Abstract
Microbes are known to withstand environmental stresses by using chromosomal toxin–antitoxin systems. MazEF is one of the most extensively studied toxin–antitoxin systems. In stressful environments, MazF toxins modulate translation by cleaving single‐stranded RNAs in a sequence‐specific fashion. Previously, a chromosomal gene located at DR0417 in Deinococcus radiodurans was predicted to code for a MazF endoribonuclease (MazFDR 0417); however, its function remains unclear. In the present study, we characterized the molecular function of MazFDR 0417. Analysis of MazFDR 0417‐cleaved RNA sites using modified massively parallel sequencing revealed a unique 4‐nt motif, UACA, as a potential cleavage pattern. The activity of MazFDR 0417 was also assessed in a real‐time fluorometric assay, which revealed that MazFDR 0417 strictly recognizes the unique tetrad UACA. This sequence specificity may allow D. radiodurans to alter its translation profile and survive under stressful conditions.
Keywords: Deinococcus radiodurans, MazEF, sequence specificity, toxin–antitoxin system
1. INTRODUCTION
Toxin–antitoxin (TA) systems are common in the bacterial and archaeal kingdoms (Pandey & Gerdes, 2005). They are typically encoded by operons comprising the genes of a stable toxin that disrupts cellular activities and an unstable antitoxin that alleviates the toxin's effect. In response to stress, antitoxins are rapidly degraded because of their labile nature, thus freeing the corresponding toxins (Page & Peti, 2016). These toxins then arrest prokaryotic growth in many different ways; for example, they can impede DNA replication, cell wall synthesis, translation, cell division, and ATP production (Schuster & Bertram, 2013). Among these mechanisms, translation inhibition caused by intracellular RNA digestion is the most common (Yamaguchi & Inouye, 2011).
The MazEF family, which is comprised of MazE antitoxin and MazF toxin, is one such representative TA system (Aizenman, Engelberg‐Kulka, & Glaser, 1996). In Escherichia coli, MazE forms a heterohexamer with MazF and inactivates the activity of MazF as a sequence‐specific endoribonuclease (Kamada, Hanaoka, & Burley, 2003). Once cells encounter specific stresses, however, unstable MazE is preferentially degraded by ClpAP protease, liberating the MazF toxin endoribonuclease (Aizenman et al., 1996; Hazan, Sat, & Engelberg‐Kulka, 2004). The released MazF then alters translation globally by cutting cellular RNAs at ACA sites (Amitai, Kolodkin‐Gal, Hananya‐Meltabashi, Sacher, & Engelberg‐Kulka, 2009; Sauert et al., 2016; Vesper et al., 2011; Zhang et al., 2003). Although the functions of prokaryotic MazF toxins remain unclear, they are thought to have various biological roles such as in virulence (Rothenbacher et al., 2012; Tiwari et al., 2015; Zhu et al., 2009), phage defense systems (Alawneh, Qi, Yonesaki, & Otsuka, 2016; Hazan & Engelberg‐Kulka, 2004), persister generation (Tripathi, Dewan, Siddique, & Varadarajan, 2014), and programmed cell death (Nariya & Inouye, 2008); an important characteristic of MazF endoribonucleases that may contribute to these physiological functions is their sequence‐specificities. In most cases, MazF toxins require strict sequences for RNA cleavages, which are typically 3–7‐nucleotide (nt) motifs (Miyamoto, Kato, Sekiguchi, Tsuneda & Noda, 2016; Miyamoto, Yokota, Tsuneda, Noda et al. 2016; Nariya & Inouye, 2008; Rothenbacher et al., 2012; Schifano et al., 2014; Schuster et al., 2013; Verma & Bhatnagar, 2014; Yamaguchi & Inouye, 2011; Yamaguchi, Nariya, Park, & Inouye, 2012; Zhang et al., 2003; Zhu et al., 2006, 2008, 2009). Thus, microbes may reprogram their translation by shutting down most translation processes or by eliminating specific transcripts to cope with unfavorable surroundings.
Deinococcus radiodurans is a Gram‐positive bacterium that inhabits a variety of environments. It is remarkably resistant to different types of stress, such as desiccation, oxidative stress, DNA damage, ionizing radiation, and ultraviolet radiation (Makarova et al., 2001). Previously, computational analysis predicted that a chromosomal gene, located at DR0417 in Deinococcus radiodurans, codes for a MazF toxin (MazFDR0417) (Chopra, Saumitra, Pathak, Bhatnagar, & Bhatnagar, 2013; Pandey & Gerdes, 2005), suggesting that D. radiodurans utilizes MazFDR0417 as a posttranscriptional regulator and regulates its translation under stressful conditions. In the present study, we found that MazFDR0417 is a toxin endoribonuclease and constitutes an authentic TA system together with its cognate antitoxin MazE, encoded by the locus DR0416 (MazEDR0416). Analysis of MazFDR0417‐cleaved RNA products using modified massively parallel sequencing revealed that MazFDR0417 cleaves RNA specifically at a unique 4‐nt motif, UACA. The indispensability of the tetrad for effective RNA cleavage was also demonstrated using a fluorescent quenching approach. The results indicate that MazFDR0417 may play a role in adaptation to stressful environments by promoting the selective degradation of intracellular RNAs.
2. MATERIALS AND METHODS
2.1. Plasmids and oligonucleotides
The expression vector pET21c was purchased from Takara Bio Service (Shiga, Japan). The pET19b expression vector encoding mazE DR0416, whose codon usage was optimized for recombinant protein expression in E. coli, was purchased from GenScript Japan (Tokyo, Japan). Double‐stranded DNA fragments, including the mazF DR0417 DNA sequence flanked by BamHI/EcoRI sites, were purchased from Life Technologies Japan Ltd. (Tokyo, Japan). The pMX‐T vector encoding D. radiodurans mazEF genes was purchased from Life Technologies Japan Ltd. Fluorescently modified oligonucleotides were purchased from Japan Bio Services (Saitama, Japan). The chemically synthesized tRNAVal oligonucleotide was purchased from Gene Design (Osaka, Japan).
