Generalized pustular psoriasis (GPP), von Zumbusch type, is an uncommon variant of psoriasis characterized by acute-onset severe inflammation and widespread sterile pustules within psoriatic plaques. GPP is a medical emergency that often requires hospitalization because of possible life-threatening complications, including heart failure, renal failure, and sepsis. Existing treatment options for GPP are largely limited to systemic immunosuppressive treatments that may be contraindicated in patients with active infection, liver disease, or malignancy.1, 2, 3 Therefore, management of GPP in patients with ≥1 of these comorbidities is challenging. We report a case of GPP successfully treated with apremilast, a phosphodiesterase-4 (PDE-4) inhibitor approved for the treatment of moderate-to-severe plaque psoriasis,4 in a patient with hepatitis C, cirrhosis, and hepatocellular carcinoma (HCC).
Case report
A 55-year-old man with active hepatitis C, decompensated cirrhosis, metastatic HCC, and a 40-year history of plaque psoriasis presented with a 1-year history of recurrent GPP flares with chills and malaise requiring multiple hospitalizations (Fig 1). The diagnosis of plaque psoriasis and new onset GPP were made clinically and a biopsy specimen was not obtained. At the time of presentation, the patient had a hepatitis C viral load >500,000 IU/L, ascites, and recurring multifocal HCC that had metastasized to his lungs. In the past year, his plaque psoriasis and GPP were treated with topical therapy and Goeckerman therapy; systemic therapies were avoided given his comorbidities. However, he continued to experience frequent GPP flares and the decision was made to start apremilast. He was treated using the standard titration dosing schedule (10 mg on day 1 and increasing the dose by 10 mg daily) until a reaching a maintenance dose of 30 mg twice daily on day 6. Improvement of his plaque psoriasis and GPP was noted after only 2 to 3 weeks of treatment. Complete clearance of plaque psoriasis and GPP was noted 6 weeks after starting apremilast with sustained remission of psoriatic plaques and pustular flares at least 9 months at the time of this writing (Fig 2). Although he initially experienced nausea, apremilast was otherwise well tolerated. Unfortunately, because of the patient's poor health with multiple comorbidities and poor prognosis, he transitioned from curative to palliative care.
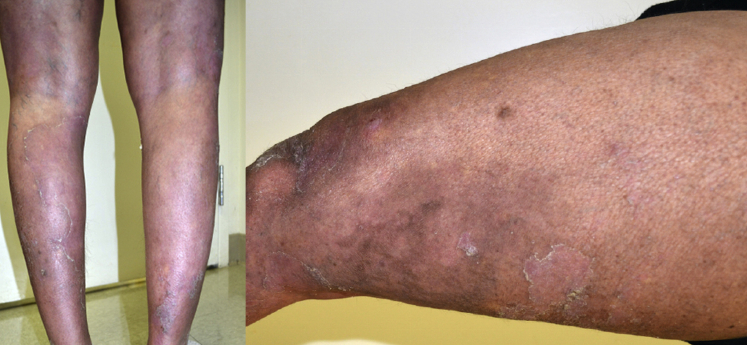
Fig 1.
Generalized pustular psoriasis with widespread areas of erythematous skin, denuded areas from pustules, and scaling.
Fig 2.
Generalized pustular psoriasis 2 to 3 weeks after the beginning treatment with apremilast. The patient had near complete clearance of his skin lesions.
Discussion
The current treatment options for GPP include first-line therapies of cyclosporine, methotrexate, acitretin, and infliximab, as well as second-line therapies of adalimumab, etanercept, psoralen plus ultraviolet A light phototherapy, and topical therapies.5 Of these, cyclosporine and infliximab are considered first line in patients with severe, acute disease because of their quick onset of action. While a recent study has suggested that biologic therapies pose a minimal to no risk of hepatitis C reactivation in low-risk patients with psoriasis,6 the outcome in patients with significant comorbidities remains unknown. Therefore, of the therapies listed above, none were appropriate for this patient because of his history of active hepatitis C infection, chronic liver disease, and active malignancy.
Apremilast is an oral small-molecule PDE-4 inhibitor that was approved by the US Food and Drug Administration for the treatment of moderate-to-severe plaque psoriasis in 2014. PDE-4 inhibition increases intracellular cyclic adenosine monophosphate levels in immune cells, leading to decreased levels of proinflammatory cytokines and chemokines tumor necrosis factor–α, interferon-γ, interleukins (ILs)-12 and -23, CXCL9, CXCL10, and CCL4, and increased levels of antiinflammatory cytokines, including IL-10.7 The pharmacokinetics of apremilast are not affected by hepatic impairment and the drug is not hepatotoxic. In addition, apremilast is not contraindicated in patients with active infection or malignancy. Therefore, apremilast was chosen for this patient.
To our knowledge, this is the first reported case of apremilast for the treatment of GPP. Because of the patient's placement into palliative care, the impact of apremilast on his hepatitis C and progression of his malignancy is uncertain, but apremilast for a psoriasis patient with hepatitis C has been previously reported.8 This case shows that apremilast can be a potentially effective and safe option for the treatment of adult-onset GPP arising from plaque psoriasis.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Frankel A.J., Van voorhees A.S., Hsu S. Treatment of psoriasis in patients with hepatitis C: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;61:1044–1055. doi: 10.1016/j.jaad.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita J., Grewal S., Langan S.M. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol. 2017;76:393–403. doi: 10.1016/j.jaad.2016.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin E.C., Debbaneh M., Koo J., Liao W. Biologic therapy in erythrodermic and pustular psoriasis. J Drugs Dermatol. 2014;13:342–354. [PubMed] [Google Scholar]
- 4.Papp K., Reich K., Leonardi C.L. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Robinson A., Van voorhees A.S., Hsu S. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279–288. doi: 10.1016/j.jaad.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Snast I., Atzmony L., Braun M., Hodak E., Pavlovsky L. Risk for hepatitis B and C virus reactivation in patients with psoriasis on biologic therapies: a retrospective cohort study and systematic review of the literature. J Am Acad Dermatol. 2017;77:88–97.e5. doi: 10.1016/j.jaad.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Vangipuram R., Alikhan A. Apremilast for the management of moderate to severe plaque psoriasis. Expert Rev Clin Pharmacol. 2017;10:349–360. doi: 10.1080/17512433.2017.1293519. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S.P., Shah V.V., Wu J.J. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017 doi: 10.1111/jdv.14301. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]