Abstract
Recently, a firmer understanding of tumor immunology and tumor escape mechanisms has led to the development of immune checkpoint inhibitors, antibodies against programmed death-1 (PD-1) and its ligand (PD-L1). Nivolumab, pembrolizumab, and atezolizumab have dramatically altered the treatment paradigm in non-small cell lung cancer (NSCLC) and have each demonstrated improvements in outcomes and quality of life when compared to chemotherapy. Enrichment strategies to better select those patients more likely to respond have identified PD-L1 staining by immunohistochemistry (IHC) to be a predictive biomarker in both treatment naïve and refractory patients. Unfortunately, many challenges exist with this strategy and underscore the need for further exploration for more reliable biomarkers. Multiple tissue and plasma-based enrichment strategies have been identified in the hope of identifying patients more likely to benefit from checkpoint inhibitors. These include tumor mutational load; the “inflamed phenotype” including tumor infiltrating lymphocytes (TILS) and immunoscore; T-cell receptor clonality; gene signatures, and several plasma biomarkers. Several studies have revealed many of these biomarkers to be reliable predictors of response to immune checkpoint inhibitors across multiple tumor types. Given the small nature of these studies, additional prospective studies are warranted to formalize and validate each of these enrichment strategies.
Keywords: Biomarkers, immunotherapy, non-small cell lung cancer (NSCLC)
Introduction
Greater understanding of tumor immunology and tumor escape mechanisms has recently led to a resurgent interest in various forms of immunotherapies for cancer. One of the greatest successes has been the development of immune checkpoint inhibitors, antibodies against programmed death-1 (PD-1) and its ligand (PD-L1). Nivolumab, pembrolizumab, and atezolizumab have dramatically altered the treatment paradigm in non-small cell lung cancer (NSCLC) and have each demonstrated improvements in outcomes and quality of life when compared to chemotherapy (1-7). In addition, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) blockade with ipilimumab in combination with PD-L1 agents has shown promising activity in both non-small cell and small cell lung cancer (8,9). Enrichment strategies to select patients more likely to respond have identified PD-L1 staining by immunohistochemistry (IHC) to be a predictive biomarker in both treatment naïve and refractory patients. Specifically, a tumor proportion score (TPS) >50% is predictive of improved response rates (RR), progression free survival (PFS) and overall survival (OS) with pembrolizumab when compared to platinum chemotherapy in treatment-naïve, advanced stage NSCLC patients (4). While this study underscores the importance of PD-L1 testing in guiding therapeutic decisions, many challenges exist with this strategy including the use of different testing platforms, utilization of different antibodies, and varying definitions of PD-L1 positivity. In addition, PD-L1 expression within tumors can be both heterogeneous and dynamic as reflected by the discrepancies in staining between primary and metastatic sites as well as the clinical observation that many patients with PD-L1 low or negative tumors still respond to these agents. Given these hurdles, there remains an urgent need for further exploration for more reliable biomarkers. Multiple tissue and plasma-based enrichment strategies have emerged to identify patients more likely to benefit from immune checkpoint inhibitors. These include tumor mutational load, the “inflamed phenotype” including tumor infiltrating lymphocytes (TILS) and immunoscore, T-cell receptor clonality, gene signatures, and several plasma biomarkers (Tables 1,2). Herein we review the available literature addressing these biomarkers and discuss the clinical implications and strategies moving forward.
Table 1. Emerging tissue-based biomarkers of immune checkpoint inhibitor response.
| Candidate biomarker | Assay | Literature support |
|---|---|---|
| TMB | Total number of mutations per DNA coding region | Melanoma: higher TMB was associated with longer overall survival, clinical benefit with anti-CTLA-4, and response to anti-PD-1/PD-L1 therapies (10-12) |
| NSCLC: higher TMB associated with improved clinical response, clinical benefit, and progression free survival to anti-PD-1/PD-L1 therapy (13-16) | ||
| Cytotoxic CD8+ T cells/TILS | Presence of CD8+ T cells within the tumor | Melanoma: higher baseline CD8+ T cell density at the tumor IM was noted in patients with anti-PD-1 response (17); baseline CD8+ TILs density was a better predictor of response than PD-L1+ cell density (17). Increase in CD8+ T cell density after anti-PD-1 therapy was associated with treatment response (17) |
| NSCLC: increase in post-treatment TILS was associated with anti-CTLA-4 response (18); CD8+ TILs in early stage lung cancer was prognostic for disease free survival and overall survival (19) | ||
| Immunoscore | A composite biomarker that integrates a combination of immune features (four IHC parameters: density of CD8+ T cells, density of CD3+ T cells, and their locations in the core of tumor or tumor infiltrating margin) into a validated scoring system | Colorectal cancer: immunoscore colon predicts the time to tumor relapse and survival better than the tumor-node-metastasis staging system (20) |
| Multiplex IHC | An advanced protein-labeling methodology that simultaneously assesses multiple proteins of interest on one FFPE slide | Melanoma: a larger PD-1/PD-L1 co-localized area was associated with anti-PD-1 response (17) |
| Pancreatic adenocarcinoma: multiplex IHC showed an association between PD-L1 up-regulation on myeloid derived cells and activated CD8+ T cells in the tumor post vaccine therapy (21) | ||
| NSCLC: multiplex IHC showed temporal influx of CD8+ T cells, with PD-1 co-expression, in the tumor after checkpoint inhibition (22) | ||
| T cell receptor clonality | Quantification of unique CDR3 region in the TCR, which determines the TCR’s antigenic recognition | Melanoma: higher TCR clonality at baseline and post-treatment was associated with anti-CTLA response (23). Ten-fold increase in expanded T cell clones was associated with anti-PD-1 response (17) |
| Breast: increase in T cell clones was shown after anti-CTLA-4 +/− cryoablation (24) | ||
| Immune gene signature | Microarray technology to evaluate multiple genes and their expression at the same time | Melanoma: high expression of immune related genes at baseline responded better to ipilimumab (25); immune gene signature appeared to predict response to combination of MAGE-3 vaccine plus immunostimulant |
| Lung: a nine gene “immune gene signature” was conserved across varying tumor types, including lung; 3 of these 9 genes were prognostic of survival in NSCLC on univariate analysis. Analysis of PRECOG showed that FOXm1 and KLRB1 co-expression were prognostic of survival on multivariable analysis |
TMB, tumor mutational burden; TILS, tumor infiltrating lymphocytes; IHC, immunohistochemistry; FFPE, formalin-fixed paraffin-embedded; CDR3, complementarity-determining region 3; TCR, T cell receptor; IM, infiltrating margin.
