Abstract
Although nivolumab is known to cause immune-related interstitial lung diseases (ILD), the detailed characteristics of ILD are still not fully understood. A 68-year-old man was treated with nivolumab because of unresectable sinonasal melanoma, he achieved a complete response soon after the initiation of the therapy and a complete response was thereafter maintained for 30 weeks until the patient experienced dyspnea of subacute onset. CT images revealed patchy infiltrates and ground-glass opacifications. The bronchoalveolar lavage fluid (BALF) contained elevated percentages of lymphocytes (53%) and neutrophils (30%). A transbronchial lung biopsy revealed intraalveolar fibrin balls without hyaline membranes, which was considered to be consistent with the pattern of acute fibrinous and organizing pneumonia (AFOP). This is the first report of AFOP induced by nivolumab.
Keywords: melanoma, nivolumab, pneumonitis, acute fibrinous and organizing pneumonia, bronchoalveolar lavage
Introduction
Nivolumab is a fully human immunoglobulin G4 monoclonal antibody which binds and blocks programmed death-1 (PD-1) receptors on cancer cell membranes, resulting in the release of cancer immune-tolerance. Its development has drastically changed the treatment of various cancers including advanced melanoma and non-small cell lung cancer (1-3). Despite its clinical benefits, nivolumab also induces a variety of immune-related adverse events. For example, clinical trials revealed that nivolumab caused interstitial lung disease (ILD) with the incidence ranging from 1.3 to 5.0% (4). However, the detailed clinical and pathological manifestations of nivolumab-induced ILD remain unclear.
On the other hand, acute fibrinous and organizing pneumonia (AFOP) is a rare type of ILD characterized by intra-alveolar fibrin balls and organizing pneumonia with a patchy distribution (5). The present report describes a patient with advanced melanoma who developed nivolumab-induced ILD, where a lung biopsy established a pathological diagnosis of AFOP.
Case Report
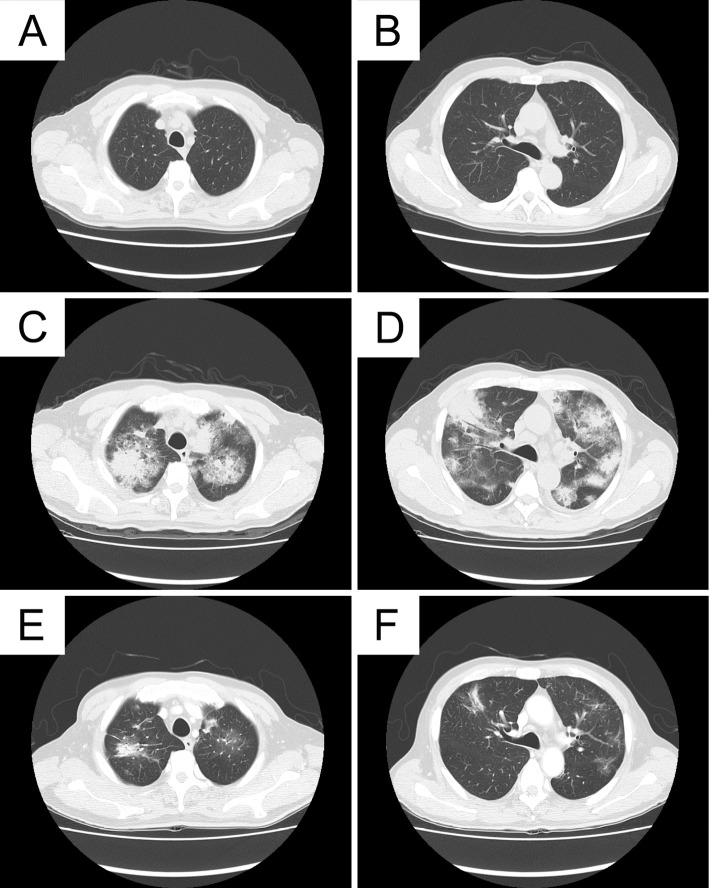
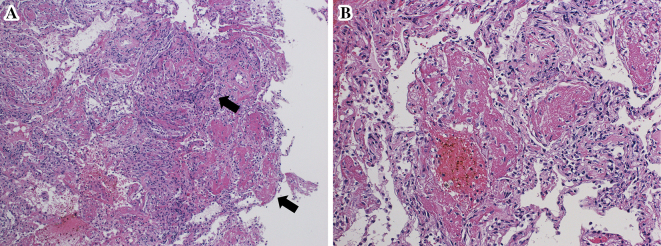
A 68-year-old man with advanced melanoma in the ethmoidal sinus was referred to our institution for treatment. He had been treated with heavy-ion particle irradiotherapy (57.6 GyE/16 fr) 9 months ago because the disease was locoreginal and unresectable. Although a combination chemotherapy regimen consisting of dacarbazine, nimustine and vincristine had been started immediately after irradiation, multiple bone and pulmonary metastases developed shortly after one cycle of chemotherapy. Then, after being referred to our institution, nivolumab at a dose of 2 mg/kg, every 3 weeks, was administered, which thus resulted in a complete response. The treatment was continued for 30 weeks until admission to our hospital because of a worsening dyspnea and non-productive cough. The onset of dyspnea developed 3 days before admission. On examination, his body temperature was 36.8℃ and oxygen saturation on room air