxynonenal (4-HNE), methylglyoxal (MGO), and others), which can become toxic at accumulated levels [115]. With a combination of diminished levels of ATP, accumulated of ROS, and toxic aldehydes, this results in accumulation of aggregated proteins and an inactive 26S proteasome, ultimately, leading to both apoptosis and necrosis [41] followed by severe cardiac dysfunction. It is no surprise, as numerous research studies have now shown, that PKC-δ inhibition will result in opposite effects to that of its activation. That is, PKC-δ inhibition at reperfusion is protective (refer to schematic diagrams in Fig. 2a, b).
Fig. 2.
The role of PKC isozymes in ischaemic heart disease. Schematic diagram showing a how ischaemic preconditioning prior to prolonged ischaemia and reperfusion provides cardioprotection by activating more PKC-ε, which translocate into the mitochondria and prevents mitochondrial dysfunction induced by prolonged ischaemia and reperfusion. b In contrast, prolonged ischaemia and reperfusion result in activation of PKC-δ more than PKC-ε, leading also to translocation of PKC-δ into the mitochondria. Mitochondrial dysfunction and increase in ROS lead to both apoptosis and necrosis and severe cardiac dysfunction
Additionally, it is now recognized that IPC consists of two (2) chronologically and patho-physiologically distinct phases comprised of an early phase and a late phase of protection. Stein et al. [116] have reported that PKC-ε activation facilitates the protective effects of late preconditioning. That is, precondition stimuli enhance the resistance of the heart to ischaemia injury 12–72 h later.
Inagaki et al. [117] have shown that PKC-δ inhibition reduces reperfusion injury to the myocardium by inhibiting both apoptosis and necrosis. Further work [110] using selective peptide inhibitors (δV1–1) has demonstrated that inhibition of PKC-δ protects the heart from ischaemic injury and further, PKC-δ activation is cardioprotective provided that there is sufficient time allowed for PKC-ε activation. These findings are in accordance with a role for PKC-δ in apoptosis as previously demonstrated by overexpression of PKC-δ [118]. It has been suggested also that inhibition of PKC-δ should be a target for drug development to prevent irreversible cardiac injury during reperfusion in humans.
Interestingly, it has been reported [109] that activated PKC-δ has two potential fates that, apparently, depend on the metabolic rate of the cell. These include (1) if the integrity of the 26S proteasome, mitochondrial function, and cellular energy is maintained, PKC-δ is effectually degraded and (2) conversely, if the aforementioned parameters are not maintained, the result is an accumulation of elevated levels of activated PKC-δ (pro-death kinase) in the mitochondria.
PKC-ε isozyme is also translocated into the mitochondria by stimuli; however, its activation has shown (in contrast to PKC-δ) to be protective [110, 119, 120] when occurring just before reperfusion. In addition, it also prevents mitochondrial dysfunction induced by prolonged ischaemia events. Mitochondrial protection is achieved by PKC-ε phosphorylation followed by activation of aldehyde dehydrogenase 2 [ALDH2]—which removes the harmful aldehyde (4HNE; MGO) and peroxidation by-products [115]. Research work by Chen et al. [121] showed that this mitochondrial enzyme (ALDH2) correlated with reduced ischaemic heart damage in rodent models, in some cases, reduced infract size by 60%. The above steps now result in lower ROS concentration, promote faster recovery of ATP and faster removal of aggregated proteins, promote an active 26S proteasome, and ultimately result in reducing cellular damage—diminished apoptosis and necrosis. In addition to the aforementioned factors and cardio benefits from PKC-ε activation, upon translocation into the mitochondria, PKC-ε isozyme is involved/initiated a number of processes that help to contribute in overall cardioprotection. These include, but not limited to, the following: firstly, opening of KATP channels—this channel opens as ATP levels fall and is inhibited when levels are high. The KATP channel, which exists in both mitochondria and sarcolemmal membrane has been recognized by Gross et al. [122] to be a likely end effector of ischaemic preconditioning, and earlier work [123] suggests that the sarcolemmal channel surface might be an important effector of the cardioprotective effects of ischemic/hypoxic preconditioning. Secondly, restrict mitochondria permeability transition pore (MPTP) from opening—this pore has been identified by Bains et al. [124] and others [125] as an effector of preconditioning. PKC-ε interacts with and inhibits the MPTP and, thus, stabilizes mitochondria in cardiac tissue during and following ischaemia. This pore, which allows water and solutes to enter the mitochondria, is closed during ischaemia and opens in the first few minutes of reperfusion [126]. It ultimately inhibits the pathological function of the pore and contributes to PKC-ε induced cardioprotection. Thirdly, increase in cytochrome c activity—PKC-ε co-immuno-precipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome c oxidase activity and cardioprotection [127]. Finally, it was reported [109] that the active proteasome that results from PKC-ε activation is capable of selectively degrading activated PKC-δ, thereby altering the ratio between PKC-ε and PKC-δ, increasing the favour of pro-survival kinase (PKC-ε), and ultimately regulating myocardial sustainability.
Cardiac hypertrophy and heart failure
Cardiac hypertrophy is a thickening of the interventricular wall and/or septum in the cells and it involves complex multiple progressive alterations of the heart geometry in response to either mechanical, electrical, or neuro-humoral stimuli such as epinephrine, norepinephrine, aldosterone, and angiotensin II. It may be further characterized with increase in cardiomyocyte size, increased protein synthesis, and changes in the organization of the sarcomeric structure. Although short-term subcellular changes (cardiomyocyte enlargement, formation of new sarcomeres, etc.) associated with cardio hypertrophy may be beneficial, however, when sustained for longer intervals, the cardiac system becomes maladaptive. This eventually leads to decompensation resulting in fibrosis, apoptosis, and cardiac remodelling among other cardiac diseases before transitioning to heart failure. Hypertrophy is therefore an early indication during clinical course of heart failure and plays an important risk factor for subsequent cardiac death.
Cardiac hypertrophy can be placed into three categories—(1) normal growth, (2) growth induced by physical condition, and (3) growth induced buy pathological stimuli—and various kinases have been identified as mediators in response to activation induced by neuro-hormone receptors [128].
Protein kinase C family have been identified as having important roles in adaptive and maladaptive cardiac responses. In cultured myocytes, it has been found that PKCs regulate contractibility and hypertrophy [128]. Studies have identified the intercellular mechanism underlying cardiac hypertrophy and PKC isozymes as potential mediators of hypertrophic stimuli [76, 129], and it has also been suggested that induced stress associated with cardiac hypertrophy coupled with PKC activation will increase PKC expression and activity [130]. As previously mentioned, PKC expression in cardiac tissue differs with species, cell type, and developmental stage. Importantly, the activity of PKCs is dependent upon its localization within the cell, expression level, and phosphorylation [131]. The following chapter focuses on the role of PKC isoforms in the aetiology of cardiac hypertrophy and heart failure.
PKC-δ and PKC-ε in cardiac hypertrophy and heart failure
In contrast with preconditioning to ischaemia in which PKC-δ and PKC-ε have opposite roles, both act in the same direction during the development of hypertrophy [110]. The activation of PKC-ε may be a factor that induces ventricular hypertrophy with its positive effect on cell growth. In this line, the relation between PKC-ε and the cytoskeleton is a mechanism that potentially initiates hypertrophy via phosphorylation of proteins in the costameres, which then transmit signalling to the Z-disk for parallel or series addition of thin filaments regulated via actin capping [132].
