Abstract
We herein report a rare case of a 79-year-old man who presented with the simultaneous occurrence of pancreatic neuroendocrine tumors (PNET) and renal cell carcinomas (RCC), without any other Von Hippel-Lindau (VHL)-associated lesions or any pertinent family history. Computed tomography showed vascular-rich solid lesions in the left kidney and the pancreatic tail, measuring 72 mm and 15 mm in size, respectively. Preoperatively, RCC with pancreatic metastasis was suspected and laparotomy was performed. However, the resected specimens revealed a different tumor histology, namely renal clear cell carcinoma (G2, pT3) and PNET (G1, pT3). The patient and his family refused genetic testing, however, so far, the patient has not developed any VHL-associated lesions for more than four years.
Keywords: pancreatic neuroendocrine tumor, renal cell carcinoma, Von Hippel-Lindau disease, diagnosis
Introduction
Both pancreatic neuroendocrine tumors (PNET) and renal cell carcinomas (RCC) are well-demarcated high-vascular solid tumors, and these tumors are sometimes recognized in patients with Von Hippel-Lindau (VHL) disease. When high vascular masses are recognized in both the kidney and pancreas, an accurate diagnosis is needed for each tumor, as the therapeutic strategy or genetic counseling may change. However, as far as we could determine, a sporadic case with PNET and RCC has not yet been reported in the previous literature.
Case Report
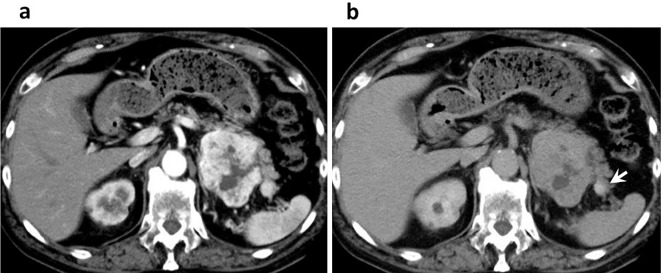
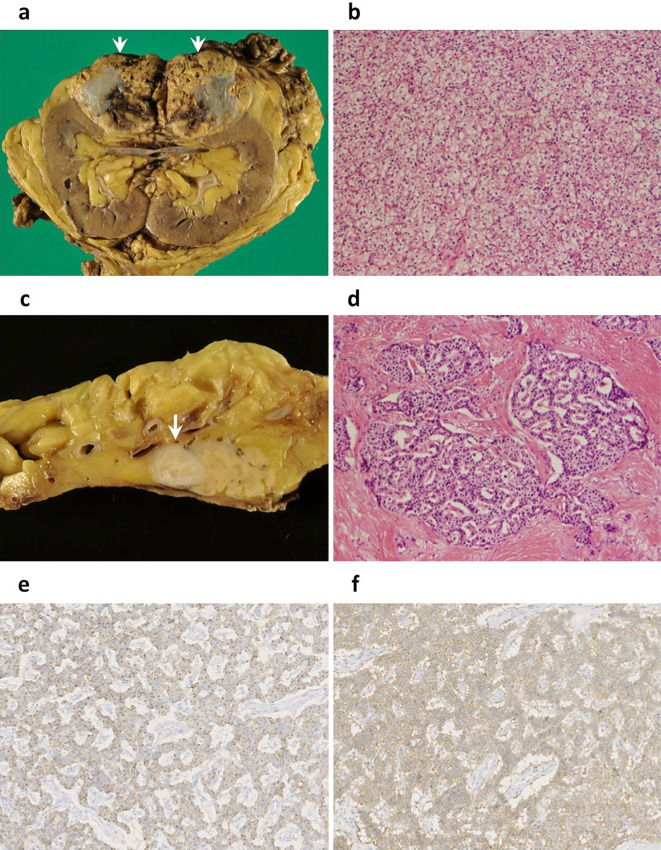
A 79-year-old man was referred to our hospital for the treatment of a renal tumor, which was detected due to symptoms of transitory back pain. He had a history of diabetes and cerebral infarction, but no lesions suggestive of hemangioblastoma were recognized on brain imaging. In his family history, a cancer history was positive, including gastric cancer (father), colon cancer (sister), and biliary cancer (second sister); however, there was no history of any lesions related to VHL or multiple endocrine neoplasia type 1 (MEN1). He had no continuous symptoms or findings suggesting a functional neuroendocrine tumor (NET), such as hypoglycemia, hyperglycemia, heartburn, nausea, and epigastralgia. A blood test revealed normal levels of calcium and intact parathyroid hormone. Computed tomography (CT) showed a marginally vascular-rich lesion, measured 72 mm in size, in the upper pole of the left kidney. In addition, a similarly well-enhanced 15 mm tumor was detected in the pancreas tail (Fig. 1a). Endoscopic ultrasonography-guided fine needle aspiration was not performed in order to avoid tumor seeding. RCC of the left kidney and metastasis to the pancreas were suspected preoperatively, and laparotomy was performed. The histology of the renal tumor indicated it to be clear-cell type RCC (G2, pT3) (Fig. 2a, b); however, the pancreatic tumor was a NET (G1, Ki-67 index: <2%, pT3) surrounded by fibrous tissue (Fig. 2c, d), and both were negative for lymph node metastasis. The PNET was positive for chromogranin A, synaptophysin, and somatostatin receptor type 2 (SSTR2) by immunostaining (Fig. 2e, f). With this histology in mind, preoperative CT images were retrospectively reviewed, and a prolonged enhancement was recognized only in the pancreatic tumor, but not in the renal tumor.
