Abstract
Ionizing radiation (IR) is one of the most widely used treatments for cancer. However, acute damage to the gastrointestinal tract or gastrointestinal acute radiation syndrome (GI-ARS) is a major dose-limiting side effect, and the mechanisms that underlie this remain unclear. Here we use mouse models to explore the relative roles of DNA repair, apoptosis, and cell cycle arrest in radiation response. IR induces DNA double strand breaks and DNA-PK mutant Prkdcscid/scid mice are sensitive to GI-ARS due to an inability to repair these breaks. IR also activates the tumor suppressor p53 to trigger apoptotic cell death within intestinal crypt cells and p53 deficient mice are resistant to apoptosis. To determine if DNA-PK and p53 interact to govern radiosensitivity, we compared the response of single and compound mutant mice to 8 Gy IR. Compound mutant Prkdcscid/scid/Trp53−/−mice died earliest due to severe GI-ARS. While both Prkdcscid/scid and Prkdcscid/scid/Trp53−/−mutant mice had higher levels of IR-induced DNA damage, particularly within the stem cell compartment of the intestinal crypt, in Prkdcscid/scid/Trp53−/−mice these damaged cells abnormally progressed through the cell cycle resulting in mitotic cell death. This led to a loss of Paneth cells and a failure to regenerate the differentiated epithelial cells required for intestinal function. IR-induced apoptosis did not correlate with radiosensitivity. Overall, these data reveal that DNA repair, mediated by DNA-PK, and cell cycle arrest, mediated by p53, cooperate to protect the stem cell niche after DNA damage, suggesting combination approaches to modulate both pathways may be beneficial to reduce GI-ARS. As many cancers harbor p53 mutations, this also suggests targeting DNA-PK may be effective to enhance sensitivity of p53 mutant tumors to radiation.
Ionizing radiation (IR) causes DNA double strand breaks (DSBs) that can trigger two distinct cellular responses: DNA repair that contributes to cell survival, versus apoptosis or programmed cell death.1 What factors regulate the decision to execute one or the other of these two responses within a given cell or between cell types, and how this dictates overall response to IR in vivo is unclear.
DNA DSBs can be repaired by homologous recombination, a pathway that predominates in cells that are in the S/G2 phase of the cell cycle or by the nonhomologous end-joining (NHEJ) pathway which predominates in cells in G0/G1.2 Stem cells are typically in G0/G1 and so these cells may be especially reliant on NHEJ.3, 4 To initiate NHEJ, two proteins, Ku70 and Ku80, bind to the broken DNA ends and recruit DNA-PKcs, the catalytic subunit of the DNA-PK holoenzyme, which together with Artemis, XLF, XRCC4 and ligase IV processes and rejoins the breaks.5 Severe combined immunodeficient mice (Prkdcscid/scid) have reduced DNA-PKcs activity due to a mutation in Prkdc at Tyr4046, resulting in impaired DNA DSB repair and radiosensitivity.6, 7 DNA DSBs can also activate p53 leading to upregulation of pro-apoptotic genes and apoptotic cell death. Transit amplifying intestinal crypt cells from Trp53−/− mice are markedly resistant to the early wave of IR-induced apoptosis which peaks at 4 h, highlighting the important role of p53 in this response.8 At 24 h post IR, a delayed wave of cell death occurs in the Trp53−/−mice9 attributed to increased mitotic catastrophe due to progression of cells with chromosomal damage through the cell cycle.10
The gastrointestinal (GI) tract is highly susceptible to the damaging effects of IR, and GI-ARS is a major limiting factor for certain radiotherapy regimens. The intestine is a tissue that exhibits high cellular turnover making it a useful model to study both stem cell biology and the response to IR.11, 12 Several markers for the identification of the small intestinal stem cell have been reported including Bmi1, HopX, mTert, Lrig1, and Lgr5+.12 Only the Lgr5+ stem cells of the GI, also called crypt base columnar cells (CBC), reside at the base of the crypt between the Paneth cells.13 The Lgr5 stem cells divide about once per day14 to generate the much more rapidly dividing transit amplifying cells located at positions +4–10 of the crypt. The transit amplifying cells in turn generate the differentiated cells of the intestine, including Paneth cells, which migrate to the crypt base adjacent to the stem cells, goblet cells and enterocytes, which migrate up the villi and slough off into the intestinal lumen. This renewal cycle of the epithelial cells takes 3–5 days and occurs throughout adult life.
Given the resistance of p53 null mice to IR-induced apoptosis, several groups have studied the role of p53 and apoptosis in GI-ARS. Kamarov et al. demonstrated that at high dose of IR, Trp53 null mice are more susceptible to GI-ARS than wild-type (WT) mice. This susceptibility was attributed to unrestrained proliferation of p53 null crypt cells leading to mitotic cell death.15 Kirsch et al.16 established that mice with intestinal-specific deletion of p53 were resistant to apoptosis but susceptible to GI-ARS, and concluded that p53 controls GI-ARS in a manner that is independent of apoptosis. In contrast, mice deficient in the p53 regulated pro-apoptotic protein PUMA are resistant to IR-induced intestinal crypt cell apoptosis, are protected from GI-ARS, and survive longer than WT mice.17
We previously reported that, although p53 null mice are resistant to apoptosis, intestinal crypt cells from compound mutant Prkdcscid/scid/Trp53−/−, Ku70−/−/Trp53−/−, and Ku80−/−/Trp53−/− mice undergo normal WT levels of IR-induced apoptosis, indicating the existence of a p53 independent apoptotic pathway that is active only in the absence of DNA-PK.18 This unexpected interaction between DNA-PK and p53 in regulating IR-induced apoptosis prompted us to examine the longer-term effects of DNA-PK and p53 on GI-ARS using Prkdcscid/scid and Trp53−/−single and compound mutant mice.