The activation of PKC-ε was shown during stretch of cardiomyocytes [133]. In isolated guinea pig hearts, stretch, one of the principal activators of ventricular hypertrophy, has been shown to induce a PKC-ε translocation to membranes that was partially inhibited by losartan [56]. In vivo, an induction of concentric cardiac hypertrophy with an overexpression of constitutively active PKC-ε [134] or with the expression of cardiac specific PKC-ε activator [135] was shown. The effect of PKC-ε is in general considered to lead to a concentric hypertrophy. However, in mice overexpressing PKC-ε [136], the evolution of hypertrophy was quite deleterious since it led to a dilated cardiomyopathy. Thus, the effect of PKC-ε may differ depending upon its level of expression.
PKC activity has been generally described as increased with different behaviours of different isozymes. In general, PKC-ε and PKC-δ increased content and translocation towards the membrane fraction was found but this is not a universal finding in all types of hypertrophy. In aortic banding in rats [137], guinea pigs [138], and in severe human aortic stenosis [67], an increased concentration of PKC-ε was found in membranes. In contrast, PKC-δ content was found as unchanged in nuclear-cytoskeletal fraction in the model of rat aortic banding [137]. Other researchers found the same translocation in a completely different type of hypertrophy, right ventricular hypertrophy induced by pulmonary hypertension due to chronic hypoxia in rats [139]. However, opposite results were described in hypertrophy or heart failure by others [66, 32, 140–142]. In human failing hearts, left ventricular PKC-ε content was decreased [67]. In rabbit left ventricular hypertrophy, researchers have found a decreased cardiac content of PKC-ε and a similar downregulation was demonstrated in a model of genetic hypertension while PKC-δ was unaffected [141]. In contrast, PKC-ε activity was found to be unchanged in rat aorto-caval fistulas while PKC-δ was increased [142]. Although PKC-ε is an actor in the development of hypertrophy, its expression in the myocardium and its translocation are not found as increased in all models. More recently, it has been suggested that PKC-ε inhibition attenuates pathological remodelling in hypertension-induced heart failure by preventing cardiac mast cell degranulation [143].
PKC-ε has been shown to bind scaffold proteins. In the heart, F-actin bound PKC-ε selectively over PKC-δ [144] and it was shown that the binding interface between PKC-ε and cardiac myofilaments was mainly on the V1 region of PKC-ε and the interface between PKC-ε and F-actin was mainly on the C1 region of PKC-ε [144].
PKC-β in cardiac hypertrophy and heart failure
PKC-β was chosen as the first isoenzyme [145] to be studied using cardiac target expression and has been shown to play an important role in cardiac hypertrophy. One of the main reasons for this being is its reactivity and expression increases in human heart failure [67]. The result showed that the calcium dependant PKC-β (stained as a triple band containing both splice variants {PKC-β1 and PKC-β11}) resides predominantly in the ventricular myocardium. They also demonstrated that in downregulation during ontogenesis in human hearts, PKC-β expression was decreased by 90%—that is, this isoform is almost totally switched off in normal adult non-failing cardiac heart. PKC-β is highly upregulated, leading to re-expression in dilated cardiomyopathy originating from severe heart failure.
Using explanted heart from patients in whom dilated cardiomyopathy was diagnosed, Bowling et al. [66] examined PKC isoforms present in these samples. Their results showed a quantitative increase of >40% in PKC-β1 and PKC-β11 membrane expression in failed human hearts compared with non-failed hearts. They also reported a reduction in membrane activity from failed hearts of 209 pmol min−1 mg−1 when a selective PKC-β inhibitor (LY333531-macrocyclic bis maleimide) was used (compared with 45.2 pmol min−1 mg−1). An important conclusion in the finding from their research is that in failed human heart, PKC-β1 and PKC-β11 expression and contribution to the total PKC activity are significantly increased.
PKC-α in cardiac hypertrophy
With respect to the conventional isoforms, PKC-α is the predominant subtype expressed in the mouse, human, and rabbit hearts, while PKC-β and PKC-γ are detectable but expressed at substantially lower levels [138, 146, 147]. Although it is the most highly expressed of the myocardial PKC isoforms, PKC-α is the least studied because unlike PKC-δ and PKC-ε, it is not regulated in acute myocardial ischaemia [148] and in contrast to PKC-β, it is not regulated in diabetes [145]. Reports have associated PKC-α activation or an increase in PKC-α expression with hypertrophy, dilated cardiomyopathy, ischaemic injury, or mitogen stimulation [128]. Increased expression of PKC-α was also observed following myocardial infarction [67]. Human heart failure has also been associated with increased activation of conventional PKC isoforms, including PKC-α [66, 67]. Thus, PKC-α fits an important criterion as a therapeutic target; its expression and activity are increased during heart disease. Initial comparative analysis of PKC isoforms [149] using wild-type or dominant inhibitory forms of PKC-α, PKC-β2, PKC-δ, and PKC-ε suggested that only PKC-α was sufficient to stimulate cell hypertrophy and only inhibition of PKC-α inhibited agonist-mediated hypertrophy. The implication of this work [149] was that PKC-α is a key regulator of cardiomyocyte hypertrophic growth.
The concept that PKC-α is of a greater importance as a regulator of myocardial contractility vs. cardiac hypertrophy was highlighted by Hahn et al. [150] using RACK binding and pseudo-RACK peptides derived from PKC-β. Previous studies [151, 152] have demonstrated that chronic activation of PKC-α diminished baseline ventricular ejection performance and, in combination with Gq-mediated hypertrophy, caused a lethal cardiomyopathy. In contrast to this, chronic PKC-α inhibition improved myocardial contractility and inhibited Gq-mediated cardiac hypertrophy [150]. The results of these studies showed that PKC-α is a critical determinant of myocardial systolic function but has minimal effects on cardiac hypertrophy.
Cardiac fibrosis
Cardiac fibrosis is the accumulation of fibroblasts that result from the expansion of the cardiac extracellular matrix proteins such as collagen, by augmented release from fibroblasts or reduced degradation of collagen. Cardiac fibrosis is crucial for scar formation after acute myocardial infarction (AMI). Ischemic injury results in increased levels of circulating cytokines, growth factors, and hormones that stimulate cell surface receptors on cardiac fibroblasts.
Fibrosis reduces the flexibility of myocardial tissue resulting in diastolic dysfunction, leading to myocardial malfunctioning (increased thickening of extracellular matrix, decreased cardiac elasticity), and consequently posing detrimental effects to failing hearts. Additionally, increased collagen content disrupts electrical connectivity between cardiomyocytes resulting in arrhythmogenesis [153].
Role of PKC isozymes in cardiac fibroblast proliferation
PKC isozymes contribute to different stages of cardiac fibroblast proliferation [153–155]. Fibroblast adhesion to the extracellular matrix has shown to be regulated through PKC-ε (via βI-integrin) while upregulation of cytokine and growth factors are mediated by PKC-α, PKC-βII, PKC-δ, PKC-ε, and PKC-ζ. In addition, PKC-δ, PKC-ε, and PKC-ζ have been demonstrated to regulate fibroblast proliferation with PKC-δ and PKC-ζ yielding opposing results in fibroblast [156].