Figure 1.
Enhanced computed tomography of the arterial phase (40 s) (a) and the late phase (4 min) (b) showing a rich enhancement at the arterial phase in both tumors of the left kidney and pancreas tail, while only retaining enhancement in the pancreas tumor in the late phase (white arrow).
Figure 2.
Pathological findings. (a) heterogenous cut surface of the renal tumor (arrow heads); (b) histology showing clear cell-type renal cell carcinoma [Hematoxylin and Eosin (H&E) staining, ×100]; (c) a yellow-whitish mass (white arrow) of the pancreas tail; (d) histology showing a neuroendocrine tumor, G1, surrounded by dense fibrosis (H&E staining, ×100); (e, f) immunostainings of chromogranin A (×200) (e) and somotostatin receptor type 2 (SSTR2) (×200) (f) diffusely positive in the pancreatic tumor.
As a coincidental occurrence of PNET and RCC is very rare, further examinations were performed to rule out VHL disease. The patient underwent magnetic resonance imaging (MRI) of the central nervous system (CNS) and ophthalmologic examinations, but no hemangioblastoma was recognized. Genetic counseling was thus carried out for the patient and his family. A genetic test of VHL gene was repeatedly recommended, but the patient consistently refused due to social reasons (possible future disadvantages for their sibs concerning marriage, employment, insurance, etc.). To date, screening has been done for this patient for more than four years, but no VHL-associated lesion has been detected either in the patient or in his two sons who are in their 50s.
Discussion
The current case raised two problems associated with the differential diagnosis: 1) double primary tumors or pancreatic metastasis of the RCC, and 2) the possibility of VHL disease in the case of double primary tumors. In Japan, the overall incidence (per 100,000 population) of PNET is 2.69 (1), while that of RCC is 5.87 (2). This rarity, relatively frequent occurrence of RCC metastatis to the pancreas (3), and the similar image findings led to our preoperative misdiagnosis, although the treatment strategy in this case was not altered because surgical resection is recommended both in double primary tumors and in RCC accompanied with isolated metastasis to the pancreas (4). When reviewing the preoperative CT images, the enhancement of the pancreatic tumor was markedly prolonged compared with the renal tumor, reflecting the dense fibrosis around the PNET (Fig. 1b) and suggesting double primary tumors. Although the possibility of seeding can not be completely ignored, for a selected situation, endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) can be recommended for the diagnosis of metastatic pancreas tumors (3).
As for the second point, the simultaneous occurrence of these rare tumors was either a coincidence or associated with some inherited disease. The patient underwent screening for CNS and no hemangioblastoma was found, and he did not meet the diagnostic criteria of VHL disease (5). Up to 20% of VHL disease is de novo, and therefore it is not possible to rule out the possibility of non-affected relatives potentially developing this disease.
However, in cases without any associated family history, hemangioblastoma is essential in the diagnosis of VHL. Besides, these tumors in VHL disease usually develop in younger subjects; from 60-80% of hemangioblastomas develop from 25-30 years of age, RCC in from 25-75% at 39 years, and PNET in from 35-75% at 36 years (5). Hence, it is quite unlikely that the patient had VHL disease. Furthermore, no VHL-related lesions were detected in their two son who were in their 50s, thus suggesting a small possibility of VHL disease.
Generally, genetic counseling and genetic testing should be recommended in cases with >1 of following four tumors; PNET, RCC, pancreatic (serous)cystadenoma, and epididymal/adnexal cystadenoma (5). As the causative mutation can be detected by genetic tests in the most VHL cases and the surveillance outcome tends to be favorable, and therefore gene examinations are thought to be quite beneficial for such suspected cases (http://www.ncbi.nlm.nih.gov/books/NBK1463/). As a rare variant of VHL could not be completely ruled out, this patient and his family should be closely followed up by medical institutions.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ito T, Lee L, Hijioka M, et al. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci 22: 574-577, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Marumo K, Kanayama H, Miyao N, et al. Prevalence of renal cell carcinoma: a nation-wide survey in Japan, 2002. Int J Urol 14: 479-482, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Hijioka S, Matsuo K, Mizuno N, et al. Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: a tertiary center experience. Pancreatology 11: 390-398, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Kitasato A, Tajima Y, Kuroki T, et al. Limited pancreatectomy for metastatic pancreatic tumors from renal cell carcinoma. Hepatogastroenterology 57: 354-357, 2010. [PubMed] [Google Scholar]
- 5. Nielsen SM, Rhodes L, Blanco I, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol 34: 2172-2181, 2016. [DOI] [PubMed] [Google Scholar]