Results
Prkdc scid/scid /Trp53 −/− mice are radiosensitive
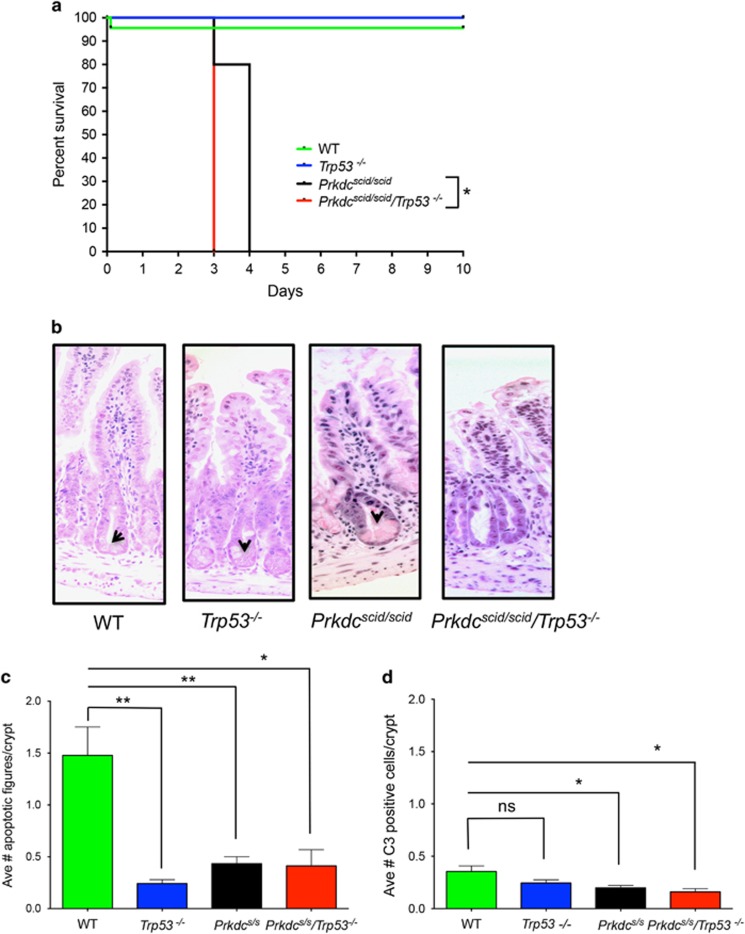
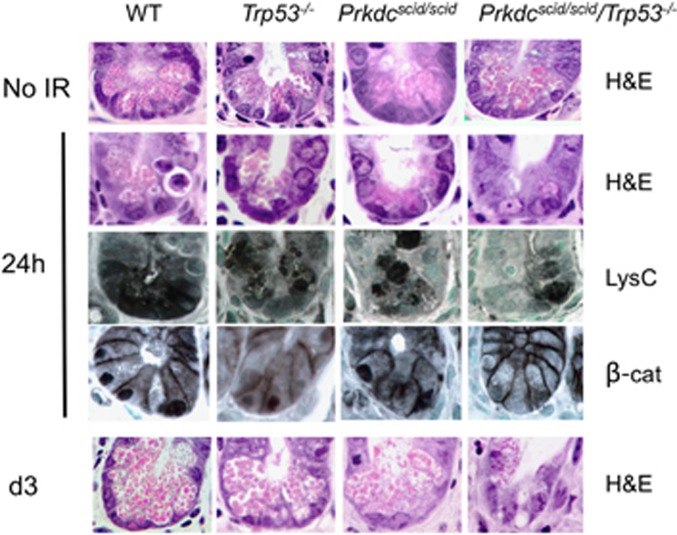
Adult Prkdcscid/scid, Prkdcscid/scid/Trp53−/−, Trp53−/−, or WT mice were exposed to a single whole-body dose of 8 Gy IR. WT and Trp53−/− mice survived >10 days with no signs of distress (Figure 1a). Prkdcscid/scid/Trp53−/− mice were the most radiosensitive, with all mice succumbing by day 3 post-IR (P<0.04 compared to Prkdcscid/scid; Figure 1a) while Prkdcscid/scid mice survived, on average, to day 4. Both Prkdcscid/scid and Prkdcscid/scid/Trp53−/− mice died from GI-ARS, marked by thinner intestines, shortening of the villi, and extensive disruption of epithelial cell integrity (Figure 1b). In addition to earlier lethality, GI-ARS was more severe in Prkdcscid/scid/Trp53−/− mice, demonstrated by depletion of Paneth cells, absence of crypts, and considerable loss of villi by day 3. Thus, the absence of p53 did not protect from and instead exacerbated the radiosensitivity of DNA-PKcs mutant mice.
Figure 1.