PKCs also regulate activity and concentrations of matrix metalloproteinase (MMP), which facilitate the motility of cardiac fibroblast [157, 158]. It has been demonstrated that PKC-θ and PKC-ζ increase activities of both MMP-2 and MMP-9 via ERK pathways in cardiac fibroblast [151]. However, in the JNK-dependent pathway, PKC-α and PKC-βI increase activity of MMP-9 and not MMP-2 [159].
Additional research that focused specifically on the critical role of PKC-ε in mediating cardiac fibrosis and the results has yielded promising insight. Mechanistic studies have demonstrated that PKC-ε forms a tight complex with β1-integrin to regulate the interaction between the cell and extra cellular matrix ECM [160, 161]. These findings help to validate a role of PKC-ε in mediating cardiac fibroblast adhesion.
Atherosclerosis
The hallmark of coronary heart disease is characterized by the development of endothelial dysfunction followed by atherosclerotic (thickening of artery wall as a result of invasion and accumulation of white blood cells) lesions in the coronary arteries leading to sustained ischaemic events and acute myocardial infarction (AMI). Atherosclerosis progression begins with low-density lipoprotein (LDL) accumulation followed by monocyte- and endothelium-mediated oxidation of LDL, monocyte extravasation, foam cell formation, and finally, formation of atherosclerotic plaque.
The role of PKCs has been shown to be intimately involved with various stages of atherosclerotic progression. Studies on human hepatic G2 cells, U-931 (human histiocytic lymphoma), and human endothelium have demonstrated isozyme-specific effects of PKC with different stages in atherosclerotic progression [162–171]. The effects and roles of PKCs in atherosclerosis and heart failure in human heart are summarized in Table 1.
Table 1.
Table showing the role of isozyme-specific PKCs in human heart failure and atherosclerosis
| PKC | Cardiac aetiology | Model | Features | Ref. |
|---|---|---|---|---|
| PKC-βII | Heart failure | Human end-stage dilated cardiac myopathy | Increase cardiac PKC-βII levels | [67] |
| PKC-βII | Heart failure | Human end-stage dilated cardiac myopathy | Increase cardiac PKC-βI levels | [66] |
| PKC-α | Atherosclerosis | Human endothelium | Increases superoxide production and inactivation of PKC-α | [162] |
| PKC-α | Atherosclerosis | HepG2 | LDL oxidation and decreased superoxide | [163] |
| PKC-α | Atherosclerosis | U-937 cells | PECAM1 expression and adhesion | [164] |
| PKC-α | Atherosclerosis | Human endothelium | Increased MMP-2 expression | [165] |
| PKC-α | Atherosclerosis | HepG2 | LDL upregulation | [166] |
| PKC-β | Atherosclerosis | HepG2 | Increased LDL activity | [167] |
| PKC-β | Atherosclerosis | Human endothelium | Induces expression of vascular cell adhesion, translocation of PKC-β | [159] |
| PKC-β | Atherosclerosis | Human endothelium | Increased MMP-1 and MMP-3 expression | [161] |
| PKC-β | Atherosclerosis | Human endothelium | Increased MMP-2 expression | [165] |
| PKC-ε | Atherosclerosis | HepG2 | Increased/decreased LDL activity | [170] |
| PKC-ε | Atherosclerosis | Human endothelium | Induces expression of vascular cell adhesion, translocation of PKC-β | [165] |
| PKC-ζ | Atherosclerosis | Human endothelium | Regulates TNF-α-induced activation of NADPH oxidase | [171] |
PKC—a target for drug development
The PKC family of kinases plays essential roles not only in CVDs but also in other diseases. This makes them an attractive target for drug development. This section will discuss areas for future investigation that may lead to drug development and novel therapeutic approaches.
The idea of PKCs as target for drug development dates back to the early 1980s, when they were first identified as the receptors for the tumour-promoter phorbol ester [172]. The central role of PKCs as tempting target for drug development is associated with the fact that these kinases are activated in a variety of diseases as evidenced in animal models and human tissue studies. In addition to heart failure and heart diseases that were extensively covered in previous sections of this review, evidence exist for the critical role of PKC in cancer [173], diabetes [174], bipolar disease [175], Parkinson’s disease [176], Alzheimer’s disease [177], psoriasis [178], kidney [179], and many other human diseases.
Researchers have been trying for years to develop PKC-specific inhibitors that are isozyme selective. Various approaches have led to development of ATP-competitive small molecules (targets the catalytic domain) [180], activators and inhibitors that mimic DG-binding (targets C1 domain) [181], and protein–protein interactions between regulatory region and RACK [182].
PKC modulation in human diseases has shown great promise but sadly, clinical trials’ results have been disappointing. Trials include transplantation clinical trial [183], bipolar disorder trials [184], oncology trials [185], diabetic trials [186], and cardiovascular trials [187]. The major challenges in clinical application of PKC modulators are due mainly to unforeseen adverse reactions, inadequate therapeutic effect, insufficient preclinical studies, absence of blood biomarkers, and lack of selectivity (PKC inhibitors also affect other kinases). Table 2 provides a summary of clinical trials with PKC regulators in human diseases.
Table 2.
Table showing summary of clinical trials of PKC regulators in various human diseases
| Disease | Drug | Mechanism | Ref. |
|---|---|---|---|
| Transplant rejection | Sotrastaurin | ↓PKC | [183] |
| Bipolar mania | Tamoxifen | ↓PKC (at high dose) | [184] |
| Diabetic retinopathy | Ruboxistaurin | ↓PKC-β | [186] |
| Oncology | Aprinocarsen Bryostatin Enzastaurin Midostaurin Tamoxifen |
↓PKC-α ↑PKC ↓PKC-β ↓PKC ↓PKC (at high dose) |
[188] [185, 189] [190, 191] [192] [193, 194] |
| Congestive heart failure | Flosequinan | ↓PKC | [187] |
| Coronary bypass grafting | Volatile anaesthetics Adenosine Acadesine |
↑PKC-ε ↑PKC-ε ↑PKC-ε |
[195, 196] [197, 198] [199, 200] |
| Acute myocardial infarction Salvage | Adenosine Delcasertib |
↑PKC-ε ↓PKC-δ |
[201] [202] |
With all the excitement around PKC as targets for drug development, both academic and pharmaceutical efforts have failed to produce a single new drug that specifically targets PKC.
A future direction for drug development has been linked to post-translational modification of PKC, based upon secondary messenger-dependent activation. These modifications include tyrosine nitration, tyrosine phosphorylation, N-acetylglucosamine O-linked (O-GlcNAc) to serines and threonines of cytosolic and nuclear proteins, oxidation of cysteine rich domain within the C1 domain, and proteolytic cleavage of the enzyme at the hinge region between the catalytic and the regulator halves [203].