2.2. Plasmid construction
Double‐stranded DNA fragment encoding mazF DR0417 gene was digested with EcoRI and BamHI (Toyobo, Osaka, Japan) and purified using a MinElute PCR purification kit (Qiagen, Hilden, Germany). Likewise, the pMX‐T plasmid encoding D. radiodurans mazEF genes (mazEF DR0416DR0417) was digested with BamHI (New England Biolabs, Ipswich, MA, USA) and cleaned with a QIAquick PCR purification kit (Qiagen). The linearized pMX‐T plasmid was then digested with EcoRI (Takara), and the DNA fragment containing mazEF DR0416DR0417 genes was recovered by using a QIAquick gel extraction kit (Qiagen). These DNA fragments were then ligated into the corresponding pET21c multiple cloning site using a DNA ligation kit (Takara), producing pET21c‐mazF DR0417 and pET21c‐mazEF DR0416DR0417, and they were introduced into the E. coli strain DH5α (Nippon Gene, Tokyo, Japan). pET21c‐mazF DR0417 and pET21c‐mazEF DR0416DR0417 were extracted using a QIAprep Spin Miniprep Kit (Qiagen) and the sequences were validated using an AB 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's protocol.
2.3. Growth effect of MazEDR0416 and MazFDR0417
The E. coli cells harboring pET21c empty vector, pET21c encoding D. radiodurans mazF or mazEF were cultivated at 37°C for 12 h in liquid LB medium supplemented with 100 μg/mL ampicillin. Turbid overnight cultures were then streaked onto the LB plates containing 100 μg/mL ampicillin and 0.2% glucose with or without 25 μmol/L IPTG at 37°C.
2.4. Protein expression
pET19b‐mazE DR0416 was introduced into the E. coli strain BL21 (DE3) (BioDynamics Laboratory Inc., Tokyo, Japan), whereas pET21c‐mazF DR0417 was introduced into the strain BL21 (DE3) (Nippon Gene) using the heat‐shock method. The E. coli cells harboring pET19b‐mazE DR0416 or pET21c‐mazF DR0417 were grown overnight in liquid LB medium supplemented with 100 μg/mL ampicillin at 37°C. These cells were inoculated into 1 L LB medium containing 100 μg/mL ampicillin. One millimolar IPTG was added to induce MazEDR0416 and MazFDR0417 when the OD600 reached 0.8 and 3.0, respectively. The cells were harvested by centrifugation at 7,000g after 5 and 3.5 h of incubation for MazEDR0416 and MazFDR0417, respectively.
2.5. Purification of MazEDR0416
Escherichia coli cells containing MazEDR0416 were thawed on ice and suspended in 18 mL of 6 mol/L urea buffer (10 mmol/L sodium phosphate buffer (pH 8.0), 150 mmol/L NaCl, 0.025% Triton X‐100, 6 mol/L urea, 2.5 mmol/L β‐mercaptoethanol, and 20 mmol/L imidazole). The suspended cells were then incubated on ice for 5 min in the presence of 0.09 mg/mL lysozyme. The cells were lysed by sonication and collected by centrifuging at 7000g. The supernatant was then filtered through a 0.45‐μm membrane (Millex, Darmstadt, Germany) and applied to a 1‐mL His‐Trap FF column (GE Healthcare, Little Chalfont, UK). Urea was then removed by gradually substituting the 6 mol/L urea buffer with binding buffer (20 mmol/L sodium phosphate buffer (pH 8.0), 300 mmol/L NaCl, 0.05% Triton X‐100, 5 mmol/L β‐mercaptoethanol, and 40 mmol/L imidazole) using an AKTA pure 25 (GE Healthcare). The following program was used for this procedure: flow rate, 1 mL/min; linear elution gradient, 20 column volumes (cv). Subsequently, the column was washed with 32 cv of binding buffer. Deca‐histidine‐tagged MazEDR0416 was selectively eluted by increasing the concentration of elution buffer (20 mmol/L sodium phosphate buffer (pH 8.0), 300 mmol/L NaCl, 0.05% Triton X‐100, 5 mmol/L β‐mercaptoethanol, and 500 mmol/L imidazole) using the following program: flow rate, 1 mL/min; linear elution gradient, 20 cv; fraction size, 0.5 mL. Molecular weight and purity were confirmed by the Agilent 2200 TapeStation P200 ScreenTape Assay (Agilent Technologies, Santa Clara, CA, USA). Protein concentration was determined using the Qubit Protein Assay Kit (Life Technologies, Carlsbad, CA, USA).
2.6. Purification of MazFDR0417
The recombinant MazFDR0417 was purified as described previously (Miyamoto et al. 2016, 2016), with slight modifications. Escherichia coli cells containing MazFDR0417 were thawed on ice and suspended in 32 mL of binding buffer (20 mmol/L sodium phosphate buffer (pH 8.0), 300 mmol/L NaCl, 5 mmol/L β‐mercaptoethanol, and 50 mmol/L imidazole). The cells were lysed by sonication and collected by centrifuging at 7000 g. The supernatant was then filtered through a 0.45‐μm membrane (Millex) and applied to a 1‐mL His‐Trap FF crude column (GE Healthcare). Nonspecifically bound proteins were removed by washing with 32 cv of binding buffer using AKTA pure 25 (GE Healthcare). Hexa‐histidine‐tagged MazFDR0417 was selectively eluted by gradually increasing the elution buffer concentration using the following program: flow rate, 1 mL/min; linear elution gradient, 20 cv; fraction size, 0.5 mL. The composition of the elution buffer was as follows: 20 mmol/L sodium phosphate buffer (pH 8.0), 300 mmol/L NaCl, 5 mmol/L β‐mercaptoethanol, and 500 mmol/L imidazole. Molecular weight and purity were confirmed by the Agilent 2200 TapeStation P200 ScreenTape Assay (Agilent Technologies). Protein concentration was determined using the Qubit Protein Assay Kit (Life Technologies).