Table 2. Emerging blood-based biomarkers of immune checkpoint inhibitor response, prognosis, or toxicity.
| Candidate biomarker | Assay | Literature support |
|---|---|---|
| TCR clonality | Quantification of unique CDR3 region in TCR, which determines the TCR’s antigenic recognition | Melanoma and prostate: increase in peripheral blood TCR diversity in patients who received anti-CTLA4 therapy (26) |
| Eosinophil count | Absolute eosinophil count calculated from peripheral blood | Melanoma: high AEC associated with improved survival at two years in patients who received ipilimumab or pembrolizumab (27); Relative eosinophil count <1.5 was associated with higher risk of death in patients who received pembrolizumab (28); baseline eosinophils may be associated with toxicity in patients who get atezolizumab, pembrolizumab, or nivolumab (29) |
| Neutrophils and lymphocytes | ALC, ANC, or NLR calculated from peripheral blood | Melanoma: pretreatment ALC and ANC appear prognostic in patients who received immunotherapy (30); NLR >3 associated with progression and death in patients who received ipilimumab (30) |
| Lung: pretreatment NLR ≥5 associated with inferior OS, PFS in patients who got nivolumab (31) | ||
| Peripheral blood cytokines | Plasma IL-6, cytoscore | Lung: plasma IL-6 and IDO elevated in tumors that expressed an inflammatory tumor microenvironment (32); a low cytoscore associated with worse survival in patients who received nivolumab or docetaxel (1) |
| Peripheral T cells | FOXP3+ Tregs, CD4and CD8 T cells | Melanoma: frequency of FOXP3+ regulatory T cells after ipilimumab associated with worse survival (27); higher FOXP3+ Treg at baseline may be associated with survival (33); increased % CD4 and CD8 T cells after ipilimumab associated with survival (34) |
TCR, T cell receptor; CDR3, complementarity-determining region 3; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; NLR, neutrophil lymphocyte ratio.
Tumor mutational burden (TMB)
Somatic mutations in the cancer genome are a consequence of multiple processes including deficiencies in DNA repair, endogenous and exogenous carcinogen exposure, and enzymatic alterations in DNA (10). The prevalence of somatic mutations among neoplasm ranges from 0.01 per megabase to more than 400 mutations per megabase, with lung and melanoma harboring high numbers of non-synonymous genetic alterations (10). Most of these mutations derive from passenger genes and lead to translation of novel peptide epitopes, or neoantigens, which are presented by the major histocompatibility complex (MHC) on the surface of malignant cells. Theoretically, the presence of neoantigens enhances the immunogenicity of the tumor by eliciting T cell repertoires that recognize these antigens as “foreign” and infiltrate the tumor microenvironment (TME). Recent advances in sequencing technology allow for whole exome interrogation and qualitative calculation of the total number of mutations per coding region or total mutation burden (TMB). Several recent studies have evaluated TMB as a candidate biomarker for immunotherapeutic approaches in both melanoma and lung cancer. In addition, given the intricate biologic link between deficiencies in DNA repair and the accumulation of genomic errors, micro-satellite instability (MSI) has also been evaluated as a potential biomarker to checkpoint inhibitors in colon cancer.
Given that immunotherapy was first approved in melanoma, TMB was initially explored as a potential biomarker to checkpoint inhibitors in this tumor type. Using massive parallel sequencing from 64 malignant melanoma patients treated with CTLA-4 blockade with either ipilimumab or tremelimumab, Snyder et al. were able to demonstrate that a mutational load of more than 100 non-synonymous mutations was associated with a longer OS than those patients with a lower TMB (P=0.04 in the discovery set, n=25; P=0.10 in the validation set, n=39) (11). Van Allen et al. carried whole exome sequencing from pretreatment biopsies in 110 patients with malignant melanoma treated with ipilimumab and demonstrated that elevated non-synonymous mutational load and neoantigen load (>100 non-synonymous somatic mutations or neoantigens) were associated with clinical benefit from ipilimumab (n=27) (P=0.0076 and 0.027, respectively) (12). Finally, utilizing hybrid capture-based next generation sequencing (NGS) on archival biopsies from advanced stage melanoma patients, Johnson et al. demonstrated that patients who responded to anti-PD-1/PD-L1 therapies had higher mutational loads in both an initial cohort (n=32; median 45.6 vs. 3.9 mutations/MB; P=0.003) and a validation cohort (n=33; 37.1 vs. 12.8 mutations/MB; P=0.002) (35). Response rate, PFS and OS were superior in the high mutational burden group compared with intermediate and low mutational load groups.
Similar to TMB in melanoma, mismatch repair deficiency has been explored, as potential biomarker for checkpoint inhibition in advanced colorectal cancer. De et al. recently carried out a phase II study evaluating 41 patients with progressive metastatic carcinoma treated with pembrolizumab and through selected microsatellite sequencing of tissue, identified a cohort of mismatch repair-deficient (n=11) and repair-proficient (n=21) colorectal cancers. Objective RR (40% vs. 0%), median PFS (NR vs. 2.2 months) and OS (NR vs. 5.0 months) were superior in mismatch repair-deficient patients versus those in repair-proficient patients (36). Whole-exome sequencing identified a mean of 1,782 somatic mutations per tumor in mismatch repair-deficient tumors as compared to only 73 in mismatch repair-proficient tumors (P=0.007).
Somatic mutation burden has been studied as a predictive biomarker in advanced stage lung cancer, following the clinical observation that a heavy smoking history can predict response to immunotherapy. Several small studies have identified a relationship between TMB and benefit to immunotherapy. Rizvi et al. performed whole exome sequencing on two independent cohorts of NSCLC patients (n=16 and 18, respectively) treated with pembrolizumab and demonstrated that higher nonsynonymous mutation burden was associated with improved objective response, durable clinical benefit, and PFS (13). More recently, Spigel et al. correlated TMB from NGS performed on more than 11,000 samples from a Foundation One database with the clinical outcomes of 64 advanced NSCLC patients from this data set who had received immunotherapy with either pembrolizumab, atezolizumab or avelumab. The amount of time on drug was used as surrogate for clinical response and was longer in those patients with high TMB versus those with low TMB (64 weeks for >15 mutations/MB vs. 17 weeks for <15 mutations/MB; P=0.010) (14). Kowanetz et al. carried out targeted genetic sequencing to calculate TMB on pretreatment tumor samples from 102 treatment naive patients (1L) and 465 treatment refractory patients (2L+) enrolled in three phase II atezolizumab monotherapy trials for advanced NSCLC (POPLAR, second/third line; BIRCH and FIR, single arm first line/second line in PD-L1+ selected patients). Atezolizumab clinical benefit correlated with higher TMB in 1L and 2L PD-L1 positive patients and was enhanced in unselected 2L+ patients from POPLAR when compared to docetaxel (15). Finally, Peters et al. recently calculated TMB scores by whole exome sequencing in a subset of patients from a randomized phase III trial comparing nivolumab to platinum chemotherapy in treatment naive, advanced stage NSCLC (Checkmate 026). In patients with high TMB, PFS was improved (median PFS of 9.7 vs. 5.8 months; HR: 0.62; 95% CI, 0.38–1.00) and objective response rate was higher with nivolumab versus chemotherapy (46.8% vs. 28.3%) (16).