was 89%. His physical examination was unremarkable. Despite normal findings at 15 weeks before the onset of dyspnea (Fig. 1A and B), computed tomography (CT) images on admission revealed patchy infiltrates and ground-glass opacifications with interlobular septal thickening (Fig. 1C and D). Laboratory tests demonstrated white blood cell counts of 12,200 /μL with 76.4% neutrophils and 8.8% lymphocytes, C-reactive protein level of 20.9 mg/dL (normal <0.3 mg/dL), serum lactate dehydrogenase (LDH) level of 215 IU/L (normal, 119-229 IU/L), and KL-6 level of 183 U/mL (normal <500 U/mL). Electrolytes, creatinine, liver function tests were normal. Rheumatoid factor, anti-nuclear antibody and proteinase 3 (PR-3)- and myeloperoxidase (MPO) anti-neutrophil cytoplasmic antibodies were negative. Anti-cytomegalovirus antibody and beta-glucan were below the detection limits. Bronchoalveolar lavage fluid (BALF) contained 600 nucleated cells/mm3, including lymphocytes (53.6%: CD4/CD8 was not evaluated because the bronchoalveolar lavage (BAL) was performed in an emergency setting), macrophages (5.7%), neutrophils (30.1%), and eosinophils (10.6%). A culture of the BALF was negative. Transbronchial lung biopsy revealed intraalveolar fibrin balls without hyaline membranes which was consistent with the pattern of AFOP (Fig. 2A). In contrast to the presence of lymphocytosis, neutrophilia and eosinophilia in the BALF, no marked infiltration of the inflammatory cells was observed in the alveolar septum, alveolar spaces and adjacent to the vascular structures. The absence of marked neutrophilia in lung tissue ruled out a diagnosis of acute respiratory distress syndrome (Fig. 2B). High dose corticosteroid administration (intravenous methylprednisolone at a dose of 1,000 mg for 3 days, followed by oral prednisolone at a dose of 1 mg/kg) was started, and the treatment was effective for ameliorating dyspnea as well as for improving the imaging findings (Fig. 1E and F). During the treatment with corticosteroid and without nivolumab, colitis with persistent diarrhea developed. Although the colitis was resolved with continuing corticosteroid and 2 shots of infliximab at a dose of 5 mg/kg, he died because of tumor relapse at the skull base in 4 months after the discontinuation of nivolumab. An autopsy was not permitted.
Figure 1.
CT images. Chest CT images after treatment with nivolumab and at 15 weeks before the onset of dyspnea show normal findings (A, B). At the onset of ILD, multiple bilateral patchy infiltrates and ground glass attenuation with interlobular septal thickening developed (C, D). After treatment with corticosteroids, these findings all improved (E, F). ILD: interstitial lung disease
Figure 2.
Pathological findings. Pathological evaluation of the lung revealed intraalveolar filling with fibrin balls (arrows) without hyaline membrane formation, thereby establishing the pathological diagnosis of acute fibrinous and organizing pneumonia [Hematoxylin and Eosin staining, original magnification of ×10 in (A) and ×20 in (B)].