2.7. Enzymatic activity of MazEDR0416 and MazFDR0417
Synthetic RNA constructs were prepared as described in our previous study (Miyamoto et al. 2016). One hundred nanograms of RNA 500‐2 was incubated with 1.2, 6, or 30 pmol of MazFDR0417 at 37°C for 2 h in MazF reaction buffer (20 mmol/L Tris‐HCl (pH 8.0), 1 mmol/L dithiothreitol, 0.01% Triton X‐100, and 4 U of recombinant RNase inhibitor (Takara)) in a 50‐μL reaction volume. As a control reaction, 30 pmol of MazFDR0417 was preincubated with 300 pmol of MazEDR0416 at room temperature for 10 min, and this mixture was incubated with 100 ng of RNA 500‐2 at 37°C for 2 h in MazF reaction buffer in a final volume of 50 μL. These RNAs were purified with RNA Clean and Concentrator™‐5 (Zymo Research, Orange, CA, USA). Next, gel loading buffer II (Ambion, Austin, TX, USA) was added to each sample. The samples were incubated at 95°C for 5 min and then separated on a 10% polyacrylamide gel containing 7 mol/L urea. The RNA was stained using SYBR Gold (Life Technologies) and then detected using a Typhoon 9210 imager (GE Healthcare).
2.8. Cleavage sequence identification
A sequencing library was constructed as described in our previous study (Miyamoto et al. 2016), with slight modifications. Briefly, five RNA mixtures were incubated with 1.5 μg of MazFDR0417 at 37°C for 30 min in MazF reaction buffer in a 20‐μL reaction volume. Phosphorylation, barcode ligation, and sequencing were performed as described previously (Miyamoto et al. 2016). The sequence data were analyzed with CLC Genomics 7.5.1., using the same parameters outlined in our previous work (Miyamoto et al. 2016). Relative coverage increase, which is defined as the coverage at the (n + 1)th position divided by the coverage at the nth position, was calculated for all reference samples. Nucleotide positions with coverage <500 were excluded from analysis. Of these nucleotide positions, those showing the overall top 15 and top 25 relative coverage increases were selected. The sequences five‐base pairs upstream and downstream of these positions were extracted and aligned using WebLogo (Crooks, Hon, Chandonia, & Brenner, 2004). These sequence data have been submitted to the DDBJ database under the accession number DRA004579.
2.9. Fluorometric analysis
Fluorometric analysis was performed as described previously (Miyamoto et al., 2016, 2016). Fifteen or 600 ng of MazFDR0417 was incubated with 10 pmol of fluorescently labeled oligonucleotides in MazF reaction buffer in a total volume of 20 μL. For control reactions, the oligonucleotides were also treated with 100 ng of RNase A (Novagen, Madison, WI, USA) in MazF reaction buffer in a final volume of 20 μL. All reactions were conducted at 37°C in triplicate. The fluorescence intensity was recorded every 1 min using a Light Cycler 480 system (Roche, Basel, Switzerland) with 483 nm excitation and 533 nm detection filters.
2.10. tRNA cleavage
Five picomoles of chemically synthesized tRNAVal was incubated with 0.1, 0.9, or 8.1 pmol of MazFDR0417 at 37°C for 30 min in MazF reaction buffer in a 20‐μL reaction volume. Gel loading buffer II (Ambion) was added to each sample. These samples were incubated at 95°C for 5 min and then separated on a 10% polyacrylamide gel containing 7 mol/L urea. The RNA was stained using SYBR Gold (Life Technologies) and then detected using a Typhoon 9210 imager (GE Healthcare).
2.11. Accession numbers
The GenBank accession numbers are as follows: MazEDR0416 (NP_294139), MazFDR0417 (NP_294140), tRNAVal (AE000513), and artificially designed RNAs 500‐2 (AB610940), 1000‐1 (AB610944), 1000‐2 (AB610945), 1000‐3 (AB610946), 1000‐4 (AB610947), and 1000‐5 (AB610948).
3. RESULTS
3.1. MazEDR0416 and MazFDR0417 constitute a TA system
Previously, the genes located at DR0416 and DR0417 were presumed to encode a TA pair, the antitoxin MazE (MazEDR0416) and the toxin MazF (MazFDR0417) (Figure S1a), which shows 42.7% and 43.6% similarity to E. coli MazE and MazF, respectively (Figure 1a) (Chopra et al., 2013; Pandey & Gerdes, 2005). To briefly assess whether these components constitute a genuine TA system, we first cloned these genes into IPTG inducible vectors and expressed them in E. coli. As shown in Figure S1b, E. coli growth was inhibited when MazFDR0417 was expressed. In contrast, the cell growth was restored in the case of MazEDR0416 coexpression (Figure S1b, right panel). Unexpectedly, we observed MazFDR0417‐mediated growth inhibition even in the absence of IPTG (Figure S1b, middle panel); this would be probably because the ‘leaky’ expression of MazFDR0417 is toxic to the cells. We next purified the recombinant proteins to study the cleavage activity of MazFDR0417. When we investigated the purity and molecular weight of the protein by gel electrophoresis, a single peak was observed (Figure S1c, lower panel), indicating that we had obtained highly purified MazFDR0417. We next coincubated MazFDR0417 with substrate RNA (RNA 500‐2), and dose‐dependent RNA fragmentation was observed (Figure 1b). Furthermore, the banding patterns of cleaved RNA were unique in length (Figure 1b, lane 5), indicating that MazFDR0417 is a functional toxin endoribonuclease possessing sequence specificity. Next, we purified MazEDR0416 (Figure S1c, upper panel) and examined the effect of MazEDR0416 on quenching of the enzymatic activity of MazFDR0417. As expected, MazFDR0417‐catalyzed cleavage was abolished by preincubation with MazEDR0416 (Figure 1b, lane 6). Therefore, MazEDR0416 and MazFDR0417 constitute an authentic TA system.
Figure 1.