In sum, strong evidence across multiple tumor types suggest TMB to be a potential biomarker for clinical benefit to checkpoint inhibitors. Given the retrospective nature of many of these trials, prospective studies will be needed for TMB validation. These trials will be aided by the breadth and depth of newer sequencing platforms that are less cost prohibitive.
Specific genotypes
In contrast to high mutational burden, patients with specific genotypes including EGFR mutations and ALK rearrangements have consistently demonstrated lack of response to checkpoint inhibitors. A recently published retrospective analysis of 28 advanced adenocarcinoma patients with EGFR mutations (n=22) or ALK rearrangements (n=6) treated with PD-L1 inhibitors demonstrated an RR of only 5% and 0%, respectively (37). In addition, a meta-analysis evaluating advanced stage patients from three randomized trials comparing checkpoint inhibitors (nivolumab, pembrolizumab) to docetaxel as second line therapy showed no OS benefit in the EGFR-mutant subgroup (n=186, HR: 1.05, P<0.81; treatment mutation interaction P=0.03) (38). Whether the lack of benefit witnessed is due to low PD-L1 expression or low TMB identified in these molecular cohorts remains unknown. Nevertheless, checkpoint inhibitors are most likely not the optimal therapy for patients with EGFR mutations or ALK rearrangements although ongoing prospective trials will hopefully address their role in combination with other novel agents in these specific genotypes.
Potential in-situ biomarkers to identify tumors with an inflamed phenotype
Within the TME, tumors are often infiltrated by a spectrum of lymphocytic and inflammatory cells—T cells, B cells, antigen presenting cells including dendritic cells, myeloid cells including macrophages and myeloid derived suppressor cells (MDSCs), and natural killer (NK) cells (39). The characterization of the specific immune cell population within in the TME have been associated with outcomes in multiple tumor types (40,41). Baseline “hot” tumors are associated with high T cell infiltration, presence of chemokine profiles that support effector CD8+ T cell influx, and type I interferon signals (42). “Hot” tumors thus have a T-cell inflamed phenotype due to immune recognition of these tumors and may be open to further immune activation and immune mediated tumor kill. Identifying in situ immune biomarkers from tissue biopsies can categorize tumors as “hot” or “cold” and may help select patients more likely to respond to immune checkpoint inhibition. Multiple studies have demonstrated that presence of CD8+ TILS, immunoscore, and multiplex IHC may identify an inflamed phenotype and thus be predictive of checkpoint inhibitor response.
CD8+ TILS
TILs found within the TME reflect the dynamic elimination phase of immune-editing in which the innate and adaptive arms of the immune system attempt to suppress tumor growth. Clinically, an extensive TIL presence has been associated with improved survival in multiple solid tumor types including melanoma (43), NSCLC (44-46), and colorectal cancer (47). Within TILs, cytotoxic (CD8+) T lymphocytes are the effectors cells of the innate immune system and their infiltration within the TME may be a surrogate for checkpoint inhibitor efficacy. Several studies have evaluated baseline or changes in intratumoral CD8+ TIL alone or in combination with PD-L1 expression as a predictive biomarker of immunotherapeutic approaches in multiple malignancies.
As with development of checkpoint inhibitors, evaluation of CD8+ TILS as predictive checkpoint biomarkers were first studied in advanced melanoma. A secondary analysis of 46 patients with metastatic melanoma treated with pembrolizumab showed a higher baseline CD8+ TIL density at the tumor invasive margin in responders versus non-responders (17). Importantly in this cohort, CD8+ TIL density determined by IHC was a better predictor of response than PD-L1+ cell density. Additionally, pembrolizumab responders had a parallel increase in CD8+ TIL density in serial post-treatment tumor biopsies, both in the tumor margin and within the tumor parenchyma, when compared to non-responders (Spearman’s r=0.71, P<0.001). An increase in serial CD8+ TIL density correlated with decrease tumor size (Spearman’s r=0.75, P=0.0002). CD8+ TIL density has also been evaluated in early stage lung cancer. In a retrospective study of 797 pathologic stage I-IIIA NSCLC, stromal CD8+ TIL density was independently prognostic of disease free survival, disease specific survival, and OS (all P<0.001) (19).
TILS have also been evaluated as a biomarker. In a phase II prospective biomarker trial including 82 melanoma patients treated with ipilimumab, an increase in post-treatment TILs was associated with response (18). Recently, investigators correlated PD-L1 expression both on tumor cells and TILS with response to atezolizumab in 277 patients with advanced solid tumors (48). In the cohort of NSCLC patients (n=53), PD-L1 expression on TILS (P=0.015) but not on tumor cells (P=0.920) correlated with response to treatment response highlighting that location of PD-L1 expression may be an important distinction in predicting response to immunotherapy.
In sum, PD-L1 expressing tumors lacking an appropriate immune infiltrate may explain the failure of checkpoint inhibition in a subset of patients. Immune infiltration parameters at baseline and after initiation of checkpoint blockade, specifically CD8+ TIL presence, present an opportunity to better select parents who would benefit from checkpoint inhibition and provide insight into to immune-editing process that may occur. Establishment of TILs, CD8+ TILS, its association with PD-L1, and clinical cutoffs of these levels in NSCLC is needed.
Immunoscore
Immunoscore is a standardized immune-based assay that measures intra- and peritumoral T cell infiltration in formalin-fixed paraffin-embedded (FFPE) tissue sections (49). Unlike single immune cell markers within the TME like CD8+ TILS, the immunoscore assay is a composite biomarker that integrates a combination of immune features into a validated scoring system that may prove to be a better predictive biomarker of checkpoint inhibition response.