Post-translational modification represents a ubiquitous and essential device for control of protein activity, localization, stability, and protein–protein interaction. The importance of this is further emphasize by the fact that covalent post-translational modification, namely serine/threonine phosphorylation of PKC along with binding of PKC to the lipid second messenger diacylglycerol, is recognized as two equally important mechanisms that regulate
PKC activity [204]. About 100 mammalian proteins, including signalling components, metabolic enzymes, and transcription factors, have been identified that carries this modification [205, 206]. However, while the modification has been known for over 30 years, and provides an alternative means of PKC activation which may play a role in disease states, no pharmacological agents have been developed yet based on second messenger-independent activation of PKC.
The PKC family still remains a desirable target for drug development. Biomarkers for specific PKC activity will play a major role for future success in developing drugs for PKC-mediated disease. There is also the need for greater and efficient drug development practices and adequate preclinical studies.
Conclusion
In conclusion, this review provides a comprehensive description of the structure, functions, and distribution of PKCs in the healthy and diseased heart with some emphasis on human heart. The study further focuses mainly on their regulation and roles in the normal healthy heart and, more so, their involvement in the development of heart failure. The regulation of the different isozymes of PKC by pharmaceutical agents may have potential benefits in the treatment of heart failure, thereby promoting a better quality of life for the patients.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The manuscript does not contain clinical studies or patient data.
References
- 1.World Health Organization (2014)- Global status report on non-communicable diseases. 10
- 2.Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131:e01–e294 [DOI] [PubMed]
- 3.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.CIR.97.3.282. [DOI] [PubMed] [Google Scholar]
- 6.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. 1. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- 7.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Phys. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 8.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 9.Nishizuka Y. Protein kinase C and lipid signalling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 10.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986;233:859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- 12.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Identification of three additional members of rat protein kinase C family: gd-, ϵ- and ξ-subspecies. FEBS Lett. 1987;226:125–128. doi: 10.1016/0014-5793(87)80564-1. [DOI] [PubMed] [Google Scholar]
- 13.Osada S, Mizuno K, Saido TC, Akita Y, Suzuki K, Kuroki T, Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990;265:22434–22440. [PubMed] [Google Scholar]
- 14.Osada S, Mizuno K, Saido TC, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKC θ, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/MCB.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selbie LA, Schmitzpeiffer C, Sheng YH, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 16.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2000;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Meco MT, Minicio MM, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype lambda/iota and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/MCB.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed S, Kozma R, Lee J, Monfries C, Harden N, Lim L. The cysteine-rich domain of human proteins, neuronal chimaerin, protein kinase C and diacylglycerol kinase binds zinc. Biochem J. 1991;280:233–241. doi: 10.1042/bj2800233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton AC. Interaction of proteins with lipid head groups: lessons from protein kinase C. Annu Rev Biophys Biomol Struct. 1993;22:1–25. doi: 10.1146/annurev.bb.22.060193.000245. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Crystal structure of the Cys2 activator-binding domain of protein kinase C δ in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-X. [DOI] [PubMed] [Google Scholar]
- 22.Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 23.Farago A, Nishizuka Y. Protein kinase C in transmembrane signalling. FEBS Lett. 1990;268:350–354. doi: 10.1016/0014-5793(90)81284-U. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowska-Patzer B, Domanska-Janik K. Increased 19 kDa protein phosphorylation and protein kinase C activity in pressure-overload cardiac hypertrophy. Basic Res Cardiol. 1991;86:402–409. doi: 10.1007/BF02191536. [DOI] [PubMed] [Google Scholar]
- 25.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLeod K, Harding SE. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J Physiol. 1991;444:481–498. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington EO, Ware JA. Diversity of the protein kinase C gene family: implications for cardiovascular disease. Trends Cardiovasc Med. 1995;5:193–199. doi: 10.1016/1050-1738(95)00058-H. [DOI] [PubMed] [Google Scholar]
- 28.Capogrossi MC, Kaku T, Filburn CR. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ Res. 1990;66:1143–1155. doi: 10.1161/01.RES.66.4.1143. [DOI] [PubMed] [Google Scholar]
- 29.Sugden PH, Bogoyevitch MA. Intracellular signalling through protein kinases in the heart. Cardiovasc Res. 1995;30:478–492. doi: 10.1016/S0008-6363(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 30.Sandhu R, Diaz RJ, Mao GD, Wilson GJ. Ischemic preconditioning: differences in protection and susceptibility to blockade with single cycle versus multicycle transient ischemia. Circulation. 1997;96:984–995. doi: 10.1161/01.CIR.96.3.984. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg SF, Goldberg M, Rybin VO. Protein kinase C isoform diversity in the heart. J Mol Cell Cardio. 1995;27:141–153. doi: 10.1016/S0022-2828(08)80014-4. [DOI] [PubMed] [Google Scholar]
- 32.Rouet-Benzineb P, Mohammadi K, Perennec J, Poyard M, Bouanani N, Crozatier B. Protein kinase C isoform expression in normal and neuro-failing rabbit hearts. Circ Res. 1996;79:153–161. doi: 10.1161/01.RES.79.2.153. [DOI] [PubMed] [Google Scholar]
- 33.Simpson PC. Beta-protein kinase C and hypertrophic signalling in human heart failure. Circulation. 1999;99:334–337. doi: 10.1161/01.CIR.99.3.334. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JA, Mochly-Rosen D. Inhibition of the spontaneous rate of contraction of neonatal cardiac myocytes by protein kinase C isozymes. A putative role for the epsilon isozyme. Circ Res. 1995;76:654–663. doi: 10.1161/01.RES.76.4.654. [DOI] [PubMed] [Google Scholar]
- 35.Ishii H, Jirousek MR, Koya D. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 36.Dekker LV, Parker PJ. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 37.Bareggi R, Narducci P, Grill V, Lach S, Martelli AM. Selective distribution of multiple protein kinase C isoforms in mouse cerebellar cortex. Biol Cell. 1996;87:55–63. doi: 10.1111/j.1768-322X.1996.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 38.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–327. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 39.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/S0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Toita R, Kim CW, Katayama Y. Protein kinase C (PKC) isozyme-specific substrates and their design. Biotechnol Adv. 2012;30(6):1662–1672. doi: 10.1016/j.biotechadv.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilon PKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Basu A, Pal D. Two faces of protein kinase C delta: the contrasting roles of PKC delta in cell survival and cell death. Sci World J. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.RES.74.2.299. [DOI] [PubMed] [Google Scholar]