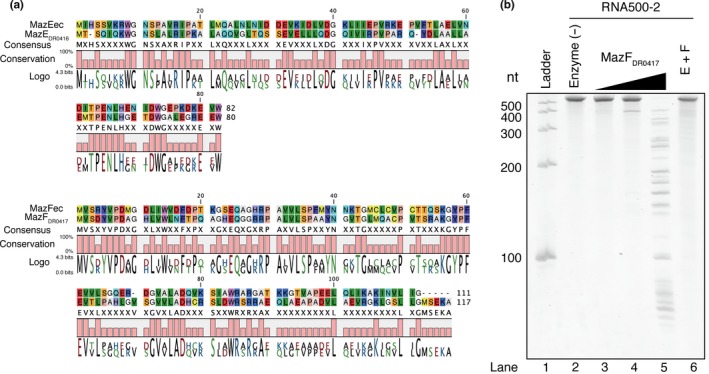
MazF endoribonuclease conserved in D. radiodurans. (a) Pairwise alignment of E. coli MazE (MazEec) and D. radiodurans MazE (MazEDR 0416) (upper panel); pairwise alignment of E.coli MazF (MazFec) and D. radiodurans MazF (MazFDR 0417) (lower panel). (b) MazFDR 0417‐mediated RNA cleavage. A 533‐nt artificially designed RNA (RNA 500‐2) was incubated with 1.2, 6, and 30 pmol of MazFDR 0417. The rightmost lane included 30 pmol of MazFDR 0417 preincubated with 300 pmol of MazEDR 0416
3.2. MazFDR0417 recognizes specific 4‐nt motifs
After confirming that mazF DR0417 encodes an endoribonuclease, we investigated its sequence specificity using a modified RNA‐seq approach recently developed in our laboratory (Miyamoto et al., 2016). In this approach, five synthetic RNA constructs (1000‐1, 1000‐2, 1000‐3, 1000‐4, and 1000‐5) were digested with MazFDR0417, and the fragmented RNA products harboring the cleavage sites at their 5′‐ends were preferentially detected by identifying the nucleotide positions with increased coverage (Figure 2a), followed by extraction of the sequences located five bases upstream and five bases downstream of the identified nucleotides (Table S1).
Figure 2.
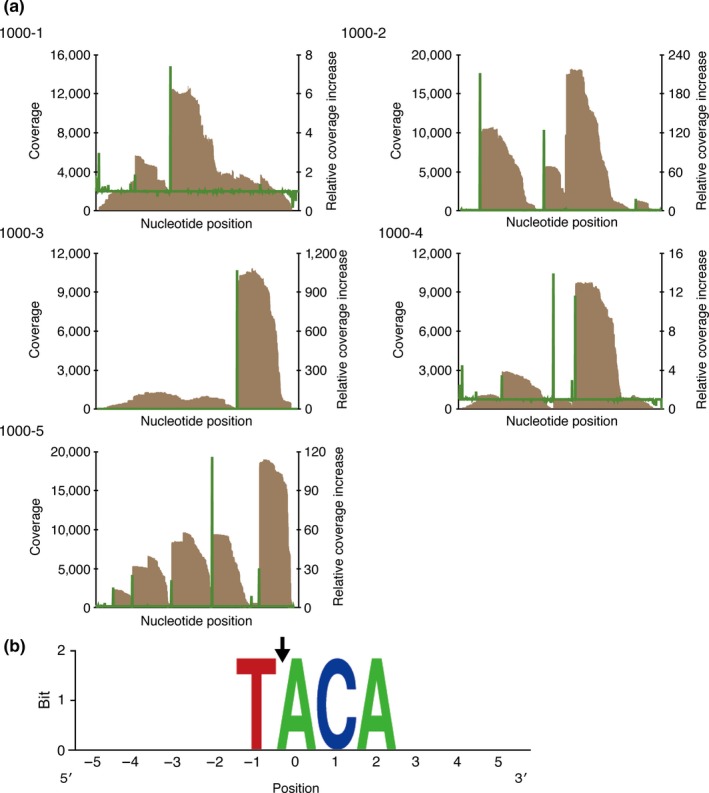
Analysis of the cleavage sequence of MazFDR 0417. (a) Graph of the coverage (brown bar) and relative coverage increase (green line). (b) Conserved sequences around the nucleotide positions with increased coverage. The nucleotide position with significant increases in coverage was set to zero. The black arrow indicates the cleavage position
As shown in Figure 2b, a strong consensus 4‐nt motif, TACA, was observed in the analysis of the overall top 15 sequences (Table S1), suggesting that UACA is the major target of MazFDR0417. However, RNA 500‐2 was degraded into many fragments (Figure 1b, lane 5), despite that it contains only two UACA sites (Table S2). Thus, we speculated that MazFDR0417, similar to other MazF homologues (Miyamoto et al., 2016; Park, Yamaguchi, & Inouye, 2011; Schifano et al., 2014; Verma & Bhatnagar, 2014; Zhu et al., 2009), possesses suboptimal cleavage sequences with relatively weak cleavage affinities. When we next analyzed the overall top 25 sequences, UACC, UACU, and AACA were additionally detected (Table S1 and Figure S2), indicating that MazFDR0417 recognizes these specific sequences with lower affinities. In all reference samples, coverage increased at the second adenine (UACA, UACC, UACU, and AACA) (Table S1). Accordingly, MazFDR0417 was likely to recognize these tetrads and cleave RNAs immediately upstream of the adenine (Figures 2b and S2).
3.3. MazFDR0417 cleaves specific tetrads in a strict manner
To further confirm that MazFDR0417 is a sequence‐specific endoribonuclease, we utilized a fluorescence quenching technique. Briefly, short oligonucleotides tagged with 6‐carboxyfluorescein and black hole quencher‐1 at the 5′‐end and 3′‐end, respectively, were treated with endoribonucleases. Because the two dyes were tethered by DNA and/or RNA nucleotides, the fluorescence of 6‐carboxyfluorescein was typically quenched. However, as oligonucleotides were cut and unbound, their fluorescence intensities increased because of the increasing amount of unquenched 6‐carboxyfluorescein (Miyamoto et al., 2016, 2016; Wang & Hergenrother, 2007). We evaluated the cleavage specificity of MazFDR0417 using the oligonucleotides listed in Table 1.
Table 1.