Discussion
Although it is challenging to diagnose drug-induced ILD because various other conditions should first be excluded, we diagnosed the present case as being nivolumab-induced. A negative culture of BALF, negative serological findings including autoantibodies, cytomegalovirus antibody and beta-glucan, and no usage of new drugs except for nivolumab might also rule out other etiologies for ILD.
The radiologic features of the present patient were characterized by patchy infiltrates and ground-glass opacifications with interlobular septal thickening. Retrospective radiology review of 27 patients with anti-PD-1/PD-L1 induced pneumonitis showed that ground-glass opacifications were the most common subtype (37%), and that interstitial pattern including interlobular septal thickening was observed in 22% of patients (6). These findings seem concordant to the radiologic characteristics of the present patient. An accumulation of over-activated T lymphocytes into the lymphatic tracts may cause an abnormal widening of the interlobular septa in this case, because interlobular septal thickening is also observed in patients with pulmonary edema, lymphangitic carcinomatosis, and pulmonary lymphoma which are related to an abnormality in lymphatic tract surrounding the pulmonary lobules. As a result, further investigation is needed to elucidate the underlying mechanism.
Among the 9 previously reported cases identified as nivolumab- or another anti-PD-1 antibody pembrolizumab-induced ILD, only 3 cases had a pathological diagnosis of ILD consisting of diffuse alveolar damage in one case and organizing pneumonia in 2 cases (Table) (7-13). Therefore, this report seems very unique in linking AFOP to a new type of nivolumab-induced ILD.
Table.
Summary of Reported Patients with Anti-PD-1 Antibody-induced ILD Including the Present Case.
| Reference | Age/sex | Cancer type | Agent | Pattern of ILD | Method of diagnosis | BALF findings | Treatment for ILD | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell counts (×105/mL) | AM (%) | Lym (%) | Neu (%) | Eos (%) | ||||||||
| (7) | 70/M | Melanoma | Nivo | ARDS | Clinical | ND | ND | ND | ND | ND | IV steroids, IV IFX | Improved |
| (7) | 38/F | Melanoma | Nivo | ARDS | Clinical | ND | ND | ND | ND | ND | IV steroids, IV IFX | Dead |
| (7) | 58/M | Melanoma | Nivo | NSIP | Clinical | ND | ND | ND | ND | ND | Oral steroids | Improved |
| (8) | 70/F | Melanoma | Nivo | OP | Biopsy | 7.7 | 38.5 | 43.5 | 13.0 | 0 | Oral steroids | Improved |
| (9) | 70/F | Melanoma | Nivo | OP | Biopsy | 2.76 | 58.9 | 37.3 | 2.3 | 1.5 | IV steroids | Improved |
| (10) | 35/F | Melanoma | Nivo | DAD | Autopsy | ND | ND | ND | ND | ND | Not described | Dead |
| (11) | 73/F | Melanoma | Nivo | DAD | Clinical | 3.1 | 77.7 | 8.7 | 14.3 | ND | mPSL pulse, IVCY | Improved |
| (12) | 64/F | Melanoma | Pembro | OP | Clinical | ND | 60.7 | 28.7 | ND | ND | IV steroids | Improved |
| (13) | 70/M | Sarcomatoid carcinoma of the lung |
Nivo | OP | Clinical | 11.7 | 65.0 | 32.5 | 2.0 | 0.5 | Oral steroids | Improved |
| Present case | 68/M | Melanoma | Nivo | AFOP | Biopsy | 6.0 | 5.7 | 53.6 | 30.1 | 10.6 | mPSL pulse | Improved |
ILD: interstitial lung disease, PD-1: programmed death-1, BALF: bronchoalveolar lavage fluid, AM: alveolar macrophages, Lym: lymphocytes, Neu: neutrophiles, Eos: eosinophiles, ND: not described, Nivo: nivolumab, Pembro: pembrolizumab, ARDS: acute respiratory distress syndrome, NSIP: nonspecific interstitial pneumonia, OP: organizing pneumonia, DAD: diffuse alveolar damage, AFOP: acute fibrinous and organizing pneumonia, IV: intravenous, IFX: infliximab, mPSL: methylprednisolone, IVCY: intravenous cyclophosphamide pulse
In 9 patients with nivolumab- or pembrolizumab-induced ILD, regardless of the pathological diagnosis, the outcome of ILD was also reported, and only 2 patients with autopsy confirmed diffuse alveolar damage (DAD) and clinically diagnosed acute respiratory distress syndrome (ARDS), respectively, died from ILD. ILD improved in 7 other patients, including 2 patients with pathologically proven organizing pneumonia. The present patient with AFOP also showed a significant improvement in his symptoms and also in the image findings after undergoing treatment with corticosteroids. Although the precise mechanisms underlying immune checkpoint inhibitor-associated pneumonitis have not yet been elucidated, a previous study reported that the infiltration and activation of T lymphocytes were commonly observed in normal tissues in patients with anti-PD-1 antibody-associated adverse events (14). Therefore, the activation of T lymphocytes may also play a role in developing pneumonitis, and this is concordant to the present patient where lymphocytosis was observed in the BALF.