Prkdcscid/scid/Trp53−/− mice are radiosensitive. (a) Prkdcscid/scid/Trp53−/− (n=3), Prkdcscid/scid (n=5), Trp53−/− (n=4), or WT (n=3) mice were subjected to 8 Gy whole body irradiation. Prkdcscid/scid/Trp53−/− mutant mice died significantly earlier from GI-ARS compared by Mantel-Cox Log rank test to all other genotypes. WT versus Prkdcscid/scidor Prkdcscid/scid/Trp53−/−: P<0.0001; Prkdcscid/scid/Trp53−/− versus Prkdcscid/scid: *P=0.04. (b) H&E-stained cross sections of irradiated gastrointestinal tracts at 3 days post 8 Gy IR. Note the loss of Paneth cells in the Prkdcscid/scid/Trp53−/− compared to Prkdcscid/scid mice; arrowheads indicate Paneth cells. (c) Average number of apoptotic figures and caspase 3 (C3) positive cells per crypt 24 h post 8 Gy IR (Unpaired t-test, **P<0.003; *P<0.02). (d) Average caspase 3 positive cells per crypt 24 h post 8 Gy IR (Unpaired t test, *P<0.02; NS=not significant); Prkdcs/s=Prkdcscid/scid
Apoptosis is not the determining factor for GI toxicity
To determine the cellular basis for the GI-ARS of Prkdcscid/scid/Trp53−/− compound mutant mice, we examined DNA damage, cell cycle parameters, and cell death at 24 h post IR. Previous studies indicate that IR-induced apoptosis in the GI crypts from WT, Prkdcscid/scid, and Prkdcscid/scid/Trp53−/− mice peaks at 4 h while Trp53−/− mice are resistant to this early wave of apoptosis.8, 18 Crypt cell apoptosis was low in all genotypes at 24 h with<2 apoptotic figures per crypt. When compared to WT mice, the other genotypes had significantly fewer apoptotic figures (Figure 1c). We next assessed levels of cleaved caspase 3, a marker of caspase-mediated apoptosis. Compared to WT mice, both Prkdcscid/scid and Prkdcscid/scid/Trp53−/−had significantly fewer caspase 3 positive cells (Figure 1d). Collectively, this indicates that apoptosis in transit amplifying cells of the crypt does not correlate with GI toxicity, as WT mice had the highest levels of apoptosis yet they survived >10 days.
Increased DNA damage in the stem cell niche of both Prkdc scid/scid and Prkdc scid/scid /Trp53 −/− mice
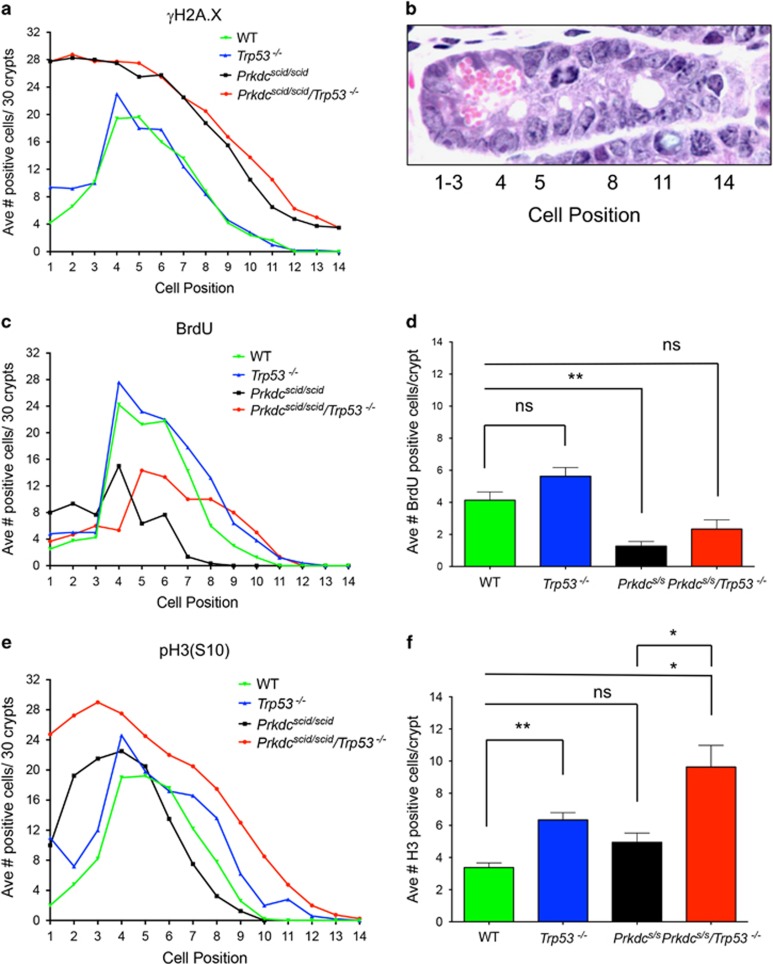
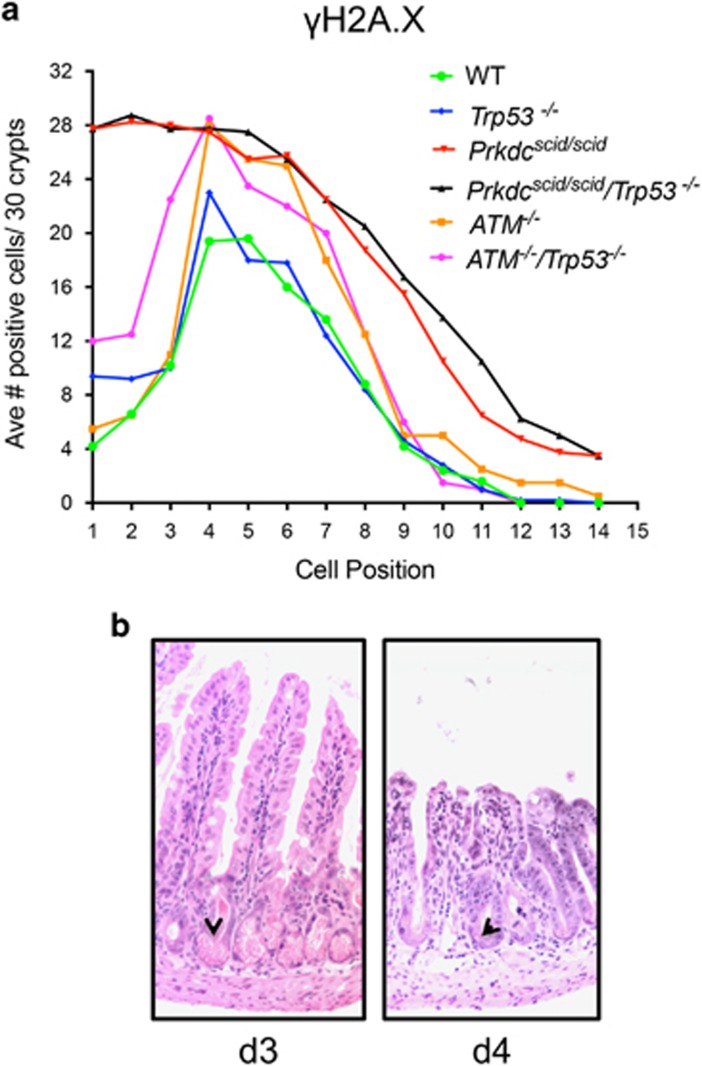
Persistent DNA damage, measured by γH2A.X staining at 24 h, was highly dependent on the cellular position within the crypt. In WT and Trp53−/− mice, DNA damage peaked in the transit amplifying zone, at cell positions 4–7 (Figures 2a and b). Few γH2A.X-positive cells were detected in the stem cell compartment at the base of the crypt or in post mitotic differentiated cells further up the crypt or in the villus. In contrast, in Prkdcscid/scid and Prkdcscid/scid/Trp53−/− mice a markedly different distribution of γH2A.X staining was observed, with the highest levels found in cells at the base of the crypt, which progressively decreased as cells moved up the crypts and onto the villi. Further, the number of cells positive for γH2A.X staining was greater at all cell positions, which would be anticipated in NHEJ deficient cells. Clearly, the greatest impact of DNA-PKcs mutation on DNA damage is seen at the base of the crypt within the stem cell compartment, suggesting these cells are particularly dependent on the NHEJ pathway of DNA repair.