Sequences of fluorescently labeled oligonucleotides
| Name | Sequence (5′ to 3′) a |
|---|---|
| DR‐14‐UACA | AAAAAUACAAAAAA |
| DR‐14‐UACC | AAAAAUACCAAAAA |
| DR‐14‐UACU | AAAAAUACUAAAAA |
| DR‐14‐AACA | AAAAAAACAAAAAA |
| D‐13‐AAA | AAAAAAAAAAAAA |
| R‐13‐GUUGU | GUUGUCAUGCCGG |
| R‐13‐UCUCG | UCUCGGUGCGUUG |
| R‐13‐UGACA | UGACACGAACCGC |
| DR‐14‐CACA | AAAAACACAAAAAA |
| DR‐14‐UAUA | AAAAAUAUAAAAAA |
| DR‐13‐ACA | AAAAAACAAAAAA |
Underlined letters represent RNA nucleotides, whereas other letters represent DNA nucleotides.
To examine whether UACA and three other sequences (UACC, UACU, and AACA) are the determinants of MazFDR0417‐mediated RNA cleavage, we synthesized DNA‐RNA chimeric oligonucleotides including corresponding RNA tetrads (DR‐14‐UACA, DR‐14‐UACC, DR‐14‐UACU, and DR‐14‐AACA). Consistent with the RNA‐seq results, DR‐14‐UACA was cleaved (Figures 3a and 4a). Meanwhile, the three other chimeric oligonucleotides (DR‐14‐UACC, DR‐14‐UACU, and DR‐14‐AACA) were cleaved less effectively, as cleavage was not observed until an excessive amount of MazFDR0417 was added (Figures 3b–d and 4b–d). These results reinforce that UACA is the major cleavage site of MazFDR0417.
Figure 3.
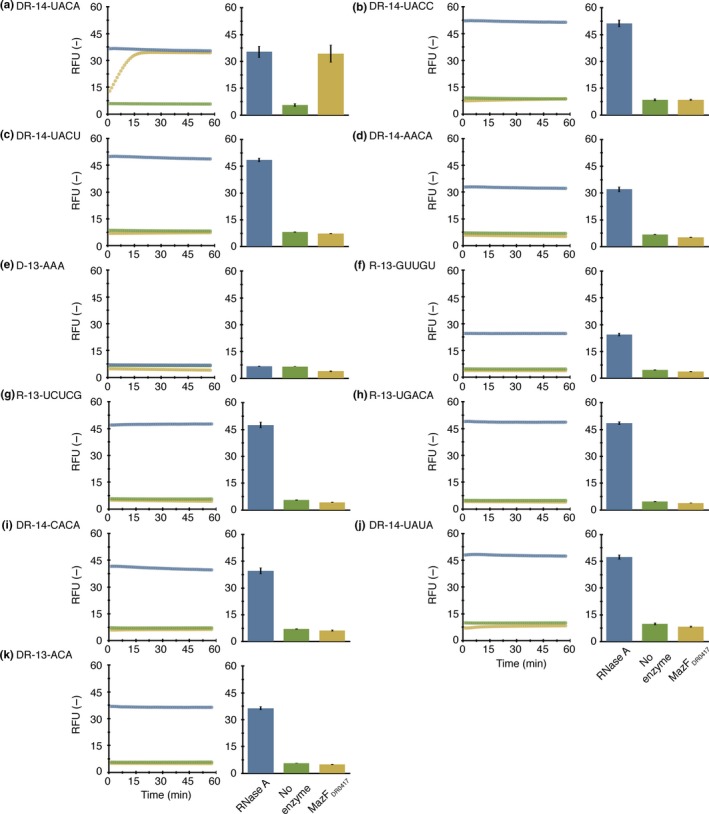
Sequence‐specific RNA cleavage with a small amount of MazFDR 0417. Fifteen nanograms of MazFDR 0417 (yellow) was incubated with 10 pmol of fluorescently modified oligonucleotides: (a) DR‐14‐UACA, (b) DR‐14‐UACC, (c) DR‐14‐UACU, (d) DR‐14‐AACA, (e) D‐13‐AAA, (f) R‐13‐GUUGU, (g) R‐13‐UCUCG, (h) R‐13‐UGACA, (i) DR‐14‐CACA, (j) DR‐14‐UAUA, and (k) DR‐13‐ACA. In the control reactions, fluorescence intensities in the presence of 100 ng of RNase A (blue) and absence of enzymes (green) at each time point (left) and the end point (right) were measured
Figure 4.
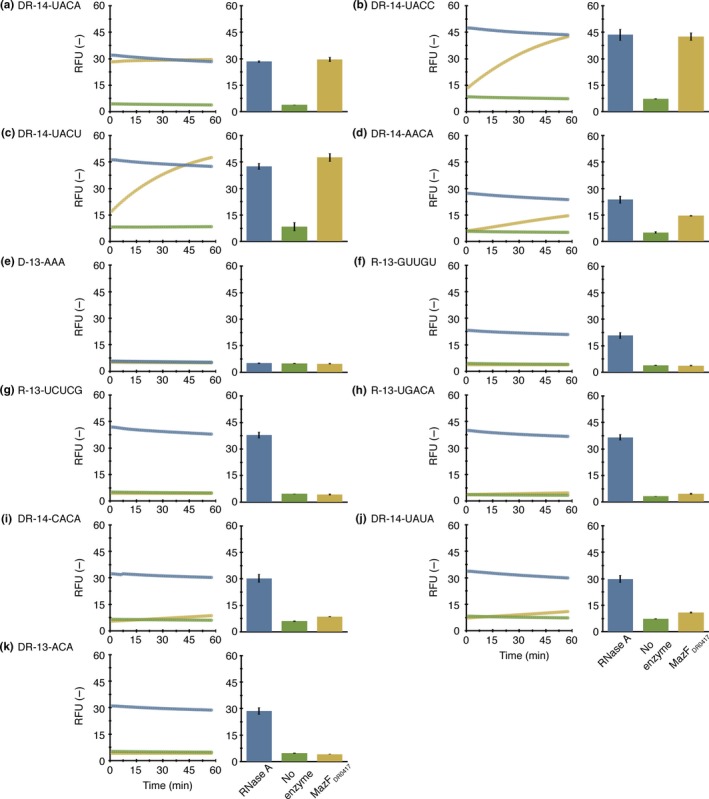
Sequence‐specific RNA cleavage with an excessive amount of MazFDR 0417. Six hundred nanograms of MazFDR 0417 (yellow) was incubated with 10 pmol of fluorescently modified oligonucleotides: (a) DR‐14‐UACA, (b) DR‐14‐UACC, (c) DR‐14‐UACU, (d) DR‐14‐AACA, (e) D‐13‐AAA, (f) R‐13‐GUUGU, (g) R‐13‐UCUCG, (h) R‐13‐UGACA, (i) DR‐14‐CACA, (j) DR‐14‐UAUA, and (k) DR‐13‐ACA. In the control reactions, fluorescence intensities in the presence of 100 ng of RNase A (blue) and absence of enzymes (green) at each time point (left) and the end point (right) were measured
Notably, there was no increase in fluorescence intensity in a DNA oligonucleotide (D‐13‐AAA) associated with MazFDR0417 treatment (Figures 3e and 4e). Furthermore, none of the three RNA oligonucleotides (R‐13‐GUUGU, R‐13‐UCUCG, and R‐13‐UGACA), the internal sequences of which were identical to that of the RNA substrate (RNA 1000‐4) used in the RNA‐seq, were cleaved by MazFDR0417 (Figures 3f–h and 4f–h). Because these oligonucleotides are devoid of specific tetrads, these results support the sequence specificity of MazFDR0417.