Immunoscore® Colon provides a risk of relapse score from 0 to 4 based on average percentages of 4 IHC parameters: density of CD8+ T cells, density of CD3+ T cells, and their location in the core of tumor (CT) or tumor infiltrating margin (IM). Immunoscore’s ability to detect decreased time to recurrence was validated using 3,800 resected colorectal specimens from an international cohort of stage I-III colorectal cancers without neoadjuvant treatment from 17 countries. Specifically in the validation set, time to relapse was shorter among 303 patients with a “low” immunoscore versus 327 patients with “high” immunoscore (HR: 0.54; 95% CI, 0.34–0.84; P=0.006). Impressively, this assay was found to prognosticate outcomes more accurately than tumor-node-metastasis (TNM) staging system and microsatellite instability status (50). Immunoscore demonstrates that the presence of a localized immune reaction in the TME can prognosticate survival in colorectal cancer. The development of an Immunoscore is currently under-investigation in melanoma (20), and more recently in NSCLC (51,52).
Multiplex immunochemistry
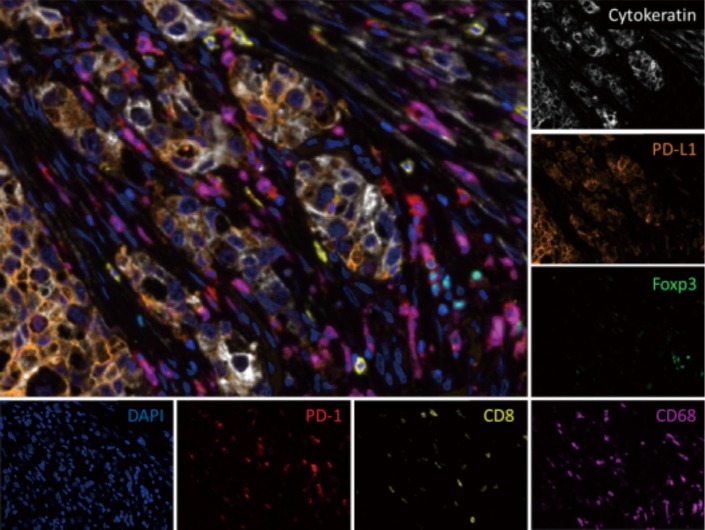
Multiplex IHC is an advanced antibody-protein labeling methodology that allows simultaneous assessment of multiple proteins of interest on one FFPE slide (Figure 1). It requires a one step process or successive cycles of a sequential IHC antibody staining and stripping process on one FFPE slide (53).
Figure 1.
Multiplex immunofluorescence (× 20) of a pulmonary adenocarcinoma shows tumor cells (white) surrounded by stromal and immune cells, including CD8+ T cells (yellow), regulatory T cells (green), and macrophages (magenta). PD-L1 (orange) is predominantly expressed on tumor cells while PD-1 (red) is seen on lymphocytes. Cell nuclei are shown in blue (DAPI). Image provided by Tricia R. Cottrell and Janis M. Taube.
Antibody-protein complexes are then linked to fluorescent dyes, to chromagens that produce color when precipitated, or to heavy-metals that can be analyzed by mass cytometry to allow visualization. Multispectral cameras or computational image analysis software are used to visualize multiplex IHC stains or to co-register and output images with overlaid IHC stains (54). Finally, analytic dedicated software such as image cytometry can be used to sort and analyze cell populations based on IHC staining within section of the slide (55). Multiplex IHC has advantages of optimizing the evaluation of the TME of limited tumor biopsy samples, mapping out the spatial/temporal resolution of immune cells and its corresponding phenotype or function in the TME, and visualizing co-expression of functional proteins on immune cells.
In melanoma, Tumeh et al. used multiplexed immunofluorescence to demonstrate that the proximity of PD-1 expressing cells and PD-L1 cells in pre-treatment samples correlated to pembrolizumab response. In analyzing 22 patients treated with pembrolizumab (n=11 responders; n=11 non-responders), a higher PD-1/PD-L1 area was found in responders (P=0.0005) (17). Multiplex immunofluorescence was also able to demonstrate co-localization of CD8 and PD-1 on a single individual cell in the TME to prove that CD8+ T cells were the primary cellular source of PD-1 receptors in this study.
Tsujikawa et al. used multiplex IHC to perform a secondary retrospective analysis of patients with pancreatic adenocarcinoma enrolled on trial receiving neoadjuvant granulocyte-macrophage-colony-stimulating tumor vaccine (GVAX therapy) prior to resection (21). The investigators showed that activated CD8+ T cells in the TME co-expressed with granzyme B, was associated with an improved OS after GVAX. An increased percentage of granzyme B+ CD8+ T cells to all T CD8+ cells present was associated with improved 2 year survival (P<0.05). Interestingly, PD-L1 status was mostly found in the CD45+ CD68+ tumor associated macrophages (TAMs) and CD45+ MHCII+ dendritic cells, and significantly correlated in TME location to activated granzyme B+ CD8+ T cells (P=0.0008) demonstrating the potential association between up-regulation of PD-L1 expression on myeloid derived cells and CD8+ T cell activation.
In NSCLC, Forde et al. presented preliminary results of a phase II trial evaluating the use of neoadjuvant nivolumab in high risk stage IB-IIIA NSCLC prior to resection at ESMO 2016 (22). In the 18 patients treated, seven patients (38%) had less than 10% residual tumor on resection and one had a complete response after neoadjuvant checkpoint inhibition. Multiplex immunofluorescence was used to demonstrate a temporal influx of CD8+ T cells, many of which expressed PD-1, in the TME after receipt of checkpoint inhibition when compared to pre-treatment tumor samples.
In sum, multiplex IHC allows for in-situ immune monitoring prior to and serially during immune checkpoint blockade using precious tumor samples. Using multiple data point captures, multiplex IHC may allow for elucidation of the plasticity and functionality of immune cells in the TME—by means of understanding complex immune cell surface protein expression profiles, cell type/location of expression, and its evolution with immune checkpoint inhibition response or resistance. Its utility in NSCLC will need to be validated in larger prospective studies.
T cell receptor (TCR) repertoire and clonality
An immune system’s TCR repertoire reflects the sum of prior unique antigen exposures to the host. Through antigen-presenting cell’s MHC-antigen complex interaction with the TCR, a naive T cell is activated and clonally expands. DNA sequencing of the TCR complementarity-determining region 3 (CDR3) domain, which uniquely encodes and determines the TCR’s antigenic recognition, allows for quantification of the T cell clonal expansion that occurs after immune stimulation (56). Expansion in TCR clonality after immune checkpoint inhibition can be detected by an increase in the frequency of a unique CDR3 variable region sequence, and has been evaluated as a potential biomarker in multiple solid tumors.