Figure 2.
DNA-PK and Trp53 cooperate to prevent aberrant mitotic entry of irradiated intestinal stem cells. (a) Average number of γH2A.X positive cells per crypt position at 24 h post 8 Gy; Prkdcscid/scid/Trp53−/− (n=4), Prkdcscid/scid (n=4), Trp53−/− (n=5), or WT (n=5). (b) H&E of crypt showing positional counting. (c) Average number of BrdU-positive cells per crypt position at 24 h post 8 Gy: Prkdcscid/scid/Trp53−/− (n=3), Prkdcscid/scid(n=3), Trp53−/− (n=5), or WT (n=4). (d) Average number of BrdU positive cells per crypt 24 h post 8 Gy IR (Unpaired t-test, **P<0.007, NS=not significant). (e). Average number of phospho-H3 (S10)-positive cells per crypt position 24 h post 8 Gy IR: Prkdcscid/scid/Trp53−/− (n=4), Prkdcscid/scid (n=4), Trp53−/− (n=5), or WT (n=5). (f) Average number of positive phospho-H3 (S10) cells per crypt 24 h post 8 Gy IR (Unpaired t test, **P<0.0015; *P<0.03; NS=not significant). Prkdcs/s=Prkdcscid/scid
In addition to DNA repair and apoptosis, IR also induces cell cycle arrest, which can affect cell survival. Cell cycle arrest allows time for DNA repair thereby preventing entry of damaged cells into mitosis, which can lead to mitotic cell death. IR-induced DNA damage activates p53, which in turn induces the CDK inhibitor p21, leading to G1 cell cycle arrest. Recent studies demonstrate DNA-PK plays a role in RPA32-phosphorylation and spindle formation; loss or inhibition of DNA-PK disrupts mitotic progression leading to mitotic catastrophe.19, 20, 21 We quantified progression into S phase by BrdU incorporation. For all genotypes, BrdU incorporation at 24 h post-IR was primarily localized to the transit amplifying cells of the crypt (Figure 2c). Trp53−/− mice had the highest number of positive cells per crypt consistent with the known role of p53 in DNA damage induced G1 arrest (Figure 2d).
Increased phospho-H3 staining in the stem cell niche of Prkdc scid/scid /Trp53 −/− mice
To discern whether cells were progressing from S phase into mitosis, phospho-H3 (S10) staining was assessed. Both WT and Trp53−/− mice had a similar distribution of phospho-H3 positive mitotic cells, with a clear peak at positions 4–7 in the transit-amplifying zone of the crypt. Little or no mitotic activity was seen at the base of the crypt in the stem cell compartment or in the upper crypt region and villi (Figure 2e). This distribution is similar to that seen in unirradiated mice indicating the spatial organization of proliferation is maintained after IR. By comparison, Prkdcscid/scid/Trp53−/− mice exhibited a marked increase in phospho-H3 positive cells throughout the entire crypt, but most notably in cells at the bottom of the crypt (Figure 2e). The average number of cells staining positive for phospho-H3 was significantly higher in both Trp53−/− and Prkdcscid/scid/Trp53−/− mice compared to WT mice (Figure 2f). We also noted a significant increase in phospho-H3 levels in Prkdcscid/scid/Trp53−/− compared to Prkdcscid/scid mice. The phospho-H3 staining pattern in Prkdcscid/scid/Trp53−/− mice exhibited prominent nuclear foci indicative of failed mitosis (data not shown).