Finally, to investigate whether MazFDR0417 recognition is strict, we prepared three mutated oligonucleotides (DR‐14‐CACA, DR‐14‐UAUA, and DR‐13‐ACA). Because the RNA‐seq results suggested that MazFDR0417 cleaves RNAs at U^ACA (where ^ denotes the position of the cleavage), we altered the bases preceding and following the second adenine to the pyrimidine bases C and U, respectively (DR‐14‐CACA and DR‐14‐UAUA). In addition, an oligonucleotide whose first uracil was removed from the tetrad was synthesized (DR‐13‐ACA). In all cases, we observed complete blockage (Figures 3i–k) or reduction in cleavage (Figures 4i–k), demonstrating that the recognition of MazFDR0417 is specific to some tetrads. From these data, we concluded that MazFDR0417 is a four‐base cutter whose prime target is UACA.
4. DISCUSSION
TA systems are common in prokaryotic chromosomes and plasmids and are frequently found in multiple loci in the same organism (Chopra et al., 2013; Pandey & Gerdes, 2005; Sevin & Barloy‐Hubler, 2007). They are activated under stressful conditions and they enhance bacterial stress resistance. Depending on the antitoxin nature and its manner of neutralizing toxin activity, TA systems are currently classified into six distinct classes (Page & Peti, 2016). Among these, the type II TA system, where a protein antitoxin inhibits the activity of its cognate protein toxin by forming a toxin‐antitoxin complex, is one of the most widely studied classes (Schuster & Bertram, 2013). Based on the similarities in the toxin sequences, these systems are further divided into several families (i.e., MazEF, VapBC, and HigBA) (Leplae et al., 2011).
In the current study, we demonstrated that a D. radiodurans chromosomal TA pair encoded by the DR0416 and DR0417 loci forms a canonical type II MazEF system (Figures 1b and S1b). Furthermore, we revealed that the MazF toxin (MazFDR0417) functions as a 4‐nt specific cutter and strictly recognizes the UACA tetrad (Figures 2, 3, 4). To the best of our knowledge, this is the first MazF that specifically recognizes the U^ACA sequence (Masuda & Inouye, 2017; Schifano & Woychik, 2017). Recently, Zorzini et al., (2016) determined the crystal structure of E. coli MazF (MazFec) in complex with the substrate analogue d(A1U2 A 3 C 4 A 5U6A7), and reinforced the notion that MazFec recognizes ACA triplet strictly and that MazFec cleaves the substrate at the position of ^ACA and A^CA (Miyamoto et al., 2016; Vesper et al., 2011; Zhang, Zhang, Hara, Kato, & Inouye, 2005a; Zhang et al., 2003). They mentioned that the MazFec recognition site could be divided into two different regions: first, the one where dU2 is located, which is called the upstream binding site; and second, the one that accommodates d(A3C4A5U6), which is called the downstream binding groove. In the upstream region, they observed a prominent cavity, and they reasoned that both purine and pyrimidine bases could be accommodated in the space; thus, any base could be located at the position of one‐base upstream of the first A (ACA). Furthermore, they mentioned that MazFec possesses two distinct positions of the cleavage sites (^ACA and A^CA), as the lack of hydrogen bonds between dU6 in the downstream binding groove and the MazFec recognition site reduces the specificity of the cleavage position. Taken together, MazFDR0417 is distinct from MazFec in the following two points: first, it recognizes the unprecedented tetrad, UACA; indeed it does not cleave ACA (Figures 3k and 4k). Second, MazFDR0417 cleaves U^ACA (Figure 2b).
It was predicted that the gene encoded at DR0662 also codes for a MazF toxin (MazFDR0662) (Pandey & Gerdes, 2005). Given that these two MazF endoribonucleases show only 22.8% similarity (Figure S3), it is not surprising that they recognize distinct sequences. In fact, although the cleavage sequence and recognition length of MazFDR0662 remain unknown, Shimada, Takayama, Asada, & Kato, (2011) previously suggested that purified MazFDR0662 cleaved the RNA oligonucleotide lacking the UACA tetrad at the UU^CCUUU site. Therefore, the difference in cleavage specificity may be beneficial for D. radiodurans to enrich specific transcripts to withstand certain environmental stresses.