- 44.Clerk A, Bogoyevitch MA, Fuller SJ, Lazou A, Parker PJ, Sugden PH. Expression of protein kinase C isoforms during cardiac ventricular development. Am J Phys. 1995;269:H1087–H1097. doi: 10.1152/ajpheart.1995.269.3.H1087. [DOI] [PubMed] [Google Scholar]
- 45.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform β 11 and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility of glycaemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rybin VO, Steinberg SF. Do adult rat ventricular myocytes express protein kinase C-α? Am J Phys. 1997;272:H2485–H2491. doi: 10.1152/ajpheart.1997.272.5.H2485. [DOI] [PubMed] [Google Scholar]
- 47.Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- 48.Qu Y, Torchia J, Phan TD, Sen AK. Purification and characterization of protein kinase C isozymes from rat heart. Mol Cell Biochem. 1991;103:171–180. doi: 10.1007/BF00227484. [DOI] [PubMed] [Google Scholar]
- 49.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Church DJ, Braconi S, Vallotton MB, Lang U. Protein kinase C-mediated phospholipase A2 activation, platelet-activating factor generation and prostacyclin release in spontaneously beating rat cardiomyocytes. Biochem J. 1993;290:477–482. doi: 10.1042/bj2900477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ Res. 1993;72:757–767. doi: 10.1161/01.RES.72.4.757. [DOI] [PubMed] [Google Scholar]
- 52.Kosaka Y, Ogita K, Ase K, Nomura H, Kikkawa U, Nishizuka Y. The heterogeneity of protein kinase C in various rat tissues. Biochem Biophys Res Commun. 1988;151:973–981. doi: 10.1016/S0006-291X(88)80461-3. [DOI] [PubMed] [Google Scholar]
- 53.Talosi L, Kranias EG. Effect of α-adrenergic stimulation on activation of protein kinase C and phosphorylation of proteins in intact rabbit hearts. Circ Res. 1992;70:670–678. doi: 10.1161/01.RES.70.4.670. [DOI] [PubMed] [Google Scholar]
- 54.Ping P, Zhang J, Qiu Y. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.RES.81.3.404. [DOI] [PubMed] [Google Scholar]
- 55.Qiu Y, Ping P, Tang XL. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul K, Ball NA, Dorn GW, II, Walsh RA. Left ventricular stretch stimulates angiotensin II-mediated phosphatidylinositol hydrolysis and protein kinase C ε isoform translocation in adult guinea pig hearts. Circ Res. 1997;81:643–650. doi: 10.1161/01.RES.81.5.643. [DOI] [PubMed] [Google Scholar]
- 57.Cai JJ, Lee HC. Protein kinase C isozyme-specific modulation of cyclic AMP-dependent phosphodiesterase in hypertrophic cadiomyopathic hamster hearts. Mol Pharmacol. 1996;1:81–88. [PubMed] [Google Scholar]
- 58.Domenech RJ, Macho P, Velez D, Sanchez G, Liu X, Dhalla NS. Tachycardia preconditions infarct size in dogs: role of adenosine and protein kinase C. Circulation. 1998;97:786–794. doi: 10.1161/01.CIR.97.8.786. [DOI] [PubMed] [Google Scholar]
- 59.Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca2+ independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Phys. 1992;263:C714–C719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- 61.Liou YM, Morgan KG. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am J Phys. 1994;267:C980–C989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- 62.Kohout TA, Rogers TB. Use of a PCR-based method to characterize protein kinase C isoform expression in cardiac cells. Am J Physiol Cell Physiol. 1993;264(5Pt.1):C1350–C1359. doi: 10.1152/ajpcell.1993.264.5.C1350. [DOI] [PubMed] [Google Scholar]
- 63.Erdbrugger W, Keffel J, Knocks M. Protein kinase C isoenzymes in rat and human cardiovascular tissues. Br J Pharmacol. 1997;120:177–186. doi: 10.1038/sj.bjp.0700877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activatedprotein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem. 1999;274:6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- 65.Hassouna AB, Matata M, Galinanes M. PKC-ε is upstream and PKC-α is downstream of mito-KATP-channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am J Phys. 2004;287:C1418–C1425. doi: 10.1152/ajpcell.00144.2004. [DOI] [PubMed] [Google Scholar]
- 66.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL. Increased protein kinase C activity and expression of Ca2+ sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.CIR.99.3.384. [DOI] [PubMed] [Google Scholar]
- 67.Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–111. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- 68.Shin HG, Barnett JV, Chang P, Reddy S, Drinkwater DC, Pierson RN. Molecular heterogeneity of protein kinase C expression in human ventricle. Cardiovasc Res. 2000;48:285–299. doi: 10.1016/S0008-6363(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg M, Steinberg SF. Tissue-specific developmental regulation protein kinase C isoforms. Biochem Pharmacol. 1996;51(8):1089–1093. doi: 10.1016/0006-2952(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 70.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 71.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Budas GR, Churchill EN, Mochly-Rosen D. Cardio-protective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Puceat M, Vassort G. Signaling by protein kinase C isoforms in the heart. Mol Cell Biochem. 1996;157:65–72. doi: 10.1007/BF00227882. [DOI] [PubMed] [Google Scholar]
- 75.Ferreira JC, Mochly-Rosen D, Boutjdir M. Regulation of cardiac excitability by protein kinase C isozymes. Front Biosci (Schol Ed) 2012;4:532–546. doi: 10.2741/s283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozyme selective inhibitors. J Mol Cell Cardiol. 2003;35:895–903. doi: 10.1016/S0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 78.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Phys. 1994;266:H1145–H1152. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 79.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circ Res. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 80.Yellon DM, Alkhulaifi AM, Browne EE, Pugsley WB. Ischaemic preconditioning limits infarct size in the rat heart. Cardiovasc Res. 1992;26:983–987. doi: 10.1093/cvr/26.10.983. [DOI] [PubMed] [Google Scholar]
- 81.Burns PG, Krunkenkamp IB, Calderone CA, Kirvaitis RJ, Gaudette GR, Levitsky S. Is the preconditioning response conserved in senescent myocardium? Ann Thorac Surg. 1996;61:925–929. doi: 10.1016/0003-4975(95)01188-9. [DOI] [PubMed] [Google Scholar]
- 82.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Phys. 1990;259:H1822–H1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 83.Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990;66:1133–1142. doi: 10.1161/01.RES.66.4.1133. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995;76:73–81. doi: 10.1161/01.RES.76.1.73. [DOI] [PubMed] [Google Scholar]
- 85.Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994;75:586–590. doi: 10.1161/01.RES.75.3.586. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Kloner RA. Does protein kinase C play a role in ischemic preconditioning in rat hearts? Am J Physiol Heart Circ Physiol. 1995;268:H426–H431. doi: 10.1152/ajpheart.1995.268.1.H426. [DOI] [PubMed] [Google Scholar]