Increased survivin staining in Prkdc scid/scid /Trp53 −/− mice
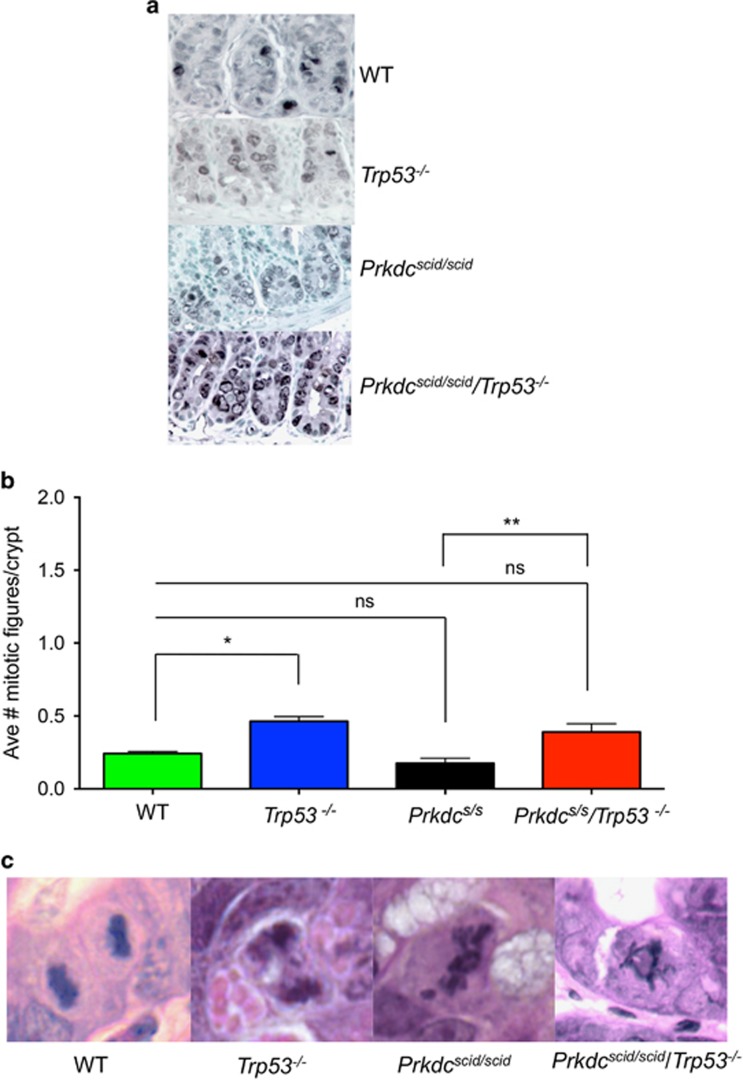
Survivin, a member of the inhibitor of apoptosis protein (IAP) family, is upregulated during G2/M to control the mitotic spindle checkpoint, and regulate cytokinesis.22 It is over-expressed in certain cancers and regulated by p53.23 Survivin staining was primarily localized in the transit amplifying cells in WT, Prkdcscid/scid and Trp53−/− mice (Figure 3a). In contrast, staining for survivin was more prominent in Prkdcscid/scid/Trp53−/− mice, and discrete, abundant nuclear foci within the stem cell compartment were evident, consistent with failed cytokinesis (Figure 3a).
Figure 3.
Survivin expression and abnormal mitotic figures. (a) Survivin staining in crypts at 24 h post 8 Gy. Large positive foci are evident in stem cells of Prkdcscid/scid/Trp53−/−mice. (b) Average number of mitotic figures per genotype at 24 h post 8 Gy IR (Unpaired t-test, *P<0.0001; ** P<0.008). (c) Representative H&E stained images of mitotic figures at 24 h post IR. Prkdcs/s=Prkdcscid/scid
Increased mitotic index and abnormal figures in Prkdc scid/scid /Trp53 −/− mice
The mitotic index at 24 h post-IR was significantly higher in Trp53−/− and Prkdcscid/scid/Trp53−/− mice compared to WT or Prkdcscid/scid mice (Figure 3b). A range of abnormalities including failure of chromosomes to align at the metaphase plate, anaphase bridges, lagging chromosomes, chromosome fragments, and tripolar mitotic figures, indicative of mitotic catastrophe were observed in mitotic figures from Prkdcscid/scid/Trp53−/− mice (Figure 3c). The percentage of abnormal mitotic figures was nearly 100% in both Prkdcscid/scisd/Trp53−/− and Prkdcscid/scidmice.
The co-localization of γH2A.X, survivin, and phospho-H3 (S10) within the stem cell compartment in irradiated Prkdcscid/scid/Trp53−/− mice along with the high number of abnormal mitotic figures indicates that, in the absence of functional DNA-PK and p53, cells with DNA damage abnormally enter S phase and progress to mitosis. The elevated mitotic index and the enhanced phospho-H3 staining highlight the significance of cell proliferation rather than apoptosis as the principal difference between the Prkdcscid/scid/Trp53−/− and the Prkdcscid/scid mice.