Sequence‐specific toxin endoribonucleases have been proposed to target messenger RNAs selectively, resulting in protein‐mediated RNA interference; therefore, these endoribonucleases were also referred to as “mRNA interferases” (Christensen‐Dalsgaard & Gerdes, 2008; McKenzie et al., 2012; Zhang, Yamaguchi, & Inouye, 2009; Zhang et al., 2005b; Zhang, Zhu, Zhang, & Inouye, 2005b; Zhang et al., 2003; 2005 Zhu et al., 2006). However, recent studies documented that some of these endoribonucleases also target transfer RNA, enabling cells to alter their translation using a different mechanism (Cruz et al., 2015; Schifano et al., 2016). In these reports, single‐stranded regions of tRNAs were cleaved by endoribonucleases, thereby blocking protein synthesis. Analysis of tRNA sequences of D. radiodurans revealed that the UACA sequence was located within the anticodon stem‐loop of tRNAVal (Figure S4a and Table S2). Thus, MazFDR0417 may also inhibit translation indirectly by inactivating the function of tRNAVal as an adaptor molecule; indeed, MazFDR0417 halved the chemically synthesized tRNAVal in vitro (Figure S4b). Future studies are necessary to validate whether native tRNAVal is a genuine target of this enzyme.
In summary, our data showed that MazFDR0417 is a functional endoribonuclease and recognizes a unique tetrad, UACA. These data indicate that this enzyme enables D. radiodurans to acclimate to environmental changes through direct and/or indirect growth modulation.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI, Grant Number 15K14241.
Miyamoto T, Ota Y, Yokota A, Suyama T, Tsuneda S, Noda N. Characterization of a Deinococcus radiodurans MazF: A UACA‐specific RNA endoribonuclease. MicrobiologyOpen. 2017;6:e501 https://doi.org/10.1002/mbo3.501
Contributor Information
Satoshi Tsuneda, Email: stsuneda@waseda.jp.
Naohiro Noda, Email: noda-naohiro@aist.go.jp.
REFERENCES
- Aizenman, E. , Engelberg‐Kulka, H. , & Glaser, G. (1996). An Escherichia coli chromosomal “addiction module” regulated by guanosine 3′, 5′‐bispyrophosphate: A model for programmed bacterial cell death. Proceedings of the National Academy of Sciences of the United States of America, 93, 6059–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawneh, A. M. , Qi, D. , Yonesaki, T. , & Otsuka, Y. (2016). An ADP‐ribosyltransferase Alt of bacteriophage T4 negatively regulates the Escherichia coli MazF toxin of a toxin‐antitoxin module. Molecular Microbiology, 99, 188–198. [DOI] [PubMed] [Google Scholar]
- Amitai, S. , Kolodkin‐Gal, I. , Hananya‐Meltabashi, M. , Sacher, A. , & Engelberg‐Kulka, H. (2009). Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genetics, 5, e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, N. , Saumitra, Pathak. A. , Bhatnagar, R. , & Bhatnagar, S. (2013). Linkage, mobility, and selfishness in the MazF family of bacterial toxins: A snapshot of bacterial evolution. Genome Biology and Evolution, 5, 2268–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen‐Dalsgaard, M. , & Gerdes, K. (2008). Translation affects YoeB and MazF messenger RNA interferase activities by different mechanisms. Nucleic Acids Research, 36, 6472–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks, G. E. , Hon, G. , Chandonia, J. M. , & Brenner, S. E. (2004). WebLogo: A sequence logo generator. Genome Research, 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, J. W. , Sharp, J. D. , Hoffer, E. D. , Maehigashi, T. , Vvedenskaya, I. O. , Konkimalla, A. , … Woychik, N. A. (2015). Growth‐regulating Mycobacterium tuberculosis VapC‐mt4 toxin is an isoacceptor‐specific tRNase. Nature Communications, 6, 7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan, R. , & Engelberg‐Kulka, H. (2004). Escherichia coli mazEF‐mediated cell death as a defense mechanism that inhibits the spread of phage P1. Molecular Genetics and Genomics, 272, 227–234. [DOI] [PubMed] [Google Scholar]
- Hazan, R. , Sat, B. , & Engelberg‐Kulka, H. (2004). Escherichia coli mazEF‐mediated cell death is triggered by various stressful conditions. Journal of Bacteriology, 186, 3663–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada, K. , Hanaoka, F. , & Burley, S. K. (2003). Crystal structure of the MazE/MazF complex: Molecular bases of antidote‐toxin recognition. Molecular Cell, 11, 875–884. [DOI] [PubMed] [Google Scholar]
- Leplae, R. , Geeraerts, D. , Hallez, R. , Guglielmini, J. , Dreze, P. , & Van Melderen, L. (2011). Diversity of bacterial type II toxin‐antitoxin systems: A comprehensive search and functional analysis of novel families. Nucleic Acids Research, 39, 5513–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova, K. S. , Aravind, L. , Wolf, Y. I. , Tatusov, R. L. , Minton, K. W. , Koonin, E. V. , & Daly, M. J. (2001). Genome of the extremely radiation‐resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiology and Molecular Biology Reviews, 65, 44–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, H. , & Inouye, M. (2017). Toxins of prokaryotic toxin‐antitoxin systems with sequence‐specific endoribonuclease activity. Toxins, 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, J. L. , Duyvestyn, J. M. , Smith, T. , Bendak, K. , MacKay, J. , Cursons, R. , … Arcus, V. L. (2012). Determination of ribonuclease sequence‐specificity using Pentaprobes and mass spectrometry. RNA, 18, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T. , Kato, Y. , Sekiguchi, Y. , Tsuneda, S. , & Noda, N. (2016. a). Characterization of MazF‐mediated sequence‐specific RNA cleavage in Pseudomonas putida using massive parallel sequencing. PLoS ONE, 11, e0149494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T. , Yokota, A. , Tsuneda, S. , & Noda, N. (2016. b). AAU‐Specific RNA cleavage mediated by MazF toxin endoribonuclease conserved in Nitrosomonas europaea . Toxins, 8, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariya, H. , & Inouye, M. (2008). MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell, 132, 55–66. [DOI] [PubMed] [Google Scholar]