- 87.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, Shimamoto K. Opening of mitochondrial KATP channel occurs downstream of PKC-ε activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 88.Kitakaze M, Funaya H, Minamino T, Node K, Sato H, Ueda Y, Okuyama Y, Kuzuya T, Hori M, Yoshida K. Role of protein kinase C-α in activation of ecto-5′-nucleotidase in the preconditioned canine myocardium. Biochem Biophys Res Commun. 1997;239:171–175. doi: 10.1006/bbrc.1997.7445. [DOI] [PubMed] [Google Scholar]
- 89.Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet. 1993;342:276–277. doi: 10.1016/0140-6736(93)91819-8. [DOI] [PubMed] [Google Scholar]
- 90.Walker DM, Walker JM, Pugsley WB, Pattison CW, Yellon DM. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol. 1995;27:1349–1357. doi: 10.1016/S0022-2828(05)82397-1. [DOI] [PubMed] [Google Scholar]
- 91.Marber M, Walker D, Yellon D. Ischaemic preconditioning. BMJ. 1994;308:1–2. doi: 10.1136/bmj.308.6920.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Speechly-Dick ME, Grover GJ, Yellon DM. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K+ channel? Studies of contractile function after simulated ischemia in an atrial in vitro model. Circ Res. 1995;77:1030–1035. doi: 10.1161/01.RES.77.5.1030. [DOI] [PubMed] [Google Scholar]
- 93.Ghosh S, Standen NB, Galinanes M. Evidence for mitochondrial KATP channels as effectors of human myocardial preconditioning. Cardiovasc Res. 2000;45:934–940. doi: 10.1016/S0008-6363(99)00407-1. [DOI] [PubMed] [Google Scholar]
- 94.Loubani M, Galinanes M. Pharmacological and ischemic preconditioning of the human myocardium: mitoKATP channels are upstream and p38MAPK is downstream of PKC. BMC Physiol. 2002;2:10. doi: 10.1186/1472-6793-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Julier K, da Silva R, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicentre study. Anesthesiology. 2003;98:1315–1327. doi: 10.1097/00000542-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Lambiase PD, Edwards RJ, Cusack MR, Bucknall CA, Redwood SR, Marber MS. Exercise-induced ischemia initiates the second window of protection in humans independent of collateral recruitment. J Am Cell Cardiol. 2003;41:1174–1182. doi: 10.1016/S0735-1097(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 97.Downey JM, Cohen MV. Arguments in favor of protein kinase C playing an important role in ischemic preconditioning. Basic Res Cardiol. 1997;92:37–39. doi: 10.1007/BF00794066. [DOI] [PubMed] [Google Scholar]
- 98.Vogt AM, Htun P, Arras M, Podzuweit T, Schaper W. Intra-myocardial infusion of tool drugs for the study of molecular mechanisms in ischemic preconditioning. Basic Res Cardiol. 1996;91:389–400. doi: 10.1007/BF00788719. [DOI] [PubMed] [Google Scholar]
- 99.Vogt A, Barancik M, Weihrauch D, Arras M, Podzuweit T, Schaper W. Protein kinase C inhibitors reduce infarct size in pig hearts in vivo. Circulation. 1994;90(suppl I):I–647. [Google Scholar]
- 100.Brooks G, Hearse DJ. Role of protein kinase C in ischemic preconditioning: player or spectator? Circ Res. 1996;79:627–630. doi: 10.1161/01.RES.79.3.628. [DOI] [PubMed] [Google Scholar]
- 101.Iliodromitis EK, Lazou A, Kremastinos DT. Ischemic preconditioning: protection against myocardial necrosis and apoptosis. Vasc Health Risk Manag. 2007;3(5):629–637. [PMC free article] [PubMed] [Google Scholar]
- 102.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 103.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–237. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rossello X, Yellon DM. Cardioprotection: the disconnect between bench and bedside. Circulation. 2016;134:574–575. doi: 10.1161/CIRCULATIONAHA.116.022829. [DOI] [PubMed] [Google Scholar]
- 105.Gersh BJ, Stone GW, White HD, Holmes DR., Jr Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293:979–986. doi: 10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 106.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 107.Brevoord D, Kranke P, Kuijpers M, Weber N, Hollmann M, Preckel B. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS One. 2012;7:e42179. doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdelnoor M, Sandven I, Limalanathan S, Eritsland J. Postconditioning in ST-elevation myocardial infarction: a systematic review, critical appraisal, and meta-analysis of randomized clinical trials. Vasc Health Risk Manag. 2014;10:477–491. doi: 10.2147/VHRM.S67154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of δPKC and εPKC. Proc Natl Acad Sci (USA) 2001;98(20):11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase C δ. Apoptosis. 2003;8:19–27. doi: 10.1023/A:1021640817208. [DOI] [PubMed] [Google Scholar]
- 112.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase C delta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 113.Li L, Lorenzo PS, Bogi K. Protein kinase C delta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/MCB.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of delta PKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 115.Armstrong JS, Whiteman M. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 2007;80:355–377. doi: 10.1016/S0091-679X(06)80018-X. [DOI] [PubMed] [Google Scholar]
- 116.Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke. 2004;35:2676–2679. doi: 10.1161/01.STR.0000143220.21382.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta protein kinase C against reperfusion injury of the ischemic heart, in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 118.Heidkamp MC, Bayer AL, Martin JL. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–890. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- 119.Dorn GW, II, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 121.Chen CH, Budas GR, Churchill ER, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 123.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.RES.84.9.973. [DOI] [PubMed] [Google Scholar]
- 124.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL. Protein kinase C epsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92(8):873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in post ischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 126.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-ε coimmuno-precipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardio-protection. Am J Physiol Heart Circ Physiol. 2007;293:H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 128.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 130.Pass JM, Zheng Y, Wead WB, Zhang J, Li RC, Bolli R. PKC epsilon activation induces dichotomous cardiac phenotypes and modulates PKCepsilon-RACK interactions and RACK expression. Am J Physiol Heart Circ Physiol. 2001;280:H946–H955. doi: 10.1152/ajpheart.2001.280.3.H946. [DOI] [PubMed] [Google Scholar]
- 131.Malhotra A, Kang BP, Opawumi D, Belizaire W, Meggs LG. Molecular biology of protein kinase C signalling in cardiac myocytes. Mol Cell Biochem. 2001;225:97–107. doi: 10.1023/A:1012261903611. [DOI] [PubMed] [Google Scholar]
- 132.Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol. 2010;48:817–823. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vincent F, Duquesnes N, Christov C, Damy T, Samuel JL, Crozatier B. Dual level of interactions between calcineurin and PKC-epsilon in cardiomyocyte stretch. Cardiovasc Res. 2006;71:97–107. doi: 10.1016/j.cardiores.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 134.Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.RES.86.12.1218. [DOI] [PubMed] [Google Scholar]
- 135.Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN. Cardiotrophic effects of protein kinase C epsilon: analysis by in vivo modulation of PKC epsilon translocation. Circ Res. 2000;86:1173–1179. doi: 10.1161/01.RES.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 136.Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL. Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res. 2004;95:424–432. doi: 10.1161/01.RES.0000138299.85648.92. [DOI] [PubMed] [Google Scholar]