Depletion of LGR5 stem cells and Paneth cells in Prkdc scid/scid /Trp53 −/− mice
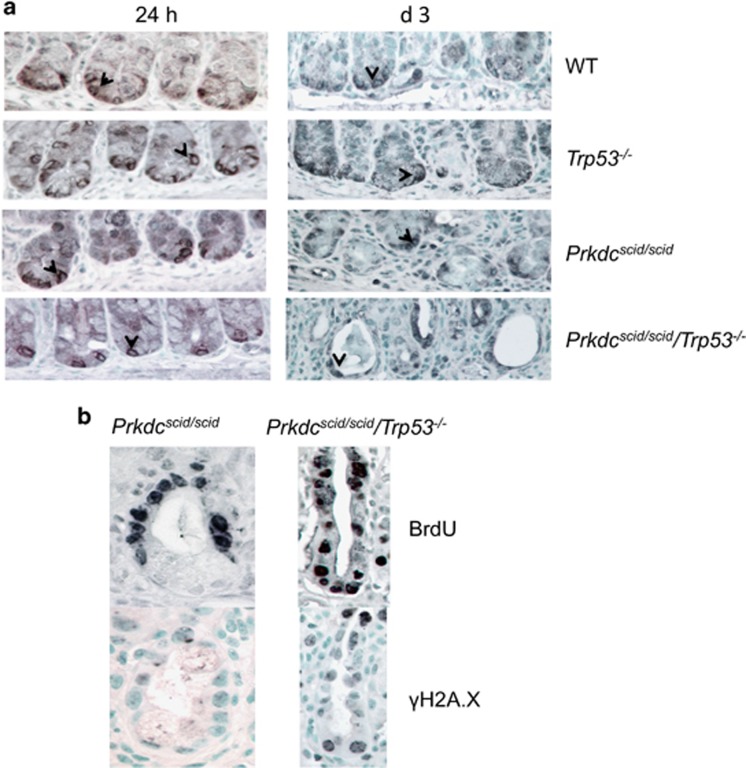
We examined Lgr5 stem cell fate using an antibody to the cell surface protein Lgr5 (GPR49).13 Lgr5+ membrane staining was evident in isolated cells at the crypt base, between the Paneth cells, consistent with the location of these stem cells (Figure 4a). Similar staining was observed prior to and at 24 h post IR for all genotypes. However, by day 3 post-IR, a marked loss of crypts with few remaining Lgr5 cells was evident in Prkdcscid/scid/Trp53−/− mice (Figures 1b and 4a). In the few crypts that remained in Prkdcscid/scid/Trp53−/− mice, cells at the base stained positive for γH2A.X and BrdU, indicating stem cells with unresolved DNA damage were entering into the cell cycle (Figure 4b).
Figure 4.
Lgr5 expression in irradiated crypts. (a) GPR49 (Lgr5) staining of crypts 24 h or 3 days after 8 Gy IR. Positive cells remain at all time points. (b) BrdU and γH2A.X staining of a remaining d 3 crypt from Prkdcscid/scid or Prkdcscid/scid/Trp53−/− mice Note more γH2A.X and BrdU staining in the crypt base of the Prkdcscid/scid/Trp53−/− mouse
Another feature of GI-ARS that was unique to Prkdcscid/scid/Trp53−/−mice was the loss of Paneth cells, evident in H&E staining at both 24 and 72 h post IR (Figures 1b and 5). Paneth cells contain abundant lysozyme C within secretory granules, and staining for lysozyme C was reduced in Prkdcscid/scid/Trp53−/−mice. Paneth cells are important for maintenance and pluripotency of the stem cells, as well as the ability of stem cells to regenerate functional daughter cells after injury.24, 25 As Wnt/ß-catenin signaling is important for Paneth cell differentiation26 and can be down-regulated by activated p53,27 we examined ß-catenin staining in irradiated tissues. Nuclear ß-catenin, evidence of active Wnt signaling, was seen in Paneth cells from WT, Trp53−/− and Prkdcscid/scid mice (Figure 5). However, little or no nuclear ß-catenin was observed in the few remaining Paneth cells of the Prkdcscid/scid/Trp53−/−mice, suggesting that their loss could be due to deregulated Wnt signaling.
Figure 5.
Loss of Paneth cells in crypts of irradiated Prkdcscid/scid/Trp53−/−mice. H&E and lysozyme C staining of secretory granules showing loss of Paneth cells in Prkdcscid/scid/Trp53−/−mice. Note reduced nuclear β-catenin staining in Paneth cells of Prkdcscid/scid/Trp53−/− mice
ATM −/− /Trp53 −/− mice have a different GI-ARS phenotype
Ataxia-telangiectasia mutated (ATM) is needed for the initiation of double-strand break repair by homologous recombination (HR). Westphal et al.28 illustrated the additional loss of p53 in ATM null mice did not alter survival after irradiation, though ATM−/− mice induce apoptosis similarly to WT while ATM−/−/Trp53−/− mutants are markedly resistant to apoptosis.18, 29 To examine the relative role of NHEJ versus HR in IR response, we compared γH2A.X staining between DNA-PK and ATM deficient crypt cells after 8 Gy IR. ATM−/− mice had a γH2A.X distribution similar to WT mice, while a sixfold increase in γH2A.X positive cells in the stem cell compartment of the crypt was evident in Prkdcscid/scid mutant mice (Figure 6). The ATM−/−/Trp53−/− mice also retained their Paneth cells, observable in the GI crypts at d 3 and d 4 post-IR. This demonstrates the NHEJ pathway of DSB repair is predominant in Lgr5 cells.
Figure 6.