A longitudinal cohort of 56 melanoma patients who received anti-CTLA-4 blockade followed by anti-PD-inhibition at the time of progression showed that higher TCR clonality at baseline (P=0.041) and on-treatment (P=0.032) was associated with response to PD-1 blockade (23). Furthermore, of the 8 patients with matched longitudinal pre-CTLA-4 and pre-PD-1 tumor samples, an increase in TCR clonality after initial CTLA-4 checkpoint inhibition was associated a response to subsequent PD-L1 blockade (n=3 of 3 anti-PD-1 responders had increase in TCR clonality versus n=1 of 5 anti-PD-1 non-responders). In another retrospective analysis of 46 patients with metastatic melanoma receiving pembrolizumab from KEYNOTE-001, sequencing of TCR beta chain CDR3 variable region in matched pre- and post-treatment tumor samples showed that responders had a ten-fold increase in expanded T cell clones compared with non-responders (17). Another small pilot study of ipilimumab, cryoablation, or both in 18 early stage breast cancer patients showed increase intra-tumoral T cells clones detected by TCR sequencing after ipilimumab +/− cryoablation (24).
Interesting, TCR clonality increase has also been shown to be a potential biomarker of ipilimumab immune toxicity (57). Investigators reviewed the peripheral blood samples of 27 advanced prostate cancer patients treated with androgen deprivation therapy plus ipilimumab in a phase II trial. An increase of ≥55 CD8+ T cell clones in the blood was associated with future development of any immune-related adverse advent (irAE) on study (OR: 1.2, 95% CI, 1.01–1.04; P=0.01).
Diversification or increase in TCR repertoire may also be a biomarker of checkpoint inhibition response. In a retrospective analysis of 25 melanoma and prostate cancer patients treated with ipilimumab on a phase I protocol, responders had an increase in the number of unique TCR clones found in peripheral blood mononuclear cells (PBMCs) 1 month after treatment. In responders, at least a 2-fold increase in TCR diversity was seen in 47% of anti-CTLA-4 therapy paired samples compared to no increase in those paired samples from nine untreated patients. A higher fold (10×) increase was found in melanoma patients (43%) with paired pre- and post-ipilimumab samples (26).
An increase in intra-tumor and peripheral blood TCR clonality or diversity may be a potential biomarker of response, and even irAE, in NSCLC. Further evaluation in NSCLC will need to be carried out in prospective studies.
Immune gene signature
Gene signatures using microarray technologies allow for multiple genes to be evaluated at the same time and can be correlated with clinical outcomes (58). Prior to the widespread use of immunotherapy agents for NSCLC, various gene expression signatures were found to have prognostic value in NSCLC (59-63). Furthermore, there is growing evidence that gene signatures may be predictive of outcome to adjuvant chemotherapy in patients with resected NSCLC (63-65). With the integration of immunotherapy agents into the armamentarium for NSCLC, interest has emerged to further study the role of immune gene signatures (3-6). Investigators are beginning to explore whether unique gene signatures could potentially be prognostic or predictive of both toxicity and response to immunotherapy in various malignancies, including NSCLC (66-68).
Immune gene signatures may be prognostic in many malignancies, including lung cancer. Chifman et al. utilized microarray expression datasets of 5,295 breast, colon, ovarian, prostate, and lung tumors and hypothesized that they would be able to identify unifying gene signatures across tumor types that were enriched for immune biological functions (69). They were able to identify nine immune-gene (“metagene”) signatures (T/NK1, T/NK2, T/NK3, B/P/T/NK, B/P, B/M/D, M/D/N1, M/D/N2, DLPS) that were conserved across all of the tumor types. Furthermore, they demonstrated on univariate analysis that the immune /NKian, prostate2 (P≤0.05), T/NK3 (P≤0.01), B/P/T/NK (P≤0.05), and B/P (P≤0.01) were prognostic of improved survival in lung cancer.
Gentles et al. also evaluated the prognostic role of immune gene signatures in multiple cancers, including lung cancer (70). The investigators compiled cancer gene expression and clinical outcome data from 18,000 patients with 39 different malignancies from the public domain into a resource for the PREdiction of Clinical Outcomes from Genomic profiles (PRECOG). They demonstrated that expression of a proto-oncogene, Forkhead box M1 (FOXM1) was an adverse prognostic factor, and KLRB1, expressed on multiple T cell subsets, was prognostic of improved survival on multivariate analysis (HR: 1.5; 95% CI, 1.3–1.8, P<0.0001) (70). The investigators demonstrated the feasibility in identifying candidate genes and tumor associated leukocyte subsets that were potentially prognostic or predictive across multiple malignancies.
Recent data has demonstrated immune gene signatures to be predictive of response to checkpoint inhibitors in multiple tumor types. In a phase II study evaluating ipilimumab in melanoma patients, gene expression profiling was performed on tumor biopsies prior to treatment and at three weeks (25). Patients with high expression levels of immune related genes responded to ipilimumab better than those patients with lower baseline expression. Similarly an immune gene signature that encompasses CCL11, CCL5, IFN-Ɣ, inducible T-cell costimulatory (ICOS), and CD20 predicted response to vaccine therapy (71-73). Specifically, analyzing gene expression signatures by mRNA microanalysis from pretreatment tumor specimens of 54 malignant melanoma patients enrolled in a phase II trial evaluating a combination of MAGE-3 vaccine plus an immune-stimulant, investigators identified a gene signature that discriminated patients with clinical benefit (GS-positive) from those without clinical benefit (GS-negative). OS was significantly higher in GS-positive (29 mo; 95% CI, 20.5–40.2 mo) patients versus GS-negative patients (16.2 mo; 95% CI, 9.0–20 mo) (73). Furthermore, when the classifier was applied to samples from early stage NSCLC patients enrolled in a randomized trial evaluating MAGE-A3 with an immune-stimulant, AS02B (n=182), those who were identified as GS-positive demonstrated improved response and survival compared to those who were GS-negative (HR: 0.39; 95% CI, 0.19–0.81; P=0.01). Finally, utilizing a Fluidigm-based expression platform, Fehrenbacher et al. analyzed immune gene expression patterns in pretreatment samples from the randomized phase II POPLAR trial evaluating second line atezolizumab versus docetaxel. Improved survival was witnessed in those patients with tumors characterized by high expression of T-effector-associated and interferon—gamma associated genes treated with atezolizumab (HR: 0.43; 95% CI, 0.24–0.77) (7).