- Page, R. , & Peti, W. (2016). Toxin‐antitoxin systems in bacterial growth arrest and persistence. Nature Chemical Biology, 12, 208–214. [DOI] [PubMed] [Google Scholar]
- Pandey, D. P. , & Gerdes, K. (2005). Toxin‐antitoxin loci are highly abundant in free‐living but lost from host‐associated prokaryotes. Nucleic Acids Research, 33, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. H. , Yamaguchi, Y. , & Inouye, M. (2011). Bacillus subtilis MazF‐bs (EndoA) is a UACAU‐specific mRNA interferase. FEBS Letters, 585, 2526–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenbacher, F. P. , Suzuki, M. , Hurley, J. M. , Montville, T. J. , Kirn, T. J. , Ouyang, M. , & Woychik, N. A. (2012). Clostridium difficile MazF toxin exhibits selective, not global, mRNA cleavage. Journal of Bacteriology, 194, 3464–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauert, M. , Wolfinger, M. T. , Vesper, O. , Müller, C. , Byrgazov, K. , & Moll, I. (2016). The MazF‐regulon : A toolbox for the post‐transcriptional stress response in Escherichia coli. Nucleic Acids Research, 44, 6660–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano, J. M. , Cruz, J. W. , Vvedenskaya, I. O. , Edifor, R. , Ouyang, M. , Husson, R. N. , … Woychik, N. A. (2016). tRNA is a new target for cleavage by a MazF toxin. Nucleic Acids Research, 44, 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano, J. M. , Vvedenskaya, I. O. , Knoblauch, J. G. , Ouyang, M. , Nickels, B. E. , & Woychik, N. A. (2014). An RNA‐seq method for defining endoribonuclease cleavage specificity identifies dual rRNA substrates for toxin MazF‐mt3. Nature Communications, 5, 3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano, J. M. , & Woychik, N. A. (2017). Cloaked dagger: tRNA slicing by an unlikely culprit. RNA Biology, 14, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, C. F. , & Bertram, R. (2013). Toxin‐antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiology Letters, 340, 73–85. [DOI] [PubMed] [Google Scholar]
- Schuster, C. F. , Park, J. H. , Prax, M. , Herbig, A. , Nieselt, K. , Rosenstein, R. , … Bertram, R. (2013). Characterization of a MazEF toxin‐antitoxin homologue from Staphylococcus equorum . Journal of Bacteriology, 195, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevin, E. W. , & Barloy‐Hubler, F. (2007). RASTA‐Bacteria: A web‐based tool for identifying toxin‐antitoxin loci in prokaryotes. Genome Biology, 8, R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, M. , Takayama, M. Asada, K. Kato, I. (2011). Endoribonuclease. U.S. Patent 8,017,356. Sep.13
- Tiwari, P. , Arora, G. , Singh, M. , Kidwai, S. , Narayan, O. P. , & Singh, R. (2015). MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nature Communications, 6, 6059. [DOI] [PubMed] [Google Scholar]
- Tripathi, A. , Dewan, P. C. , Siddique, S. A. , & Varadarajan, R. (2014). MazF‐induced growth inhibition and persister generation in Escherichia coli . Journal of Biological Chemistry, 289, 4191–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S. , & Bhatnagar, R. (2014). MoxT toxin of Bacillus anthracis exhibits sequence specific ribonuclease activity. Biochemical and Biophysical Research Communications, 450, 998–1004. [DOI] [PubMed] [Google Scholar]
- Vesper, O. , Amitai, S. , Belitsky, M. , Byrgazov, K. , Kaberdina, A. C. , Engelberg‐Kulka, H. , & Moll, I. (2011). Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli . Cell, 147, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. R. , & Hergenrother, P. J. (2007). A continuous fluorometric assay for the assessment of MazF ribonuclease activity. Analytical Biochemistry, 371, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, Y. , & Inouye, M. (2011). Regulation of growth and death in Escherichia coli by toxin‐antitoxin systems. Nature Reviews Microbiology, 9, 779–790. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y. , Nariya, H. , Park, J. H. , & Inouye, M. (2012). Inhibition of specific gene expressions by protein‐mediated mRNA interference. Nature Communications, 3, 607. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Yamaguchi, Y. , & Inouye, M. (2009). Characterization of YafO, an Escherichia coli toxin. Journal of Biological Chemistry, 284, 25522–25531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang, J. , Hara, H. , Kato, I. , & Inouye, M. (2005. a). Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. Journal of Biological Chemistry, 280, 3143–3150. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Zhu, L. , Zhang, J. , & Inouye, M. (2005b). Characterization of ChpBK, an mRNA interferase from Escherichia coli . Journal of Biological Chemistry, 280, 26080–26088. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang, J. , Hoeflich, K. P. , Ikura, M. , Qing, G. , & Inouye, M. (2003). MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli . Molecular Cell, 12, 913–923. [DOI] [PubMed] [Google Scholar]
- Zhu, L. , Inoue, K. , Yoshizumi, S. , Kobayashi, H. , Zhang, Y. , Ouyang, M. , … Inouye, M. (2009). Staphylococcus aureus MazF specifically cleaves a pentad sequence, UACAU, which is unusually abundant in the mRNA for pathogenic adhesive factor SraP. Journal of Bacteriology, 191, 3248–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Phadtare, S. , Nariya, H. , Ouyang, M. , Husson, R. N. , & Inouye, M. (2008). The mRNA interferases, MazF‐mt3 and MazF‐mt7 from Mycobacterium tuberculosis target unique pentad sequences in single‐stranded RNA. Molecular Microbiology, 69, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Zhang, Y. , Teh, J. S. , Zhang, J. , Connell, N. , Rubin, H. , & Inouye, M. (2006). Characterization of mRNA interferases from Mycobacterium tuberculosis . Journal of Biological Chemistry, 281, 18638–18643. [DOI] [PubMed] [Google Scholar]
- Zorzini, V. , Mernik, A. , Lah, J. , Sterckx, Y. G. J. , De Jonge, N. , Garcia‐Pino, A. , … Loris, R. (2016). Substrate recognition and activity regulation of the Escherichia coli mRNA endonuclease MazF. Journal of Biological Chemistry, 291, 10950–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