- 137.Gu X, Bishop SP. Increased protein kinase C and isozyme redistribution in pressure-overload cardiac hypertrophy in the rat. Circ Res. 1994;75:926–931. doi: 10.1161/01.RES.75.5.926. [DOI] [PubMed] [Google Scholar]
- 138.Takeishi Y, Bhagwat A, Ball NA, Kirkpatrick DL, Periasamy M, Walsh RA. Effect of angiotensin-converting enzyme inhibition on protein kinase C and SR proteins in heart failure. Am J Phys. 1999;276:H53–H62. doi: 10.1152/ajpheart.1999.276.1.H53. [DOI] [PubMed] [Google Scholar]
- 139.Morel OE, Buvry A, Le Corvoisier P, Tual L, Favret F, Leon-Velarde F. Effects of nifedipine-induced pulmonary vasodilatation on cardiac receptors and protein kinase C isoforms in the chronically hypoxic rat. Pflugers Arch. 2003;446:356–364. doi: 10.1007/s00424-003-1034-y. [DOI] [PubMed] [Google Scholar]
- 140.Mohammadi K, Rouet-Benzineb P, Laplace M, Crozatier B. Protein kinase C activity and expression in rabbit left ventricular hypertrophy. J Mol Cell Cardiol. 1997;29:1687–1694. doi: 10.1006/jmcc.1997.0411. [DOI] [PubMed] [Google Scholar]
- 141.Fryer LG, Holness MJ, Decock JB, Sugden MC. Cardiac protein kinase C expression in two models of cardiac hypertrophy associated with an activated cardiac reninangiotensin system: effects of experimental hyperthyroidism and genetic hypertension (the mRen-2 rat) J Endocrinol. 1998;158:27–33. doi: 10.1677/joe.0.1580027. [DOI] [PubMed] [Google Scholar]
- 142.Braun MU, La-Rosee P, Simonis G, Borst MM, Strasser RH. Regulation of protein kinase C isozymes in volume overload cardiac hypertrophy. Mol Cell Biochem. 2004;262:135–143. doi: 10.1023/B:MCBI.0000038229.23132.9f. [DOI] [PubMed] [Google Scholar]
- 143.Palaniyandi SS, Inagaki K, Mochly-Rosen D. Mast cells and epsilon PKC: a role in cardiac remodeling in hypertension-induced heart failure. J Mol Cell Cardiol. 2008;45:779–786. doi: 10.1016/j.yjmcc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang X, Walker JW. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. J Cell Sci. 2004;117:1971–1978. doi: 10.1242/jcs.01044. [DOI] [PubMed] [Google Scholar]
- 145.He Z, King GL. Protein kinase C beta isoform inhibitors: a new treatment for diabetic cardiovascular diseases. Circulation. 2004;110:7–9. doi: 10.1161/01.CIR.0000133428.02295.6C. [DOI] [PubMed] [Google Scholar]
- 146.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dorn GW, Mochly-Rosen D. Intracellular transport mechanisms of signal transducers. Annu Rev Physiol. 2002;64:407–429. doi: 10.1146/annurev.physiol.64.081501.155903. [DOI] [PubMed] [Google Scholar]
- 149.Braz JC, Bueno OF. De Windt LJ, Molkentin JD. PKC alpha regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2) J Cell Biol. 2002;156:905–919. doi: 10.1083/jcb.200108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., II Protein kinase C alpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 151.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., II Transgenic G(alphaq) overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dorn GW, II, Tepe NM, Wu G, Yatani A, Liggett SB. Mechanisms of impaired beta adrenergic receptor signaling in G(alphaq)-mediated cardiac hypertrophy and ventricular dysfunction. Mol Pharmacol. 2000;57:278–287. [PubMed] [Google Scholar]
- 153.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Ann Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 154.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40(2):352–363. doi: 10.1016/S0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 155.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozymeselective inhibitors. J Mol Cell Cardiol. 2003;35:895–903. doi: 10.1016/S0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 157.Reuben PM, Cheung HS. Regulation of matrix metalloproteinase (MMP) gene expression by protein kinases. Front Biosci. 2006;11:1199–1215. doi: 10.2741/1873. [DOI] [PubMed] [Google Scholar]
- 158.Xie B, Laouar A, Huberman E. Fibronectin-mediated cell adhesion is required for induction of 92-kDa type IV collagenase/gelatinase (MMP-9) gene expression during macrophage differentiation. The signaling role of protein kinase C-beta. J Biol Chem. 1998;273:11576–11582. doi: 10.1074/jbc.273.19.11576. [DOI] [PubMed] [Google Scholar]
- 159.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1beta. J Biol Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 160.Heidkamp MC, Bayer AL, Scully BT, Eble DM, Samarel AM. Activation of focal adhesion kinase by protein kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H1684–H1696. doi: 10.1152/ajpheart.00016.2003. [DOI] [PubMed] [Google Scholar]
- 161.Ivaska J, Whelan RD, Watson R, Parker PJ. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fleming I, Mohamed A, Galle J, Turchanowa L, Brandes RP, Fisslthaler B. Oxidized low-density lipoprotein increases superoxide production by endothelial nitric oxide synthase by inhibiting PKCα. Cardiovasc Res. 2005;65(4):897–906. doi: 10.1016/j.cardiores.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 163.Li Q, Subbulakshmi V, Fields AP, Murray NR, Cathcart MK. Protein kinase C alpha regulates human monocyte O-2 production and low-density lipoprotein lipid oxidation. J Biol Chem. 1999;274(6):3764–3771. doi: 10.1074/jbc.274.6.3764. [DOI] [PubMed] [Google Scholar]
- 164.Gong N, Wei H, Chowdhury SH, Chatterjee S. Lactosylceramide recruits PKCα/ε and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells. Proc Natl Acad Sci U S A. 2004;101(17):6490–6495. doi: 10.1073/pnas.0308684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mountain DJ, Singh M, Menon B, Singh K. Interleukin-1β increases expression and activity of matrix metalloproteinase-2 in cardiac microvascular endothelial cells: role of PKCα/β1 and MAPKs. Am J Physiol Cell Physiol. 2007;292(2):C867–C875. doi: 10.1152/ajpcell.00161.2006. [DOI] [PubMed] [Google Scholar]
- 166.Kumar A, Chambers TC, Cloud-Heflin BA, Mehta KD. Phorbol ester induced low-density lipoprotein receptor gene expression in HepG2 cells involves protein kinase C-mediated p42/44 MAP kinase activation. J Lipid Res. 1997;38(11):2240–2248. [PubMed] [Google Scholar]
- 167.Kapoor GS, Golden C, Atkins B, Mehta KD. Pp90RSK- and protein kinase C-dependent pathway regulates p42/44MAPK-induced LDL receptor transcription in HepG2 cells. J Lipid Res. 2003;44(3):584–593. doi: 10.1194/jlr.M200302-JLR200. [DOI] [PubMed] [Google Scholar]
- 168.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114(7):681–687. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 169.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107(4):612–617. doi: 10.1161/01.CIR.0000047276.52039.FB. [DOI] [PubMed] [Google Scholar]
- 170.Mehta KD, Radominska-Pandya A, Kapoor GS, Dave B, Atkins BA. Critical role of diacylglycerol- and phospholipid-regulated protein kinase C epsilon in induction of low-density lipoprotein receptor transcription in response to depletion of cholesterol. Mol Cell Biol. 2002;22(11):3783–3793. doi: 10.1128/MCB.22.11.3783-3793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCζ regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90(9):1012–1019. doi: 10.1161/01.RES.0000017631.28815.8E. [DOI] [PubMed] [Google Scholar]
- 172.Castagna M, Takai Y, Kaibuchi K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein-kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 173.Toton E, Ignatowicz E, Skrzeczkowska K, Rybczynska M. Protein kinase Cepsilon as a cancer marker and target for anticancer therapy. Pharmacol Rep. 2011;63:19–29. doi: 10.1016/S1734-1140(11)70395-4. [DOI] [PubMed] [Google Scholar]