Given the rare, yet fatal adverse events related to immunotherapy, predicting immune-related toxicity to checkpoint inhibitors will be valuable in optimizing therapeutic decisions. Immune gene signatures may play a role in this area but published data is restricted to other malignancies (74). Shahabi et al. evaluated candidate biomarkers from 162 advanced melanoma patients on two phase II clinical trials that could potentially predict for immune related gastrointestinal (GI) toxicities after the use of ipilimumab (74). The mean cell cycle and immune related gene expression was significantly higher in patients who experienced grade 2 or higher GI toxicity compared to those who did not experience GI toxicity after ipilimumab (n patients who experienced g Additionally, they identified higher pre-treatment to post treatment increases in immunoglobulin related gene expression in patients who experienced GI toxicity compared to those who did not.
Serum/plasma markers
The accessibility and convenience of serum and blood makes plasma-based biomarkers an attractive enrichment strategy for patients receiving checkpoint inhibitors (Table 2). Peripheral blood counts are readily available and utilization of eosinophil, lymphocyte, and neutrophil counts for prognostic or predictive purposes is a growing area of study. Relative eosinophil counts, for example, have been evaluated in patients with melanoma who have been treated with either ipilimumab or pembrolizumab (27,28). Martens performed an analysis on prospectively collected blood from 209 melanoma patients who received ipilimumab and identified that a high absolute eosinophil count (AEC) ≥50/µL was associated with improved survival compared to patients with AEC <50/µL at 2 years (27.2% vs. 6.7%, P=5.1×10−5) (34). Similarly, in a retrospective analysis of 616 melanoma patients who received pembrolizumab, relative eosinophil count (REC) <1.5% was independently associated with increased risk of death (HR: 2.0, P<0.001) (28). Finally, a preliminary analysis of 44 patients treated with either atezolizumab, nivolumab, or pembrolizumab, suggests that high levels of baseline eosinophils is associated with increased risk of toxicity (OR: 6, P=0.014) (29).
There is also great interest in serum neutrophils and lymphocytes as a potential biomarker for immunotherapy. Absolute neutrophil or lymphocyte count (ANC, ALC), or the neutrophil lymphocyte ratio (NLR) calculated by dividing ANC by ALC are prognostic in many malignancies and at various stages of disease, including lung cancer. In a retrospective analysis of 98 consecutive advanced melanoma patients, pre-treatment ALC ≥1,000/µL (HR: 0.40, P=0.004) and ANC <4,000/µL (HR 0.46, P=0.014) were significantly associated with OS on multivariate analysis (33). Ferrucci et al. found that an NLR >3 was associated with increased risk of progression (HR: 2.03; 95% CI, 1.66–2.47; P<0.0001) and death (HR: 2.29; 95% CI, 1.86–2.82; P<0.0001) in 720 melanoma patients treated with ipilimumab (30).
In lung cancer, NLR has been studied in the patients receiving immune checkpoint inhibitors. A retrospective study of 175 patients with advanced NSCLC treated with nivolumab demonstrated that a pre-treatment NLR ≥5 was independently associated with inferior OS and PFS compared to a pre-treatment NLR <5 (OS: 5.5 vs. 8.4 mo; HR: 2.07; 95% CI, 1.3–3.3; P=0.002; PFS: 1.9 vs. 2.8 mo, HR: 1.43; 95% CI, 1.02–2.0; P=0.04) (31). Multiple other retrospective analyses have started to evaluate and demonstrate a prognostic and predictive role of NLR in lung cancer patients receiving immune checkpoint inhibitors. However, these studies have all been small and retrospective and further prospective evaluation is warranted (75-79).
Peripheral cytokines are another potential biomarker that has been evaluated in lung cancer patients receiving immune checkpoint inhibitors. In an analysis evaluating gene expression profiles from lung samples from the Cancer Genome Atlas (TCGA) and two MD Anderson datasets, investigators found that that plasma IL-6 and indoleamine 2,3-dioxygenase (IDO) were elevated in tumors that expressed an inflammatory TME (32). Borghaei et al. has also presented early data from two phase III clinical trials evaluating advanced lung cancer patients who received either nivolumab or docetaxel and developed a “cytoscore” in attempts to quantify the effect of various cytokines on OS (1). In this exploratory analysis, squamous cell lung cancer patients with a high cytoscore had improved survival relative to low cytoscore patients in both patients who received nivolumab (15.6 vs. 5.3 mo; HR 0.48; 95% CI, 0.36–0.64; P<0.0001) and docetaxel (9.1 vs. 4.9 mo, HR 0.39; 95% CI, 0.27–0.56; P<0.0001). Additionally, those with non-squamous histology and a high cytoscore demonstrated superior survival than low cytoscore in both patients receiving nivolumab (17.9 vs. 5.9 mo; HR: 0.52; 95% CI, 0.39–0.71; P<0.0001) and docetaxel (11.5 vs. 8.5 mo, HR: 0.60; 95% CI, 0.45–0.79; P=0.0001).
Another biomarker of interest in the context of immune checkpoint inhibitors is peripheral T cells (80). High frequency of FoxP3+ regulatory T cells (≥1.5%) has been associated with improved PFS and OS in patients receiving ipilimumab for melanoma (27,33,34). In an analysis of 95 patients with advanced melanoma treated with ipilimumab, increased levels of FoxP3+ regulatory T cells after treatment with ipilimumab was significantly associated with an inferior survival compared to stable or decreased FoxP3+ cells (5.3 vs. 15.8 mo, P=0.03) (33). A larger analysis of 209 patients receiving ipilimumab for advanced melanoma demonstrated that higher FoxP3+ Treg frequency (≥1.5%) at baseline was also associated with improved 1-year survival rate on univariate analysis (43.3% vs. 7.1%, P=8.7×10−5) but this was no longer significant in a multivariate analysis (27). Similarly, in another exploratory analysis of 82 patients with advanced melanoma receiving ipilimumab, increases in the percentage of CD4 T and CD8 T cells at 14 weeks after ipilimumab was associated with a superior 2-year OS (31.1% vs. 8.3%; P=0.020 and 41.4% vs. 5.3%; P=0.020) (34).
Limited emerging data in lung cancer also suggests that peripheral T cells may be a useful biomarker. In a small analysis presented in abstract form, investigators observed that following two cycles of neoadjuvant ipilimumab with carboplatin and paclitaxel, CD4+ and CD8+ T cells were highly activated in NSCLC patients, demonstrated by increased frequency of Tregs, defined by CD4+, CD25+, and FoxP3 expression (81). Another small analysis was performed using next-generation sequencing for TCR alpha/beta chain in 54 NSCLC patients receiving anti-PD1 therapy and suggested that early clonal T cell expansion within tumor and PBMC correlated with response to therapy (82). Further study of peripheral T cells in NSCLC with the use of immune checkpoint inhibitors is warranted.