The present patient also seems to be important in light of the pathogenesis of the rare disease of AFOP. The fact that this disease was evoked as an adverse event of an immune-check point inhibitor, and that marked lymphocytosis and neutrophilia in situ were confirmed by BAL, may suggest an immunological roles in the development of AFOP. Neutrophilia in BALF has also been reportedly observed in AFOP of idiopathic (15-17), drug-induced (18), lung transplantation-induced (19), and hematopoietic stem cell transplantation-induced (20).
In conclusion, the present report provides important evidence that nivolumab-induced ILD contains AFOP. The findings presented therein may provide some valuable insight into the pathogenesis of AFOP. Further elucidating nivolumab-induced ILD is therefore warranted.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was financially supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology in Japan (Kiban-C #26461182).
References
- 1. Robert C, Long GV, Brady B, et al. . Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372: 320-330, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis 10: 183-193, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med 126: 1064-1070, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Naidoo J, Wang X, Woo KM, et al. . Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016 Sep 19. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 373: 288-290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakashima K, Naito T, Omori S, et al. . Organizing pneumonia induced by nivolumab in a patient with metastatic melanoma. J Thorac Oncol 11: 432-433, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Sano T, Uhara H, Mikoshiba Y, et al. . Nivolumab-induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol 46: 270-272, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Koelzer VH, Rothschild SI, Zihler D, et al. . Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer 4: 13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe S, Kimura H, Takato H, et al. . Severe pneumonitis after nivolumab treatment in a patient with melanoma. Allergol Int 65: 487-489, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S, Koumarianou A. A case of organizing pneumonia (OP) associated with pembrolizumab. Drug Target Insights 10: 9-12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gounant V, Brosseau S, Naltet C, et al. . Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer 99: 162-165, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol 33: 2092-2099, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damas C, Morais A, Moura CS, Marques A. Acute fibrinous and organizing pneumonia. Rev Port Pneumol 12: 615-620, 2006. [PubMed] [Google Scholar]
- 16. Rafii R, Murin S, Morrissey BM. A case report of idiopathic acute fibrinous pneumonia and a review of the literature. Chest 138: 47A, 2010.20154074 [Google Scholar]
- 17. Hara Y, Shinkai M, Kanoh S, et al. . Clinico-pathological analysis referring hemeoxygenase-1 in acute fibrinous and organizing pneumonia patients. Respir Med Case Rep 14: 53-56, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasu TS, Cavallazzi R, Hirani A, Marik PE. A 64-year-old male with fever and persistent lung infiltrate. Respir Care 54: 1263-1265, 2009. [PubMed] [Google Scholar]
- 19. Alici IO, Yekeler E, Yazicioglu A, et al. . A case of acute fibrinous and organizing pneumonia during early postoperative period after lung transplantation. Transplant Proc 47: 836-840, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen LP, Ahdoot S, Sriratanaviriyakul N, et al. . Acute fibrinous and organizing pneumonia associated with allogenic hematopoietic stem cell transplant successfully treated with corticosteroids: a two-patient case series. J Investig Med High Impact Case Rep 4: 2324709616643990, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]