- 174.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23:569–582. doi: 10.2165/00023210-200923070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang D, Anantharam V, Kantehasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson’s disease. J Pharmacol Exp Ther. 2007;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- 177.Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16:1982–1984. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- 178.Maioli E, Rottlerin VG. Bases for a possible usage in psoriasis. Curr Drug Metab. 2010;11:425–430. doi: 10.2174/138920010791526097. [DOI] [PubMed] [Google Scholar]
- 179.Li J, Gobe G. Protein kinase C activation and its role in kidney disease. Nephrology (Carlton) 2006;11:428–434. doi: 10.1111/j.1440-1797.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 180.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wender PA, Baryza JL, Brenner SE, DeChristopher BA, Loy BA, Schrier AJ, Verma VA. Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity. Proc Natl Acad Sci U S A. 2011;108:6721–6726. doi: 10.1073/pnas.1015270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nature Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 183.Friman S, Arns W, Nashan B, Vincenti F, Banas B, Budde K, Cibrik D, Chan L, Klempnauer J, Mulgaonkar S, Nicholson M, Wahlberg J, Wissing KM, Abrams K, Witte S, Woodle ES. Sotrastaurin, a novel small molecule inhibiting protein-kinase C: randomized phase II study in renal transplant recipients. Am J Transplant. 2011;11:1444–1455. doi: 10.1111/j.1600-6143.2011.03538.x. [DOI] [PubMed] [Google Scholar]
- 184.Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65:255–263. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]
- 185.Philip PA, Rea D, Thavasu P, Carmichael J, Stuart NS, Rockett H, Talbot DC, Ganesan T, Pettit GR, Balkwill F. Phase I study of bryostatin 1: assessment of interleukin 6 and tumor necrosis factor alpha induction in vivo The Cancer Research Campaign Phase I Committee. J Natl Cancer Inst. 1993;85:1812–1818. doi: 10.1093/jnci/85.22.1812. [DOI] [PubMed] [Google Scholar]
- 186.PKC-DRS Study Group, T The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 187.Packer M, Narahara KA, Elkayam U, Sullivan JM, Pearle DL, Massie BM, Creager MA. Double-blind, placebo-controlled study of the efficacy of flosequinan in patients with chronic heart failure. Principal Investigators of the REFLECT Study. J Am Coll Cardiol. 1993;22:65–72. doi: 10.1016/0735-1097(93)90816-J. [DOI] [PubMed] [Google Scholar]
- 188.Advani R, Lum BL, Fisher GA, Halsey J, Geary RS, Holmlund JT, Kwoh TJ, Dorr FA, Sikic BI. A phase I trial of aprinocarsen (ISIS 3521/LY900003), an antisense inhibitor of protein kinase C-alpha administered as a 24-hour weekly infusion schedule in patients with advanced cancer. Investig New Drugs. 2005;23:467–477. doi: 10.1007/s10637-005-2906-0. [DOI] [PubMed] [Google Scholar]
- 189.Gonzalez R, Ebbinghaus S, Henthorn TK, Miller D, Kraft AS. Treatment of patients with metastatic melanoma with bryostatin-1—a phase II study. Melanoma Res. 1999;9:599–606. doi: 10.1097/00008390-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 190.Robertson MJ, Kahl BS, Vose JM, de Vos S, Laughlin M, Flynn PJ, Rowland K, Cruz JC, Goldberg SL, Musib L, Darstein C, Enas N, Kutok JL, Aster JC, Neuberg D, Savage KJ, LaCasce A, Thornton D, Slapak CA, Shipp MA. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:1741–1746. doi: 10.1200/JCO.2006.09.3146. [DOI] [PubMed] [Google Scholar]
- 191.Morschhauser F, Seymour JF, Kluin-Nelemans HC, Grigg A, Wolf M, Pfreundschuh M, Tilly H, Raemaekers J, van’t Veer MB, Milpied N, Cartron G, Pezzutto A, Spencer A, Reyes F, Dreyling M. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann Oncol. 2008;19:247–253. doi: 10.1093/annonc/mdm463. [DOI] [PubMed] [Google Scholar]
- 192.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E, Ehninger G, Feldman EJ, Schiller GJ, Klimek VM, Nimer SD, Gilliland DG, Dutreix C, Huntsman-Labed A, Virkus J, Giles FJ. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Robins HI, Won M, Seiferheld WF, Schultz CJ, Choucair AK, Brachman DG, Demas WF, Mehta MP. Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro-Oncology. 2006;8:47–52. doi: 10.1215/S1522851705000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Couldwell WT, Hinton DR, Surnock AA, DeGiorgio CM, Weiner LP, Apuzzo ML, Masri L, Law RE, Weiss MH. Treatment of recurrent malignant gliomas with chronic oral high-dose tamoxifen. Clin Cancer Res. 1996;2:619–622. [PubMed] [Google Scholar]
- 195.Guarracino F, Landoni G, Tritapepe L, Pompei F, Leoni A, Aletti G, Scandroglio AM, Maselli D, De Luca M, Marchetti C, Crescenzi G, Zangrillo A. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth. 2006;20:477–483. doi: 10.1053/j.jvca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 196.Tritapepe L, Landoni G, Guarracino F, Pompei F. Cardiac protection by volatile anaesthetics: a multicentre randomized controlled study in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Eur J Anaesthesiol. 2007;24:323–331. doi: 10.1017/S0265021506001931. [DOI] [PubMed] [Google Scholar]
- 197.Jin Z, Duan W, Chen M, Yu S, Zhang H, Feng G, Xiong L, Yi D. The myocardial protective effects of adenosine pretreatment in children undergoing cardiac surgery: a randomized controlled clinical trial. Eur J Cardiothorac Surg. 2011;39:e90–e96. doi: 10.1016/j.ejcts.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 198.Mentzer RM, Jr, Birjiniuk V, Khuri S, Lowe JE, Rahko PS, Weisel RD, Wellons HA, Barker ML, Lasley RD. Adenosine myocardial protection: preliminary results of a phase II clinical trial. Ann Surg. 1999;229:643–649. doi: 10.1097/00000658-199905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mangano DT, Miao Y, Tudor IC, Dietzel C. Post-reperfusion myocardial infarction: long-term survival improvement using adenosine regulation with acadesine. J Am Coll Cardiol. 2006;48:206–214. doi: 10.1016/j.jacc.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 200.Newman MF, Ferguson TB, White JA, Ambrosio G, Koglin J, Nussmeier NA, Pearl RG, Pitt B, Wechsler AS, Weisel RD, Reece TL, Lira A, Harrington RA, RED-CABG Steering Committee and Investigators Effect of adenosine-regulating agent acadesine on morbidity and mortality associated with coronary artery bypass grafting: the RED-CABG randomized controlled trial. JAMA. 2012;308:157–164. doi: 10.1001/jama.2012.7633. [DOI] [PubMed] [Google Scholar]
- 201.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 202.Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, Zelizko M, Kleiman N, White H, McErlean E, Erlinge D, Laine M, Dos Santos Ferreira JM, Goodman S, Mehta S, Atar D, Suryapranata H, Jensen SE, Forster T, Fernandez-Ortiz A, Schoors D, Radke P, Belli G, Brennan D, Bell G, Krucoff M, Investigators PROTECTIONAMI. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J. 2014;35(37):2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- 203.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shirai Y, Saito N. Activation mechanisms of protein kinase C: maturation, catalytic activation and targeting. J Biochemist. 2002;132:663–668. doi: 10.1093/oxfordjournals.jbchem.a003271. [DOI] [PubMed] [Google Scholar]
- 205.Wells L, Whelan SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–441. doi: 10.1016/S0006-291X(03)00175-X. [DOI] [PubMed] [Google Scholar]
- 206.Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. doi: 10.1016/S0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]