Discussion
As we advance outcomes for patients with lung cancer, the success of therapies will continue to be predicated on the identification of reliable predictive biomarkers. While delivery to genotype driven agents to patients with actionable mutations has been emblematic of precision oncology, there remains many outstanding issues related to the optimal biomarkers for checkpoint inhibitors. Given the cost and selective toxicities of these agents along with the challenges of PD-L1 testing, enhanced enrichment strategies are needed.
Ongoing efforts have identified several tissue and plasma based-biomarkers predictive of checkpoint inhibitors in solid tumors including NSCLC. While many of the studies have been small and retrospective, they have provided further insights into how these agents can be best optimized. Amongst the candidate biomarkers reviewed here, TMB seems promising as its predictive utility has been demonstrated across multiple tumor types although further validation will be needed in larger studies. Plasma based markers also remain attractive and are aided by the convenience and the capability to evaluate longitudinal samples with relative ease. Given the complex interplay between the adaptive immune system and the TME, further enrichment efforts may employ combination strategies in which two or more methods are combined as a composite biomarker. This has been recently demonstrated in a phase I/II trial evaluating the anti-PD-L1 agent durvalumab in advanced NSCLC patients in which pretreatment biopsies were analyzed for PD-L1 by IHC and immune gene expression, including interferon gamma. Patients who were both PD-L1 positive (>25%) and expressed interferon gamma had a response rate of 46% which was greater than those patients who were only PD-L1 positive (RR: 27%) or only expressed interferon gamma (RR: 33%) (83). We look forward future biomarker enrichment efforts rooted in scientific rationale that will help better define those patients more likely to respond and exclude patients that otherwise receive no benefit with added potential toxicity from these agents.
Acknowledgements
We sincerely thank Drs. Tricia R. Cottrell and Janis M. Taube for the image used in Figure 1 of the review.
Footnotes
Conflicts of Interest: J Feliciano: Consulting from Takeda; research funding from Merck and Genentech. B Levy: Consulting from Astra-Zeneca, Celgene, Eli Lilly, Bristol Myers Squib, Genentech, and Takeda. The other authors have no conflicts of interest to declare.
References
- 1.Borghaei H, Brahmer J, Horn L, et al. P2.35: Nivolumab vs Docetaxel in Advanced NSCLC: CheckMate 017/057 2-Y Update and Exploratory Cytokine Profile Analysis: Track: Immunotherapy. J Thorac Oncol 2016;11:S237-S238. 10.1016/j.jtho.2016.08.10627676569 [DOI] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 10.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350:207-11. 10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34:abstr 9017.
- 15.Kowanetz M, Zou W, Shames D, et al. Tumor Mutation Burden (TMB) is Associated with Improved Efficacy of Atezolizumab in 1L and 2L+ NSCLC Patients. J Thorac Oncol 2017;12:S321-S322. 10.1016/j.jtho.2016.11.343 [DOI] [Google Scholar]
- 16.Peters S, Creelan B, Hellmann MD, et al. CT082 - Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage iv or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026, AACR Annual Meeting, Washington, DC, 2017. [Google Scholar]
- 17.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011;9:204. 10.1186/1479-5876-9-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell Density-A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:2635-43. 10.1158/1078-0432.CCR-14-1905 [DOI] [PubMed] [Google Scholar]
- 20.Galon J, Fox BA, Bifulco CB, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med 2016;14:273. 10.1186/s12967-016-1029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujikawa T, Kumar S, Borkar RN, et al. Quantitative Multiplex Immunohistochemistry Reveals Myeloid-Inflamed Tumor-Immune Complexity Associated with Poor Prognosis. Cell Rep 2017;19:203-17. 10.1016/j.celrep.2017.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forde PM, Smith KN, Chaft JE, et al. Neoadjuvant anti-PD1, nivolumab, in early stage resectable non-small-cell lung cancer. Ann Oncol 2016;27:1-36. 10.1093/annonc/mdw435.38 [DOI] [Google Scholar]
- 23.Roh W, Chen PL, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page DB, Yuan J, Redmond D, et al. Deep Sequencing of T-cell Receptor DNA as a Biomarker of Clonally Expanded TILs in Breast Cancer after Immunotherapy. Cancer Immunol Res 2016;4:835-44. 10.1158/2326-6066.CIR-16-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019-31. 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med 2014;6:238ra70. 10.1126/scitranslmed.3008211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens A, Wistuba-Hamprecht K, Foppen MG, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016;22:2908-18. 10.1158/1078-0432.CCR-15-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487-96. 10.1158/1078-0432.CCR-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olmedo E, Del Toro JM, Gorospe L, et al. Could Blood Levels of Lymphocytes and Eosinophils Help Us to Identify the Efficacy or Toxicity of Immunotherapy? J Thorac Oncol 2017;12:S1322-3. 10.1016/j.jtho.2016.11.1871 [DOI] [Google Scholar]
- 30.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732-8. 10.1093/annonc/mdw016 [DOI] [PubMed] [Google Scholar]
- 31.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 32.Lou YD, Cuentas ER, Denning W, et al. ORAL13.07 - EMT is Associated with an Inflammatory Tumor Microenvironment with Elevation off Immune Checkpoints and Suppressive Cytokines in Lung Cancer. International Association for the Study of Lung Cancer 2016. [Google Scholar]
- 33.Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014;63:675-83. 10.1007/s00262-014-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens A, Wistuba-Hamprecht K, Yuan J, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res 2016;22:4848-58. 10.1158/1078-0432.CCR-16-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson DB, Frampton GM, Rioth MJ, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res 2016;4:959-67. 10.1158/2326-6066.CIR-16-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Finn OJ. Cancer immunology. N Engl J Med 2008;358:2704-15. 10.1056/NEJMra072739 [DOI] [PubMed] [Google Scholar]
- 40.Tsou P, Katayama H, Ostrin EJ, et al. The Emerging Role of B Cells in Tumor Immunity. Cancer Res 2016;76:5597-601. 10.1158/0008-5472.CAN-16-0431 [DOI] [PubMed] [Google Scholar]
- 41.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605-12. 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 42.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 2013;31:4252-9. 10.1200/JCO.2013.51.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglesia MD, Parker JS, Hoadley KA, et al. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. J Natl Cancer Inst 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- 46.Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223-30. 10.1200/JCO.2015.63.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012;147:366-72. 10.1001/archsurg.2012.35 [DOI] [PubMed] [Google Scholar]
- 48.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermitte F. Biomarkers immune monitoring technology primer: Immunoscore(R) Colon. J Immunother Cancer 2016;4:57. 10.1186/s40425-016-0161-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galon J, Mlecnik B, Marliot F, et al. Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J Clin Oncol 2016;34:abstr 3500.