ATM−/−/Trp53−/− mice are radiosensitive, but have a different phenotype compared to Prkdcscid/scid/Trp53−/− mice. (a) Positional counts of γH2A.X positive cells 24 h after 8 Gy; ATM−/−(n=2), ATM−/−/Trp53−/−(n=2). Data from other genotypes are replotted here from Figure 2. (b) H&E staining of ATM−/−/p53−/− mouse at day 3 left, and day 4 right. Note evidence of Paneth cells at both time points (arrowheads)
Discussion
These studies reveal a functional interaction exists between DNA-PK and p53 within the stem cell compartment that governs GI-ARS. While DNA-PK mutant mice are radiosensitive, simultaneous loss of p53 exacerbated the radiosensitivity, characterized by loss of crypts and Paneth cells, absence of villi, intestinal collapse and earlier lethality. Reduced DNA-PK activity leads to persistent DNA damage, most notably in the stem cell compartment, and this coupled with loss of p53-mediated cell cycle arrest, results in aberrant progression of damaged cells into mitosis and ensuing mitotic catastrophe. In agreement with previous studies, p53 dependent apoptosis in transit amplifying cells did not correlate with GI-ARS.15, 16, 28 Rather, the cell cycle arrest function of p53, especially in the context of excessive DNA damage, affects the overall tissue response to IR damage.30 PUMA knockout mice are resistant to apoptosis and protected from succumbing to early GI-ARS at high doses of IR; notably, the mice did eventually die from GI-ARS, albeit several days after WT.17 This is indicative that it is not only the initial apoptotic response that is important to GI-ARS, but also later cellular repair and mitotic integrity.
Stem cells need to persist throughout the life of the organism to maintain and regenerate damaged tissues, and so must have robust mechanisms to ensure their survival and to maintain genomic integrity. Paneth cells produce factors critical for maintaining gastrointestinal stem cells in their quiescent state; selective depletion of Paneth cells in mouse models leads to loss of stem cells.24 Lgr5+ stem cells are required for IR-induced intestinal regeneration, and Lgr5+-depleted mice are highly radiosensitive.31 Loss of crypt Paneth cells was already apparent by 24 h post IR in the Prkdcscid/scid/Trp53−/− mice, indicating failure to produce or maintain these differentiated cells, and by day 3, crypts were largely absent in Prkdcscid/scid/Trp53−/− mice. Overall, this leads to failure to maintain functional crypts and differentiated intestinal epithelial cells, highlighting the importance of DNA-PK in maintaining crypt homeostasis.
We also noted a marked increase in persistent DNA damage in Prkdcscid/scid mutant compared to ATM−/− or WT mice, particularly within the stem cell compartment at the base of crypt. Overall, this indicates these cells are especially reliant on DNA-PK mediated DNA DSB repair, consistent with NHEJ as the primary DNA DSB repair pathway in G0/G1 cells. Other studies demonstrated increased DNA repair activity in stem cells, which may serve to protect these long-lived cells from endogenous and environmental DNA damage.4, 32, 33
In addition to its well-known DNA repair function, DNA-PK also plays a role in mitotic spindle formation and mitotic progression.21 Inhibition or depletion of DNA-PK leads to chromosome misalignment, multipolar spindles and polyploidy.20, 34 Cells lacking DNA-PKcs have defective replication checkpoint arrest and sustained H2A.X phosphorylation that persists in cells entering mitosis, indicating inappropriate mitotic entry with unrepaired damage,19, 35 which likely contributes to the GI-ARS phenotype observed.
The radiosensitive phenotype of DNA-PK mutant cells exacerbated by p53 loss is indicative of a functional interaction between DNA-PK and p53. In addition to an increase in persistent DNA damage in the stem cell compartment, irradiated Prkdcscid/scid/Trp53−/− mice abnormally progress through the cell cycle, apparent by increased BrdU incorporation, phospho-H3 staining, and abnormal mitotic figures. While reduced DNA-PK activity leads to persistent DNA damage, this coupled with loss of p53 mediated cell cycle arrest results in aberrant progression of damaged cells into mitosis and ensuing mitotic catastrophe, effectively depleting the stem and Paneth cells, eventually leading to tissue failure.
These results highlight the importance of both in vivo cellular hierarchies and genetic interactions in dictating overall tissue response to DNA damaging agents such as IR. The functional interaction between DNA-PK and p53 that affects stem cell fate after damage may be useful in designing treatments for GI-ARS and p53 mutant cancers.
Materials and methods
Mice
C57BL/6 (Prkdcscid/scid) mice (Jackson Laboratory, Bar Harbor, ME, USA) were crossed with C57BL/6 Trp53 knockout mice36 to generate single and compound mutant mice and WT littermate controls. Generation of ATM−/−/Trp53−/− mice was as previously described.29 Genotyping was done using established protocols available on request. All mice were kept in microisolator cages. Adult mice received a single dose of 8 Gy from a Mark I Cesium irradiator. Mice were killed at 24 h or when they became moribund. Some mice were injected with BrdU (100 mg/kg i.p., Sigma Aldrich, St. Louis, MO, USA) 1 h before sacrifice. All procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee.
Immunohistochemistry
Tissues were fixed in neutral buffered formalin, and then processed to paraffin. Sections (4 μm) were deparaffinized and rehydrated and stained with hematoxylin and eosin (H&E), or subjected to immunohistochemistry for specific proteins or BrdU using a three step ABC technique with the following pre-treatments. Sections stained for phosphorylated histone H3 (S10; Cell Signaling Technology, Danvers, MA, USA), phosphorylated H2A.X (S139; Cell Signaling Technology), cleaved caspase 3 (Asp175; Cell Signaling), lysozyme (Abcam, Cambridge, MA, USA), β-catenin (NeoMarkers, Fremont, CA, USA), GPR49 (Lgr5; Abbiotec, San Diego, CA) or survivin (Cell Signaling Technology) were steamed for 20 min in pH 6.0 citrate buffer. Sections stained for BrdU (Dako, Carpinteria, CA, USA) were treated with HCl and trypsin. All sections were blocked for peroxidase with 3% H2O2, stained with the primary antibody followed by a biotinylated secondary antibody (Vector, Southern Biotech) and streptavidin ABC (Vector Laboratories, Burlingame, CA, USA). Slides were developed using DAB/NiCl (Sigma-Aldrich, Saint Louis, MO, USA) and counterstained with methylgreen (Sigma-Aldrich).
Quantification
Ten × 40 fields were analyzed and positively stained cells were counted and expressed as positive cells per crypt. For cell position counts, 30 crypts from four to five mice were counted and results shown as the average number of positive cells per cell position.
Statistics
Survival curves were compared using log rank (Mantel-Cox) test. Samples were compared via unpaired, two-tailed Student’s t tests using GraphPad Prism software version 6 (San Diego, CA, USA). When variances significantly differed, Welch’s correction was used.
Acknowledgments
This work was funded by NCI grants U01 CA176303, U54CA132383 and U54 CA132381.
Footnotes
Edited by M Oren
The authors declare no conflict of interest.
References
- Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer 2016; 16: 20–33. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012; 47: 497–510. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 2007; 447: 686–690. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol 2011; 12: 198–202. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Jette N, Lees-Miller SP. Non-homologous end joining: emerging themes and unanswered questions. DNA Repair (Amst) 2014; 17: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann KA, Sun JR, Giaccia AJ, Tosto LM, Brown JM. Scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA 1991; 88: 1394–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danska JS, Holland DP, Mariathasan S, Williiams KM, Guidos CJ. Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol Cell Biol 1996; 16: 5507–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Lane DP, Hall PA. The role of spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53 deficient mice. Cancer Res 1994; 54: 614–617. [PubMed] [Google Scholar]
- Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 1997; 14: 2759–2766. [DOI] [PubMed] [Google Scholar]
- Bouffler SD, Kemp CJ, Balmain A, Cox R. Spontaneous and ionizing radiation-induced chromosomal abnormalities in p53-deficient mice. Cancer Res 1995; 55: 3883–3889. [PubMed] [Google Scholar]
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 2004; 161: 123–136. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 2012; 11: 452–460. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 1974; 141: 537–561. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 2004; 23: 3265–3271. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Santiago PM, di TE, Sullivan JM, Hou WS, Dayton T et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 2010; 327: 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008; 2: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley KE, Moser R, Gu Y, Hasty P, Kemp CJ. DNA-PK suppresses a p53-independent apoptotic response to DNA damage. EMBO Rep 2009; 10: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley AK, Shrivastav M, Nie J, Amerin C, Troksa K, Glanzer JG et al. DNA-PK phosphorylation of RPA32 Ser4/Ser8 regulates replication stress checkpoint activation, fork restart, homologous recombination and mitotic catastrophe. DNA Repair (Amst) 2014; 21: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R, Liu XD et al. Inactivation of DNA-dependent protein kinase leads to spindle disruption and mitotic catastrophe with attenuated checkpoint protein 2 Phosphorylation in response to DNA damage. Cancer Res 2010; 70: 3657–3666. [DOI] [PubMed] [Google Scholar]
- Jette N, Lees-Miller SP. The DNA-dependent protein kinase: a multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol 2015; 117: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580–584. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wei Y, Xiong L, Yang Y, Wu JR. Differential regulation of survivin by p53 contributes to cell cycle dependent apoptosis. Cell Res 2005; 15: 361–370. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry L, Young M, El MF, Clarke AR. Evidence for a crucial role of paneth cells in mediating the intestinal response to injury. Stem Cells 2013; 31: 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S et al. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol 2008; 324: 288–296. [DOI] [PubMed] [Google Scholar]
- Sadot E, Geiger B, Oren M, Ben-Ze'ev A. Down-regulation of beta-catenin by activated p53. Mol Cell Biol 2001; 21: 6768–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal CH, Rowan S, Schmaltz C, Elson A, Fisher DE, Leder P. Atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet. 1997; 16: 397–401. [DOI] [PubMed] [Google Scholar]
- Gurley KE, Kemp CJ. Atm is not required for p53 induction and apoptosis in irradiated epithelial tissues. Mol Cancer Res 2007; 5: 1312–1318. [DOI] [PubMed] [Google Scholar]
- Wei L, Leibowitz BJ, Wang X, Epperly M, Greenberger J, Zhang L et al. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Invest 2016; 126: 4076–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014; 14: 149–159. [DOI] [PubMed] [Google Scholar]
- Seita J, Rossi DJ, Weissman IL. Differential DNA damage response in stem and progenitor cells. Cell Stem Cell 2010; 7: 145–147. [DOI] [PubMed] [Google Scholar]
- Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012; 143: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Lin YF, Chou HY, Yajima H, Fattah KR, Lee SC et al. Involvement of DNA-dependent protein kinase in normal cell cycle progression through mitosis. J Biol Chem 2011; 286: 12796–12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Opiyo SO, Manthey K, Glanzer JG, Ashley AK, Amerin C et al. Distinct roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint activation in response to replication stress. Nucleic Acids Res 2012; 40: 10780–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992; 356: 215–221. [DOI] [PubMed] [Google Scholar]