- 51.Paulsen EE, Kilvaer T, Khanehkenari MR, et al. CD45RO(+) Memory T Lymphocytes--a Candidate Marker for TNM-Immunoscore in Squamous Non-Small Cell Lung Cancer. Neoplasia 2015;17:839-48. 10.1016/j.neo.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol 2016;27:225-32. 10.1093/annonc/mdv560 [DOI] [PubMed] [Google Scholar]
- 53.van der Loos CM. Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem 2008;56:313-28. 10.1369/jhc.2007.950170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stack EC, Wang C, Roman KA, et al. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014;70:46-58. 10.1016/j.ymeth.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 55.Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 2014;11:417-22. 10.1038/nmeth.2869 [DOI] [PubMed] [Google Scholar]
- 56.Ruggiero E, Nicolay JP, Fronza R, et al. High-resolution analysis of the human T-cell receptor repertoire. Nat Commun 2015;6:8081. 10.1038/ncomms9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A 2016;113:11919-24. 10.1073/pnas.1611421113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liotta L, Petricoin E. Molecular profiling of human cancer. Nature Reviews Genetics 2000;1:48-56. 10.1038/35049567 [DOI] [PubMed] [Google Scholar]
- 59.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med 2002;8:816-24. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y, Lemon W, Liu P-Y, et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLoS Med 2006;3:e467. 10.1371/journal.pmed.0030467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raponi M, Zhang Y, Yu J, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res 2006;66:7466-72. 10.1158/0008-5472.CAN-06-1191 [DOI] [PubMed] [Google Scholar]
- 62.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression–based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 2008;14:822-7. 10.1038/nm.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu CQ, Ding K, Strumpf D, et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non–small-cell lung cancer. J Clin Oncol 2010;28:4417-24. 10.1200/JCO.2009.26.4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shahid M, Choi TG, Nguyen MN, et al. An 8-gene signature for prediction of prognosis and chemoresponse in non-small cell lung cancer. Oncotarget 2016;7:86561-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen DT, Hsu YL, Fulp WJ, et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J Natl Cancer Inst 2011;103:1859-70. 10.1093/jnci/djr420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seiwert TY, Burtness B, Weiss J, et al. Inflamed-phenotype gene expression signatures to predict benefit from the anti-PD-1 antibody pembrolizumab in PD-L1+ head and neck cancer patients. American Society of Clinical Oncology 2015. [Google Scholar]
- 67.Shankaran V, Muro K, Bang YJ, et al. Correlation of gene expression signatures and clinical outcomes in patients with advanced gastric cancer treated with pembrolizumab (MK-3475). American Society of Clinical Oncology 2015. [Google Scholar]
- 68.Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J Clin Oncol 2015;33:abstr 3001.
- 69.Chifman J, Pullikuth A, Chou JW, et al. Conservation of immune gene signatures in solid tumors and prognostic implications. BMC Cancer 2016;16:911. 10.1186/s12885-016-2948-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tahara H, Sato M, Thurin M, et al. Emerging concepts in biomarker discovery; the US-Japan Workshop on Immunological Molecular Markers in Oncology. J Transl Med 2009;7:45. 10.1186/1479-5876-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bedognetti D, Wang E, Sertoli MR, et al. Gene-expression profiling in vaccine therapy and immunotherapy for cancer. Expert Rev Vaccines 2010;9:555-65. 10.1586/erv.10.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013;31:2388-95. 10.1200/JCO.2012.44.3762 [DOI] [PubMed] [Google Scholar]
- 74.Shahabi V, Berman D, Chasalow SD, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013;11:75. 10.1186/1479-5876-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zer A, Sung M, Walia K, et al. Correlation of Neutrophil to Lymphocyte Ratio (NLR) with Clinical Benefit from Checkpoint Inhibitors in Advanced Lung Cancer. J Thorac Oncol 2017;12:S1323-4. 10.1016/j.jtho.2016.11.1872 [DOI] [Google Scholar]
- 76.Galetta D, Logroscino AF, Misino A, et al. Neutrophil/Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer: Correlation with Prognosis and Response to Anti-PD1 Therapy. J Thorac Oncol 2017;12:S1329-30. 10.1016/j.jtho.2016.11.1881 [DOI] [Google Scholar]
- 77.Preeshagul I, Sullivan K, Paul D, et al. The Utilization of Pre-Treatment Neutrophil to Lymphocyte Ratio as a Predictive Marker for Efficacy of Immunotherapy in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:S1325 10.1016/j.jtho.2016.11.1874 [DOI] [Google Scholar]
- 78.Mezquita L, Charrier M, Auclin E, et al. Neutrophil-To-Lymphocyte and Other Ratios as Prognostic and Predictive Markers of Immune Checkpoint Inhibitors in Advanced NSCLC Patients. J Thorac Oncol 2017;12:S1315-6. 10.1016/j.jtho.2016.11.1860 [DOI] [Google Scholar]
- 79.Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-Lymphocyte Ratio Is a Prognostic Marker in Patients with Locally Advanced (Stage IIIA and IIIB) Non-Small Cell Lung Cancer Treated with Combined Modality Therapy. Oncologist 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyerly HK, editor. Biomarkers and Correlative Endpoints for Immunotherapy Trials: What Can We Learn in Lung Cancer from Other Tumor Types? American Society of Clinical Oncology 2013. [DOI] [PubMed] [Google Scholar]
- 81.Osborn R. MINI26.05 - Immunophenotyping of Circulating T cells and TILs with Chemotherapy and Phased Ipilimumab in Non-small cell lung cancer. International Association for the Study of Lung Cancer 2016. [Google Scholar]
- 82.Olugbile S, Kiyotani K, Inoue H, et al. In-Depth Molecular Characterization of T Cell Clonal Expansion Induced by Anti-PD1 Therapy in NSCLC. J Thorac Oncol 2017;12:S1310-1. 10.1016/j.jtho.2016.11.1853 [DOI] [Google Scholar]
- 83.Higgs BW, Morehouse C, Streicher K, et al. Relationship of baseline tumoral IFNγ mRNA and PD-L1 protein expression to overall survival in durvalumab-treated NSCLC patients. J Clin Oncol 2016;34:abstr 3036.